Abstract
In the present study, Sugar Remedy, a polyherbal formulation (manufactured by Umalaxmi Organics Pvt Ltd, Jodhpur, Rajasthan, India) was evaluated for its antihyperglycemic, antihyperlipidemic, and antioxidant effects against normal and streptozotocin (STZ)-induced diabetic rats. Type II diabetes was induced in male Wistar rats by administration of a single intraperitoneal (IP) injection of STZ at a dose of 60 mg/kg. Effects of three different doses of Sugar Remedy suspension (185, 370, and 740 mg/kg/day, orally) and Metformin (500 mg/kg/day, orally) administered for 21 days were studied on parameters such as blood glucose, lipid profile, and antioxidant levels. Results were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett's test. No significant changes were noticed in blood glucose, serum lipid levels, and kidney parameters in normal rats treated with Sugar Remedy suspension alone. The efficacy of Sugar Remedy as an antihyperglycemic, antihyperlipidemic, and antioxidant agent in STZ-induced diabetes was comparable to that of the standard, 500 mg/kg of Metformin. Present findings provide experimental evidence that Sugar Remedy has significant antihyperglycemic, antihyperlipidemic, and antioxidative effects in diabetic experimental rats. Hence, Sugar Remedy may be regarded as a promising natural and safe remedy for the prevention or delay of diabetic complications.
Keywords: Ayurveda, Diabetes mellitus, Hyperlipidemia, Streptozotocin, Sugar remedy
INTRODUCTION
Polyherbal drugs as Ayurvedic medicines are considered to be more effective for the management of diabetes. Diabetes mellitus (DM) represents a syndrome of metabolic disorders and complex pathophysiological interactions between hyperglycemia, insulin resistance, and dysfunction of the β-cells of pancreas. The available antidiabetic measures, such as oral hypoglycemic agents and insulin, do not effectively control the delayed diabetic complications like nephropathy, neuropathy, retinopathy, and cardiovascular diseases.[1] Oxidative tissue injury has been suggested to play an important role in the pathogenesis of diabetes and associated complications.[2] The antihyperglycemic effects of various plants are credited to their ability to restore the function of pancreatic tissues by causing an increase in the insulin output or by inhibiting the intestinal absorption of glucose or by the facilitation of metabolites in insulin-dependent processes.[3] Herbal products are generally considered to be least toxic and free from side effects when compared with their synthetic counterparts.[4]
Sugar Remedy is claimed to be a unique formulation that helps in holistic management of blood glucose and diabetes-related complications. Each 100 g of Sugar Remedy contains the following as the major constituents: Bitter melon extract: 50 g; gudmar extract: 16.7 g; ashwagandha extract: 8.4 g; jamun extract: 4.2 g; shilajit extract: 4.2 g; fenugreek extract: 4.2 g; triphala extract: 4.2 g; cinnamon extract: 4.2 g; and vijaysar extract: 4.2 g. The combined role of the key ingredients present in Sugar Remedy [Table 1] in lowering the blood glucose level and, thus, improving the hepatic and renal functions, lipid profile, and antioxidant activity is evidenced from previous studies performed on individual herbs, revealing their activities which are the following: Karela by insulin secretion, inhibition of glucose reabsorption in the gut, and increase of peripheral glucose utilization;[5] gudmar by regulation of β-cell function and by its antioxidant activity;[6,7,8] fenugreek and cinnamon by improving digestion, metabolism and reduction in insulin resistance and increasing hepatic glycogenesis;[9] jamun by increasing the pancreatic secretion of insulin;[10] shilajit by improving the neurogenic function associated with diabetes and through its pancreatotrophic action;[11] and vijaysar by pancreatic β-cell regranulation.[12]
Table 1.
