Abstract
Background
The optimal treatment for ischemic mitral regurgitation (IMR) remains actively debated. Our objective was to evaluate the relationship between IMR treatment strategy and survival.
Methods and Results
We retrospectively reviewed patients at our institution diagnosed with significant coronary artery disease and moderate or severe IMR from 1990–2009, categorized by medical treatment alone (MED), percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), or CABG + mitral valve repair or replacement (MVRR). Kaplan-Meier methods and multivariable Cox proportional hazard analyses were performed to assess the relationship between treatment strategy and survival, using propensity scores to account for nonrandom treatment assignment. A total of 4,989 patients were included: MED = 36%, PCI = 26%, CABG = 33%, and CABG+MVRR = 5%. Median follow-up was 5.37 years. Compared to MED, significantly lower mortality was observed in patients treated with PCI [adjusted hazard ratio (AHR): 0.83, 95% confidence interval (CI): 0.76 – 0.92, p=0.0002], CABG (AHR: 0.56, CI: 0.51 – 0.62, p<0.0001), and CABG+MVRR (AHR: 0.69, CI: 0.57 – 0.82, p<0.0001). There was no significant difference in these results based on MR severity.
Conclusions
Patients with significant coronary artery disease and moderate or severe IMR undergoing CABG alone demonstrated the lowest risk of death. CABG with or without mitral valve surgery was associated with lower mortality than either PCI or MED.
Keywords: Ischemia, Mitral valve, Revascularization, Stents, Valvuloplasty
INTRODUCTION
Over 600,000 patients underwent hospital admission for acute myocardial infarction (MI) in the United States in 2010 and over 7.9 million Americans have a history of MI.1, 2 Ischemic mitral regurgitation (IMR) has been reported to occur in more than 50% of patients after an acute MI, representing a distinct clinical entity from degenerative structural causes of mitral valve (MV) insufficiency.3–8 The presence of IMR is associated with poor outcomes,9 and while outcomes are worse with increasing IMR severity, even mild IMR portends a significantly increased risk of heart failure and death.10–16
Important contributions in the management of mitral regurgitation date back several decades,17 yet the optimal treatment strategy for IMR remains the subject of active debate, with increasing controversy regarding appropriate therapy for this patient population.18, 19 Several studies including a 2009 meta-analysis have reported no survival benefit to adding MV repair to coronary artery bypass grafting (CABG) for patients with IMR;20–25 however, conflicting reports exist including results from a multicenter randomized trial reported by Deja et al suggesting that MV repair may improve survival compared with CABG alone.26–32 The utility of the current body of evidence in guiding clinical management of IMR is further limited by the preponderance of small patient samples,21, 22, 25, 27, 30 outdated studies inadequately capturing current IMR assessment techniques and perioperative surgical risk,20, 23, 26 and lack of comparison groups sufficient to capture the full range of treatment modalities, including medical management, percutaneous coronary intervention (PCI) and CABG with and without MV repair or replacement.26–30 Given the lack of sufficient evidence to build consensus in treating IMR, multiple investigators have called for randomized trials to better support clinical decision making.4, 5, 11, 13, 14, 18, 25, 26
Medical management has recently been advocated as the standard of care for functional MR.33, 34 This is in contrast to an evaluation of the Duke Cardiovascular Disease Database for patients treated from 1986 to 2001 which demonstrated that revascularization (PCI or CABG with or without mitral valve surgery) provides a significant longevity benefit compared to medical therapy as an initial strategy.20 Our objective in this study was to extend these observations to include advances in PCI technology and mitral valve surgical techniques, and to extend the duration of follow-up for this important manifestation of ischemic heart disease.
METHODS
This study was approved by the Institutional Review Board of Duke University Medical Center.
Data Source
The Duke Databank of Cardiovascular Disease (DDCD) was used for this study. This is a prospective clinical database of over 200,000 patients who have undergone cardiac catheterization at Duke University Medical Center since 1969.35 The DDCD includes baseline variables from the patients’ history, physical examination, laboratory studies, imaging and diagnostic studies as well as the results of procedures including PCI and cardiac surgery.
Patient follow-up was conducted by the Duke Clinical Research Institute Follow-up Services Group, which is responsible for collecting annual follow-up data on death and other clinical events for patients in the DDCD. Annual surveys collect data on survival, hospitalizations, myocardial infarction, stroke, cardiac procedures, and medication use. Patients are surveyed 6 months after their index catheterization and yearly thereafter by means of a mailed, self-administered questionnaire. Non-responders are surveyed by telephone. For mortality data, the response rate is 95% and there is an annual search of the National Death Index for patients who are lost to follow-up (2%) or who have requested not to be contacted (3%). Information on death is collected through next-of-kin interviews, reviews of hospital discharge summaries and death certificates, and the National Death Index, which provides the cause of death according to the International Classification of Diseases, 10th Revision.
Study Design
We performed a retrospective cohort analysis of all patients undergoing cardiac catheterization at our institution between January 1, 1990 and June 7, 2009 who had significant coronary artery disease (CAD) (≥ 75% lesion in one or more coronary vessels) and moderate or severe (≥2+) mitral regurgitation (MR) based on the maximum MR grade by echocardiography or left ventriculography. For patients with both echocardiographic data and ventriculography, the echocardiogram was given higher priority in determining MR grade. For a small number of patients (<3%), MR grade was determined by cardiac catheterization reports for outside our institution. Patients were excluded if there was no evidence of significant coronary artery disease, prior history of CABG, history of aortic or pulmonic valvular disease, concomitant valve disease (excluding tricuspid regurgitation), MV prolapse, congenital heart disease, myxomatous or rheumatic MV disease, or endocarditis.
Patients were analyzed according to whether they initially underwent medical management, PCI, CABG alone, or CABG + mitral valve repair or replacement (MVRR) within 30 days of catheterization. To minimize the influence of waiting-time bias, patients who died in the medical management group within 5 days of catheterization were excluded. Operative strategies for PCI and CABG were at the discretion of the treating physician. Similarly, the decision to perform mitral valve repair or replacement, and the choice of mitral prosthesis, was at the discretion of the attending surgeon. Once a patient was assigned to a treatment category, he or she was maintained in that group until death or the end of the follow-up period, regardless of therapeutic crossovers. The primary predictor variable was IMR treatment strategy and the outcome variable was overall survival.
Statistical Analysis
Baseline characteristics were described for the overall population and by treatment strategy using medians, 25th and 75th percentiles for continuous variables, and proportions for discrete variables. Comparisons for continuous and ordered categorical variables were made using Kruskal-Wallis tests and unordered categorical variables were compared using the Pearson Chi-square test. Survival curves were constructed for each group using the Kaplan-Meier method, and comparisons were made using the log-rank test. Cox proportional hazard models were used to evaluate the univariable association of relevant patient and disease characteristics (Table 1) with overall survival. Multivariable adjusted Cox proportional hazard modeling was used to assess the independent effect of treatment strategy on survival. Covariates included for risk adjustment in the multivariable model were determined by stepwise, linear regression with the entry criterion of p<0.10 from the univariable analysis. Additionally, to account for nonrandom treatment assignment, factors favoring selection of one treatment strategy over another were adjusted for by propensity scores. The propensity score for an individual, defined as the conditional probability of a given treatment conditionally on (or only on the basis of) the individual’s covariate values, can be used to balance the covariates between groups and thus reduce bias.36 In order to estimate the likelihood of each treatment modality, pairwise propensity score models between each combination of treatment pairs were created using all possible clinically relevant variables. No interactions were included in the propensity score models. We created a propensity score for each treatment pair (MED vs. PCI, MED vs. CABG, MED vs. CABG+MV, PCI vs. CABG, PCI vs. CABG+MV, and CABG vs. CABG+MV). Linearity of the propensity scores was tested, and there was no significant violation of linearity. As such, the propensity scores were then included as additional covariates in the risk-adjusted Cox proportional hazard model without stratification into quintiles given the test of linearity as noted above. As verification of this approach, analysis using propensity score quintiles was also performed to ensure no difference in results (data not shown). The adjusted survival curve for each group was then constructed by applying its estimated baseline hazard function, along with covariate Cox model parameter estimates, to all patients in the cohort and then averaged over all patients at each time point. The resulting curves represent a survival estimate that would have been realized had all patients been in each treatment group.
