Abstract
Folium mori (桑葉 Sāng Yè, leaf of Morus alba L.; FM) is known to possess hypoglycemic effects, and 1-deoxynojirimycin (1-DNJ) has been proposed as an important functional compound in FM. However, the hypoglycemic activity of purified 1-DNJ has been rarely studied. It is also not known how FM and 1-DNJ affect the development of DM nephropathy. This study compared the antidiabetic effect of a commercial FM product with that of purified 1-DNJ in streptozotocin-induced diabetic rats. Seven days after induction, the diabetic rats were gavaged with FM (1, 3, 10, and 30 mg/kg/day), 1-DNJ (30 mg/kg/day), or vehicle (distilled deionized water; 2 ml/kg/day) for 7 days. All doses of FM ameliorated fasting and post-prandial blood glucose concomitantly with an increase in peripheral and pancreatic levels of insulin and improved homeostasis model assessment (HOMA-IR) in diabetic rats in a dose-dependent manner. Increased thiobarbituric acid reactive substances (TBARS) and nitrate/nitrite levels in the kidney, liver, and muscle of diabetic rats were reversed by all doses of FM. The renal function of the diabetic rats was normalized by all doses of FM, while blood pressure changes were reversed by FM at doses of 3 mg/kg and above. Moreover, most of the above-mentioned parameters were improved by FM at doses of 3 mg/kg and above to a similar extent as that of 1-DNJ. The results showed superior antidiabetic potential of the commercial FM product for glycemic control and protection against the development of diabetic nephropathy.
Keywords: 1-Deoxynojirimycin, Diabetes mellitus, Folium mori, Nephropathy, Streptozotocin
INTRODUCTION
White mulberry (桑白皮 Sāng Bái Pí; Morus alba L.) is well known as a traditional Chinese herb for the treatment of various diseases and has been approved in China as an antidiabetic herbal drug.[1,2] The earliest report on the property and use of folium mori (桑葉 Sāng Yè; FM), leaf of M. alba L., appeared in Han dynasty (from 25th to 27th century BC) in the Divine Husbandman's Herbal Foundation Canon (神農本草經 Shén Nóng Běn Cǎo Jing). Traditional use of FM has been to relieve wind-heat exterior syndrome, to moisten the lung effectively to relieve cough and lung dryness, to treat dizziness and headache caused by liver heat, to remove liver heat to improve eyesight, and to cool blood and stop bleeding. In the late 16th century, Li Shi-Zhen (李時珍 Lǐ Shízhēn), a Ming botanist, pharmacologist, and the author of the Compendium of Materia Medica (本草綱目 Běn Cǎo Gāng Mù), reported FM tea being a useful therapy for diabetes. In Taiwan, white mulberry tree is cultivated in large areas, and the commercial product of its leaf preparation is popular as a complementary/alternative medicine for disorders including hyperglycemia, hypertension, and dyslipidemia. Recent scientific evidences have confirmed that the dried powder, water extract, and ethanol extract of the FM (leaf of M. alba L.) possess diverse biological activities, including neuroprotective, antimicrobial, antioxidant, anti-inflammatory, anti-tumor, anti-atherosclerotic, and hypoglycemic actions.[3]
Diabetes is a common chronic and systemic disorder that causes disability and threatens the lives of people worldwide. In the field of research on natural products as anti-diabetic remedies, FM has raised considerable interest. Studies with diabetic animals have demonstrated that treatment with FM can acutely or chronically lower blood glucose levels in hyperglycemic animals and humans.[4,5,6,7,8] An earlier understanding of the mechanisms that underlie FM's function as a hypoglycemic agent includes its ability to curb the desire for food under diabetic conditions,[5] to inhibit the activities of intestinal enzymes involved in the digestion of carbohydrates,[6] and to inhibit glucose absorption in the small intestine.[8] Limited studies on human beings revealed that the activities of sucrase and certain enzymes involved in the digestion of other disaccharides were largely inhibited by FM,[6] and that in control and type 2 diabetes mellitus (DM) patients, co-ingestion of mulberry extract with sucrose significantly reduced the increase in postprandial blood glucose levels.[9]
The components of FM that may contribute to its hypoglycemic activity have been proposed to be the following: total flavonoids as it was found that this fraction is able to inhibit small intestine disaccharidases in diabetic rats,[10] polysaccharides as it was reported that this fraction is able to scavenge hydroxyl radicals and superoxide anion radical in vivo,[11] or iminosugars [i.e. 1-deoxynojirimicin (1-DNJ)] as it was observed that 1-DNJ is able to inhibit the activity of α-glucosidase.[12,13] In addition, Hunyadi et al. summarized that chlorogenic acid and rutin account for as much as half the observed anti-diabetic activity of FM.[14]
We are fascinated by the idea that 1-DNJ in FM is an important anti-diabetic com pound, because certain efforts have been made by researchers using various approaches to enrich 1-DNJ in FM preparations in order to improve their anti-diabetic activity.[15,16,17] Studies on human beings have revealed that a 1-DNJ–enriched FM preparation is an effective hypoglycemic agent in control and type 2 DM patients.[9,18] However, a limited number of studies have been conducted on the hypoglycemic activity of purified 1-DNJ in diabetic animals.[11,19] From the practical point of view, the question that arises in our mind is how important it is to emphasize the antidiabetic role of 1-DNJ in FM if the raw extract of FM already possesses significant activity. Consequently, we compared the anti-diabetic activity of FM with that of purified 1-DNJ. In this study, we also aimed to investigate the effect of these FM preparations on the development of diabetic nephropathy, which, to the authors’ knowledge, has not been revealed thus far.