Formulated herbal drugs with antidiabetic properties
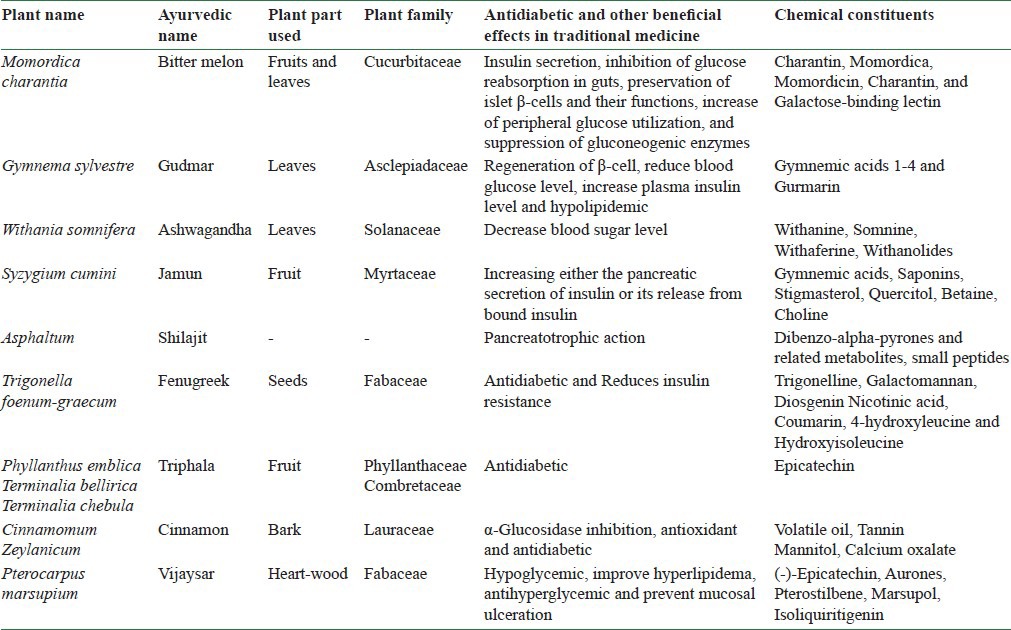
In the present study, attempts have been made to establish the scientific validity for the antihyperglycemic property of Sugar Remedy using streptozotocin (STZ)-induced diabetic model in rats. The results of the study can serve as a step toward the development of an antihyperglycemic herbal therapy for diabetes.
MATERIALS AND METHODS
Animals and grouping
Male Wistar rats, 7-8 weeks old and weighing 150-220 g, were used for the present study. The rats were randomly distributed to different groups with six animals in each. Animals were provided with standard pellets and drinking water ad libitum and were maintained at 12 h light and dark cycle. The protocol of the experiment (1258/ac/09/CPCSEA) was approved by the Institutional Animal Ethics Committee (IAEC), and the experiments were conducted in accordance with the guidelines as per the “Guide for the Care and Use of Laboratory Animals” and with permission from the “Committee for the Purpose of Control and Supervision of Experiments on Animals” (CPCSEA).
Drugs and chemicals
Sugar Remedy was obtained from Umalaxmi Organics Pvt Ltd, Jodhpur, India. The formulation was suspended in water for the preparation of an oral dosage formulation and administered by per oral (PO) route in different doses, twice daily for 21 days, while the control group was treated with water. STZ was obtained Sigma Aldrich Co, Mumbai, India. Metformin (Glyciphage, Franco-Indian Pharmaceuticals Pvt Ltd) was obtained from Rina's Pharma, Jodhpur, India.
Induction of diabetes
Diabetes was induced in overnight fasted rats by the intraperitoneal (IP) injection of STZ dissolved in freshly prepared citrate buffer (0.1 M, pH 4.5) in a volume of 1 ml/kg body weight at a dose of 60 mg/kg body weight.[13] Diabetes was confirmed 72 h after the injection by determining the blood glucose concentration. Only those animals with blood glucose level of >200 mg/dl (mild diabetes)[14,15] were used for the experiment. The diabetic animals were allowed free access to tap water and pelleted diet and were maintained at room temperature in plastic cages.
Acute toxicity studies
To study any possible toxic effects, mortality, and/or changes in the behavioral pattern, acute toxicity studies were performed according to Organisation for economic co-operation and development (OECD) guidelines 423, December 2001. Experiments were carried out in normal rats and they were kept under closed observation for 24 h. All symptoms including changes in awareness, mood, motor activity, posture activity, and mortality were recorded. No toxicity or mortality was observed with Sugar Remedy up to a dose of 1000 mg/kg body weight.[16]
Biochemical estimations
Blood glucose estimation was done using a glucometer and with Trinder's enzymatic method using an autoanalyzer.[17] Lipid profile was checked with an autoanalyzer using the following methods: Cholesterol oxidase-phenol + aminophenazone (CHOD-PAP) end point method was used for cholesterol estimation, phosphotungstic acid end point method for high density lipoprotein (HDL) estimation, and glycerol-3-phosphate oxidase (GPO)-Trinder end point method was used for triglyceride estimation.[18,19] Very low density lipoprotein (VLDL) and low density lipoprotein (LDL) fractions were calculated by using Friedewald's equation as follows:[20]
VLDL = triglycerides/5
LDL = TC − (HDL + VLDL)
Physiological profile of kidneys was estimated by Jaffe's method Initial Rate for creatinine and modified Trinder's method, end point for uric acid, using autoanalyzer.[21]
Antioxidant activity in liver tissue homogenate
By the end of the treatment period, on the next day of administering the last dose of the respective treatments, immediately after collection of blood, the animals were euthanized with an overdose of ether and their livers were excised, washed with ice-cold normal saline, and weighed. Then, a 10% liver tissue homogenate was prepared by homogenizing in ice-chilled phosphate-buffered saline (PBS; pH 7.4) using a tissue homogenizer. The homogenate was centrifuged at 10,000 rpm for 4°C using a refrigerated centrifuge and the supernatant was used for the determination of various antioxidant parameters like reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), and malondialdehyde (MDA) levels.[22,23,24,25]
Statistical analysis
Results are expressed as mean ± standard error of mean (SEM) and statistical difference was evaluated using one-way analysis of variance (ANOVA) followed by Dunnett's test. Data were considered statistically significant at a P ≤ 0.01 and highly significant at a P < 0.001. All the above statistical analyses were done on Prism software.
