Summary
Cancer immunotherapy has great promise, but is limited by diverse mechanisms used by tumors to prevent sustained antitumor immune responses. Tumors disrupt antigen presentation, T/NK–cell activation, and T/NK–cell homing through soluble and cell-surface mediators, the vasculature, and immunosuppressive cells such as myeloid-derived suppressor cells and regulatory T cells. However, many molecular mechanisms preventing the efficacy of antitumor immunity have been identified and can be disrupted by combination immunotherapy. Here, we examine immunosuppressive mechanisms exploited by tumors and provide insights into the therapies under development to overcome them, focusing on lymphocyte traffic.
INTRODUCTION
Cancer immunotherapy is a rapidly developing field that has yielded impressive breakthroughs. Although numerous approaches are under development, two stand out. The use of monoclonal antibodies (mAb) blocking key inhibitory receptors of T cells has led to robust antitumor immune response activation and has proved effective across multiple tumor types (1). Second, robust clinical responses have been seen with the adoptive transfer of tumor-specific autologous T cells, harvested from tumors (an approach that has been specifically tested in melanoma) or generated ex vivo through the insertion of exogenous receptors that recognize cancer cells, such as cloned T-cell receptors (TCR) or chimeric antigen receptors (CAR; ref. 2). However, despite recent successes, many patients with cancer fail to respond. In some cases, it is possible that lack of therapeutic response is due to a failure of effector T cells to reach into tumors. Tumors develop vascular barriers to T-cell homing and can thus dampen the efficacy of immunotherapy.
T-CELL INFILTRATION IS IMPORTANT FOR OVERALL SURVIVAL
In tumor immunology, size matters. Key limiting factors of the tumor-containing capacity of antitumor immune effector cells are their actual numbers, relative frequency, and functional capabilities in tumors. The killer (effector) to target ratio is crucial for the fraction of tumor cells eventually killed both in vitro and in vivo. Importantly, the baseline infiltration by T lymphocytes has important prognostic implications for different types of solid tumors. Many groups have demonstrated that the presence of intratumoral CD8+ and CD4+ T cells is independently associated with a good clinical outcome (3). It is unclear what drives spontaneous T-cell infiltration within tumors, but epigenetic changes and/or mutations could control these phenotypes. In colorectal cancer, it has been shown that a T cell–based cellular and molecular tumor profile predicts survival better than either surgical staging or histologic evaluation for other risk factors such as invasiveness. In particular, transcriptomic footprints in tumors denoting antigen-experienced T cells and T-cell activation have been shown to be advantageous (4). In this line, it has been shown that response to immunotherapy is better in melanoma patients with baseline T-cell infiltration (5). Therefore, it is quite plausible that T-cell infiltration and circulation into tumors (as depicted in Fig. 1) can be a key limiting factor for immunotherapy.
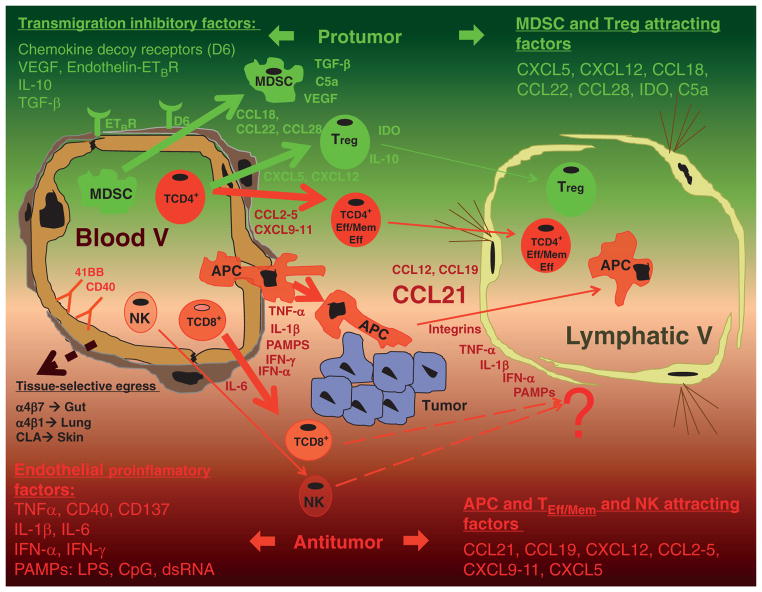
Figure 1.