MATERIALS AND METHODS
Plant material and extraction
The spray-drie d FM (桑葉 Sāng Yè) water extract preparation used in this study was kindly provided by Chin Ang Pharmaceutical Co., Ltd (Chiayi, Taiwan). Fresh M. alba L. leaves were collected from a farm in Chiayi, a county in central Taiwan. One gram of the spray-dried FM water extract preparation was generated from 10 g of dried white mulberry (桑白皮 Sāng Bái Pí) leaf, and was composed of 1.22% 1-DNJ, according to a high-performance liquid chromatography (HPLC) analysis as described by Ouyang et al.,[20] with modifications. Briefly, 1-DNJ in FM was extracted with 0.05 mol/l of HCl and made to react with fluorenylmethoxycarbonyl (FMOC)-Cl at 20°C for 20 min, followed by the addition of 0.1 mol/l of glycine and 0.1% acetic acid aqueous solution (v/v). After filtration through a 0.45 μm filter, the DNJ–FMOC derivative in the sample was separated on SUPELCO Ascentis 5-μm C18-A column (Waters, Milford, MA, USA) at 25°C. The mobile phase consisted of acetonitrile: 0.1% aqueous acetic acid (55:45) with a flow rate of 1.0 ml/min. The fluorescence detector (Waters 2475 Multil λ Fluorescence Detector; Waters) was operated at λex = 254 nm and λem = 322 nm. The spectrum of HPLC analysis of 1-DNJ standard and of 1-DNJ in FM extract is shown in Figure 1.
Figure 1.
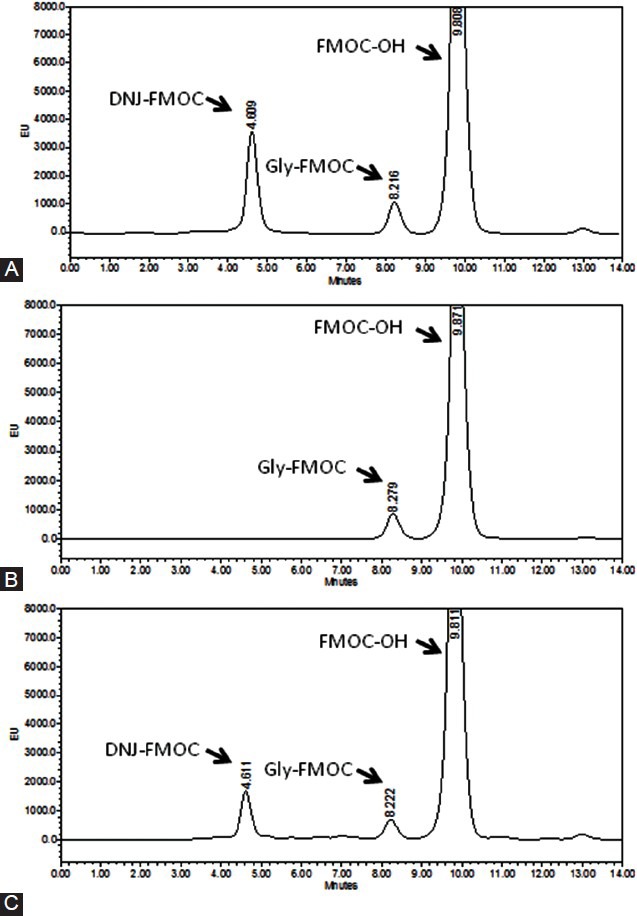
High-performance liquid chromatography (HPLC) spectrum of (A) 1-DNJ standard, (B) blank, and (C) FM preparation. 1-DNJ in the FM preparation was extracted with 0.05 mol/l HCl, made to react with fluorenylmethoxycarbonyl (FMOC)-Cl to generate the DNJ–FMOC derivative, and separated on the SUPELCO Ascentis 5-μm C18-A column at 25°C. The mobile phase consisted of acetonitrile:0.1% aqueous acetic acid (55:45) with a flow rate of 1.0 ml/min. The fluorescence detector was operated at λex = 254 nm and λem = 322 nm
Animals and experimental procedures
Four-week-old weanling male Wistar rats were purchased from the National Animal Breeding and Research Center (Taipei, Taiwan). The animals were maintained under a 12 h light–dark cycle at an ambient temperature of 23°C, and were given free access to water and standard rat feed (Rodent Diet 5001; Purina Mills, Richmond, IN, USA). All animals were allowed to adapt to the environment for 1 week after their arrival, before beginning the experiment. Diabetes was induced by injecting streptozotocin (Sigma, St Louis, MO, USA) (i.v., 65 mg/kg body weight), and the control rats were injected with the same volume of vehicle as described by Liu et al.[21] One week after the injection, the diabetic animals were randomly assigned to six groups which received FM extract (1, 3, 10, or 30 mg/kg body weight/day), 1-DNJ (Tocris Bioscience; Bristol, UK) (30 mg/kg body weight/day), or vehicle (distilled and deionized water; 2 ml/kg body weight/day) by gavage, respectively, for seven consecutive days. The control rats received the vehicle only. During the experimental period, the animals were housed in metabolic cages and were given free access to water and a powdered diet (Rat Diet 5012; Purina Mills). Food and water intake and urine excretion were measured. Li et al. reported that 1-DNJ was able to improve glycemic control in alloxan-induced diabetic mice at doses of 50 and 100 mg/kg.[10,11] In the present study, a dose of 30 mg/kg 1-DNJ was used for comparison purposes because our preliminary study showed that FM was effective in improving fasting blood glucose levels in STZ-induced diabetic rats in a dose-dependent manner between the doses of 1 and 30 mg/kg.
The oral glucose tolerance test (OGTT) and blood pressure determination of the animals were conducted on days 11 and 13 after induction, respectively. The animals were then starved overnight before they were sacrificed by carbon dioxide euthanasia on day 14 after injection. Urine collected during the last 24 h of the animal's life was used to measure creatinine concentrations. Blood collected immediately after the animals were sacrificed was used to measure the concentrations of glucose, insulin, creatinine, and urea nitrogen. At the time the animals were sacrificed, the liver, kidney, soleus muscle, extensor digitorum longus muscle, and gastrocnemius muscle were isolated and weighed, and the ratio of organ tissue to body weight was calculated. Kidney weight was defined as the sum of weights of the right and left kidneys for each animal. Housing conditions and experimental procedures were in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the ethical committee for animal experimentation at Chung Shan Medical University, Taichung, Taiwan.
Oral glucose tolerance test
The OGTT was performed by administering a solution of 10% (w/v) glucose (1 g/kg body weight) by oral gavage. Blood samples were withdrawn from the lateral tail vein immediately before and 15, 30, 45, 60, 90, and 120 min after the bolus glucose loading. Heparin-containing blood samples were immediately centrifuged, and the plasma was separated and frozen at −20°C until it was analyzed for glucose and insulin. The area under the curve (AUC) of the blood glucose and insulin response to oral glucose loading was calculated by the trapezoidal rule.