RESULTS
Effect of sugar remedy on blood glucose levels
STZ treatment produced significant increase in blood glucose levels (347.50 ± 21.90 mg/dl) with respect to the control group (109.47 ± 3.46 mg/dl). The hyperglycemia was pronounced after 21st day of administering STZ. As shown in Table 2, the administration of 740 mg/kg of Sugar Remedy or 500 mg/kg of Metformin significantly reversed (129.72 ± 8.25 mg/dl and 128.2 ± 12.46 mg/dl, respectively) the increase in blood glucose concentration induced by STZ. Such an effect was more obvious with high dose of Sugar Remedy (740 mg/kg body weight).
Table 2.
Effect of Sugar remedy on serum glucose levels

Effect of sugar remedy on the lipid profile
STZ produced significant increases in serum triglycerides (153.63 ± 3.77 vs. 78.61 ± 7.35 mg/dl in normal control rats), serum cholesterol (123.17 ± 0.78 vs. 76.35 ± 3.06 mg/dl in normal control rats), LDL (80.96 ± 1.02 vs. 30.04 ± 1.90 mg/dl in normal control rats), and VLDL (30.73 ± 0.72 vs. 16.67 ± 1.37 mg/dl in normal control rats), as well as marked reduction in serum HDL levels (11.48 ± 0.67 vs. 29.60 ± 1.10 mg/dl in normal control rats). As shown in Table 3, treatment with 740 mg/kg of Sugar Remedy reduced the levels of serum cholesterol, triglycerides, VLDL, and LDL (81.20 ± 1.64, 102.35 ± 1.55, 20.47 ± 0.31, and 36.95 ± 1.37 mg/dl, respectively), which was comparable to the levels in control group (76.35 ± 3.06, 78.61 ± 7.35, 16.67 ± 1.37, and 30.04 ± 1.90 mg/dl, respectively). Metformin at a dose of 500 mg/kg also produced the same effect (80.16 ± 1.01, 99.63 ± 3.01, 19.92 ± 0.60, and 33.97 ± 0.85 mg/dl, respectively). Increase in HDL levels was also much pronounced in animals treated with 740 mg/kg of Sugar Remedy or 500 mg/kg of Metformin (23.78 ± 1.00 and 26.26 ± 0.56 mg/dl, respectively) which was comparable to the normal control animals (29.60 ± 1.10 mg/dl).
Table 3.
Effect of Sugar remedy on lipid profile

Effect of sugar remedy on kidney and liver parameters
STZ-diabetic (DC) rats exhibited higher serum creatinine (2.55 ± 0.18 mg/dl) and uric acid (9.26 ± 0.18 mg/dl) levels as compared to those of normal control rats (0.86 ± 0.05 and 3.26 ± 0.09 mg/dl, respectively) [Table 4]. Chronic treatment with 740 mg/kg of Sugar Remedy significantly reduced the elevated creatinine as well as uric acid levels in diabetic rats (1.01 ± 0.24 mg/dl and 4.33 ± 0.39 mg/dl, respectively), which was comparable to the levels in Metformin (500 mg/kg) treated animals (1.69 ± 0.21 and 6.09 ± 1.17 mg/dl, respectively).
Table 4.
Effect of sugar remedy on serum creatinine and uric acid levels
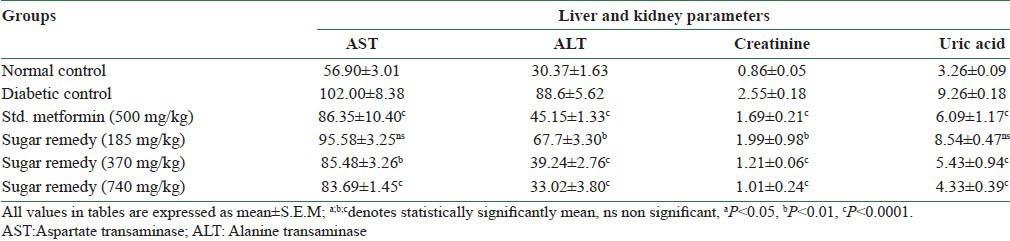
Aspartate transaminase (AST) level was significantly reduced in rats treated with 740 mg/kg of Sugar Remedy (83.69 ± 1.45 U/l) or 500 mg/kg of Metformin (86.35 ± 10.40 U/l) when compared to diabetic control rats (102.00 ± 8.38 U/l). Similarly, alanine transaminase (ALT) level was significantly reduced in rats treated with 740 mg/kg of Sugar Remedy or 500 mg/kg of Metformin (33.02 ± 3.80 U/l and 45.15 ± 1.33 U/l, respectively), when compared to diabetic control rats (88.60 ± 5.62 U/l). This indicates that Sugar Remedy also improved the liver physiology and may have hepatoprotective effects [Table 4].