Schematic representation of tumor microenvironment with leukocytes entering through blood capillaries and exiting via afferent lymphatic vessels. Antitumor mechanisms favoring the entrance of tumor-destroying leukocytes are depicted in green. Protumor mechanisms based on the interference with effector and memory T-cell entrance, entrance of Tregs, and MDSCs are represented in red. Efferent lymphatic (V) vessels control traffic of DCs ferrying antigen cargo to draining lymph nodes and may play a vital role in recirculation of memory T lymphocytes. dsRNA, double-stranded RNA; LPS, lipopolysaccharide; APC, antigen-presenting cells; PAMP, pathogen associated molecular pattern; IDO, indoleamine 2,3-dioxygenase; DC, dendritic cell; MDSC, myeloid-derived suppressor cell.
NK cells are infrequently seen in tumor biopsies. However, several studies have shown that intratumoral NK cells can also be associated with increased survival (6). Thus, strategies that would increase infiltration of NK lymphocytes into tumors would be a plausible strategy to enhance antitumor efficacy. Such strategies would expectedly also enhance treatments with antitumor mAbs that elicit NK cell–based antibody-dependent cell-mediated cytotoxicity (ADCC), for example, Herceptin or cetuximab. However, NK-cell presence may not be always beneficial, as NK cells could suppress CD8+ T-cell responses during chronic infections (7, 8).
Unraveling the mechanisms of T and NK cell and recruitment can provide important therapeutic targets that can be pharmacologically validated. Robust examples of targeting T-cell trafficking come from the field of autoimmunity and transplantation, with antibodies against VLA-4, α7β1, and pharmacologic antagonists for S1PRs. Contrary strategies aimed at enhancing inbound leukocyte traffic to tumors hold promise for immune tumor rejection.
MOLECULAR TRICKS PLAYED BY TUMORS TO ATTRACT PROTUMOR LEUKOCYTES AND REPRESS EFFECTOR T-CELL RESPONSES
Tumors manipulate the immune system through diverse mechanisms, shaping it to promote tumor growth and dissemination. This is achieved by disruption of the generation of an immune response, inhibition of antitumor T-cell activation, and blockade of effector T-cell recruitment, and through recruitment of immunosuppressive cells.
A primary mechanism of immune evasion is through inhibiting normal dendritic cell (DC) activation and maturation, while promoting a tolerogenic DC phenotype. Tumors secrete a number of factors that contribute to this role, and chief among them is VEGF-A (9). Tumor-derived VEGF prevents DC maturation in patients and mice and impairs the generation of an antitumor response. A growing body of evidence has also implicated a number of additional tumor-derived factors that can disrupt normal DC functions, including TGF-β and interleukin (IL)-10, as well as physiologic stimuli such as hypoxia and lactic acid.
An additional mechanism limiting the antitumor immune response is the deregulation of immune-cell recruitment. Particularly, the recruitment of suppressive myeloid cells, such as myeloid-derived suppressor cells (MDSC), tumor-associated macrophages (TAM), immature DCs, and immunosuppressive neutrophils, is a chief mechanism of immunosuppression (10). Recruitment is mediated by diverse chemokines that are overexpressed by tumor cells due to genetic alterations or physiologic stimuli such as hypoxia. Tumors can also alter the chemistry of certain chemokines to preferentially recruit myeloid cells such as the nitrosylated CCL2, which selectively recruits MDSCs to tumor sites, while eliminating the ability to recruit CTLs and Th1 effector cells (11). Recruitment of regulatory T cells (Treg) is an additional mechanism of immunosuppression, and under hypoxic stress, tumors secrete the chemokine CCL28, which selectively attracts Tregs and promotes tumor angiogenesis (12).