Systolic blood pressure determination
Systolic blood pressure was determined in the conscious state by the indirect tail cuff method using a Model MK-2000 BP monitor for rats and mice (Muromachi Kikai, Tokyo, Japan) according to the manufacturer's instructions. The measurement was performed under room temperature conditions (24°C).
Biochemical analysis of blood and tissue/organ samples
For glucose analysis, plasma was deproteinized and glucose concentrations were determined enzymatically.[20] Plasma insulin concentration was determined spectrophotometrically with a rat insulin enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions. The insulin resistance index, as assessed by homeostasis model assessment (HOMA-IR), was calculated to estimate peripheral insulin resistance after treatment according to the following formula as described by Matthews et al.:
fasting plasma glucose (mg/dl) × fasting plasma insulin (μU/ml)/405.[22]
Immediately following the removal of the pancreas, the organ was irrigated with cold phosphate buffered saline (PBS) (pH 7.2) containing 1 mM phenylmethylsulfonyl fluoride to inhibit protease activity and was stored at −80°C until it was assayed for insulin with the rat insulin ELISA kit (Mercodia, Uppsala, Sweden) as stated above. Immediately following the removal of the liver, gastrocnemius muscle, and kidney, the tissues/organs were clamped in liquid nitrogen and then stored at −80°C until the lipid peroxidation level and nitrate/nitrite content were determined. The lipid peroxidation level was determined by measuring thiobarbituric acid reactive substances (TBARS) using a fluorescence spectrophotometer (Hitachi F4500; Hitachi Ltd, Tokyo, Japan). The nitrate/nitrite levels in the samples were measured spectrophotometrically using the nitrate/nitrite kit (Cayman, Ann Arbor, MI, USA) according to the manufacturer's instructions and were analyzed with a micro-plate reader (VersaMax; Molecular Devices Ltd, Sunnyvale, CA, USA). Protein assays were performed by using Bio-Rad protein assay kits (Bio-Rad Laboratories, Richmond, CA, USA).
Creatinine concentrations in the plasma and urine were determined with the Creatinine Reagent Set Kit (Teco Diagnostics, Anaheim, CA, USA), and blood urea nitrogen (BUN) was determined with the QuantiChrom™ Urea Assay Kit (DIUR-500) (BioAssay Systems, Hayward, CA, USA) according to the manufacturer's instructions. The results were analyzed with the micro-plate reader (VersaMax; Molecular Devices Ltd). Creatinine clearance rate (CCR) was calculated using the standard formula to determine the capacity of glomerular filtration. The glomerular filtration rate (GFR) was also expressed as GFR1 or GFR2 by dividing the CCR by the kidney weight or body weight, respectively.
Histological analysis of glomeruli
Kidneys fixed in 10% neutral buffered formalin were embedded in paraffin, sectioned into 5-μm sections, and stained with hematoxylin and eosin to evaluate the glomerular morphology by light microscopy.
Statistical analysis
The data were expressed as mean ± SD. The data were analyzed by one-way analysis of variance. Student's t-test was used to detect differences between the means of the control and diabetic groups. Duncan's multiple comparison test was used to detect differences in the means among the STZ-injected groups. P < 0.05 were considered statistically significant. All statistical analyses were performed with commercially available software (SPSS Inc., Chicago, IL, USA).
RESULTS
Animal characteristics
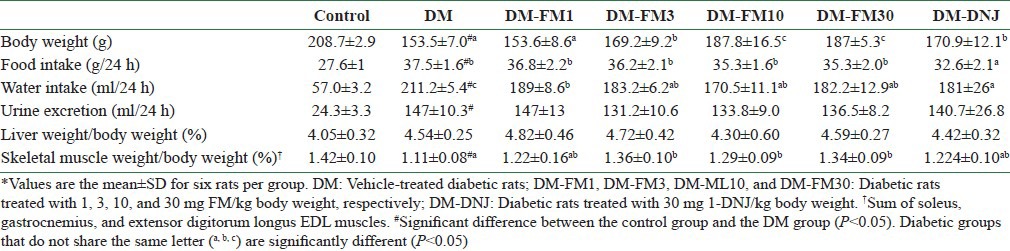
Induction of diabetes with STZ (DM group) was associated with the development of a slower rate of body weight gain, greater food and water intake, and greater urine excretion [Table 1]. A significant muscle loss was also observed in the DM group, suggesting that nitrogen was lost due to a catabolic condition [Table 1]. Compared with the DM group, the diabetic rats that received treatment with FM (桑葉 Sāng Yè) at a dose of 3 mg/kg and above showed significantly improved body weight (P < 0.05). The water intake of the diabetic rats was lowered significantly by all tested doses of FM (P < 0.05). Similarly, 1-DNJ elevated the body weight and lowered the water intake in diabetic rats. In addition, 1-DNJ significantly reduced the food intake in diabetic rats (P < 0.05), which remained unaffected by FM. FM at a dose equal to or higher than 3 mg/kg significantly reversed the skeletal muscle loss in diabetic rats (P < 0.05). However, 1-DNJ did not show a significant beneficial effect on skeletal muscle loss in diabetic rats, compared to FM.
Table 1.
Metabolic characteristics and growth characteristics of control rats or streptozotocin-induced diabetic rats that did or did not receive FM or 1-DNJ*
Fasting plasma glucose and plasma and pancreatic insulin levels
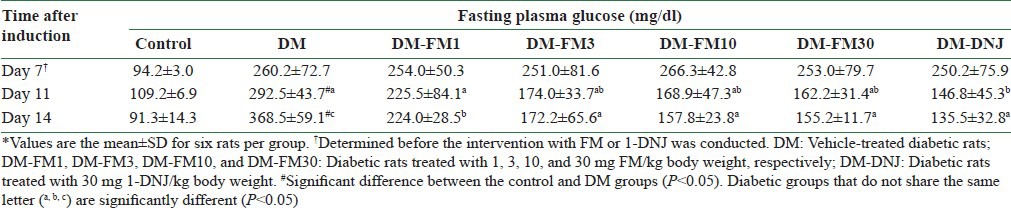
The STZ injections caused a significantly elevated fasting plasma glucose level at day 7 before the first dose of FM or 1-DNJ was administered to the diabetic rats (P < 0.05) [Table 2]. In the DM group, the fasting plasma glucose level worsened in a time-dependent manner during the 7-day investigation period. At day 11 after the induction of DM, or 4 days after the intervention began, 1-DNJ significantly lowered the fasting plasma glucose level by 49.8% compared with the DM group (P < 0.05), while FM lowered the fasting plasma glucose level less than that caused by 1-DNJ during the same period. At day 14 after the induction of DM, or 7 days after the intervention began, all tested doses of FM significantly lowered the fasting plasma glucose level (P < 0.05) in a dose-dependent manner. 1-DNJ lowered the fasting plasma glucose level to a greater extent than FM at day 14, but the effect was not significantly different compared to that of 3, 10, or 30 mg/kg FM [Table 2].
