Abstract
Neoplasms with histology and immunohistochemistry similar to gastrointestinal stromal tumors may occur primarily outside the gastrointestinal tract, usually in the omentum and mesentery. These are referred to as extragastrointestinal stromal tumors (EGISTs). Retroperitoneum is a very rare site for such neoplasms. We report a patient with EGIST in the retroperitoneum, elaborating the cross-sectional imaging and histopathologic findings.
Keywords: CD117, extragastrointestinal stromal tumor, gastrointestinal stromal tumor, retroperitoneum
INTRODUCTION

Classical gastrointestinal stromal tumor (GIST) is a non-epithelial neoplasm arising from the muscularis propria layer of the gastrointestinal tract leading to its tendency for exophytic growth. This is in contrast to other mesenchymal tumors like leiomyomas that involve the muscularis mucosae. GISTs most frequently occur in the stomach (70%), followed by the small intestine (20-30%), anorectum (7%), colon, and esophagus.[1]
Extragastrointestinal stromal tumors (EGISTs) are neoplasms with histopathologic features similar to GIST, but are found outside the gastrointestinal tract with no evidence of a concurrent neoplasm in the gastrointestinal tract. This distinction from GIST is essential since extragastrointestinal spread of a proven GIST has been frequently documented. Only 58 cases of pathologically proven EGIST in retroperitoneal location have been documented in current English medical literature and less than 10 of these had a preoperative imaging with confirmed histopathology.[2,3]
A 40-year-old female patient presented to the surgical outpatient department of our institution with a 4-month history of a gradually increasing generalized abdominal swelling, more on the left side. No history of similar complaints or previous abdominal pain or fever was present. On palpation, generalized distension of the abdomen was noted, especially involving the left side quadrants. No evidence of focal tenderness or guarding was noted.
RADIOLOGIC FEATURES
On the basis of clinical assessment, the patient was referred for cross-sectional imaging. Patient underwent a contrast-enhanced computed tomography (CECT) scan on a 16-slice CT scanner (General Electric Brightspeed, Milwaukee, WI, USA). CECT was performed by injecting 90 ml of intravenous contrast Iohexol (Omnipaque, General Electric Healthcare Milwaukee, WI, USA) through an 18-gauge needle in the antecubital vein at a rate of 3.5 ml/s. Scanning parameters used were tube current of 105 mA s and peak tube voltage of 130 kilovoltage (kVp). Acquisition was done at a slice thickness of 5 mm. In addition to a baseline non-enhanced scan, image acquisition was done during venous phase (70 s) and the excretory phase (3 min). The 5-mm-thick axial images were reformatted into thinner sections in three orthogonal planes (0.6 mm thick).
Non-enhanced computed tomography (NECT) images showed a very large well-defined mass in the abdominal cavity, predominantly on the left side. These scans showed the lesion had predominantly low attenuation with fine linear calcification forming a reticular pattern. Few foci of punctuate calcification were also seen in the posterior part of the lesion [Figure 1]. On post-contrast images, multiple enhancing septae appeared to radiate out from a central soft attenuation enhancing structure. Rest of the lesion showed areas of non-enhancing fluid attenuation [Figure 2]. Few septae in the periphery of the mass appeared thicker with tiny calcified foci [Figure 3]. The mass caused compression of liver, spleen [Figure 3], pancreas [Figure 4], and descending colon. However, no evidence of infiltration or invasion was noted [Figure 5] (negative embedded organ sign demonstrated, for comparison see [Figure 6]). The pancreas was noted as being displaced anteriorly and to the right of the midline and lay along the right lateral margin of the mass. A subtle but distinct flat plane was noted between the mass and pancreatic tissue. Above findings show that the mass did not originate from any of the structures in close contact, indicating a lesion of retroperitoneal origin. Both kidneys demonstrated normal parenchymal enhancement and contrast excretion, suggesting absence of distal obstruction [Figures 4 and 5].
Figure 1.
