Abstract
The signaling lymphocytic activation molecule-associated adaptor Ewing's sarcoma's-activated transcript 2 (EAT-2) is primarily expressed in dendritic cells, macrophages and natural killer cells. Including EAT-2 in a vaccination regimen enhanced innate and adaptive immune responses toward pathogen-derived antigens, even in the face of pre-existing vaccine immunity. Herein, we investigate whether co-vaccinations with two recombinant Ad5 (rAd5) vectors, one expressing the carcinoembryonic antigen (CEA) and one expressing EAT-2, can induce more potent CEA-specific cytotoxic T lymphocyte (CTL) and antitumor activity in the therapeutic CEA-expressing MC-38 tumor model. Our results suggest that inclusion of EAT-2 significantly alters the kinetics of Th1-biasing proinflammatory cytokine and chemokine responses, and enhances anti-CEA-specific CTL responses. As a result, rAd5-EAT2-augmented rAd5-CEA vaccinations are more efficient in eliminating CEA-expressing target cells as measured by an in vivo CTL assay. Administration of rAd5-EAT2 vaccines also reduced the rate of growth of MC-38 tumor growth in vivo. Also, an increase in MC-38 tumor cell apoptosis (as measured by hematoxylin and eosin staining, active caspase-3 and granzyme B levels within the tumors) was observed. These data provide evidence that more efficient, CEA-specific effector T cells are generated by rAd5 vaccines expressing CEA, when augmented by rAd5 vaccines expressing EAT-2, and this regimen may be a promising approach for cancer immunotherapy in general.
Keywords: cancer vaccines, EAT-2, immune modulation, innate immunity, SLAM receptors, SAP adaptors, carcinoembryonic antigen
INTRODUCTION
Cancer patients many times suffer from the absence of efficient tumor-specific immunity as a result of inadequate antigen-presenting cell (APC) function or T-cell tolerance toward tumor-associated antigens (TAAs).1 TAAs are sometimes self-antigens, which many times are overexpressed in cancer cells.2 Although TAAs are weak immunogens, many preclinical as well clinical antitumor vaccine studies have confirmed a critical role for TAA-specific T-cell responses in antitumor immunity.3–6 Development of an effective, therapeutic cancer vaccine will rely heavily upon the potential of a respective vaccine platform to generate potent antitumor immunity by breaking self-tolerance to TAAs. Carcinoembryonic antigen (CEA) is a well-known TAA that is a member of the immunoglobulin supergene family. CEA is expressed during fetal development7 and is also overexpressed in a variety of adenocarcinomas, including colorectal, lung, breast and pancreatic cancers.8,9 CEA is recognized by the National Cancer Institute (NCI) as an extremely attractive target for immunotherapy because it has been demonstrated to be immunogenic in a variety of patient populations, among other reasons.2,9–11 CEA has also been a target in active vaccination strategies against diverse CEA-expressing cancers because of its expression profile on oncofetal tissues and its role in tumorigenesis.9,10 CEA-specific T cells have been induced in vitro and in vivo, and have been shown to suppress the growth of CEA-positive cancers in mouse studies12,13 as well as in human clinical trials.14–17 Immunotherapy directed at CEA has been reported to be beneficial in some patients, resulting in antitumor responses and may prolong survival.14,18,19 Induction of potent cellular immune responses toward targeted TAAs such as CEA may advance the immunotherapy approach against many CEA-overexpressing cancers.
