Abstract
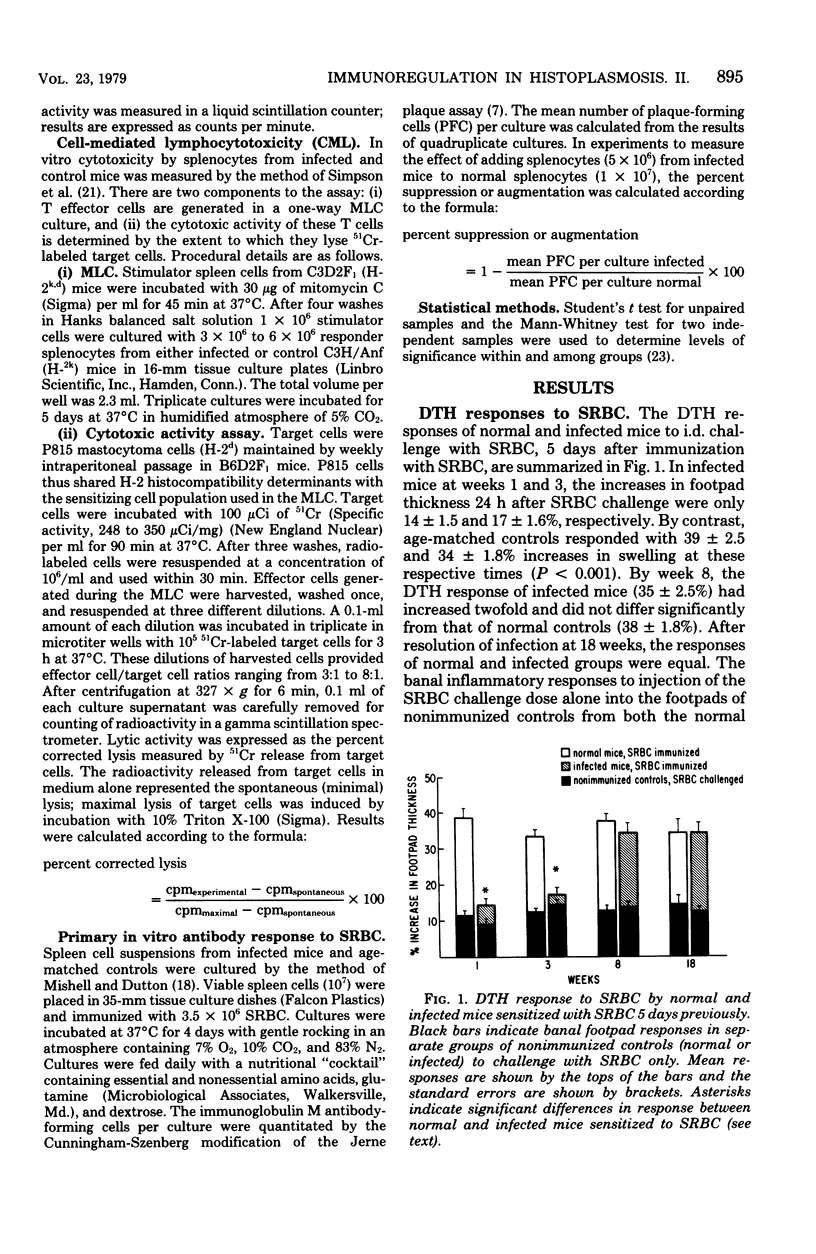
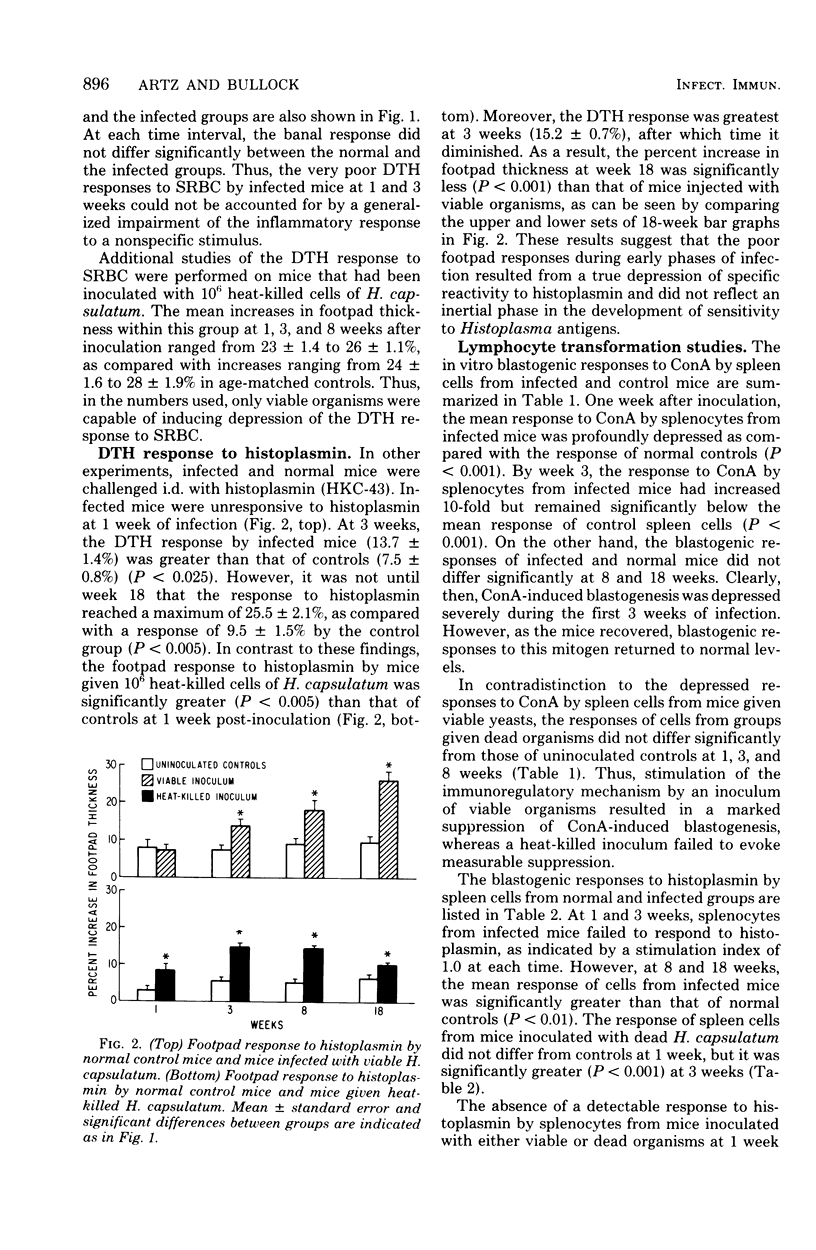
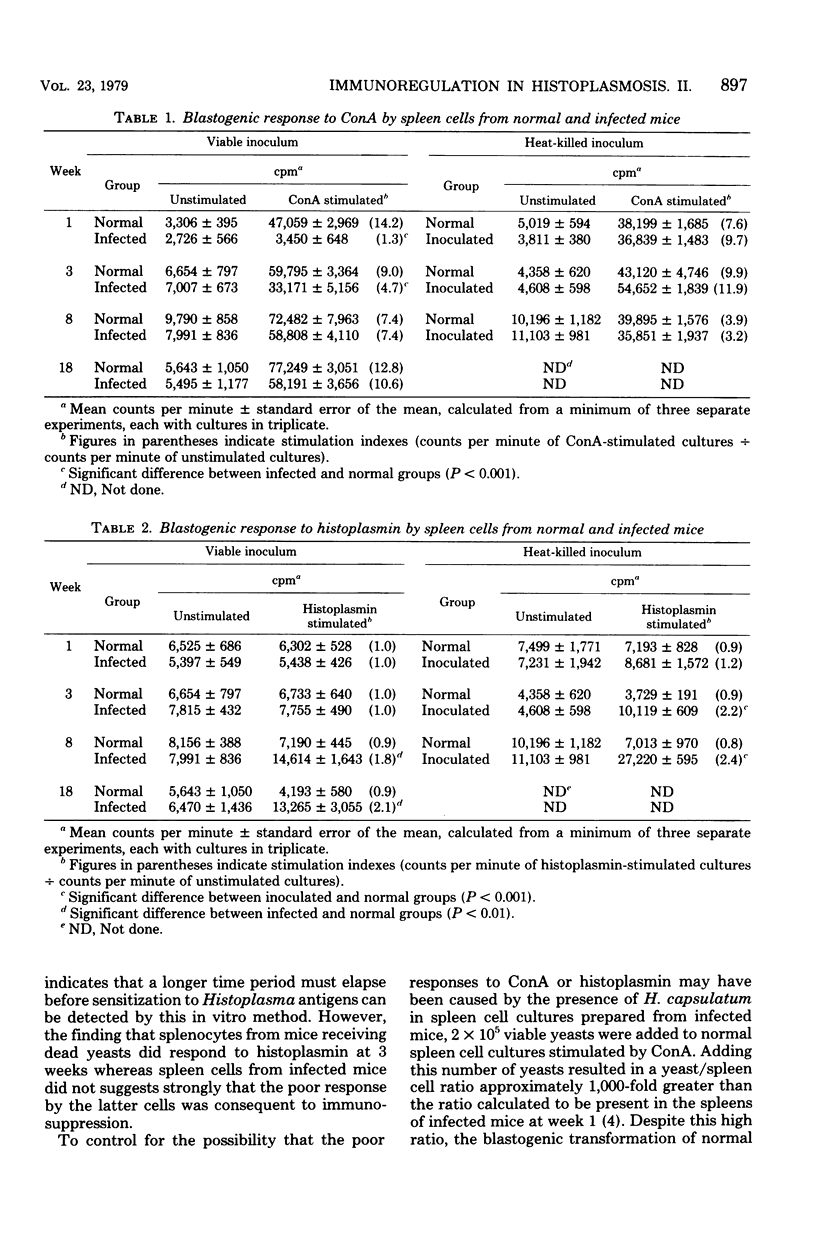
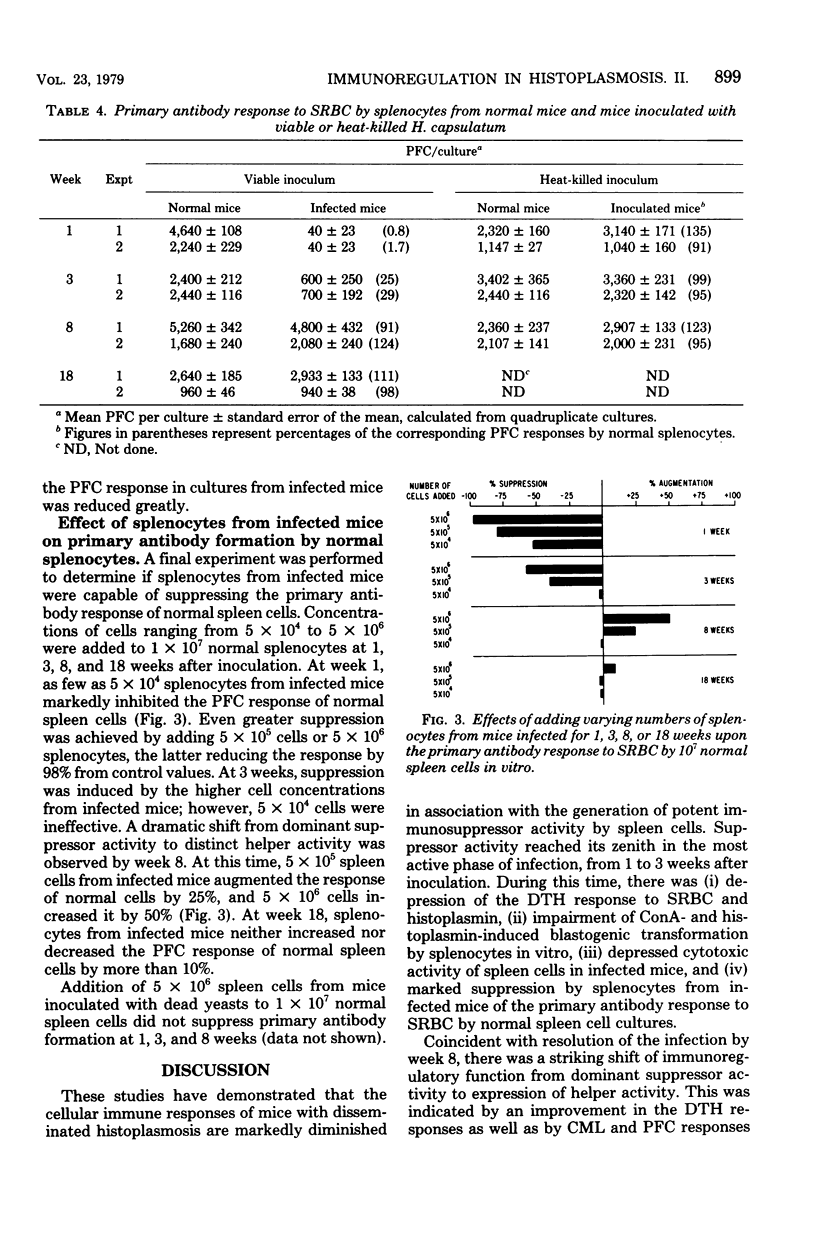
The cellular immune responses of mice with disseminated histoplasmosis are markedly diminished in association with the generation of potent immunosuppressor activity by spleen cells. The zenith of suppressor activity was observed during most active infection, from 1 to 3 weeks after inoculation. During this time there was: (i) depression of the delayed-type hypersensitivity response to sheep erythrocytes and histoplasmin, (ii) impairment of concanavalin A- and histoplasmin-induced blastogenic transformations by splenocytes in vitro, (iii) depressed cytotoxic activity of spleen cells from infected mice, and (iv) marked suppression by splenocytes from infected mice of the primary antibody response to sheep erythrocytes by normal spleen cell cultures. With resolution of the infection by week 8, there was a shift of immunoregulatory function from dominant suppressor activity to expression of helper activity. At this time, delayed-type hypersensitivity responses to the above antigens were vigorous; furthermore, the cytotoxic activity