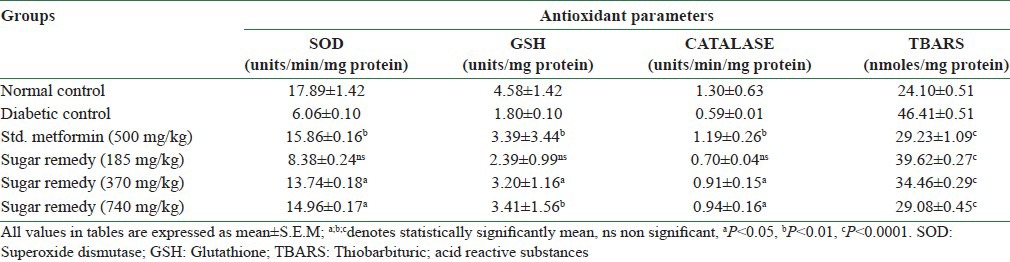
Effect of sugar remedy on the antioxidant parameters in liver
DC rats showed a significant decrease in SOD, CAT, and GSH levels (6.06 ± 0.10, 0.59 ± 0.01 units/min/mg protein and 1.80 ± 0.10 units/mg protein, respectively) when compared with the normal control animals (17.89 ± 1.42, 1.30 ± 0.63 units/min/mg protein and 4.58 ± 1.42 mg/g protein, respectively). Thiobarbituric acid reactive substances (TBARS) significantly increased following STZ administration (46.41 ± 0.51 nmol/mg protein) in DC rats when compared to normal control rats (24.10 ± 0.51 nmol/mg protein). Administration of Sugar Remedy in various doses for 21 days produced a marked increase in the antioxidant (SOD, GSH, and CAT) levels, whereas it produced a significant decrease in the prooxidant parameter (TBARS) [Table 5].
Table 5.
Effect of sugar remedy on antioxidant parameters
DISCUSSION
DM is one of the most common chronic diseases, and is associated with hyperlipidemia and co-morbidities such as obesity and hypertension. Hyperlipidemia is a metabolic complication of both clinical and experimental diabetes.[26] STZ, a β-cytotoxin, induces “chemical diabetes” in a wide variety of animal species, including rats, by selectively damaging the insulin-secreting β-cells of the pancreas. IP injection of STZ produces fragmentation of DNA of the β-cells of pancreas, which stimulates poly (ADP-ribose) and depletes nicotinamide adenine dinucleotide (NAD) ultimately leading to the destruction of β-cells. It is evidenced by the clinical symptoms of hyperglycemia and hypoinsulinemia. The serum glucose, lipid, and cholesterol values for the rats are in agreement with those expected for STZ-diabetic rats.[27,28,29,30]
Decrease in blood glucose levels was found to be more effective with Sugar Remedy in doses of 370 and 740 mg/kg. Metformin showed rapid normalization of blood glucose due to its insulin releasing effects. Treatment with polyherbal formulation Sugar Remedy caused significant decrease in fasting serum glucose (FBG) level near to that of healthy control rats. The plant extracts may involve one or more compounds which decrease the blood glucose levels, suggesting that the natural constituents could act synergistically to induce a hypoglycemic effect as described by Marles et al.[31,32,33] These effects might be achieved by facilitating insulin release from pancreatic β-cells, inhibiting glucose absorption in the gut, stimulating glycogenesis in the liver, and/or increasing glucose utilization by the body. These compounds also exhibited antioxidant and hypolipidemic activities, restored the enzymatic functions, and helped in repair and regeneration of pancreatic islets and the alleviation of liver and renal damage.[31,34,35,36,37,38,39,40,41,42]
Insulin deficiency may be responsible for dyslipidemia because insulin has an inhibitory action on 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase, a key enzyme that is rate limiting in the metabolism of cholesterol-rich LDL particles.[43] The development of hypertriglyceridemia in uncontrolled diabetes in humans (possibly in insulin-deficient STZ-diabetic rats) may be due to a number of metabolic abnormalities that occur sequentially. Acute insulin deficiency initially causes an increase in free fatty acid mobilization from the adipose tissue, resulting in increased secretion of VLDL and triglycerides from the liver.[44] In diabetic rats, there is a decrease in lipoprotein lipase activity,[45] resulting in impaired clearance of VLDL and chylomicrons from the plasma.[46]