Upon arrival within the tumor environment, antitumor T cells face the important task of overcoming local immunosuppression. A number of soluble and cell-surface molecules expressed by tumors as well as resident cells, such as MDSCs, inhibit effector T-cell functions that can control tumor growth. T-cell killing, cytokine elaboration, and survival are all negatively affected within the tumor environment. For example, the programmed death-1 (PD-1) receptor and its ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC), are among the most potent T-cell suppression pathways. These are only a few of the first examples of the panoply of molecular mechanisms likely to be discovered. In this regard, it is likely that inhibitory molecules expressed at the tumor microenvironment would ultimately deregulate chemokine circuitries that attract Th1 cells and cytotoxic lymphocytes (CTL).
BLOCKING T-CELL ENTRANCE AND FUNCTIONS IN CANCER
Tumors exploit a number of mechanisms to limit T-cell homing and infiltration. Particularly for patients without preexisting tumor-infiltrating lymphocytes (TIL), these are likely effective barriers to successful immunotherapy. Below, we focus on the ways tumors disrupt T-cell trafficking.
Disruption of T-Cell Homing by Tumors
Leukocyte trafficking across vascular endothelial barriers into tissues is highly regulated. In nonlymphoid tissues, resting endothelial cells lining capillaries and venules are normally refractory to leukocyte entry. Transmigration into tissue is achieved only occasionally by monocytes and a few recirculating T lymphocytes. However, when septic or sterile inflammation is elicited, endothelial cells, tissue-resident leukocytes, parenchymal cells, and epithelial cells produce chemokines, which along with proteolytic fragments, as well as complement anaphylatoxins (e.g., C5a), attract leukocyte subsets. Extravasation of leukocytes is elicited by gradients of chemokines expressed on endothelial cells and within the extracellular matrix. Upon exposure to chemokines (as well as proinflammatory cytokines or antigen), integrins expressed on activated leukocytes acquire an active conformation conferring high affinity for their cognate ligands. A parallel event is the induction of surface ICAM-1 and VCAM on endothelial cells, which is mediated by factors that activate NF-κB (e.g., TNFα). LFA-1 (CD11a/CD18) and VLA-4 (α4β1) are the principal mediators of leukocyte extravasation recognizing endothelial ICAM-1 and VCAM. Selectins and selectin ligands mediate additional but weaker adhesive interactions, based on lectin to carbohydrate chain recognition.
Tumors with high numbers of T cells express high amounts of established T cell–attracting chemokines, including chemokine (C–C motif) ligand (CCL) 2, CCL3, CCL4, CCL5, chemokine (C–X–C motif) ligand (CXCL) 9, and CXCL10 (13). The initiating events are unclear, but the pattern of T-cell infiltration in mouse models suggests that a few T cells infiltrate tumors initially, followed by a large influx of both specific and nonspecific T cells (14). There are several ways in which tumors interfere with chemokine signaling. In skin tumors, aberrant EGF receptor (EGFR)–RAS signaling has been shown to suppress the production of CCL27, a chemokine constitutively expressed by normal keratinocytes, and disruption of CCL27 expression in vivo prevented T-cell homing to skin tumors and accelerated tumor outgrowth in a mouse model (15). In addition, CCL2, an important chemokine for the recruitment of CTLs to the tumor site, undergoes nitrosylation induced by reactive nitrogen species in the tumor microenvironment, which abrogates its ability to attract tumor-specific CTLs (11). Furthermore, altered proteolytic processing of CXCL11, an important chemokine recruiting CXCR3+ effector T cells, can impair binding and signaling of the chemokine, ultimately reducing lymphocyte migration (16). Thus, deregulation of chemokine expression is an important mechanism preventing T-cell infiltration and homing.
The Aberrant Vasculature of Tumors
The vascular system develops through the coordinated actions of both vasculogenesis and angiogenesis. Physiologic angiogenesis typically occurs during development and wound healing, and proceeds through vessel destabilization, sprouting, endothelial migration, and proliferation, followed by resolution and stabilization of the new vessel. Pathologic angiogenesis, a key feature of tumor biology, shares many of the same processes, but it is characterized by a failure of the resolution phase, which leads to the generation of a highly disorganized vascular network.