and plaque-forming cell response of splenocytes from 8-week-infected mice were equal to or greater than normal control values. The shift in the immunoregulatory response from a suppressor to a helper mode indicated that the net amount of help or suppression measured at any given time during infection represented the algebraic sum of both helper and suppressor activities mediated by different populations or subpopulations of cells within the splenic microenvironment of infected mice.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford R. H., Goodwin R. A. Patterns of immune response in chronic pulmonary histoplasmosis. J Infect Dis. 1972 Mar;125(3):269–275. doi: 10.1093/infdis/125.3.269. [DOI] [PubMed] [Google Scholar]
- Artz R. P., Bullock W. E. Immunoregulatory responses in experimental disseminated histoplasmosis: lymphoid organ histopathology and serological studies. Infect Immun. 1979 Mar;23(3):884–892. doi: 10.1128/iai.23.3.884-892.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E., Carlson E. M., Gershon R. K. The evolution of immunosuppressive cell populations in experimental mycobacterial infection. J Immunol. 1978 May;120(5):1709–1716. [PubMed] [Google Scholar]
- Bullock W. E., Jr Perturbation of lymphocyte circulation in experimental murine leprosy. I. Description of the defect. J Immunol. 1976 Oct;117(4):1164–1170. [PubMed] [Google Scholar]
- Bullock W. E., Jr Perturbation of lymphocyte circulation in experimental murine leprosy. II. Nature of the defect. J Immunol. 1976 Oct;117(4):1171–1178. [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Regulation of cellular and humoral immune responses by T-cell subclasses. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):23–32. doi: 10.1101/sqb.1977.041.01.006. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Davies S. F., Khan M., Sarosi G. A. Disseminated histoplasmosis in immunologically suppressed patients. Occurrence in a nonendemic area. Am J Med. 1978 Jan;64(1):94–100. doi: 10.1016/0002-9343(78)90183-3. [DOI] [PubMed] [Google Scholar]
- Durkin H. G., Carboni J. M., Waksman B. H. Antigen-induced increase in migration of large cortical thymocytes (regulatory cells?) to the marginal zone and red pulp of the spleen. J Immunol. 1978 Sep;121(3):1075–1081. [PubMed] [Google Scholar]