Adenovirus vectors have been shown to induce potent cellular and humoral immune responses toward the antigens they express.12,20–23 Although promising, considerable effort is required to generate large numbers of effector T cells in vivo that also overcome the immunosuppressive environment that can be present in many solid tumors. To overcome these barriers, incorporation of adjuvants into vaccine formulations may improve the induction of tumor antigen-specific adaptive immune responses.24–26 Pro-active induction and/or harnessing of beneficial innate immune responses may be the mechanism underlying the effectiveness of certain adjuvants to significantly contribute to the ability of vaccines to generate adaptive immune responses.27,28 In recent years, there has been accumulating evidence implicating a critical role for the signaling lymphocytic activation molecules (SLAM) family of receptors and their adaptors in regulating both innate and adaptive immunity.29 SLAM receptors have been shown to function as adhesion and co-stimulatory molecules on the surface of several hematopoietic cells.30 In addition, signaling downstream of SLAM receptors initiate distinct signal-transduction networks that orchestrate T cells, B cells, natural killer (NK) cells, dendritic cells (DCs), and macrophage effector and regulatory functions.31 As Ewing's sarcoma's-activated transcript 2 (EAT-2) is the only known SLAM-associated adaptor protein expressed in innate immune cells (DCs, macrophages and NK cells), it has been proposed that EAT-2 facilitates SLAM-dependent proinflammatory cytokine expression in these cell types.32 We previously reported development of a novel adenovirus-based vaccine platform, one that expresses the SLAM family of receptors adaptor EAT-2. Ad5 vectors expressing the EAT-2 adaptor are superior to conventional Ad5 vectors.20,33,34 Specifically, we found that administration of a recombinant Ad5 vector-expressing EAT-2 (rAd5-EAT2) improved early activation of innate immune responses, responses that are characterized by increased activation of innate immune cells (NK cells, NK T cells), enhanced maturation of APCs (DCs and macrophages) and enhanced production of beneficial cytokines and chemokines.20,34 As a result of the enhanced innate immune profile prompted by expression of EAT-2, EAT-2-expressing Ad vaccines also improved the induction of adaptive immune responses generated against co-expressed target antigens, including the induction of superior effector memory (TEM)-biased T-lymphocyte immune responses.34
In this study, we evaluated the potential of improving cancer vaccine efficacy by presenting a target TAA, CEA, while simultaneously activating the SLAM family of receptors signaling by the EAT-2 adaptor. We hypothesized that EAT-2 overexpression at the time of immunization with CEA-expressing Ad5 vectors would enhance the innate immune responses and also result in more potent CEA-specific adaptive immune responses. We also investigated the CEA-specific antitumor effects induced by rAd5-EAT2 in experimental animals and present the results of these studies herein.
MATERIALS AND METHODS
Vector construction
The rAd5-GFP and rAd5-EAT2 viruses were purified as previously described.20,35 Briefly, the open reading frame of the EAT-2 gene, (Genbank Accession no. NM_012009), http://www.ncbi.nlm.nih.gov/nuccore/148747581, was excised using primers flanked by XhoI and XbaI restriction endonucleases (NEB, Ipswich, MA, USA) from a plasmid (Open Biosystem, Huntsville, AL, USA) and subcloned into the pShuttle vector, which contains a CMV expression cassette. The resulting pshuttle-EAT-2 shuttle plasmid was linearized with PmeI restriction enzyme and homologously recombined with the pAdEasyI Ad5 vector genome, as previously described,36 yielding pAd5-EAT2. HEK293 cells were transfected with PacI-linearized plasmid and viable virus was obtained and amplified after several rounds of expanding infection. rAd5-EAT2 virus was purified using a CsCl2 gradient, as previously described.37 Direct sequencing and restriction enzyme mapping were carried out to confirm the integrity of the EAT-2 sequence. rAd5 vectors expressing human CEA were constructed and produced12 using a modified human CEA containing the highly immunogenic epitope CAP-1 (6D) mutation as the transgene insert, as previously described.38 Briefly, the CEA complementary DNA was subcloned into the E1 region of the Ad5 vectors using a previously described homologous recombination.39,40 The replication-deficient rAd5-CEA virus was then prepared as described above. All viruses were found to be replication-competent adenovirus (RCA)-free both by RCA PCR (E1 region amplification) and direct sequencing methods, as previously described.41 All Ads have also been tested for the presence of bacterial endotoxin, as previously described,41 and were found to contain <0.15 EU per ml. The infectivity of all Ad vectors used in our studies was confirmed by infectious (TCID50) and transducing units titer assays. The viral particle per tissue culture infectivity dose (vp/TCID) was similar between all the vectors. The TCID50 for the viral vectors was as follows: Ad-CEA = 4.4 × 1010 TCID50 per ml; Ad-EAT2 = 3.16 × 1010 TCID50 per ml; Ad-Null = 4.1 × 1010 ICID50 per ml; Ad-GFP = 4.09 × 1010 TCID50 per ml. Furthermore, viral particle (VP) titers were determined by spectrophotometry and validated by SDS-polyacrylamide gel electrophoresis of purified Ads followed by silver staining and/or western blotting.