Table 1.
Patient and disease characteristics for univariable and multivariable analysis.
| Demographics: | Comorbidities: |
| Age | Congestive heart failure |
| Sex | Previous myocardial infarction |
| Race | Peripheral vascular disease |
| Cerebrovascular disease | |
| Year of catheterization | Diabetes |
| Number of diseased vessels at catheterization | Hypertension |
| MR grade | Hyperlipidemia |
| Body mass index | |
| Hemodynamic and renal function | |
| parameters: | Chronic obstructive pulmonary disease |
| Systolic and diastolic blood pressure | Liver disease |
| Heart rate | Peptic ulcer disease |
| Ejection fraction | Prior malignancy |
| Estimated glomerular filtration rate | Connective tissue disease |
| End stage renal disease requiring dialysis | |
| New York Heart Association class | Dementia |
| Non-cardiac Charlson Comorbidity Index | |
| Family history of coronary artery disease | |
| Smoking history |
Sensitivity Analysis Based on Mitral Regurgitation Severity
A sensitivity analysis was performed to assess differences in survival endpoints based on the degree of MR severity. This analysis incorporated a cross-product term (or interaction term) designed to assess for a non-additive simultaneous influence two independent variables (MR severity and treatment category) on the dependent variable of overall survival.37 The interaction term of treatment category and MR severity [dichotomized as moderate MR (2+ or 3+) and severe MR (4+)] was forced into the above described Cox proportional hazard model.
A p-value ≤0.05 was used to indicate statistical significance for all comparisons and analyses. All p-values are two-sided. Statistical analyses were performed using SAS version 8.2 (SAS Institute Inc., Cary, NC).
RESULTS
Study Population
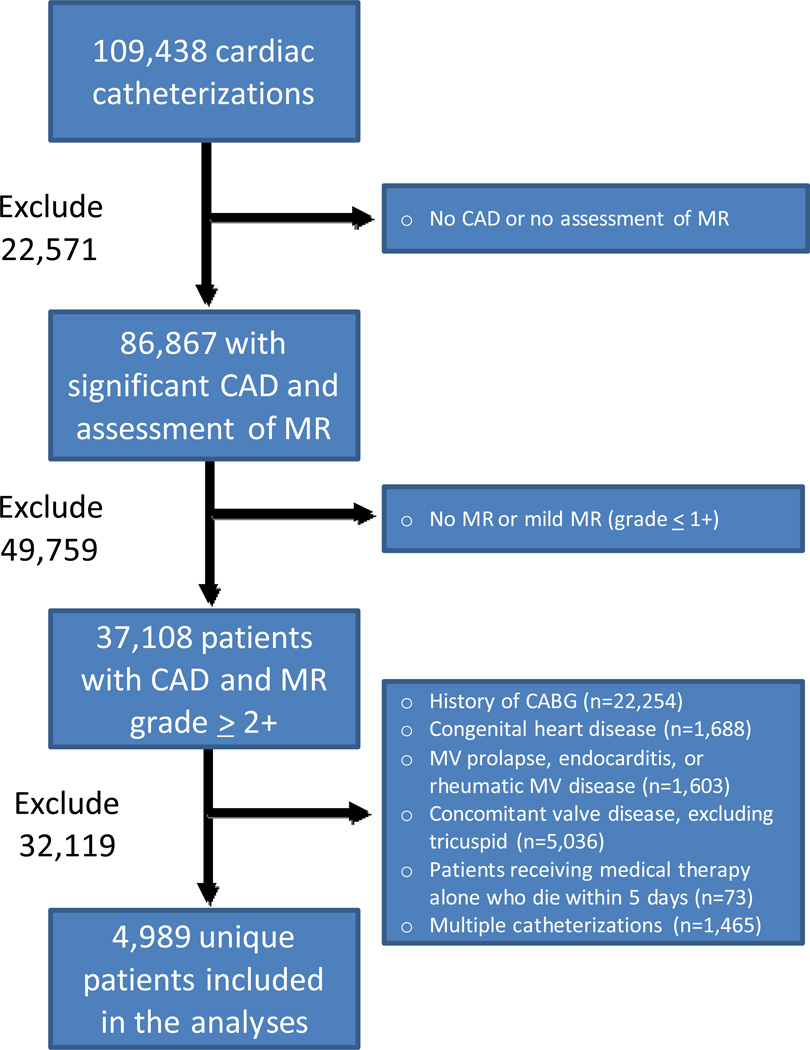
A total of 109,438 cardiac catheterizations were performed at our institution within the study period. Of these, 22,571 cases were excluded due to the absence of CAD or lack of MR assessment, 49,759 due to MR grade ≤1, and 32,119 due to pre-existing conditions, concomitant disease, or patients in the medical management group who died within 5 days of catheterization (n=73) (Figure 1). This resulted in 4,989 patients included in our study population, of which 1,800 (36%) received medical management, 1,295 (26%) underwent PCI, 1,651 (33%) were treated with CABG alone, and 243 (5%) underwent CABG+MVRR. The assigned MR grade was determined by echocardiography for 68% of the study population (n=3,394) with 32% (n=1,595) determined by left ventriculography.
Figure 1. Study inclusion algorithm.
CAD = coronary artery disease; MR = mitral regurgitation; IMR = ischemic mitral regurgitation; CABG = coronary artery bypass grafting; MV = mitral valve.
Baseline Characteristics
The median age for our study population was 67 years [25th – 75th percentiles (Q1–Q3) 58 – 74] with 44.6% female and 73.1% white ethnicity. The majority of patients had three vessel coronary disease (43.1% of the study population) and 2+ MR (70.0% of the study population).
Patients undergoing medical management were significantly older when compared to patients receiving other treatment modalities (median 68 years, Q1–Q3 59–76) and had a higher incidence of diabetes (39.3%), chronic obstructive pulmonary disease (10.4%), peripheral vascular disease (16.3%), and cerebrovascular disease (17.6%), with a lower GFR (median 63.1 mL/min/1.73 m2, Q1––Q3 43.5 – 79.5) and EF (median 40%, Q1–Q3 26 – 60%) as well as higher incidence of end stage renal disease requiring dialysis (2.7%) (Table 2). Patients undergoing PCI had a significantly higher BMI when compared to the other groups (median 27.3 kg/m2, Q1–Q3 24.1 – 31.0), were more frequently female (51.0%), and were more likely to have 1 or 2 vessel coronary disease (51.0% and 33.6%, respectively). Patients undergoing CABG alone were more likely to be white (78.4%), have three-vessel coronary disease (70.9%), hyperlipidemia (52.9%), or moderate (2+) MR (77.8%), while patients undergoing CABG+MVRR were more likely to have greater than 2+ MR (93.4%) or be NYHA Class II – IV (54.4%). There was no significant difference in the incidence of hypertension, smoking, or family history of CAD.
Table 2.