Table 2.
Fasting blood glucose concentration of control rats or streptozotocin-induced diabetic rats that did or did not receive FM or 1-DNJ during the treatment period from day 7 through day 14 after induction*
The data in Figure 2A show that the pancreatic insulin content was significantly lowered in the DM group, but was reversed by FM at a dose equal to or greater than 10 mg/kg, and 30 mg/kg 1-DNJ had a similar effect (P < 0.05). The peripheral insulin level showed that the DM-lowered insulin level was significantly alleviated by 1-DNJ and all tested doses of FM [Figure 2B]. The greater fasting insulin level in the peripheral blood than in the pancreas of the DM group compared to the control group reflected increased insulin secretion that was stimulated by the hyperglycemic condition in the DM rats.
Figure 2.
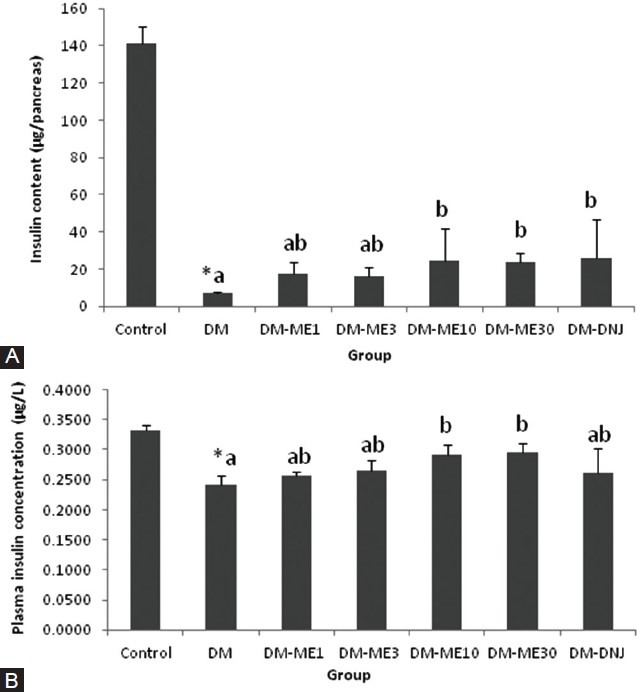
Effects of FM and 1-DNJ on insulin levels in the pancreas (A) and peripheral blood (B) of diabetic rats. The data are the mean ± SD for six rats in each group. #Significantly different from the control rats (P < 0.05). Diabetic groups that do not share the same letter (A, B) are significantly different (P < 0.05)
The HOMA-IR calculated using the fasting plasma insulin and glucose levels showed that STZ-induced diabetes caused reduced insulin sensitivity compared to the controls (5.73 ± 0.27 for DM vs. 2.13 ± 0.42 for controls, P < 0.05). While all tested doses of FM significantly improved the insulin sensitivity in DM rats in a dose-dependent manner, the HOMA-IR values for 1, 3, 10, and 30 mg/kg body weight FM-treated DM rats were 3.33 ± 0.40, 2.83 ± 0.76, 2.14 ± 0.34, and 1.90 ± 0.28, respectively. The HOMA-IR value of 1-DNJ–treated DM rats was also significantly improved (1.99 ± 0.74).
Oral glucose tolerance test
Blood samples were collected immediately before and during the 120-min period after glucose loading. The responses and the integral values of the AUC of plasma glucose during the investigation period were calculated and are presented in Figure 3A and B, respectively. The AUC of glucose was significantly elevated in the diabetic group compared to the control group (P < 0.05), and this increase was attenuated by treatment with all tested doses of FM (P < 0.05). 1-DNJ also improved the glucose tolerance significantly and to an extent similar to that of FM [Figure 3B].
Figure 3.
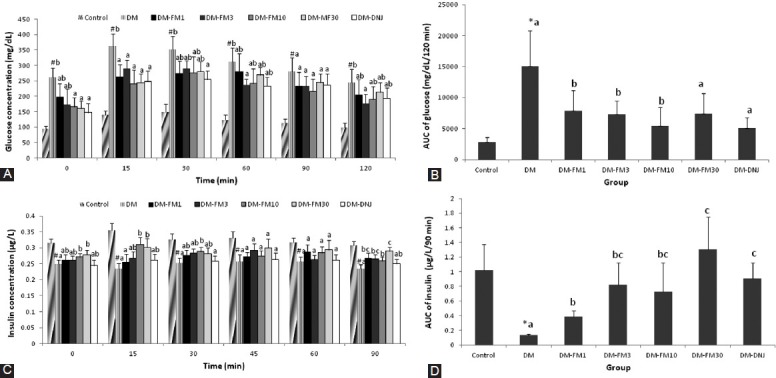
Effects of FM and 1-DNJ on the plasma glucose as a function of time (A), AUC of plasma glucose (B), plasma insulin as a function of time (C), and AUC of plasma insulin (D) in response to an oral glucose bolus (1 g/kg body weight) in diabetic rats. The data in (B) and (D) are the increment of glucose and insulin which are being calculated from the AUCs of (A) and (C), respectively. The data are the mean ± SD for six rats in each group. #Significantly different from the control (P < 0.05). Diabetic groups that do not share the same letter (A, B, C) are significantly different (P < 0.05)
The responses and the integral values of the AUC of plasma insulin during the investigation period were calculated and are presented in Figure 3C and D, respectively. The AUC of plasma insulin during the OGTT period for the DM group was only 13.0% of the control value [Figure 3D]. However, FM treatment at all tested doses significantly improved the insulin response to oral glucose loading (P < 0.05) [Figure 3D]. 1-DNJ showed an improved AUC of insulin during the OGTT to an extent similar to that of FMat a dose of 3 mg/kg and above (P < 0.05) [Figure 3D].