Administration of Sugar Remedy decreased the levels of tissue free fatty acids and phospholipids. Accumulation of triglycerides is one of the risk factors in coronary heart disease (CHD). The significant increase in the level of triglycerides in the liver and kidney of diabetic control rats may be due to the lack of insulin. Under normal conditions, insulin activates the enzyme lipoprotein lipase and hydrolyzes triglycerides. Sugar Remedy reduces triglycerides in the tissues of STZ-induced diabetic rats and may prevent the progression of CHD.[47]
The decreased activities of CAT and SOD in the diabetic group may be a response to the increased production of H2O2 and O2 by the auto-oxidation of glucose and nonenzymatic glycation. Hepatic SOD and CAT activities were reduced during diabetes and this may result in a number of harmful effects due to the accumulation of hydrogen peroxides and superoxide radicals. Administration of Sugar Remedy caused decreased lipid peroxidation, which is associated with increased SOD and CAT activities, indicating that Sugar Remedy can reduce reactive oxygen free radicals and improve the activities of the hepatic antioxidant enzymes. The antioxidative activity of Sugar Remedy gains further evidence from the quantification of TBARS in hepatic tissues, as an inverse relationship was found between the activities of antioxidant enzymes and the quantity of free radicals, which is in agreement with previous reports.[48,49] In diabetes, AST and ALT activities are increased,[50] which may be due to the cellular damage.[51] The plant extract was observed to normalize the levels of these enzymes, which indicates that it has a promising antidiabetic effect without inducing toxicity.
Administration of Sugar Remedy and Metformin reduced the lipid peroxidative markers in the liver and kidney tissues of diabetic rats. This indicates that Sugar Remedy inhibits oxidative damage due to the antiperoxidative effect of its ingredients. This could be correlated with previous studies reporting that Momordica charantia,[52] Gymnema sylvestre,[53] Withania somnifera,[54] Syzygium cumini,[55] Asphaltum,[11] Trigonella foenum-graecum,[56] Triphala,[57] Cinnamomum zeylanicum,[58] and Pterocarpus marsupium[59] (ingredients of Sugar Remedy) have antiperoxidative and antihyperlipidemic effects in diabetic animals.
The antidiabetic and antihyperlipidemic effects of Sugar Remedy may be due to the effect of active constituents of different plants, viz., momorcharin and momordicin isolated from M. charantia; gymnemic acids 1-4 and gurmarin from G. sylvestre; withanine, somnine, withaferine, and withanolides from W. somnifera; gymnemic acids, saponins, stigmasterol, quercitol, betaine, and choline from S. cumini; dibenzo-alpha-pyrones and related metabolites, and small peptides from Asphaltum; trigonelline and scopoletin from T. foenum-graecum; epicatechin from P. emblica; volatile oil, tannin, mannitol, and calcium oxalate from C. zeylanicum; and (−)-epicatechin, aurones, pterostilbene, marsupol, and isoliquiritigenin from P. marsupium, which may be responsible for scavenging the free radicals liberated by STZ in diabetic rats.[9,10,11,60,61,62]
CONCLUSION
On the basis of the aforementioned results, it may be concluded that Sugar Remedy has significant antihyperglycemic, antihyperlipidemic, and antioxidative effects in diabetic experimental rats. Hence, Sugar Remedy may be regarded as a promising natural and safe remedy for the prevention or delay of diabetic complications.
ACKNOWLEDGMENTS
We are grateful to the Faculty of Pharmaceutical Sciences, Jodhpur National University for their assistance in completing the research project. We would also like to thank the animal house staff and animal care takers for their help and hard work.