- Eardley D. D., Hugenberger J., McVay-Boudreau L., Shen F. W., Gershon R. K., Cantor H. Immunoregulatory circuits among T-cell sets. I. T-helper cells induce other T-cell sets to exert feedback inhibition. J Exp Med. 1978 Apr 1;147(4):1106–1115. doi: 10.1084/jem.147.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford W. L. Lymphocyte migration and immune responses. Prog Allergy. 1975;19:1–59. doi: 10.1159/000313381. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Eardley D. D., Naidorf K. F., Ptak W. The hermaphrocyte: a suppressor-helper T cell. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):85–91. doi: 10.1101/sqb.1977.041.01.012. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Lance E. M., Kondo K. Immuno-regulatory role of spleen localizing thymocytes. J Immunol. 1974 Feb;112(2):546–554. [PubMed] [Google Scholar]
- Huldt G., Gard S., Olovson S. G. Effect of Toxoplasma gondii on the thymus. Nature. 1973 Aug 3;244(5414):301–303. doi: 10.1038/244301a0. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H. Suppressor cells in experimentally trypanosomiasis. Nature. 1977 Feb 10;265(5594):539–541. doi: 10.1038/265539a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kauffman C. A., Israel K. S., Smith J. W., White A. C., Schwarz J., Brooks G. F. Histoplasmosis in immunosuppressed patients. Am J Med. 1978 Jun;64(6):923–932. doi: 10.1016/0002-9343(78)90445-x. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry W. M., Jr, Chandler J. W., Jr, Chin T. D., Kirkpatrick C. H. Immunology of the mycoses. I. Depressed lymphocyte transformation in chronic histoplasmosis. J Immunol. 1968 Feb;100(2):436–443. [PubMed] [Google Scholar]
- Reddy P., Gorelick D. F., Brasher C. A., Larsh H. Progressive disseminated histoplasmosis as seen in adults. Am J Med. 1970 May;48(5):629–636. doi: 10.1016/0002-9343(70)90014-8. [DOI] [PubMed] [Google Scholar]
- Simpson E., Gordon R., Taylor M., Mertin J., Chandler P. Micromethods for induction and assay of mouse mixed lymphocyte reactions and cytotoxicity. Eur J Immunol. 1976 Jul;5(7):451–455. doi: 10.1002/eji.1830050705. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Utz J. P. Progressive disseminated histoplasmosis. A prospective study of 26 patients. Ann Intern Med. 1972 Apr;76(4):557–565. doi: 10.7326/0003-4819-76-4-557. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Paul S., Van Scoy R. E., Hermans P. E. Suppressor thymus-derived lymphocytes in fungal infection. J Clin Invest. 1976 Feb;57(2):319–328. doi: 10.1172/JCI108283. [DOI] [PMC free article] [PubMed] [Google Scholar]