Mounting experimental evidence indicates that the tumor vasculature can be a substantial barrier to the extravasation of the tumor-reactive T cells and to the success of immunotherapies. Although activated T cells could be documented in the periphery in experimental models of cancer immunotherapy, they often fail to infiltrate the tumor itself (17). The prohibitive nature of the tumor endothelium is likely maintained by the coordinated actions of immunosuppressive, proangiogenic growth factors such as VEGF, as well as angiogenesis-associated myeloid cells (MDSCs and TAMs) that directly suppress T-cell functions and promote pathologic angiogenesis (18). Under the influence of these factors, the tumor endothelium downregulates the expression of adhesion molecules, limiting extravasation of T cells. For instance, tumor endothelial cells can express high levels of the endothelin B receptor (ETBR) and, under the influence of the cognate ligand endothelin-1 produced by tumor cells, develop an “anergic” phenotype in which expression of key homing adhesion molecules for T cells, such as ICAM-1, is deregulated (18). In a similar vein, VEGF and basic fibroblast growth factor signaling on endothelial cells can repress adhesion molecule expression and prevent T-cell infiltration. In addition, there is evidence that the tumor endothelium can contribute to the composition of T-cell infiltration in tumors, selectively allowing homing of specific lymphocyte subsets according to their “polarization” (Th1 vs. Th2, Th17 or Treg), phenotype, or activation status.
It is emerging that the tumor vasculature can also shape the nature of T-cell infiltration in tumors through direct immunosuppression. Endothelial cells can express a number of mediators that suppress or kill effector lymphocytes such as TRAIL, PD-L1, PD-L2, B7-H3, galectin-1, IL-10, TGF-β, and PGE2. Metabolic disruption of T cells through indoleamine 2,3-dioxygenase (IDO) expression and arginase-I by endothelial cells has also been suggested, but whether these mechanisms are active in tumors is unknown. Whatever the mechanism, it has been shown that a primary function of tumor endothelial cells is largely immunosuppressive, maintained by tumor cells through paracrine mechanisms (19).
HOW TO MODIFY T-CELL INFILTRATION TO HELP IMMUNOTHERAPY
Despite the large number of immunosuppressive mechanisms operating in tumor microenvironments, a large number of recent clinical successes that rely on immunotherapy clearly indicate the power of targeting these pathways. There are presently numerous ways to target immunosuppressive pathways and mobilize antitumor immunity in vivo or ex vivo: (1) Effective antitumor T cells can be expanded ex vivo from natural TILs, or from T cells transduced with exogenous cloned TCRs or CARs, and the host can be conditioned by lymphodepletion to optimize their engraftment; (2) endogenous T cells can be effectively activated by pharmacologic checkpoint inhibitors; (3) the vasculature can be disrupted by low-dose metronomic chemotherapy or normalized by drugs targeting angiogenesis; (4) soluble immunosuppressive factors, such as IDO, IL-6, IL-10, TGF-β, and PGE2, can be individually blocked by specific pharmacologic inhibitors; (5) suppressor cells can be depleted by specific chemotherapy drugs such as cyclophosphamide (for Tregs) or gemcitabine (for MDSCs); (6) endogenous tumor-specific T-cell immunity can be effectively boosted by exogenous vaccines; and (7) tumor-associated antigen-presenting cells (APC) can be activated by specific drugs, including Toll-like receptor agonists, whereas tumor antigens can be released in situ by immunogenic cell death induced by specific chemotherapy drugs or radiation.
Tumors with Preexisting TILs
In tumors with preexisting TILs, one can assume that the tumor microenvironment does not offer major barriers to T-cell homing. For such patients, the use of therapies aimed at relieving local immunosuppression or enhancing the homing of adoptively transferred T cells would be key. For example, the use of CAR T cells or ex vivo–expanded TILs that have introduced expression of homing receptors, such as CCR2 (20), could significantly enhance the success of adoptive cell transfer (ACT) in these patients. In addition, the use of PD–PD-L1–PD-L2 axis and/or CTLA-4 inhibitors could significantly promote antitumor immunity in patients with preexisting TILs. For example, ipiliumab (anti–CTLA-4) has shown an overall survival benefit in two randomized, phase III trials in patients with advanced melanoma. The response rates were modest, between 10% and 15%, but a significant survival advantage was observed in the treated populations. PD-1 is an additional coinhibitory receptor expressed on activated T cells. In early clinical studies using either anti-PD1 or anti–PD-L1 antibody, a large number of patients have demonstrated objective clinical responses (21). There have been encouraging preclinical results with dual blockade of PD-1 and CTLA-4 (22), and clinical data are still being collected. The fact that many patients still do not respond to CTLA-4 and/or PD-1 blockade may suggest that additional signals are required to effectively rescue TIL function. A variety of additional coinhibitory (LAG-3, TIM-3, BTLA, 2B4, KLRG-1, CD160, etc.) and costimulatory receptors (CD28, 4-1BB, OX-40, GITR, etc.) have been identified. The additional blockade or agonist stimulation of these targets could enhance immunotherapy (1).