Oxidative stress and inflammation indices in organs/tissues
Compared with the levels in controls rats, lipid peroxidation (as measured by the levels of TBARS) was significantly higher in the liver, skeletal muscle, and kidney in the DM group (P < 0.05) [Figure 4A]. Treatment with FM significantly reversed the elevated lipid peroxidation in these tissues/organs (P < 0.05), and this reversal occurred in a dose-dependent manner. 1-DNJ also significantly alleviated the level of lipid peroxidation in these tissues/organs in the DM group; however, the effect occurred to a lesser extent, especially in skeletal muscle, compared with the same dose (30 mg/kg) of FM [Figure 4A].
Figure 4.
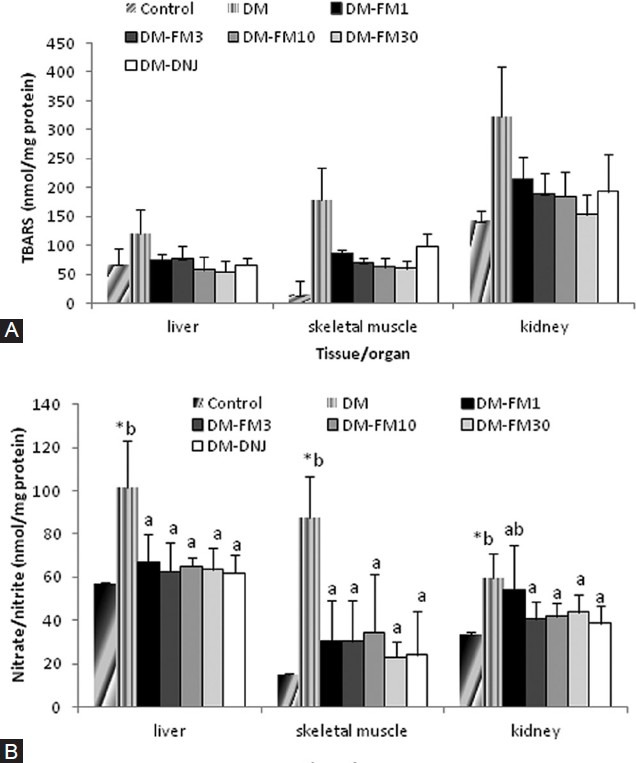
Effects of FM and 1-DNJ on lipid peroxidation (A) and nitrate/ nitrite content in various tissues/organs (B) in diabetic rats. The data are the mean ± SD for six rats in each group. #Significantly different from the control (P < 0.05). Diabetic groups that do not share the same letter (A, B) are significantly different (P < 0.05)
However, STZ-induced diabetes significantly elevated the nitrate/nitrite content in the liver, skeletal muscle, and kidney, compared with the control rats (P < 0.05) [Figure 4B], and the levels were all significantly suppressed by treatment with FM and 1-DNJ [Figure 4B].
Renal function
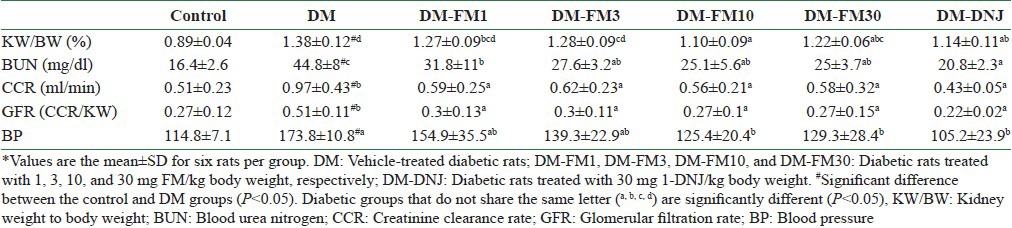
As shown in Table 3, the higher kidney weight to body weight ratio in the vehicle-treated diabetic animals than in the controls indicated the development of kidney hypertrophy in the diabetic animals (P < 0.05). The CCR was 90.2% higher and the BUN level was 173.2% higher in the vehicle-treated diabetic animals than in the controls [Table 3]. The GFR that was calculated based on kidney weight demonstrated higher values compared with the control rats (P < 0.05), suggesting that the glomeruli of the DM group were undergoing a hyperfiltration period. The BUN level was also significantly higher in the DM group than in the controls (P < 0.05), which may reflect both a renal problem and a muscle wasting condition in diabetes. FM significantly improved the kidney hypertrophy, GFR, and BUN in the diabetic animals at a dose of 1 mg/kg and above [Table 3]. 1-DNJ ameliorated the kidney hypertrophy, CCR, and GFR more than that caused by same dose (30 mg/kg) of FM. Blood pressure was elevated by 51.4% in the DM group compared with the controls, but was lowered significantly by FM at 10 and 30 mg/kg by 27.8% and 25.6%, respectively. 1-DNJ lowered the blood pressure in diabetic rats by 39.5%, which was more than the effect produced by FM [Table 3].
Table 3.
* Ratio of kidney weight to body weight (KW/BW), blood urea nitrogen (BUN), creatinine clearance rate (CCR), glomerular filtration rate (GFR), and blood pressure (BP) for control rats and rats with streptozotocin-induced diabetes that did or did not receive FM or 1-DNJ
Glomerular morphology
The present study collected the kidney for the morphological investigation as well as renal function at the time when the rats were induced to be diabetes for 2 weeks; therefore, the development of diabetic nephropathy was most likely at its initial stages. Compared with the normal glomerular morphology in the controls [Figure 5], the renal histology of the DM group showed signs of pathological changes, such as greater basal membrane thickness (reflected by a less transparent basement) and higher density of mesangial cells (prominent glomerular hypercellularity) that represents moderate proliferation of these cells. However, the morphology of the glomerulus was improved by FM treatment. We observed that the area of basal membrane thickness and the glomerular cellularity were slightly reduced by 1 and 3 mg/kg FM, and were largely reduced by 10 and 30 mg/kg FM. 1-DNJ also reversed the renal morphology, and the basal membrane thickness and glomerular cellularity were similar to those of the controls [Figure 5].