Summary of baseline characteristics.†
| Characteristic | Total (n = 4989) |
Medicine (n = 1800) |
PCI (n = 1295) |
CABG (n = 1651) |
CABG + MVRR (n = 243) |
P Value |
|---|---|---|---|---|---|---|
| Patient age | 67 (58 – 74) | 68 (59 – 76) | 66 (56 – 74) | 66 (58 – 73) | 66 (58 – 74) | <.0001 |
| Femal gender | 2227 (44.64) | 878 (48.78) | 661 (51.04) | 577 (34.95) | 111 (45.68) | <.0001 |
| White ethnicity‡ | 3635 (73.15) | 1239 (69.18) | 924 (71.57) | 1291 (78.43) | 181 (75.1) | <.0001 |
| Year of catheterization | <.0001 | |||||
| 1990 – 1994 | 1246 (24.97) | 566 (31.45) | 304 (23.46) | 325 (19.69) | 51 (21) | |
| 1995 – 1999 | 1387 (27.79) | 563 (31.28) | 339 (26.17) | 429 (25.98) | 56 (23.05) | |
| 2000 – 2004 | 1488 (29.82) | 436 (24.22) | 404 (31.18) | 570 (34.52) | 78 (32.1) | |
| 2005 – 2009 | 868 (17.4) | 235 (13.06) | 248 (19.15) | 327 (19.8) | 58 (23.88) | |
| Number of diseased vessels | <.0001 | |||||
| 1 | 1493 (29.93) | 664 (36.89) | 661 (51.04) | 123 (7.45) | 45 (18.52) | |
| 2 | 1346 (26.98) | 486 (27) | 435 (33.59) | 357 (21.62) | 68 (27.98) | |
| 3 | 2150 (43.09) | 650 (36.11) | 199 (15.37) | 1171 (70.93) | 130 (53.5) | |
| MR grade | <.0001 | |||||
| 2+ | 3494 (70.03) | 1188 (66) | 1006 (77.68) | 1284 (77.77) | 16 (6.58) | |
| 3+ | 1113 (22.31) | 443 (24.61) | 244 (18.84) | 327 (19.81) | 99 (40.74) | |
| 4+ | 381 (7.64) | 169 (9.39) | 44 (3.4) | 40 (2.42) | 128 (52.67) | |
| BMI (kg/m2)‡ | 26.9 (23.8 – 30.5) | 26.5 (23.3 – 30.3) | 27.3 (24.1 – 31) | 27.1 (24.2 – 30.7) | 27.1 (23.2 – 29.6) | <.0001 |
| Hypertension | 3385 (67.85) | 1226 (68.11) | 877 (67.72) | 1133 (68.63) | 149 (61.32) | 0.1527 |
| Diabetes | 1772 (35.52) | 708 (39.33) | 390 (30.12) | 590 (35.74) | 84 (34.57) | <.0001 |
| Hyperlipidemia | 2523 (50.57) | 848 (47.11) | 684 (52.82) | 874 (52.94) | 117 (48.15) | 0.0014 |
| COPD | 395 (7.92) | 187 (10.39) | 92 (7.1) | 101 (6.12) | 15 (6.17) | <.0001 |
| Smoker | 2637 (52.86) | 964 (53.56) | 672 (51.89) | 880 (53.3) | 121 (49.79) | 0.5973 |
| Peripheral vascular disease | 662 (13.27) | 294 (16.33) | 137 (10.58) | 209 (12.66) | 22 (9.05) | <.0001 |
| Cerebrovascular disease | 653 (13.09) | 316 (17.56) | 121 (9.34) | 190 (11.51) | 26 (10.7) | <.0001 |
| History of CHF¥ | 1952 (39.67) | 977 (54.67) | 367 (28.63) | 461 (28.6) | 147 (61.51) | <.0001 |
| NYHA Class¥ | ||||||
| None | 3112 (64.01) | 877 (49.74) | 953 (75.76) | 1186 (74.03) | 96 (40.17) | <.0001 |
| I | 329 (6.77) | 150 (8.51) | 67 (5.33) | 99 (6.18) | 13 (5.44) | |
| II | 373 (7.67) | 184 (10.44) | 62 (4.93) | 91 (5.68) | 36 (15.06) | |
| III | 541 (11.13) | 267 (15.14) | 108 (8.59) | 117 (7.3) | 49 (20.5) | |
| IV | 507 (10.43) | 285 (16.17) | 68 (5.41) | 109 (6.8) | 45 (18.83) | |
| GFR (mL/min/1.73m2) | 66.3 (49 – 82.2) | 63.1 (43.5 – 79.5) | 69 (51.6 – 84.4) | 69.4 (52.7 – 83.7) | 63.8 (48 – 85.2) | <.0001 |
| Requiring dialysis | 107 (2.14) | 49 (2.72) | 33 (2.55) | 20 (1.21) | 5 (2.06) | 0.0133 |
| Mild/moderate liver disease | 18 (0.36) | 7 (0.39) | 2 (0.15) | 7 (0.42) | 2 (0.82) | 0.3616 |
| Severe liver disease | 7 (0.14) | 5 (0.28) | 1 (0.08) | 1 (0.06) | 0 (0) | 0.274 |
| Peptic ulcer disease | 100 (2) | 43 (2.39) | 19 (1.47) | 36 (2.18) | 2 (0.82) | 0.1547 |
| Dimentia | 22 (0.44) | 10 (0.56) | 8 (0.62) | 4 (0.24) | 0 (0) | 0.2592 |
| HIV/AIDS | 5 (0.1) | 2 (0.11) | 1 (0.08) | 1 (0.06) | 1 (0.41) | 0.44 |
| Ejection fraction | 46 (30 – 60) | 40 (26 – 60) | 50 (40 – 60) | 48 (35 – 60) | 45 (31 – 60) | <.0001 |
Continuous variables are reported as median (25th percentile - 75th percentile); Discrete variables are reported as n (%).
Indicates variable with missing data for < 1% of patient sample.
Indicates variable with missing data for < 3% of patient sample.
Abbreviations: PCI = percutaneous coronary intervention; CABG = coronary artery bypass grafting; MVRR = mitral valve repair or replacement; MR = mitral regurgitation; BMI = body mass index; COPD = chronic obstructive pulmonary disease; CHF = congestive heart failure; NYHA = New York Heart Association; GFR = glomerular filtration rate.
Trends in Treatment Modality
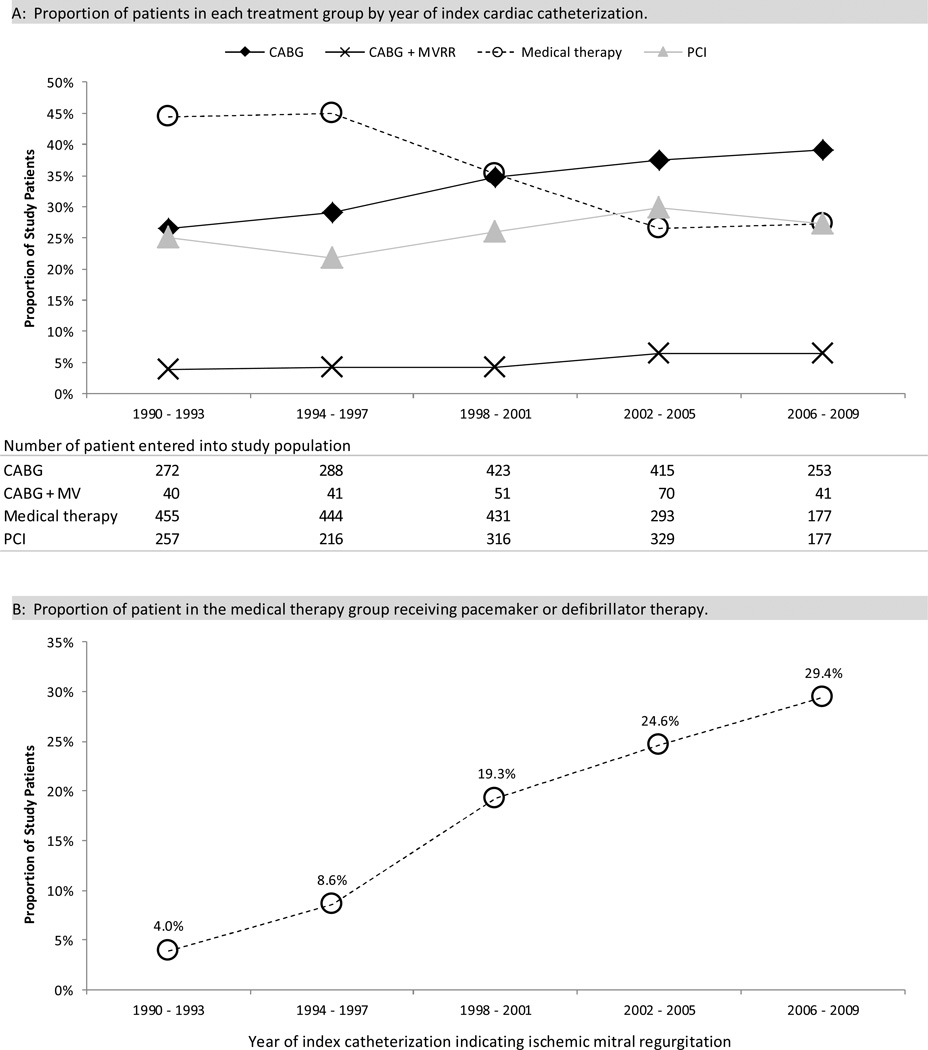
During the first 10 years of the study period more patients received medical management compared to the other treatment modalities, whereas in the latter 10 years of the study CABG alone was the most common treatment strategy (Figure 2A). The proportion of patients treated medically declined from 44.4% in the first five years of the study to 27.3% in the last five years. The proportion of patients treated with PCI rose from 25.1% to 27.3%, CABG rose 26.6% to 39.0%, and CABG+MVRR 3.9% to 6.3%, The use of pacemaker or defibrillator therapy in the medical management group steadily increased from 4.0% in the first five years of the study to 29.4% in the latter five years (Figure 2B).