Tumors Lacking Preexisting TILs
Tumors with preexisting TILs seem intrinsically more immunogenic and easier to approach therapeutically, whereas tumors lacking TILs may be more complex. A prohibitive tumor endothelial barrier is likely a primary reason why T cells may not be able to infiltrate tumors. Proangiogenic signals can deregulate adhesive properties of the tumor endothelium and, thus, antiangiogenesis therapy could be contributory to the activation of antitumor immune response (18). However, the fact that VEGF inhibition has not been beneficial in many human solid tumor types (23) indicates that combinatorial approaches will likely be required, such as the use of VEGF inhibitors, or additional inhibitors of angiogenesis pathways, and ACT.
Several methods to transform the tumor microenvironment so as to become infiltrated by T cells have proved effective in mouse cancer models. Local intratumoral delivery of cytokines, chemokines, or pathogen associated molecular patterns (PAMP) is a plausible approach (24). Among the cytokines that best induce chemoattractants, the IL-12–IFN-γ–CXCL10/11 axis stands out, together with type I IFN-α and IFN-β. Furthermore, administration of radiotherapy and/or chemotherapy could feasibly alter the tumor microenvironment by releasing danger signals from necrotic tumor cells (25).
An alternative approach would be to manipulate the tumor endothelium, allowing for the infiltration of tumor-reactive T cells. This could be achieved through selective blockade of known factors that limit T-cell infiltration. For instance, blockade of the endothelin receptor ETB R leads to increased T-cell infiltration and limited tumor growth by reducing the effects of ET-1 on adhesion molecule inhibition (17).
Activated T lymphocytes are a major source of proinflammatory cytokines and chemokines, and these functions are among those derepressed by mAbs acting as checkpoint inhibitors. In addition, some immunostimulatory mAbs, such as anti-CD40 and anti-41BB (CD137), may act directly on tumor-associated capillary vessels via the induction of adhesion molecules and chemokines, further promoting antitumor activity (26).
It is important to consider that activated T cells in the tumor microenvironment are or may become powerful sources of chemokines attracting sister T cells. Conceivably, once some properly activated or derepressed T lymphocytes are behind the cancerous enemy lines, they can recruit larger numbers of effector lymphocytes from circulation in a positive feedback loop. Unfortunately, some of these lymphocyte-produced chemo-attractants bring Tregs and MDSCs into the tumor tissue.
Another fascinating discovery is the fact that T lymphocytes remember the site where they first recognized antigen and continue to express chemokine receptors and homing receptors enabling them to recirculate through the same tissue, presumably patrolling for residual antigen (27). This behavior is imprinted in T cells by tissue-resident DCs and is probably maintained as a result of epigenetic regulation of gene expression. It is becoming clear that tissues can recruit lymphocytes in a specific manner using chemokine combinations. For example, CLA, CCR4, and CCR10 drive skin-homing lymphocytes, CCR4 and VLA-1 drive lymphocytes to the respiratory tract, and α4β7 and CCR9 direct lymphocytes to the gut. This phenomenon has been compared with bar coding, whereby memory T lymphocytes with the appropriate specificities recirculate to the peripheral and lymphoid tissues in which antigen is more likely to appear. This has profound implications for the route of vaccination with cancer antigens (28) and for the priming of T cells to be used in adoptive transfer therapy.