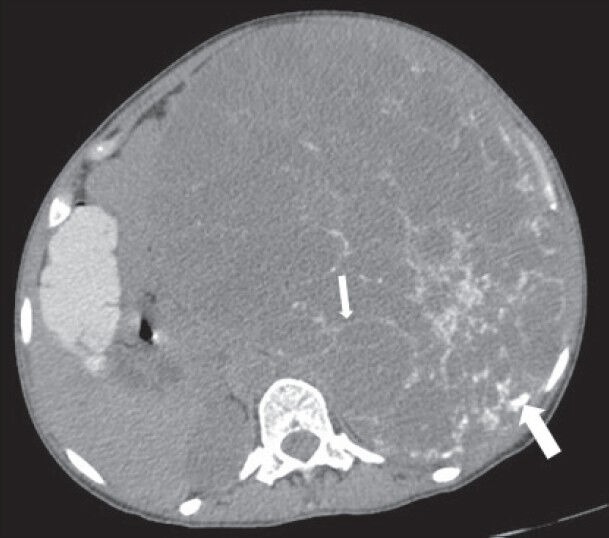
40-year-old female with abdominal mass subsequently diagnosed as extragastrointestinal stromal tumor (EGIST) in the retroperitoneum. Nonenhanced computed tomography of abdomen (axial section) shows a large well-defined lesion with low attenuation areas and reticular pattern fine linear calcification (small arrow) involving the abdominal cavity, predominantly on the left side. Few rounded foci of calcification are also noted in the posterior part of the lesion (thick white arrow).
Figure 2.
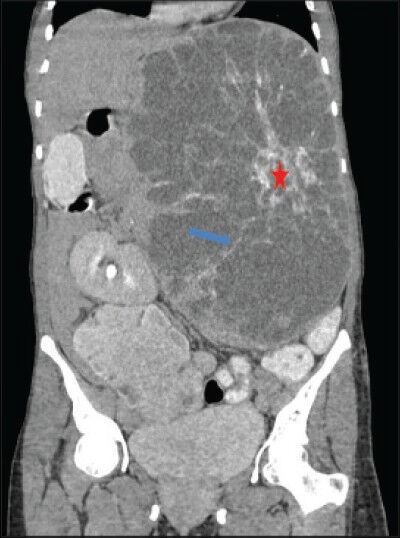
40-year-old female with abdominal mass subsequently diagnosed as EGIST in the retroperitoneum. Contrast-enhanced computed tomography (CECT) of abdomen (coronal section) during venous phase demonstrates multiple enhancing septae (blue arrow) radiating out from a central enhancing soft tissue attenuation structure (red asterisk). No enhancement of the low attenuation areas is noted.
Figure 3.
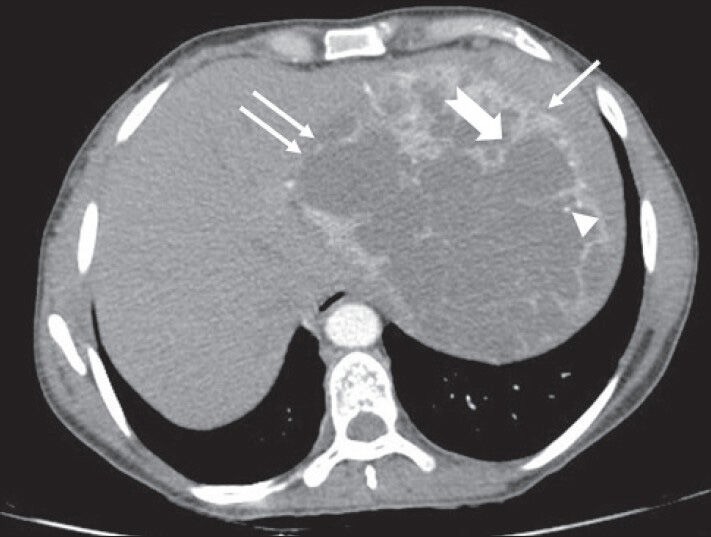
40-year-old female with abdominal mass subsequently diagnosed as EGIST in the retroperitoneum. CECT abdomen (axial section) during venous phase shows the lesion compressing spleen (solid arrow), left lobe of liver (solid double arrows) without invasion (negative embedded organ sign). Few septae in the periphery appear thicker (notched arrow). Few tiny foci of calcification are also noted in the periphery (arrowhead)
Figure 4.