Figure 2. Summary of treatment modality by year of index cardiac catheterization.
Abbreviations: CABG = coronary artery bypass grafting; MVRR = mitral valve repair or replacement; PCI = percutaneous coronary intervention;
Long-Term Survival
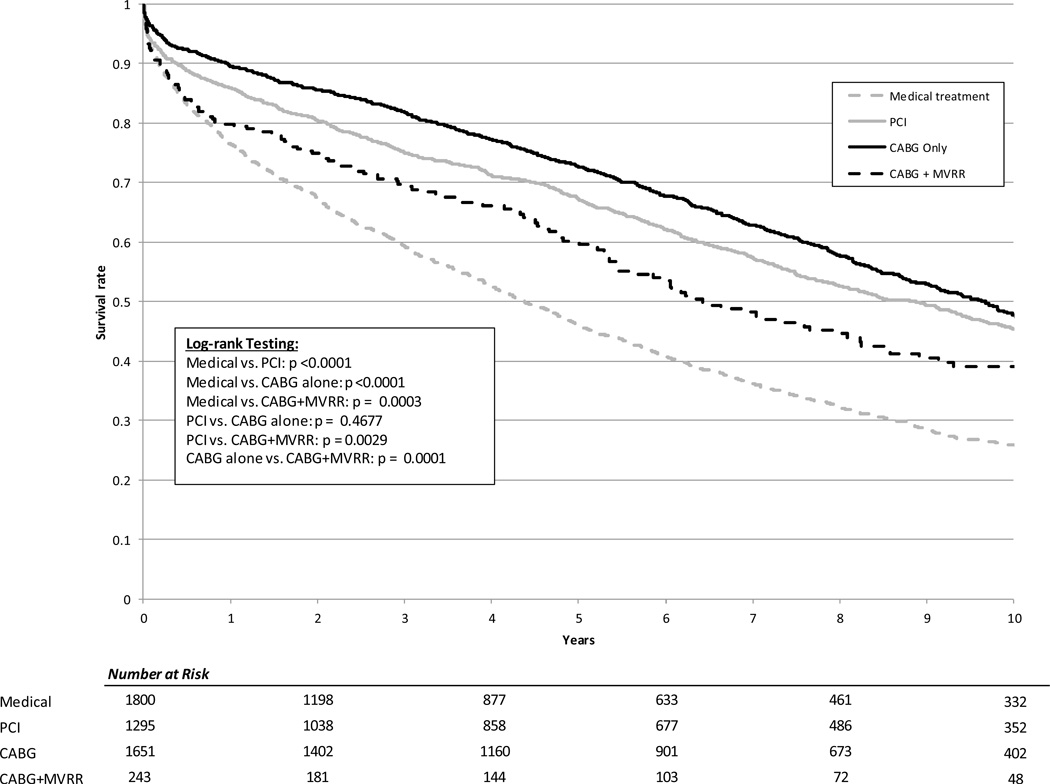
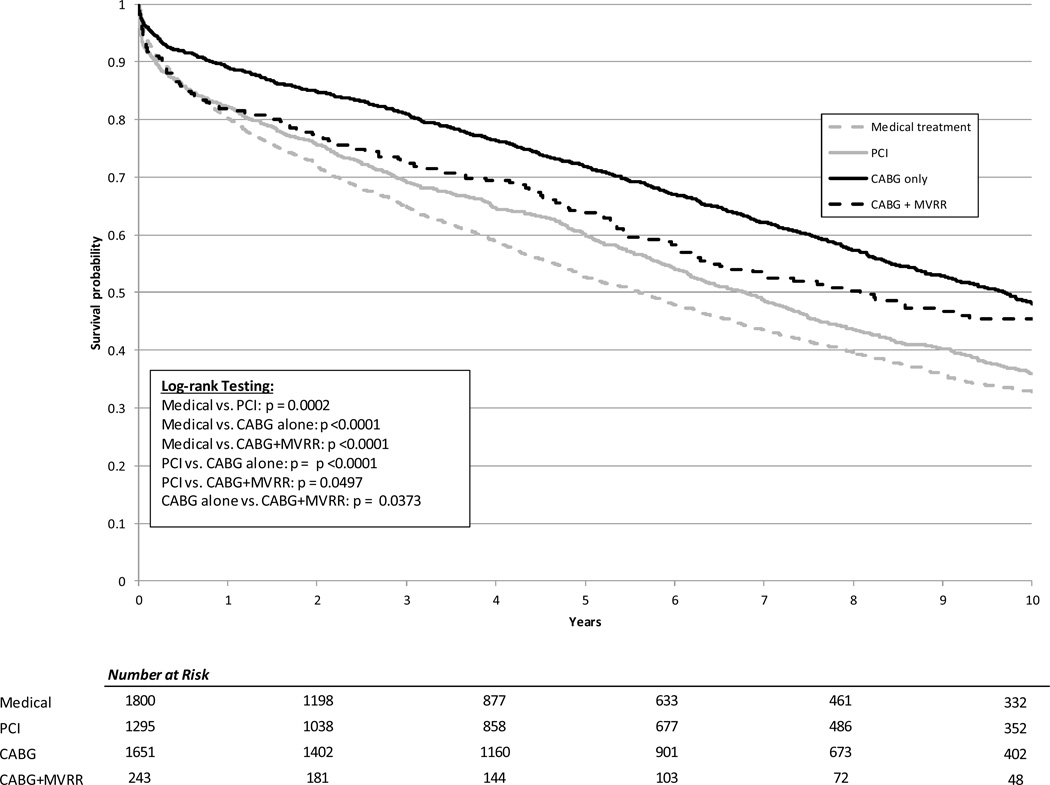
The median duration of follow-up for the study was 5.37 years. Using Kaplan-Meier methods, the median unadjusted survival was 4.4 years for patients undergoing medical management, 8.9 years for patients treated with PCI, 9.7 years for patients undergoing CABG alone, and 6.4 years for the CABG+MVRR group (Figure 3). The median adjusted survival was 5.6 years for patients undergoing medical management, 6.8 years for patients treated with PCI, 9.7 years for patients undergoing CABG alone, and 8.1 years for the CABG+MVRR group (Figure 4)
Figure 3. Unadjusted Kaplan-Meier survival curves by treatment category.