REFERENCES
- 1.Mallick C, Mandal S, Barik B, Bhattacharya A, Ghosh D. Protection of testicular dysfunctions by MTEC, a formulated herbal drug, in streptozotocin induced diabetic rat. Biol Pharm Bull. 2007;30:84–90. doi: 10.1248/bpb.30.84. [DOI] [PubMed] [Google Scholar]
- 2.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 3.Malviya N, Jain S, Malviya S. Review Antidiabetic potential of medicinal plants. Acta Pol Pharm. 2010;67:113–8. [PubMed] [Google Scholar]
- 4.Perl Meir. The biochemical basis of the hypoglycemic effect of some plant extracts. In: Craker LE, Simon JE, editors. herbs, spices and medicinal plants; Recent advances in botany, horticulture and pharmacology. Vol. 3. USA: Oxford Press; 1998. [Google Scholar]
- 5.Singh J, Cumming E, Manoharan G, Kalasz H, Adeghate E. Medicinal chemistry of the anti-diabetic effects of Momordica charantia: Active constituents and modes of actions. Open Med Chem J. 2011;5(Suppl):70–7. doi: 10.2174/1874104501105010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramkumar KM, Lee AS, Krishnamurthi K, Devi SS, Chakrabarti T, Kang KP, et al. Gymnema montanum H. protects against alloxan-induced oxidative stress and apoptosis in pancreatic beta-cells. Cell Physiol Biochem. 2009;24:429–40. doi: 10.1159/000257480. [DOI] [PubMed] [Google Scholar]
- 7.Al-Romaiyan A, King AJ, Persaud SJ, Jones PM. A novel extract of Gymnema sylvestre improves glucose tolerance in vivo and stimulates insulin secretion and synthesis in vitro. Phytother Res. 2013;27:1006–11. doi: 10.1002/ptr.4815. [DOI] [PubMed] [Google Scholar]
- 8.Al-Romaiyan A, Liu B, Asare-Anane H, Maity CR, Chatterjee SK, Koley N, et al. A novel Gymnema sylvestre extract stimulates insulin secretion from human islets in vivo and in vitro. Phytother Res. 2010;24:1370–6. doi: 10.1002/ptr.3125. [DOI] [PubMed] [Google Scholar]
- 9.Chang CL, Lin Y, Bartolome AP, Chen YC, Chiu SC, Yang WC. Herbal therapies for type 2 diabetes mellitus: Chemistry, biology, and potential application of selected plants and compounds. Evid Based Complement Alternat Med 2013. 2013:1–33. doi: 10.1155/2013/378657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bnouham M, Ziyyat A, Mekhfi H, Tahri A, Legssyer A. Medicinal plants with potential antidiabetic activity-a review of ten years of herbal medicine research (1990-2000) Int J Diabetes Metab. 2006;14:1–25. [Google Scholar]
- 11.Trivedi NA, Mazumdar B, Bhatt JD, Hemavathi KG. Effect of shilajit on blood glucose and lipid profile in alloxan-induced diabetic rats. Indian J Pharmacol. 2004;36:373–6. [Google Scholar]
- 12.Chakravarty BK, Gupta S, Gambhir SS, Gode KD. Pancreatic β-cell regeneration. A novel antidiabetic mechanism of Pterocarpus marsupium Roxb. Indian J Pharmacol. 1980;12:123–7. [Google Scholar]
- 13.Siddique O, Sun Y, Lin JC, Chien YW. Facilitated transdermal transport of insulin. J Pharm Sci. 1987;76:341. doi: 10.1002/jps.2600760416. [DOI] [PubMed] [Google Scholar]
- 14.Ewart RB, Kornfeld S, Kipnis DM. Effect of lectins on hormone release from isolated rat islets of langerhans. Diabetes. 1975;24:705–14. doi: 10.2337/diab.24.8.705. [DOI] [PubMed] [Google Scholar]
- 15.Cetto AA, Weidenfeld H, Revilla MC, Sergio IA. Hypoglycemic effect of Equisetum mriochaetum aerial parts on STZ diabetic rats. J Ethnopharmacol. 2000;72:129–33. doi: 10.1016/s0378-8741(00)00218-x. [DOI] [PubMed] [Google Scholar]
- 16.OECD Guideline for testing of Chemicals-Acute Oral Toxicity – Acute Toxic Class Method-423. [Adopted: 17th December 2001]; [Google Scholar]
- 17.Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol. 1969;22:158–61. doi: 10.1136/jcp.22.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siedel J, Hagele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983;29:1075–80. [PubMed] [Google Scholar]
- 19.Assmann G, Schriewer H, Schmitz G, Hägele EO. Quantification of high-density-lipoprotein cholesterol by precipitation with phosphotungstic Acid/MgCI2. Clin Chem. 1983;29:2026–30. [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Cholongitas E, Marelli L, Kerry A, Senzolo M, Goodier DW, Nair D, et al. Different methods of creatinine measurement significantly affect MELD scores. Liver Transpl. 2007;13:523–9. doi: 10.1002/lt.20994. [DOI] [PubMed] [Google Scholar]