Figure 5.
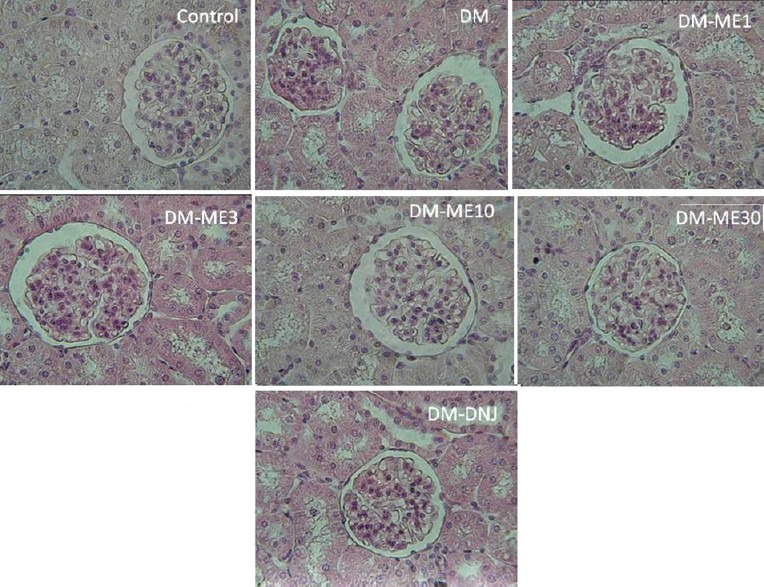
Histological examination showing the effect of FM and 1-DNJ on kidney. Cross sections of glomeruli were stained with hematoxylin and eosin (original magnification×100)
DISCUSSION
Various forms of FM (桑葉 Sāng Yè) products are beneficial for glycemic control in diabetic animals and diabetic patients.[4,5,6,7,8] The published data have shown that the effective doses of FM extract for glycemic control and reducing the blood pressure in diabetic animals were equivalent to a dose of 500-1000 mg/kg/day of dried leaf, but not to 250 mg/kg/day, in STZ-induced diabetic rats.[23,24] Among the proposed hypoglycemic FM components, 1-DNJ is one of the mostly studied compounds due to its α-glucosidase inhibitory role which is well established.[16,25] Subsequently, a great effort has been made to improve the yield of 1-DNJ from natural resources such as the mulberry leaf and other microorganisms.[17] The present study reported the anti-diabetic activity of a commercial FM product to be effective at a dose as low as 1 mg/kg, which is equivalent to 10 mg/kg dried white mulberry (桑白皮 Sāng Bái Pí) leaf and is a much lower dose than what has been previously reported. When comparing the anti-diabetic effect of FM and purified 1-DNJ, the FM extract appeared to restore glycemic control and renal function in STZ-induced diabetic rats in a dose-dependent manner and functioned similar to that of purified 1-DNJ when administered at the same dose (30 mg/kg) or even at lower doses (1-10 mg/kg). Moreover, our data also implicated certain differential advantages between FM and 1-DNJ when applied as anti-diabetic agents.
In addition to 1-DNJ, various components in FM have been shown to possess hypoglycemic activity, including flavonoids,[14] polysaccharides,[11,14] chlorogenic acid, and rutin.[14] Hunyadi et al. reported that chlorogenic acid and rutin account for as much as half the observed anti-diabetic activity of FM.[14] Thus, the hypoglycemic activity of FM found in the present study may be a combined effect of these compounds. However, 1-DNJ has been demonstrated to improve the glycemic control in alloxan-induced diabetic mice at doses of 50 and 100 mg/kg and in STZ-induced diabetic rats at a dose of 50 mg/kg.[11,19] The present study demonstrated the effectiveness of 1-DNJ as an anti-diabetic agent at a dose of 30 mg/kg, which is lower than what has been previously reported. Another novel finding of the present study is that despite the fact that 1-DNJ lowered fasting blood glucose to a greater extent than the same dose of FM, the latter improved body weight and skeletal muscle weight to a greater extent than the former, suggesting an overall beneficial effect of FM on energy metabolism.
FM is composed of flavonoids that are known for their antioxidant and anti-inflammatory activities, such as quercetin-3-(6-malonylglucoside), quercetin, rutin, and β-carotene.[2,3] Thus, FM may be beneficial in DM patients as it can prevent the glucose toxicity that is attributed to DM progression and the development of complications. Aqueous fractions of FM were able to reverse the tumor necrosis factor (TNF)-α–induced activation of nuclear factor-kappaB and the phosphorylation of an inhibitory factor of NF-kappaB-α in a time- and concentration-dependent manner.[26] FM has been demonstrated to ameliorate the blood glucose level in db/db mice, in association with decreased expression of pro-inflammatory cytokines in the adipose tissue and decreased levels of lipid peroxides in the adipose tissue and liver.[27] Naowaboot et al. reported that the administration of an ethanolic extract of FM decreased blood glucose; lowered lipid peroxidation in the liver, kidney, heart, and aorta; and restored vascular reactivity in STZ-induced diabetic rats.[23,24] FM has also been reported to suppress resistin-induced human endothelial activation partly via antioxidant mechanisms.[28] A polysaccharide fraction of FM was shown to scavenge hydroxyl radicals and superoxide anion radical effects in vitro and could protect alloxan-induced pancreatic islets from damage by scavenging the free radicals and repairing the destroyed pancreatic β-cells.[11] Consistent with these previous findings, the present study found that FM dramatically lowered the level of lipid peroxidation and NO content in the liver, skeletal muscle, and kidney. Further, it was observed in the present study that the antioxidant and anti-inflammatory effects of FM were associated with improved insulin sensitivity and renal function in STZ-induced diabetic rats. However, we found that 1-DNJ also significantly lowered the level of lipid peroxidation and NO in these tissues/organs, although to a lesser extent than the same dose of FM. No reports have indicated a direct antioxidant or anti-inflammatory role for 1-DNJ; however, these results can be explained as being at least partly due to the amelioration of blood glucose through 1-DNJ's known inhibitory effect on α-glucosidase. To our knowledge, this report is the first to demonstrate the antioxidant and anti-inflammatory roles of 1-DNJ in diabetes. This result warrants further investigation into the regulatory role of this compound on antioxidant and immune systems.