PCI = percutaneous coronary intervention; CABG = coronary artery bypass; MVRR = mitral valve repair or replacement.
Figure 4. Adjusted survival curves by treatment category.
PCI = percutaneous coronary intervention; CABG = coronary artery bypass; MVRR = mitral valve repair or replacement.
The results of the Cox-proportional hazard analysis identifying the independent relationship between clinical variables and mortality are shown in Table 3. After adjusting for other important clinical characteristics, the PCI, CABG alone, and CABG+MVRR groups all demonstrated significantly lower risk of death when compared to medical therapy. Compared to patients receiving medical therapy, the CABG alone group demonstrated the lowest risk of death [adjusted hazard ratio (AHR): 0.56, 95% confidence interval (CI): 0.51 – 0.62, p<0.0001], followed by CABG+MVRR (AHR: 0.69, CI: 0.57 – 0.82, p<0.0001) and PCI (AHR 0.83, 95% CI: 0.76 – 0.92, p=0.0002). There was no significant difference in these results when stratifying the propensity score by quintiles (data not shown).
Table 3.
Cox proportional hazard independent predictors of mortality.
| Covariate | Wald X2 | HR (95% CI) | P Value |
|---|---|---|---|
| Treatment category* | |||
| PCI† | 13.6 | 0.83 (0.76 – 0.92) | 0.0002 |
| CABG Only† | 141.1 | 0.56 (0.51 – 0.62) | <.0001 |
| CABG + MVRR† | 16.6 | 0.69 (0.57 – 0.82) | <.0001 |
| Patient age (HR per 10 year increase) | 272.1 | <.0001 | |
| Linear component below | 60 | 1.17 (1.05 – 1.29) | |
| Linear component above | 60 | 1.6 (1.49 – 1.73) | |
| Number of diseased vessels | 11.5 | 1.19 (1.08 – 1.32) | 0.0007 |
| BMI (HR per 1 unit increase) | 30.6 | <.0001 | |
| Linear component below | 28 | 0.96 (0.95 – 0.98) | |
| Linear component above | 28 | 1.02 (1.01 – 1.03) | |
| Diabetes | 12.5 | 1.2 (1.08 – 1.32) | 0.0004 |
| Hyperlipidemia | 16.6 | 0.83 (0.76 – 0.91) | <.0001 |
| History of smoking | 21.3 | 1.22 (1.12 – 1.32) | <.0001 |
| History of CHF | 24.1 | 1.3 (1.17 – 1.44) | <.0001 |
| GFR (HR per 5 unit increase)‡ | 190.2 | 0.94 (0.93 – 0.95) | <.0001 |
| Non-cardiac Charlson Index | 84.3 | 1.22 (1.17 – 1.28) | <.0001 |
| Heart rate (HR per 10 unit increase)‡ | 28.3 | 1.11 (1.07 – 1.15) | <.0001 |
| Diastolic BP (HR per 10 unit increase)¥ | 18.4 | 0.92 (0.89 – 0.96) | <.0001 |
| Ejection fraction (HR per 5% increase) | 58.8 | 0.94 (0.93 – 0.96) | <.0001 |
Propensity score for the respective treatment categories included as covariate.
Medical therapy as treatment reference group.
Upper limit of GFR and heart rate truncated at 90.
Upper limit of diastolic blood pressure truncated at 80.
Abbreviations:X2 = Chi Square; HR = hazard ratio; CI = confidence interval; PCI = percutaneous coronary intervention; CABG = coronary artery bypass grafting; MVRR = mitral valve repair or replacement; BMI = body mass index; COPD = chronic obstructive pulmonary disease; MI = myocardial CHF = congestive heart failure; GFR = glomerular filtration rate; BP = blood pressure.
Sensitivity Analysis Based on Mitral Regurgitation Severity
The cross-product interaction term of MR severity and treatment group was not statistically significance when analyzed as part of the multivariable Cox proportional hazard model (p=0.61), indicating that the adjusted risk of death based on treatment group did not differ significantly in cases of moderate versus severe MR.
DISCUSSION
Typically, post-infarct LV remodeling results in apical and inferior papillary muscle displacement and mitral annular dilatation, with the combination of these geometric changes resulting in the restriction of otherwise structurally normal mitral leaflets and thus IMR.16, 38, 39 Due to the combined effects of ischemia and mitral regurgitation, these patients may have significant heart failure symptoms and can pose significant clinical management challenges. Because of the complex and dynamic nature of ischemic mitral regurgitation, there is controversy as to appropriate management of these patients. The current study increases the body of evidence for clinical decisions in IMR treatment by reporting the largest real-world series from the modern era including the full spectrum of treatment strategies.
This study is an extension of previous research from our institution demonstrating that ischemic MR patients have increased longevity with revascularization by any means (with or without valve surgery in the CABG group) compared to an initial medical treatment strategy.20 In the current analysis, we have increased the study population from 2,757 to 4,989, extended median follow-up from 3.2 years to 5.37 years, and included patients treated with PCI and drug-eluting stents. In the previously reported study, we were not able to discriminate between the revascularization methods. The current analysis confirms the superiority of revascularization to medical therapy. Additionally, these data are now able to demonstrate that CABG alone may provide optimal survival and that surgical therapy regardless of the treatment of the mitral valve itself is superior to PCI. These findings are aligned with observational and randomized trials comparing CABG and PCI, suggesting that the treatment of the coronary disease dominates the effect on longevity outcome and that ischemic MR and associated mortality is predominantly driven by ventricular pathology.
Long-Term Survival
In our investigation, treating IMR patients with revascularization (CABG alone or PCI) was associated with significantly higher long-term survival compared to medical management after adjusting for patient demographics, comorbidities, and disease characteristics. This is in contrast to current literature advocating medical management as the standard of care for functional MR.33, 34 The inclusion of patients initially managed medically makes our dataset unique in its ability to further advance the debate on this issue by demonstrating superior survival with surgical intervention, which ongoing randomized clinical trials evaluating IMR would likely be underpowered to detect.40, 41 These findings are consistent with other studies in the literature that have proposed that revascularization alone may improve LV wall motion and geometry, restoring valvular coaptation and thereby improving IMR.20, 23 Importantly, our study demonstrates that CABG with or without mitral valve surgery is associated with better outcomes than PCI in patients with IMR. While some of the better survival seen by the CABG alone group can be explained by the survival advantage of CABG compared to PCI for all patients with coronary artery disease,42 this relationship in our study was particularly pronounced. Campwala et al43 previously reported progression of IMR in cases of incomplete coronary revascularization, which may in part explain the better survival of patients undergoing CABG compared to PCI in the current study. It is important to note that MR in our study population was determined by echocardiography and/or ventriculography, excluding structural or degenerative causes of mitral regurgitation, as has been previously established in the literature.20, 26, 27, 44 All echocardiograms were performed at Duke, reviewed at the time of the study, and prospectively databased for mitral valve pathology and MR etiology. These data were accessed directly for this study. Ventriculography performed outside of the Duke system (<3%) were interpreted by the treating physicians, and categorical data regarding these features were also prospectively entered into the database. Neither Duke studies nor external studies (unavailable to the authors) were independently reviewed in preparing this manuscript. Detailed information as to the morphology of the mitral valve to confirm leaflet tethering and/or wall motion abnormalities is not available in our dataset; however, data from the Surgical Treatment for Ischemic Heart Failure (STICH) Trial demonstrate that measurements associated with ischemic MR severity are complex and heterogeneous in nature and therefore quantifying MR based on valve morphology would also be subject to limitations.45 Our results should therefore be analyzed in coordination with smaller prospective studies incorporating valve morphology information.