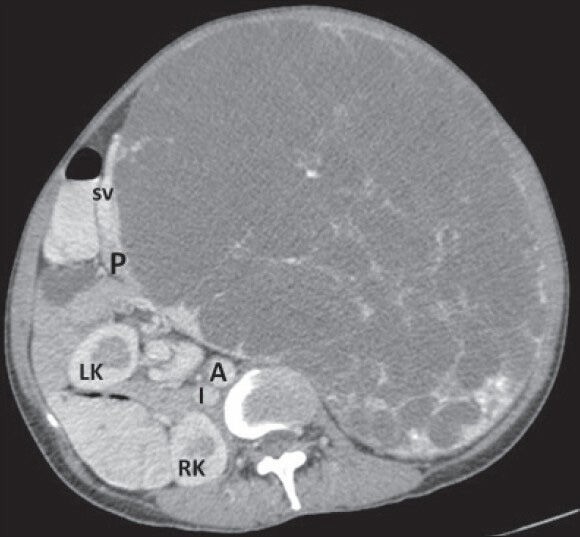
40-year-old female with abdominal mass subsequently diagnosed as EGIST in retroperitoneum. CECT abdomen (axial section) during venous phase shows the lesion compressing head and body of pancreas (P) without infiltration (negative embedded organ sign) and a distinct intervening fat plane with abdominal aorta (A). LK: Left kidney, RK: Right kidney, I: Inferior vena cava, SV: Splenic vein.
Figure 5.
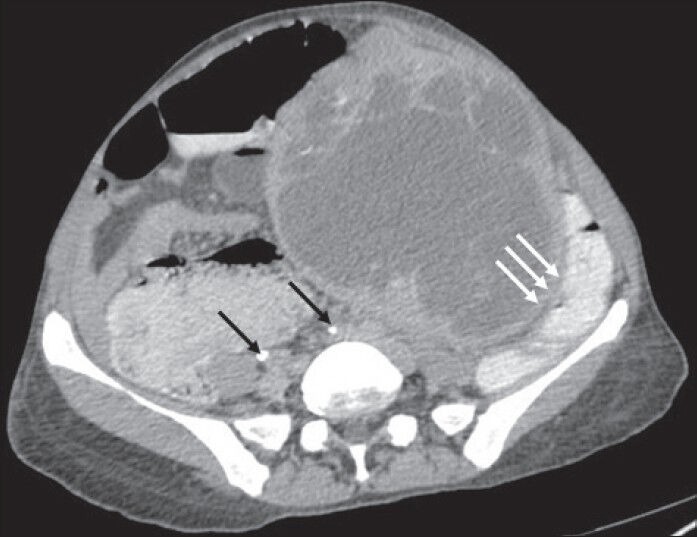
40-year-old female with abdominal mass subsequently diagnosed as EGIST in retroperitoneum. CECT abdomen (axial section) in excretory phase demonstrates contrast excretion with adequate opacification of ureters (thin black arrows). The mass lesion shows negative embedded organ sign with descending colon (solid triple white arrows).
Figure 6.
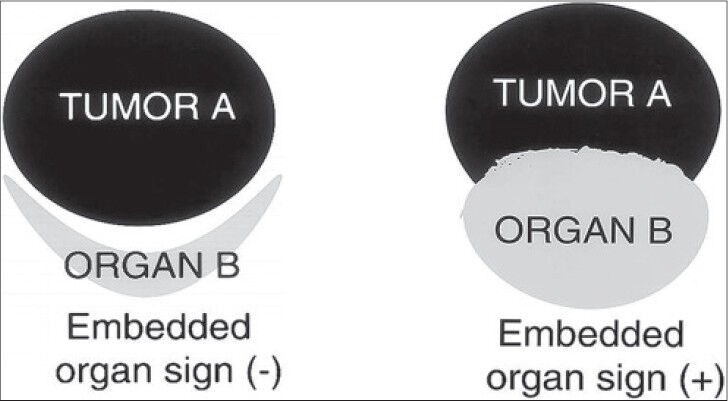
Embedded organ sign, schematic diagram (reproduced with permission from Ref. [4). Schematic diagram shows embedded organ sign, negative and positive.