The kidney has become the focus of investigation in studies on diabetic complications because many of the same factors are involved in the development of diabetic nephropathy and other common diabetic complications such as microvascular disease and retinopathy. The early clinical course of diabetic nephropathy includes an initial increase in the GFR that correlates with hypertrophy, and thus an increased filtration surface of the glomeruli.[29,30] Such dysfunction of the kidney can also be reflected by the histological characteristics of early diabetic nephropathy, including mesangial expansion, diffuse glomerular basement membrane thickening, and increased mesangial cellularity.[31] In the present study, renal hypertrophy and hyperfiltration in DM rats occurred at the end of 2 weeks, but were reversed by treatment with FM. The hypoglycemic effect of FM may at least partially explain the ameliorated renal function in the FM-treated diabetic rats. However, because systemic hypertension also contributes to the development of diabetic nephropathy via associated glomerular hypertension,[32] we cannot exclude the possibility that FM may benefit renal function in diabetes by lowering the blood pressure. Interestingly, we found that the blood pressure of diabetic rats was significantly lowered by FM only at a dose of 10 mg/kg and above. Nevertheless, FM dramatically improved the GFR at a dose as low as 1 mg/kg, suggesting that the protective effect of FM on renal function is mainly via its activity rather than by lowering the blood pressure at lower doses (e.g. FM dramatically improved blood glucose control and oxidative and inflammatory conditions in the kidney). However, we found that 1-DNJ improved the GFR concomitantly with lowered blood pressure to a similar extent as the same dose of FM. Although the improved renal function by 1-DNJ and high doses of FM may be at least partly due to the improved glycemic control, the role of the blood pressure regulatory effect of 1-DNJ and higher doses of FM may not be excluded and warrants further investigation.
CONCLUSION
In conclusion, the present study demonstrated that a commercial FM product possesses hypoglycemic activity similar to that of same dose of purified 1-DNJ, and this effect is associated with improved insulin secretion and insulin sensitivity in diabetic animals. The present study demonstrated the renoprotective effect of both FM and 1-DNJ in diabetes for the first time and provided data for the possible role of these two white mulberry (桑白皮 Sāng Bái Pí) preparations in regulating blood pressure. The anti-diabetic effects of both FM and 1-DNJ are associated with alleviated oxidative stress and inflammatory conditions in diabetic animals.
ACKNOWLEDGMENTS
This work was supported by a grant from the NSC foundation of the National Science Council (94-2320-B-040-035-). We acknowledge Chin Ang Pharmaceutical Co., Ltd (Chiayi, Taiwan) for providing the F. mori preparation.
REFERENCES
- 1.Jia W, Gao W, Tang L. Antidiabetic herbal drugs officially approved in China. Phytother Res. 2003;17:1127–34. doi: 10.1002/ptr.1398. [DOI] [PubMed] [Google Scholar]
- 2.Zafar MS, Muhammad F, Javed I, Akhtar M, Khaliq T, Aslam B, et al. White mulberry (Morus alba): A brief phytochemical and pharmacological evaluations account. Int J Agric Biol. 2013;15:612–20. [Google Scholar]
- 3.Butt MS, Nazir A, Sultan MT, Schroën K, Morus alba L. Nature's functional tonic. Trends Food Sci Technol. 2008;19:505–12. [Google Scholar]
- 4.Chen F, Nakashima N, Kimura I, Kimura M. Hypoglycemic activity and mechanisms of extracts from mulberry leaves (Folium Mori) and Cortex Mori radic in streptozotoin-induced diabetic mice. Yakugaku Zasshi. 1995;115:476–82. doi: 10.1248/yakushi1947.115.6_476. [DOI] [PubMed] [Google Scholar]
- 5.Jang MH, Kim H, Shin MC, Lim BV, Lee TH, Jung SB, et al. Administration of Folium mori extract decreases nitric oxide synthase expression in the hypothalamus of streptozotocin-induced diabetic rats. Jpn J Pharmacol. 2002;90:189–92. doi: 10.1254/jjp.90.189. [DOI] [PubMed] [Google Scholar]
- 6.Oku T, Yamada M, Nakamura M, Sadamori N, Nakamura S. Inhibitory effects of extractives from leaves of Morus alba on human and rat small intestinal disaccharidase activity. Br J Nutr. 2006;95:933–8. doi: 10.1079/bjn20061746. [DOI] [PubMed] [Google Scholar]
- 7.Mohammadi J, Naik PR. Evaluation of hypoglycemic effect of Morus alba in an animal model. Indian J Pharmacol. 2008;40:15–8. doi: 10.4103/0253-7613.40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JM, Bong HY, Jeong HI, Kim YK, Kim JY, Kwon O. Postprandial hypoglycemic effect of mulberry leaf in Goto-Kakizaki rats and counterpart control Wistar rats. Nutr Res Pract. 2009;3:272–8. doi: 10.4162/nrp.2009.3.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mudra M, Ercan-Fang N, Zhong L, Furne J, Levitt M. Influence of mulberry leaf extract on the blood glucose and breath hydrogen response to ingestion of 75 g sucrose by type 2 diabetic and control subjects. Diabetes Care. 2007;30:1272–4. doi: 10.2337/dc06-2120. [DOI] [PubMed] [Google Scholar]
- 10.Yu LY, Li XR, Fang X. Inhibitory effect of total flavonoids from mulberry tree leaf on small intestine disaccharidases in diabetic rats. Chin J Endocrinol Metab. 2002;18:313–5. [Google Scholar]