Concomitant Mitral Valve Surgery
Interestingly, our analysis revealed no better survival associated with concomitant mitral valve surgery compared to CABG alone, with no difference in these results based on MR severity or when excluding MV replacement from the analysis (data not shown). It has been hypothesized that the correction of MR may be of little benefit due to the remaining presence of the underlying ischemic injury, implying that MR may reflect ischemia or advanced left-ventricular dysfunction and therefore not have an independent impact on survival.3, 5, 30, 46
Although several retrospective studies using propensity score-matched cohorts of patients showed that CABG combined with MV repair reduced MR, these studies have not demonstrated a survival advantage.6, 21, 47 However, an analysis of the impact of MR on survival in the STICH trial reported observational data in 91 patients with moderate to severe MR randomized to CABG with (n = 49) or without (n = 42) MV surgery at the discretion of the surgeon.31 Their results demonstrated a survival advantage in adding mitral valve surgery compared to CABG alone after adjusting for baseline variables; however, a limitation noted by the authors is the potential for surgeons to perform CABG alone in higher risk patients as well as the relatively small sample size.
Additionally, these opposing findings from the STICH trial compared to the current study could relate to a lower EF in the STICH population (median 25% compared to 48% in the current study). Of note, 6 of 7 perioperative deaths in their cohort occurred in patients receiving CABG alone. The authors note the need for additional observations in larger numbers of patients undergoing CABG with moderate to severe MR to confirm whether failure to repair MR in this patient population decreases long-term survival, and our results of 1,651 patients undergoing CABG alone argue against this hypothesis. However; it is important to note that compared to the CABG group the CABG+MVRR group contained a much higher proportion of patients with MR grade 4+ (52.7% vs. 2.4%) and NYHA Class III-IV (39.3% vs. 14.1%). This could indicate a higher degree of illness in patients treated with CABG+MVRR, which may not entirely be accounted for by statistical methods. Additionally, these data may indicate that MR was the inciting event leading to an intervention for the CAGB+MVRR group as opposed to cardiac ischemia, which we cannot completely discern in our dataset; however, these data do not indicate that MR was the most frequent trigger for surgical intervention in the CABG group (without MV surgery) as the CABG group had a lower proportion of patients with MR grade 3+ or 4+ (22.23%) compared to both medical management (34%) and PCI (22.24%).
Additional Outcome Considerations
Even without a survival advantage, however, correction of MR may affect other import endpoints, such as sequelae of heart failure,29, 30, 48 with important implications for both patient quality of life as well as the utilization of healthcare resources.9 Moreover, postoperative improvement of LV dysfunction by CABG alone cannot be predicted reliably,23 and some have advocated that correction of MR may allow for significant reverse LV remodeling after surgery.32, 49, 50 The Randomized Ischemic Mitral Evaluation (RIME) Trial (ClinicalTrials.gov NCT00413998) has provided additional support for potential benefits of MV repair in addition to CABG by demonstrating greater improvement in functional capacity as measured by peak oxygen consumption, and greater left ventricular reverse remodeling as measured by the left ventricular end-systolic volume index, reduction in mitral regurgitation severity, and B-type natriuretic peptide levels, compared with the CABG alone.40 There was, however, a trend towards higher complications in patients undergoing CABG with MVR and current data are limited to 1-year results.
Future Contributions in IMR Management
These results further underscore the need for continued randomized trials to provide more definitive guidance in managing patients with IMR. The RIME trial has recently reported 1-year results.40 This multicenter, single-blinded, randomized, controlled trial evaluating the role of mitral valve repair during CABG in patients with moderate IMR demonstrated improved functional capacity and symptoms at 1 year; however, with only 73 patients, the study was underpowered to assess survival and longer term results are still pending. The Cardiothoracic Surgical Trials Network (CSTN) Surgical Interventions for Moderate Ischemic Mitral Regurgitation Trial comparing CABG alone to CABG with MV repair for moderate IMR (ClinicalTrials.gov NCT00806988) completed enrollment in April 2013 with anticipated results April 2014.41 This study included 301 patients with moderate IMR randomized to CABG or CABG with MV repair. An important limitation to the current study in determining the appropriateness of adding mitral valve surgery to CABG is the absence of functional and quality of life metrics, for which the CSTN results are anxiously awaited for additional information pertaining to these endpoints. Furthermore, clinical equipoise exists in the decision to repair or replace the mitral valve in treatment of severe IMR, and as such CSTN is also conducting a trial to evaluate CABG plus mitral repair versus replacement for severe IMR, and has completed enrollment of 251 patients (ClinicalTrials.gov NCT00807040). Results from these trials will further elucidate the optimal treatment algorithm for patients with IMR; however, as with other cardiac surgery trials in the past, these will be limited by insufficient power to fully detect causal relationships with low-rate events such as mortality,24 suggesting a continued role for large observational studies to guide clinical decisions in managing this disease process.
Limitations
The results of this investigation should be interpreted in the context of the study’s limitations. First, this was an observational study and therefore allocation to a treatment strategy was not randomized. Modeling techniques including propensity score adjustment may not entirely account for the lack of randomization between cohorts. Imbalances in the baseline characteristics between treatment groups were present and may have affected our results due to selection bias and unmeasured confounders. It is important to note that the medical management group were older with higher rates of chronic obstructive pulmonary disease, peripheral vascular disease and cerebrovascular disease, while the CABG group demonstrated a higher ischemic burden based on the number of diseased vessels and the CABG+MVRR group likely suffered more mitral valve disease based on MR severity and NYHA class. These differences may not be entirely accounted for by statistical methods of comparison. Additionally, outcome measures other than survival were not analyzed, such as quality of life or subsequent use of resources to achieve the demonstrated survival. Furthermore, out study population excludes patients with a previous CABG. A significant portion of these patients could develop IMR and may warrant further study specific to IMR following surgical revascularization. Despite these limitations, however, our series of a 20-year longitudinal analysis represents the largest clinical study comparing the full spectrum of treatment strategies for patients with moderate to severe IMR. While there are inherent limitations to this analysis, randomized trials with equivalent power to that of the current study would not be feasible and level B evidence will likely remain an important component of the literature guiding decisions in treating ischemic MR.
Conclusion
Compared to medical management, revascularization with or without treatment of the mitral valve is associated with significantly longer survival for patients with IMR. CABG alone demonstrated the lowest risk of death and CABG with or without mitral valve surgery was associated with better outcomes than either PCI or medical therapy.
Supplementary Material
Acknowledgments
We would like to thank Dr. Michael J. Mack, M.D., FACC for his critical review and constructive criticism in preparing this manuscript.
Funding Sources: Drs. Smith, Alexander, Williams, and Mathew are supported in part by grant U01-HL088953 from the National Institutes of Health Cardiothoracic Surgical Trials Network.
Footnotes
Conflict of Interest Disclosures: Dr. Milano is a consultant for Thoratec Corporation and HeartWare Inc. Dr Velazquez has received grant funding from Abbott Vascular Structural Heart. Dr. Lopes has received grant funding from Bristol-Myers Squibb. The other authors report no conflicts.