In order to better characterize the lesion, patient was further evaluated using non-contrast magnetic resonance imaging (MRI). On T1-weighted spin-echo (SE) images, the mass showed areas of low signal intensity (isointense to skeletal muscle) and multiple markedly hypointense (dark) linear bands and punctuate foci [Figure 7].
Figure 7.
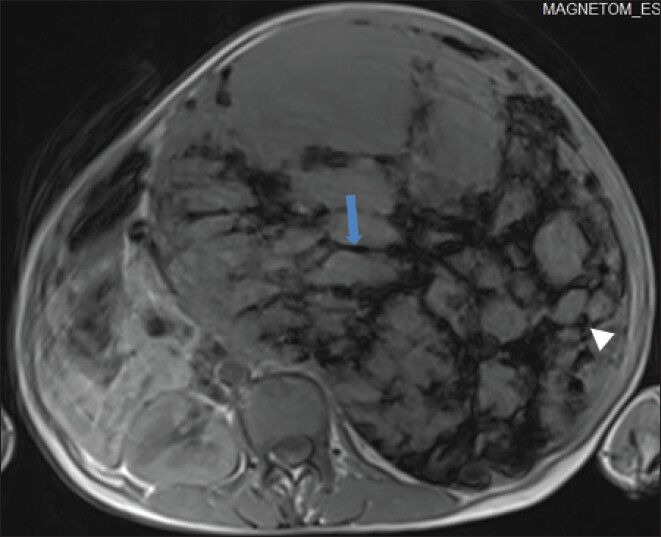
40-year-old female with abdominal mass subsequently diagnosed as EGIST in retroperitoneum. Non-enhanced magnetic resonance imaging (MRI) (axial section) of abdomen shows the mass with low signal intensity areas, multiple markedly hypointense linear bands (blue arrow) in a reticular pattern, and few hypointense round foci (white arrowhead) on T1-weighted spin echo (SE) images.
T2-weighted fast spin echo (FSE) images demonstrated an extremely heterogeneous lesion composed of multiple well-defined loculations of varying sizes and signal intensity. Most of the locules demonstrated iso- to mildly hyperintense signal intensity (isointense to renal parenchyma, hyperintense to liver parenchyma) [Figure 8], but few of these also showed cyst-like bright fluid signal intensity [Figure 9]. The locules showed predominantly thin walls. Overall, the lesion gave a bubbly appearance. The central area of the lesion showed irregular markedly hypointense signal intensity [Figure 9].
Figure 8.
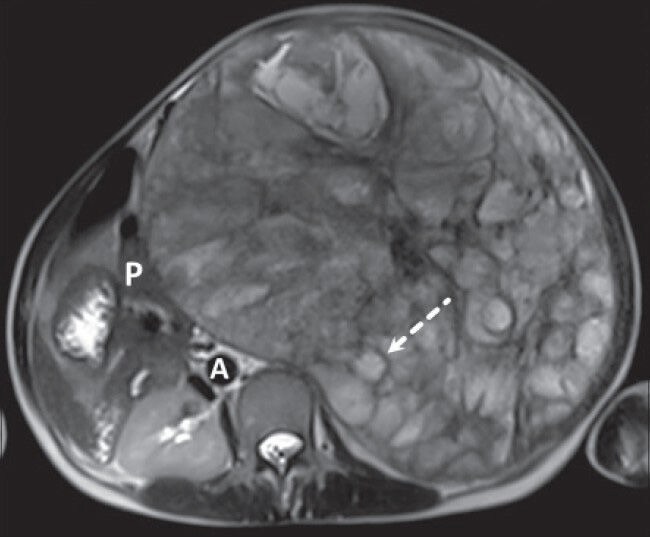
40-year-old female with abdominal mass subsequently diagnosed as EGIST in retroperitoneum. MRI (axial section) of abdomen shows multiple loculated structures with iso- to mildly hyperintense signal intensity on T2-weighted fast spin echo (FSE) image (dashed arrow) [pancreas (P), aorta (A)].