- 22.Ellman GL. Tissue sulfhydral groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 23.Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–95. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 24.Luck H, Catalase . Methods of enzyme analysis. In: Bergmeyer HU, editor. New York: Academic Press; pp. 885–93. [Google Scholar]
- 25.Wills ED. Mechanism of lipid peroxide formation in animal's tissue. Biochem J. 1996;99:667–76. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bierman EL, Amaral JA, Balknap BH. Hyperlipidemia and diabetes mellitus. Diabetes. 1975;25:509–15. [Google Scholar]
- 27.Rodrigues B, McNeill JH. Cardiac function in spontaneously hypertensive diabetic rats. Am J Physiol. 1986;251:H571–80. doi: 10.1152/ajpheart.1986.251.3.H571. [DOI] [PubMed] [Google Scholar]
- 28.Hoftiezer V, Carpenter AM. Comparison of STZ induced diabetes in rats, including volumetric quantization of pancreatic islet cells. Diabetologia. 1973;9:178–84. doi: 10.1007/BF01219780. [DOI] [PubMed] [Google Scholar]
- 29.Murali B, Goyal RK. Effect of chronic treatment with losartan on streptozotocin induced diabetic rats. Indian J Exp Biol. 2002;40:31–4. [PubMed] [Google Scholar]
- 30.Gangadevi T, Subramoniam A. Antidiabetic activity of ethanol extract of Cassia Kleinii leaf in streptozotocin induced diabetic rats and isolation of an active fraction and toxicity evaluation of the extract. Indian J Pharmacol. 2003;35:290–6. [Google Scholar]
- 31.Marles JR, Farnsworth NR. Antidiabetic plants and their active constituents. Phytomedicine. 1995;2:137–89. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- 32.Sy GY, Cisse A, Nongonierma RB, Sarr M, Mbodj NA, Faye B. Hypoglycaemic and antidiabetic activity of acetonic extract of Veronia colorata leaves in normoglycemic and alloxan induced diabetic rats. J Ethnopharmacol. 2005;98:171–5. doi: 10.1016/j.jep.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Roy S, Sehgal R, Padhy BM, Kumar VL. Antioxidant and protective effect of latex of Calotropis procera against alloxan-induced diabetes in rats. J Ethnopharmacol. 2005;102:470–3. doi: 10.1016/j.jep.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 34.Tabatabaei-Malazy O, Larijani B, Abdollahi M. A systematic review of in vitro studies conducted on effect of herbal products on secretion of insulin from langerhans islets. J Pharm Pharm Sci. 2012;15:447–66. doi: 10.18433/j32w29. [DOI] [PubMed] [Google Scholar]
- 35.Li WL, Zheng HC, Bukru J, De Kimpe N. Natural Medicines used in traditional Chinese medicine system for therapy of diabetes mellitus. J Ethnopharmacol. 2004;92:1–21. doi: 10.1016/j.jep.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 36.Tanko Y, Yaro AH, Isa AI, Yerima M, Saleh MI, Mohammed A. Toxicological and hypoglycemic studies on the leaves of Cissampelos Mucronata (Menispermaceae) on blood glucose levels of streptozocin-induced diabetic wistar rats. J Med Plants Res. 2007;1:113–6. [Google Scholar]
- 37.Sharma RD, Sarkhar A, Hazra DK, Misra B, Singh JB, Maheshwari BB. Toxicological evaluation of Fenugreek seeds: A Long Term Feeding Experiment in Diabetic Patients. Phytother Res. 1996;10:519–20. [Google Scholar]
- 38.Sikarwar MS, Patil MB. Antidiabetic activity of Crateva nurvala stem bark extracts in alloxan-induced diabetic rats. J Pharm Bioallied Sci. 2010;2:18–21. doi: 10.4103/0975-7406.62700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saxena A, Vikram NK. Role of selected Indian plants in management of type 2 diabetes: A review. J Altern Complement Med. 2004;10:369–78. doi: 10.1089/107555304323062365. [DOI] [PubMed] [Google Scholar]
- 40.Grover JK, Yadev S, Vats V. Medicinal plants of India with antidiabetic potential. J Ethnopharmacol. 2002;81:81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 41.Sezik E, Aslan M, Yesilada E, Ito S. Hypoglycemic activity of Gentiana olivieri and isolation of the active constituent through bioassay-directed fractionation techniques. Life Sci. 2005;76:1223–38. doi: 10.1016/j.lfs.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 42.Mukherjee PK, Maiti K, Mukherjee K, Houghton PJ. Leads from Indian medicinal plants with hypoglycemic potentials. J Ethnopharmacol. 2006;106:1–28. doi: 10.1016/j.jep.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 43.Gold AH. The effect of diabetes and insulin on liver glycogen synthetase activation. J Biol Chem. 1970;245:903–5. [PubMed] [Google Scholar]