References
- 1.HCUPnet. Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; 2010. [Accessed July 15, 2012]. http://hcupnet.ahrq.gov/ [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 3.Birnbaum Y, Chamoun AJ, Conti VR, Uretsky BF. Mitral regurgitation following acute myocardial infarction. Coron Artery Dis. 2002;13:337–344. doi: 10.1097/00019501-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Bursi F, Enriquez-Sarano M, Jacobsen SJ, Roger VL. Mitral regurgitation after myocardial infarction: a review. Am J Med. 2006;119:103–112. doi: 10.1016/j.amjmed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 5.Carabello BA. The current therapy for mitral regurgitation. J Am Coll Cardiol. 2008;52:319–326. doi: 10.1016/j.jacc.2008.02.084. [DOI] [PubMed] [Google Scholar]
- 6.Magne J, Sénéchal M, Dumesnil JG, Pibarot P. Ischemic mitral regurgitation: a complex multifaceted disease. Cardiology. 2009;112:244–259. doi: 10.1159/000151693. [DOI] [PubMed] [Google Scholar]
- 7.Daneshmand MA, Milano CA, Rankin JS, Honeycutt EF, Swaminathan M, Shaw LK, Smith PK, Glower DD. Mitral Valve Repair for Degenerative Disease: A 20-Year Experience. Ann Thorac Surg. 2009;88:1828–1837. doi: 10.1016/j.athoracsur.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Foster E. Clinical practice. Mitral regurgitation due to degenerative mitral-valve disease. N Engl J Med. 2010;363:156–165. doi: 10.1056/NEJMcp0906782. [DOI] [PubMed] [Google Scholar]
- 9.Badiwala MV, Verma S, Rao V. Surgical management of ischemic mitral regurgitation. Circulation. 2009;120:1287–1293. doi: 10.1161/CIRCULATIONAHA.108.836627. [DOI] [PubMed] [Google Scholar]
- 10.Feinberg MS, Schwammenthal E, Shlizerman L, Porter A, Hod H, Friemark D, Matezky S, Boyko V, Mandelzweig L, Vered Z, Behar S, Sagie A. Prognostic significance of mild mitral regurgitation by color Doppler echocardiography in acute myocardial infarction. Am J Cardiol. 2000;86:903–907. doi: 10.1016/s0002-9149(00)01119-x. [DOI] [PubMed] [Google Scholar]
- 11.Mallidi HR, Pelletier MP, Lamb J, Desai N, Sever J, Christakis GT, Cohen G, Goldman BS, Fremes SE. Late outcomes in patients with uncorrected mild to moderate mitral regurgitation at the time of isolated coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2004;127:636–644. doi: 10.1016/j.jtcvs.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Bursi F Nkomo VTJS, Weston SA, Meverden RA, Roger VL, Enriquez-Sarano M. Heart Failure and Death After Myocardial Infarction in the Community: The Emerging Role of Mitral Regurgitation. Circulation. 2005;111:295–301. doi: 10.1161/01.CIR.0000151097.30779.04. [DOI] [PubMed] [Google Scholar]
- 13.Lam B-K, Blackstone EH ea, Gillinov A M. Importance of moderate ischemic mitral regurgitation. Ann Thorac Surg. 2005;79:462–470. doi: 10.1016/j.athoracsur.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 14.Schroder JN, Hata JA ea, Williams ML. Impact of Mitral Valve Regurgitation Evaluated by Intraoperative Transesophageal Echocardiography on Long-Term Outcomes After Coronary Artery Bypass Grafting. Circulation. 2005;112 doi: 10.1161/CIRCULATIONAHA.104.523472. I-293-298. [DOI] [PubMed] [Google Scholar]
- 15.Aronson D, Zukermann R ea, Goldsher N. Ischemic mitral regurgitation and risk of heart failure after myocardial infarction. Arch Intern Med. 2006;166:2362–2368. doi: 10.1001/archinte.166.21.2362. [DOI] [PubMed] [Google Scholar]
- 16.Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. 2009;373:1382–1394. doi: 10.1016/S0140-6736(09)60692-9. [DOI] [PubMed] [Google Scholar]
- 17.Enriquez-Sarano M, Schaff HV, Orszulak TA, Tajik AJ, Bailey KR, Frye RL. Valve repair improves the outcome of surgery for mitral regurgitation. A multivariate analysis. Circulation. 1995;91:1022–1028. doi: 10.1161/01.cir.91.4.1022. [DOI] [PubMed] [Google Scholar]
- 18.Raja SG, Berg GA. Moderate ischemic mitral regurgitation: to treat or not to treat? J Card Surg. 2007;22:362–369. doi: 10.1111/j.1540-8191.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- 19.Murphy MO, Rao C, Punjabi PP, Athanasiou T. In patients undergoing mitral surgery for ischaemic mitral regurgitation is it preferable to repair or replace the mitral valve? Interact Cardiovasc Thorac Surg. 2011;12:218–227. doi: 10.1510/icvts.2010.245191. [DOI] [PubMed] [Google Scholar]
- 20.Trichon BH, Shaw LKCC, Anstrom KJ, Felker GM, O’Connor CM, Glower DD. Survival After Coronary Revascularization, With and Without Mitral Valve Surgery, in Patients With Ischemic Mitral Regurgitation. Circulation. 2003;108:103–110. doi: 10.1161/01.cir.0000087656.10829.df. [DOI] [PubMed] [Google Scholar]
- 21.Diodato MD, Moon MR, Pasque MK, Barner HB, Moazami N, Lawton JS, Bailey MS, Guthrie TJ, Meyers BF, Damiano RJ., Jr Repair of ischemic mitral regurgitation does not increase mortality or improve long-term survival in patients undergoing coronary artery revascularization: A propensity analysis. Ann Thorac Surg. 2004;78:794–799. doi: 10.1016/j.athoracsur.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y-H, Czer LSC, Soukiasian HJ, De Robertis M, Magliato KE, Blanche C, Raissi SS, Mirocha J, Siegel RJ, Kass RM, Trento A. Ischemic mitral regurgitation: revascularization alone versus revascularization and mitral valve repair. Ann Thorac Surg. 2005;79:1895–1901. doi: 10.1016/j.athoracsur.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Kang DH, Kim MJ, Kang SJ, Song JM, Song H, Hong MK, Choi KJ, Song JK, Lee JW. Mitral valve repair versus revascularization alone in the treatment of ischemic mitral regurgitation. Circulation. 2006;114(1 Suppl):I499–I503. doi: 10.1161/CIRCULATIONAHA.105.000398. [DOI] [PubMed] [Google Scholar]
- 24.Benedetto U, Melina G, Roscitano A, Fiorani B, Capuano F, Sclafani G, Comito C, Nucci GDd, Sinatra R. Does combined mitral valve surgery improve survival when compared to revascularization alone in patients with ischemic mitral regurgitation? A meta-analysis on 2479 patients. J Card Med. 2009;10:109–114. doi: 10.2459/JCM.0b013e32831c84b0. [DOI] [PubMed] [Google Scholar]
- 25.Goland S, Czer LSC, Siegel RJ, DeRobertis MA, Mirocha J, Zivari K, Kass RM, Raissi S, Fontana G, Cheng W, Trento A. Coronary revascularization alone or with mitral valve repair: outcomes in patients with moderate ischemic mitral regurgitation. Tex Heart Inst J. 2009;36:416–424. [PMC free article] [PubMed] [Google Scholar]
- 26.Aklog L, Filsoufi F, Flores KQ, Chen RH, Cohn LH, Nathan NS, Byrne JG, Adams DH. Does Coronary Artery Bypass Grafting Alone Correct Moderate Ischemic Mitral Regurgitation? Circulation. 2001;104 doi: 10.1161/hc37t1.094706. I-68-75. [DOI] [PubMed] [Google Scholar]
- 27.Fukushima S, Kobayashi J, Bando K, Niwaya K, Tagusari O, Nakajima H, Kitamura S. Late outcomes after isolated coronary artery bypass grafting for ischemic mitral regurgitation. Jpn J Thorac Cardiovasc Surg. 2005;53:354–360. doi: 10.1007/s11748-005-0049-z. [DOI] [PubMed] [Google Scholar]