Figure 9.
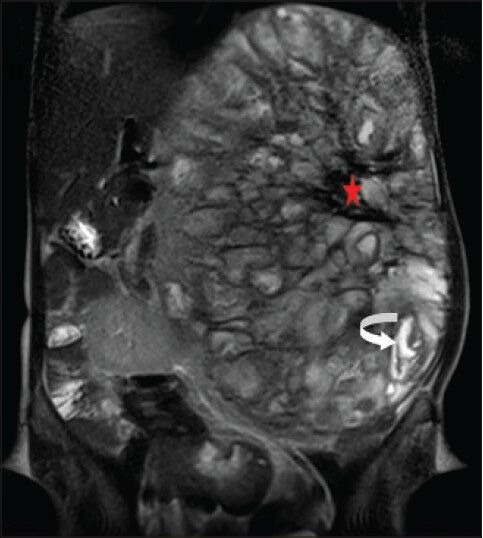
40-year-old female with abdominal mass subsequently diagnosed as EGIST in retroperitoneum. MRI (coronal section) of abdomen shows a few of the loculated structures with bright fluid signal intensity (curved white arrow) and the central region of the lesion with large hypointense signal (red asterisk) on T2-weighted FSE images.
PATHOLOGIC FEATURES
Subsequently, the patient underwent exploratory laparotomy with enucleation of the tumor. Intraoperatively, after mobilizing the descending colon and splenic flexure, the relationship of the mass with pancreas, left kidney, and surrounding vessels was noted. Pancreas could be lifted up from the mass. Pancreatic body and tail was freely dissected from the tumor with the help of blunt and sharp dissection. Small feeding vessels in between were ligated and divided. There was no infiltration of mass into the retroperitoneal structures including left kidney and pancreas. Grossly, the lesion appeared well-defined. On cut section, the mass appeared grayish white to brownish in color showing solid areas and partly necrotic surface.
Histopathologic examination using hematoxylin and eosin stain at (×10) magnification, revealed extensive hyalinization, scanty cellular areas with areas of hemorrhage, and foci of osseous metaplasia [Figure 10] probably corresponding to the calcification in the lesion. Further analysis of the stained sample at (×40) magnification showed fascicles of uniform spindle cells with pale eosinophilic cytoplasm and perinuclear vacuoles indenting the nucleus [Figure 11] suggestive of a spindle cell sarcoma of low to intermediate grade - possibly leiomyosarcoma.
Figure 10.
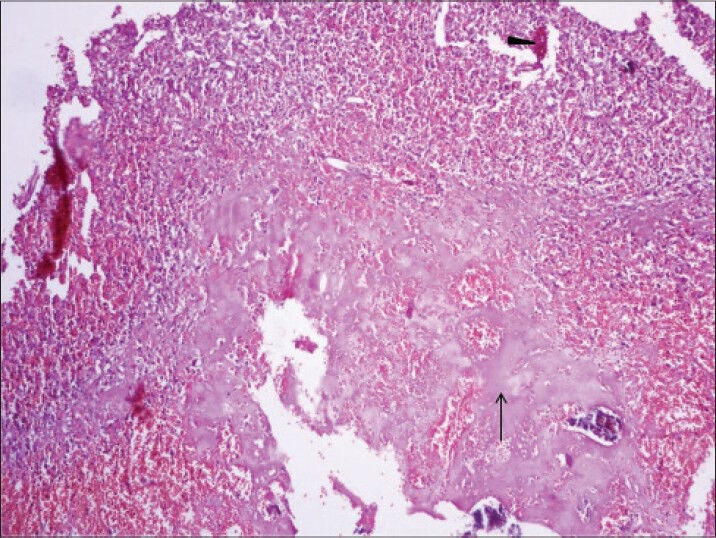
40-year-old female with abdominal mass subsequently diagnosed as EGIST in retroperitoneum. Photomicrograph [hematoxylin and eosin (H and E) stained sample (×10)] shows extensive hyalinization, scanty cellular areas with few areas of hemorrhage (black arrowhead), and foci of osseous metaplasia (thin long black arrow).