- 11.Li YG, Ji DF, Zhong S, Lv ZQ, Lin TB, Chen S, et al. Hybrid of 1-deoxynojirimycin and polysaccharide from mulberry leaves treat diabetes mellitus by activating PDX-1/insulin-1 signaling pathway and regulating the expression of glucokinase, phosphoenolpyruvate carboxykinase and glucose-6-phosphatase in alloxan-induced diabetic mice. J Ethnopharmacol. 2011;134:961–70. doi: 10.1016/j.jep.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Asano N, Oseki K, Kaneko E, Matsui K. Enzymic synthesis of α- and β-d-glucosides of 1-deoxynojirimycin and their glycosidase inhibitory activities. Carbohydr Res. 1994;258:255–66. doi: 10.1016/0008-6215(94)84091-1. [DOI] [PubMed] [Google Scholar]
- 13.Yatsunami K, Ichida M, Onodera S. The relationship between 1-deoxynojirimycin content and alpha-glucosidase inhibitory activity in leaves of 276 mulberry cultivars (Morus spp.) in Kyoto, Japan. J Nat Med. 2008;62:63–6. doi: 10.1007/s11418-007-0185-0. [DOI] [PubMed] [Google Scholar]
- 14.Hunyadi A, Martins A, Hsieh TJ, Seres A, Zupkó I. Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS One. 2012;7:e50619. doi: 10.1371/journal.pone.0050619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vichasilp C, Nakagawa K, Sookwong P, Suzuki Y, Kimura F, Higuchi O, et al. Optimization of 1-deoxynojirimycin extraction from mulberry leaves by using response surface methodology. Biosci Biotechnol Biochem. 2009;73:2684–9. doi: 10.1271/bbb.90543. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi H, Onose S, Kitahara E, Chumchuen S, Takasaki M, Konishi H, et al. Effect of environmental conditions on the α-glucosidase inhibitory activity of mulberry leaves. Biosci Biotechnol Biochem. 2011;75:2293–6. doi: 10.1271/bbb.110407. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa K. Studies targeting α-glucosidase inhibition, antiangiogenic effects, and lipid modification regulation: Background, evaluation, and challenges in the development of food ingredients for therapeutic purposes. Biosci Biotechnol Biochem. 2013;77:900–8. doi: 10.1271/bbb.120908. [DOI] [PubMed] [Google Scholar]
- 18.Kimura T, Nakagawa K, Kubota H, Kojima Y, Goto Y, Yamagishi K, et al. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J Agric Food Chem. 2007;55:5869–74. doi: 10.1021/jf062680g. [DOI] [PubMed] [Google Scholar]
- 19.Li YG, Ji DF, Zhong S, Lin TB, Lv ZQ, Hu GY, et al. 1-deoxynojirimycin inhibits glucose absorption and accelerates glucose metabolism in streptozotocin-induced diabetic mice. Sci Rep. 2013;3:1377. doi: 10.1038/srep01377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouyang Z, Li YH, Chen J. Determination of 1-deoxynojirimycin in leaves of Morus alba by high performance liquid chromatography with fluorescence detection. Zhongguo Zhong Yao Za Zhi. 2005;30:682–5. [PubMed] [Google Scholar]
- 21.Liu CT, Wong PL, Lii CK, Hse H, Sheen LY. Antidiabetic effect of garlic oil but not diallyl disulfide in rats with streptozotocin-induced diabetes. Food Chem Toxicol. 2006;44:1377–84. doi: 10.1016/j.fct.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and b-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Naowaboot J, Pannangpetch P, Kukongviriyapan V, Kongyingyoes B, Kukongviriyapan U. Antihyperglycemic, antioxidant and antiglycation activities of mulberry leaf extract in streptozotocin-induced chronic diabetic rats. Plant Foods Hum Nutr. 2009;64:116–21. doi: 10.1007/s11130-009-0112-5. [DOI] [PubMed] [Google Scholar]
- 24.Naowaboot J, Pannangpetch P, Kukongviriyapan V, Kukongviriyapan U, Nakmareong S, Itharat A. Mulberry leaf extract restores arterial pressure in streptozotocin-induced chronic diabetic rats. Nutr Res. 2009;29:602–8. doi: 10.1016/j.nutres.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Kimura T, Nakagawa K, Saito Y, Yamagishi K, Suzuki M, Yamaki K, et al. Determination of 1-deoxynojirimycin in mulberry leaves using hydrophilic interaction chromatography with evaporative light scattering detection. J Agric Food Chem. 2004;52:1415–8. doi: 10.1021/jf0306901. [DOI] [PubMed] [Google Scholar]
- 26.Shibata Y, Kume N, Arai H, Hayashida K, Inui-Hayashida A, Minami M, et al. Mulberry leaf aqueous fractions inhibit TNF-α-induced nuclear factor κB (NF-κB) activation and lectin-like oxidized LDL receptor-1 (LOX-1) expression in vascular endothelial cells. Atherosclerosis. 2007;193:20–7. doi: 10.1016/j.atherosclerosis.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Sugimoto M, Arai H, Tamura Y, Murayama T, Khaengkhan P, Nishio T, et al. Mulberry leaf ameliorates the expression profile of adipocytokines by inhibiting oxidative stress in white adipose tissue in db/db mice. Atherosclerosis. 2009;204:388–94. doi: 10.1016/j.atherosclerosis.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 28.Pirvulescu MM, Gan AM, Stan D, Simion V, Calin M, Butoi E, et al. Curcumin and a Morus alba extract reduce pro-inflammatory effects of resistin in human endothelial cells. Phytother Res. 2011;25:1737–42. doi: 10.1002/ptr.3463. [DOI] [PubMed] [Google Scholar]
- 29.Hirose K, Tsuchida H, Østerby R, Gundersen HJG. A strong correlation between glomerular filtration rate and filtration surface in diabetic kidney hyperfunction. Lab Invest. 1980;43:434–7. [PubMed] [Google Scholar]
- 30.Rudberg S, Persson B, Dahlquist G. Increased glomerular filtration rate as a predictor of diabetic nephropathy—An 8-year prospective study. Kidney Int. 1992;41:822–8. doi: 10.1038/ki.1992.126. [DOI] [PubMed] [Google Scholar]
- 31.Alsaad KO, Herzenberg AM. Distinguishing diabetic nephropathy from other causes of glomerulosclerosis: An update. J Clin Pathol. 2007;60:18–26. doi: 10.1136/jcp.2005.035592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Buren PN, Toto R. Hypertension in diabetic nephropathy: Epidemiology, mechanisms, and management. Adv Chronic Kidney Dis. 2011;18:28–41. doi: 10.1053/j.ackd.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