CONCLUDING REMARKS
In our opinion, T-cell and NK-cell trafficking is a largely neglected strategy to enhance the efficacy of tumor immunotherapy. As a monotherapy it may be weak, but it offers clear potential to act synergistically. Synergy could be achieved with immunostimulatory mAbs (anti–PD-1/PD-L1; anti–CTLA-4, anti-CD137, anti-CD40, or anti-OX40), with adoptive T-cell therapy (with TILs or engineered lymphocytes), with cytotoxic monoclonal antibodies, and with cancer vaccines. Next-generation cancer immunotherapy should harness the trafficking of immune cells to malignant tissue to achieve superior cancer therapies.
Acknowledgments
Grant Support
This study has been financially supported by Ministerio de Economia SAF2011-22831, Redes temáticas de investigación cooperativa (RETIC) RD06/0020/0065, and EU 7th Framework Program IACT (to I. Melero); and NIH Transformative R01 CA156695 and ERC 322875 (to G. Coukos).
Footnotes
Disclosure of Potential Conflicts of Interest
I. Melero is a consultant/advisory board member of Bristol-Myers Squibb, Merck Serono, and Medimmune. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Melero I, Grimaldi A, Perez Gracia J, Ascierto P. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013;19:997–1008. doi: 10.1158/1078-0432.CCR-12-2214. [DOI] [PubMed] [Google Scholar]
- 2.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39:49–60. doi: 10.1016/j.immuni.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 4.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 5.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J. 2010;16:399–403. doi: 10.1097/PPO.0b013e3181eacbd8. [DOI] [PubMed] [Google Scholar]
- 6.Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–8. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 7.Lang PA, Lang KS, Xu HC, Grusdat M, Parish IA, Recher M, et al. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci U S A. 2012;109:1210–5. doi: 10.1073/pnas.1118834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook KD, Whitmire JK. The depletion of NK cells prevents T cell exhaustion to efficiently control disseminating virus infection. J Immunol. 2013;190:641–9. doi: 10.4049/jimmunol.1202448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 10.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–62. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–30. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 13.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–85. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204:345–56. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pivarcsi A, Müller A, Hippe A, Rieker J, van Lierop A, Steinhoff M, et al. Tumor immune escape by the loss of homeostatic chemokine expression. Proc Natl Acad Sci U S A. 2007;104:19055–60. doi: 10.1073/pnas.0705673104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proost P, Mortier A, Loos T, Vandercappellen J, Gouwy M, Ronsse I, et al. Proteolytic processing of CXCL11 by CD13/aminopeptidase N impairs CXCR3 and CXCR7 binding and signaling and reduces lymphocyte and endothelial cell migration. Blood. 2007;110:37–44. doi: 10.1182/blood-2006-10-049072. [DOI] [PubMed] [Google Scholar]
- 17.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 18.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11:702–11. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- 19.Mulligan JK, Day TA, Gillespie MB, Rosenzweig SA, Young MR. Secretion of vascular endothelial growth factor by oral squamous cell carcinoma cells skews endothelial cells to suppress T-cell functions. Hum Immunol. 2009;70:375–82. doi: 10.1016/j.humimm.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon EK, Carpenito C, Sun J, Wang LC, Kapoor V, Predina J, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res. 2011;17:4719–30. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–12. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebos JM, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8:210–21. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28:4324–32. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 26.Palazón A, Teijeira A, Martínez-Forero I, Hervás-Stubbs S, Roncal C, Peñuelas I, et al. Agonist anti-CD137 mAb act on tumor endothelial cells to enhance recruitment of activated T lymphocytes. Cancer Res. 2011;71:801–11. doi: 10.1158/0008-5472.CAN-10-1733. [DOI] [PubMed] [Google Scholar]
- 27.Mikhak Z, Strassner JP, Luster AD. Lung dendritic cells imprint T cell lung homing and promote lung immunity through the chemokine receptor CCR4. J Exp Med. 2013;210:1855–69. doi: 10.1084/jem.20130091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandoval F, Terme M, Nizard M, Badoual C, Bureau MF, Freyburger L, et al. Mucosal imprinting of vaccine-induced CD8+ T cells is crucial to inhibit the growth of mucosal tumors. Sci Transl Med. 2013;5:172ra20. doi: 10.1126/scitranslmed.3004888. [DOI] [PMC free article] [PubMed] [Google Scholar]