Figure 11.
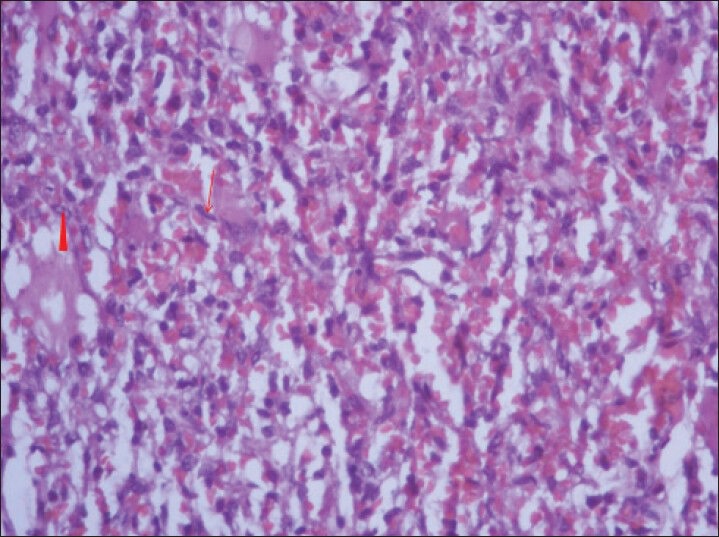
40-year-old female with abdominal mass subsequently diagnosed as EGIST in retroperitoneum. Photomicrograph of hematoxylin and eosin (H and E) stained sample (×40) shows fascicles of uniform bland spindle cells (thin long red arrow) with pale eosinophilic fibrillary cytoplasm and extravasated red blood cells. Perinuclear vacuoles indent the nucleus (red arrowhead).
Immunohistochemical staining revealed diffuse positivity for vimentin, but the actin and desmin markers were found to be negative. The strong and diffuse immunoreactivity for CD117 (proto-oncogene c-KIT) [Figure 12] confirmed the lesion as GIST in retroperitoneal location.
Figure 12.
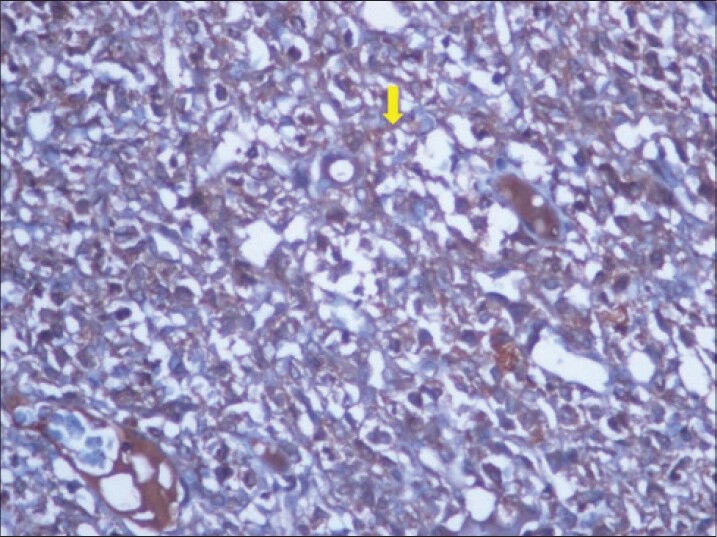
40-year-old female with abdominal mass subsequently diagnosed as EGIST in retroperitoneum. Immunohistochemical staining for CD117 (C-KIT) (×40) shows diffuse reactivity for tumor cells (thick yellow arrow).