- 44.Balasse EO, Bier DM, Havel RJ. Early effects of anti-insulin serum on hepatic metabolism of plasma free fatty acids in dogs. Diabetes. 1972;21:280–8. doi: 10.2337/diab.21.5.280. [DOI] [PubMed] [Google Scholar]
- 45.Nikkilä EA, Huttunen JK, Ehnholm C. Postheparin plasma lipoprotein lipase and hepatic lipase in diabetes mellitus. Relationship to plasma triglyceride metabolism. Diabetes. 1977;26:11–21. doi: 10.2337/diab.26.1.11. [DOI] [PubMed] [Google Scholar]
- 46.Bagdade JD, Porte D, Jr, Bierman EL. Acute insulin withdrawal and the regulation of plasma triglyceride removal in diabetic subjects. Diabetes. 1968;17:127–32. doi: 10.2337/diab.17.3.127. [DOI] [PubMed] [Google Scholar]
- 47.Frayn KN. Insulin resistance and lipid metabolism. Curr Opin Lipidol. 1993;4:197–204. [Google Scholar]
- 48.Pari L, Saravanan G. Antidiabetic effect of Congent db, a herbal drug in alloxan-induced diabetes mellitus. Comp Biochem Physiol C Toxicol Pharmacol. 2002;131:19–25. doi: 10.1016/s1532-0456(01)00259-9. [DOI] [PubMed] [Google Scholar]
- 49.Mallick C, Chatterjee K, Mandal U, Ghosh D. Antihyperglycemic, antilipidperoxidative and antioxidative effects of extracts of Musa paradisiaca and Coccinia indica in Streptozotocin-Induced Diabetic Rat. Ethiop Pharm J. 2007;25:9–22. [Google Scholar]
- 50.Balaraman AK, Singh J, Dash S, Maiti TK. Antihyperglycemic and hypolipidemic effects of Melothria maderaspatana and Coccinia indica in streptozotocin induced diabetes in rats. Saudi Pharm J. 2010;18:173–8. doi: 10.1016/j.jsps.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McAnuff MA, Omoruyi FO, Morrison EY, Asemota HN. Hepatic function enzymes and lipid peroxidation in streptozotocin induced diabetic rats fed bitter yam (Dioscorea polygonoides) steroidal sapogenin extract. Diabetol Croat. 2003;32:17–22. [Google Scholar]
- 52.Ahmed I, Lakhani MS, Gillett M, John A, Raza H. Hypotriglyceridemic and hypocholesterolemic effects of anti-diabetic Momordica charantia (karela) fruit extract in streptozotocin-induced diabetic rats. Diabetes Res Clin Pract. 2001;51:155–61. doi: 10.1016/s0168-8227(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 53.Patel SS, Shah RS, Goyal RK. Antihyperglycemic, antihyperlipidemic and antioxidant effects of Dihar, a polyherbal ayurvedic formulation in streptozotocin induced diabetic rats. Indian J Exp Biol. 2009;47:564–70. [PubMed] [Google Scholar]
- 54.Andallu B, Radhika B. Hypoglycemic, diuretic and hypocholesterolemic effect of winter cherry (Withania somnifera, Dunal) root. Indian J Exp Biol. 2000;38:607–9. [PubMed] [Google Scholar]
- 55.Gupta R, Saxena AM. Hypoglycemic and anti-hyperglycemic activities of Syzygium cumini (Linn.) skeels whole fruit, in normal and streptozotocin-induced diabetic rats. Asian J Phar Biol Res. 2011;1:267–72. [Google Scholar]
- 56.Xue WL, Li XS, Zhang J, Liu YH, Wang ZL, Zhang RJ. Effect of Trigonella foenum-graecum (fenugreek) extract on blood glucose, blood lipid and hemorheological properties in streptozotocin-induced diabetic rats. Asia Pac J Clin Nutr. 2007;16(Suppl 1):422–6. [PubMed] [Google Scholar]
- 57.Sabu MC, Kuttan R. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J Ethnopharmacol. 2002;81:155–60. doi: 10.1016/s0378-8741(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 58.Hassan SA, Barthwal R, Nair MS, Haque SS. Aqueous bark extract of Cinnamomum Zeylanicum: A potential therapeutic agent for streptozotocin-induced type 1 diabetes mellitus (T1DM) rats. Trop J Pharm Res. 2012;11:429–35. [Google Scholar]
- 59.Dhanabal SP, Kokate CK, Ramanathan M, Kumar EP, Suresh B. Hypoglycaemic activity of Pterocarpus marsupium Roxb. Phytother Res. 2006;20:4–8. doi: 10.1002/ptr.1819. [DOI] [PubMed] [Google Scholar]
- 60.Patel D, Prasad S, Kumar R, Hemalathaet S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed. 2012;2:320–30. doi: 10.1016/S2221-1691(12)60032-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chawla R, Thakur P, Chowdhry A, Jaiswal S, Sharma A, Goel R, et al. Evidence based herbal drug standardization approach in coping with challenges of holistic management of diabetes: A dreadful lifestyle disorder of 21st century. J Diabetes Metab Disord. 2013;12:35. doi: 10.1186/2251-6581-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Subash-Babu P, Ignacimuthu S. Antihyperlipidemic and antioxidant effect of hyponidd in the brain of streptozotocin induced diabetic rat. Int J Biol Chem. 2007;1:196–204. [Google Scholar]