- 28.Milano Ca, Daneshmand Ma, Rankin JS, Honeycutt E, Williams ML, Swaminathan M, Linblad L, Shaw LK, Glower DD, Smith PK. Survival prognosis and surgical management of ischemic mitral regurgitation. Ann Thorac Surg. 2008;86:735–744. doi: 10.1016/j.athoracsur.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Grossi EA, Woo YJ, Patel N, Goldberg JD, Schwartz CF, Subramanian VA, Genco C, Goldman SM, Zenati MA, Wolfe JA, Mishra YK, Trehan N. Outcomes of coronary artery bypass grafting and reduction annuloplasty for functional ischemic mitral regurgitation: a prospective multicenter study (Randomized Evaluation of a Surgical Treatment for Off-Pump Repair of the Mitral Valve) J Thorac Cardiovasc Surg. 2011;141:91–97. doi: 10.1016/j.jtcvs.2010.08.057. [DOI] [PubMed] [Google Scholar]
- 30.Kang D-H, Sun BJ, Kim D-H, Yun S-C, Song J-M, Choo SJ, Chung CH, Song J-K, Lee J-W, Park S-W, Park S-J. Percutaneous Versus Surgical Revascularization in Patients With Ischemic Mitral Regurgitation. Circulation. 2011;124:S156–S162. doi: 10.1161/CIRCULATIONAHA.110.011254. [DOI] [PubMed] [Google Scholar]
- 31.Deja MA, Grayburn PA, Sun B, Rao V, She L, Krejca M, Jain AR, Chua YL, Daly R, Senni M, Mokrzycki K, Menicanti L, Oh JK, Michler R, Wrobel K, Lamy A, Velazquez EJ, Lee KL, Jones RH. Influence of Mitral Regurgitation Repair on Survival in the Surgical Treatment for Ischemic Heart Failure Trial. Circulation. 2012;125:2639–2648. doi: 10.1161/CIRCULATIONAHA.111.072256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bax J, Somer S, Braun J. Restrictive annuloplasty and coronary revascularization in ischemic mitral regurgitation results in reverse left ventricular remodelling. Circulation. 2004;110:II-103–II-108. doi: 10.1161/01.CIR.0000138196.06772.4e. [DOI] [PubMed] [Google Scholar]
- 33.Schofer J, Siminiak T, Haude M, Herrman JP, Vainer J, Wu JC, Levy WC, Mauri L, Feldman T, Kwong RY, Kaye DM, Duffy SJ, Tubler T, Degen H, Brandt MC, Van Bibber R, Goldberg S, Reuter DG, Hoppe UC. Percutaneous mitral annuloplasty for functional mitral regurgitation: results of the CARILLON Mitral Annuloplasty Device European Union Study. Circulation. 2009;120:326–333. doi: 10.1161/CIRCULATIONAHA.109.849885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 35.Rosati RA, McNeer JF, Starmer CF, Mittler BS, Morris JJ, Jr, Wallace AG. A new information system for medical practice. Arch Intern Med. 1975;135:1017–1024. [PubMed] [Google Scholar]
- 36.D'Agostino RB., Jr Propensity scores in cardiovascular research. Circulation. 2007;115:2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 37.Neter J, Wasserman W, Kutner MH. Applied linear statistical models : regression, analysis of variance, and experimental designs. Homewood, IL: Irwin; 1990. [Google Scholar]
- 38.Kishon Y, Oh JK, Schaff HV, Mullany CJ, Tajik AJ, Gersh BJ. Mitral valve operation in postinfarction rupture of a papillary muscle: immediate results and long-term follow-up of 22 patients. Mayo Clin Proc. 1992;67:1023–1030. doi: 10.1016/s0025-6196(12)61116-1. [DOI] [PubMed] [Google Scholar]
- 39.Yiu SF, Enriquez-Sarano M, Tribouilloy C, Seward JB, Tajik AJ. Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: A quantitative clinical study. Circulation. 2000;102:1400–1406. doi: 10.1161/01.cir.102.12.1400. [DOI] [PubMed] [Google Scholar]
- 40.Chan KM, Punjabi PP, Flather M, Wage R, Symmonds K, Roussin I, Rahman-Haley S, Pennell DJ, Kilner PJ, Dreyfus GD, Pepper JR, Investigators R. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation. 2012;126:2502–2510. doi: 10.1161/CIRCULATIONAHA.112.143818. [DOI] [PubMed] [Google Scholar]
- 41.Smith PK, Michler RE, Woo YJ, Alexander JH, Puskas JD, Parides MK, Hahn RT, Williams JB, Dent JM, Ferguson TB, Moquete E, Rose EA, Pagé P, Jeffries NO, O'Gara PT, Ascheim DD. Design, rationale, and initiation of the Surgical Interventions for Moderate Ischemic Mitral Regurgitation Trial: a report from the Cardiothoracic Surgical Trials Network. J Thorac Cardiovasc Surg. 2012;143:111–117. doi: 10.1016/j.jtcvs.2011.05.006. 117.e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weintraub WS, Grau-Sepulveda MV, Weiss JM, O'Brien SM, Peterson ED, Kolm P, Zhang Z, Klein LW, Shaw RE, McKay C, Ritzenthaler LL, Popma JJ, Messenger JC, Shahian DM, Grover FL, Mayer JE, Shewan CM, Garratt KN, Moussa ID, Dangas GD, Edwards FH. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366:1467–1476. doi: 10.1056/NEJMoa1110717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campwala SZ Wang N, Bansal R C. Mitral regurgitation progression following isolated coronary artery bypass surgery: frequency, risk factors, and potential prevention strategies. Eur J Cardio-Thorac Surg. 2006;29:348. doi: 10.1016/j.ejcts.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Pastorius CA, Henry TD, Harris KM. Long-term outcomes of patients with mitral regurgitation undergoing percutaneous coronary intervention. Am J Cardiol. 2007;100:1218–1223. doi: 10.1016/j.amjcard.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 45.Golba K, Mokrzycki K, Drozdz J, Cherniavsky A, Wrobel K, Roberts BJ, Haddad H, Maurer G, Yii M, Asch FM, Handschumacher MD, Holly TA, Przybylski R, Kron I, Schaff H, Aston S, Horton J, Lee KL, Velazquez EJ, Grayburn PA. Mechanisms of Functional Mitral Regurgitation in Ischemic Cardiomyopathy: A Transesophageal Echocardiography Substudy of the Surgical Treatment for Ischemic Heart Failure (STICH) Trial. Am J Cardiol. 2013;112:1812–1818. doi: 10.1016/j.amjcard.2013.07.047. Epub 2013 Sep 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan V, Ruel M, Mesana TG. Mitral valve replacement is a viable alternative to mitral valve repair for ischemic mitral regurgitation: a case-matched study. Ann Thorac Surg. 2011;92:1358–1365. doi: 10.1016/j.athoracsur.2011.05.056. discussion 1365-1356. [DOI] [PubMed] [Google Scholar]
- 47.Mihaljevic T, Rajeswaran J, Lam BK. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol. 2007;49:2191–2201. doi: 10.1016/j.jacc.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 48.Szalay ZA, Civelek A, Hohe S, Brunner-LaRocca HP, Klovekorn WP, Knez I, Vogt PR, Bauer EP. Mitral annuloplasty in patients with ischemic versus dilated cardiomyopathy. Eur J Cardiothorac Surg. 2003;23:567–572. doi: 10.1016/s1010-7940(02)00864-3. [DOI] [PubMed] [Google Scholar]
- 49.Bolling SF, Smolens IA, Pagani FD. Surgical alternatives for heart failure. J Heart Lung Transplant. 2001;20:729–733. doi: 10.1016/s1053-2498(01)00239-x. [DOI] [PubMed] [Google Scholar]
- 50.Westenberg JJ, van der Geest RJ, Lamb HJ, Versteegh MI, Braun J, Doornbos J, de Roos A, van der Wall EE, Dion RA, Reiber JH, Bax JJ. MRI to evaluate left atrial and ventricular reverse remodeling after restrictive mitral annuloplasty in dilated cardiomyopathy. Circulation. 2005;112:I437–I442. doi: 10.1161/CIRCULATIONAHA.104.525659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.