DISCUSSION
By definition, the term GIST applies only to those gastrointestinal mesenchymal neoplasms with immunoreactivity for CD117 marker, normally expressed on the myenteric plexus and interstitial cells of Cajal in normal adult gastrointestinal tract. Retroperitoneal EGIST, like typical GIST, also shows positive staining for CD117 for confirmation. Staining for other immunological markers is variable: B-cell lymphoma 2 (BCL-2) (80%), CD34 (70%), smooth muscle actin (35%), S100 (10%), and desmin (5%).[1,2,3]
Retroperitoneal EGISTs have been shown to have two main histological patterns - spindle cell variety (cigar-shaped cells with elongated nuclei) and epithelioid type (polygonal cells with centrally placed nuclei).[1,2] EGISTs in the retroperitoneum grow silently and consequently are only discovered once they start causing compression symptoms.[3]
Understanding of the embedded organ sign (both positive and negative) helps in localizing the lesion to its possible organ of origin. This has been illustrated using the schematic Figure 6 (reproduced with permission).[4]
GIST of primary origin in the retroperitoneum has been often discussed in the surgical and pathologic literature, but rarely in the radiologic literature.[5]
On cross-sectional imaging, retroperitoneal EGISTs mostly appear as well-defined, non-infiltrative, inhomogenous soft-tissue density masses with heterogeneous contrast enhancement.[5,6] However, they may rarely appear as homogenous lesions also.[3] They may show areas of cystic degeneration, necrosis, or calcification.[5] The calcification pattern in the case reported by Takao et al., was peripheral rim-like,[5] in contrast to the reticular pattern noted in our patient. No calcification has been seen in other previously reported cases.
The general pathologic consensus for grading and assessing metastasis risk of classical gastric GISTs is based on tumor size and mitotic count. No such consensus appears to exist for retroperitoneal EGIST as of yet, since metastases have been rarely reported.[1,2,7]
On the basis of imaging findings alone it may be very difficult to distinguish GIST from other retroperitoneal mesenchymal neoplasms such as malignant fibrous histiocytoma, liposarcoma, leiomyosarcoma, and fibrosarcoma.[5]
CONCLUSION
On cross-sectional imaging, when a large well-defined retroperitoneal mass lesion with atypical calcification patterns (peripheral rim like or reticular) is encountered, GIST should be considered in the differential diagnosis, even if the lesion shows poor contrast enhancement.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2014/4/1/34/135484
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: Radiologic features with pathologic correlation. Radiographics. 2003;23:283–304. doi: 10.1148/rg.232025146. 456; quiz 532. [DOI] [PubMed] [Google Scholar]
- 2.Abuduwayite R, Muhemaiti A, Biekemituofu H, Mohemaiti P. Malignant retroperitoneal extra-gastrointestinal stromal tumor: A case report. Br J Med Med Res. 2002;2:142–9. [Google Scholar]
- 3.Casella C, Villanacci V, D’Adda F, Codazzi M, Salerni B. Primary extra-gastrointestinal stromal tumor of retroperitoneum. Clin Med Insights Oncol. 2012;6:189–97. doi: 10.4137/CMO.S9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishino M, Hayakawa K, Minami M, Yamamoto A, Ueda H, Takasu K. Primary retroperitoneal neoplasms: CT and MR imaging findings with anatomic and pathologic diagnostic clues. Radiographics. 2003;23:45–57. doi: 10.1148/rg.231025037. [DOI] [PubMed] [Google Scholar]
- 5.Takao H, Yamahira K, Doi I, Watanabe T. Gastrointestinal stromal tumor of the retroperitoneum: CT and MR findings. Eur Radiol. 2004;14:1926–9. doi: 10.1007/s00330-004-2404-3. [DOI] [PubMed] [Google Scholar]
- 6.Takizawa I, Morishita H, Matsuki S, Komeyama T, Emura I, Hara N. Primary gastrointestinal stromal tumor in the retroperitoneum. Int J Urol. 2006;13:1245–8. doi: 10.1111/j.1442-2042.2006.01545.x. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–65. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]