Animal procedures
All animal procedures were approved by the Michigan State University institutional animal care and use committee. Adult male wild-type (WT) C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Intravenous injection of animals (8–10 weeks old) consisted of injection (via the retro-orbital sinus) of 200 μl of a phosphate-buffered saline solution (PBS, pH 7. 4) containing 7.5 × 1010 total virus particles of either Ad-Null or Ad5-EAT2, as previously described.42 Plasma and tissue samples were obtained and processed at the indicated times post injection, as previously described.42 Intramuscular injections were completed by injection of the indicated virus particles in a total volume of 20 μl into the tibialis anterior of the right hindlimb.
Cytokine and chemokine analysis
A mouse 23-plex multiplex-based assay was used to determine the indicated cytokine/chemokine plasma concentrations as per the manufacturer's instructions (Bio-Rad, Hercules, CA, USA) using Luminex 100 technology (Luminex, Austin, TX, USA) essentially, as previously described.42 The lower and upper detection limits for each cytokine and chemokine are shown in Supplementary Table 1.
Enzyme-linked immunosorbent spot (ELISPOT) assay analysis
Splenocytes were collected from individual mice and red blood cells were lysed using ammonium-chloride-potassium lysis buffer (Invitrogen, Carlsbad, CA, USA). Ninety-six-well Multiscreen high-protein-binding Immobilon-P membrane plates (Millipore, Billerica, MA, USA) were pretreated with ethanol (EtOH), coated with mouse anti-interferon (IFN)-γ, interleukin (IL)-2 or IL-4 capture antibodies, incubated overnight and blocked before the addition of 5 × 105 splenocytes per well. Ex vivo stimulation included the incubation of splenocytes in 100 ml of media alone (unstimulated) or media containing 4 mg per ml of human CEA-specific peptides pool (15 mers with 11 aa overlap covering full-length CEA with the 6D modification) (was kindly provided by Dr Michael Morse, Duke Medical Center, Durham, NC, USA) for 24 h in a 37 °C, 5% CO2 incubator.
Staining of plates was completed as per the manufacturer's protocol. Spots were counted and photographed by an automated ELISPOT reader system (Cellular Technology, Cleveland, OH, USA). Ready-set Go IFNγ, IL-2 and IL-4 mouse ELISPOT kits were purchased from eBioscience (San Diego, CA, USA).
Cell staining and flow cytometry
To evaluate the intracellular cytokine responses following Ad5-CEA and Ad-EAT2 co-vaccination, intracellular staining was performed as previously described.20 Briefly, cells were surface stained with APC-Cy7-CD3, Alexa Floure700-CD8a and CD16/32 Fc-block antibodies, fixed with 2% formaldehyde (Polysciences, Warrington, PA, USA), permeabilized with 0.2% Saponin (Sigma-Aldrich, St Louis, MO, USA) and stained for intracellular cytokines with PE-Cy7-TNFα, APC-granzyme B, FITC-IFNγ, PE Perforin (4 μg ml; all obtained from BD Biosciences, San Diego, CA, USA) and PerCpCy5.5-IL-2 (4 mg ml; BioLegend, San Diego, CA, USA). We included the violet fluorescent reactive dye (ViViD, Invitrogen) as a viability marker to exclude dead cells from the analysis.43
In vivo cytotoxic T lymphocyte assay
C57Bl/6 mice (N = 6) were co-vaccinated with equivalent doses of Ad5-CEA with either Ad5-GFP or Ad5-EAT2 (totaling 1 × 109 VPS). At 14 days, syngeneic splenocytes were isolated and either pulsed with an irrelevant peptide specific to the Plasmodium falciparum circumsporozoite antigen (NYDNAGTNL) or with the CEA-specific peptide pool for 1 h at 37 °C. Irrelevant peptide-pulsed cells were subsequently stained with 1 μm carboxyfluorescein succinimidyl ester (CFSE; (CFSELow), whereas CEA peptide-pulsed cells were stained with 10 μm CFSE (CFSEHigh). Naive and immunized mice were injected with equivalent amount of both CFSELow and CFSEHigh-stained cells (8 × 106 cells) via the retro-orbital sinus. After 20 h, mice were terminally killed and splenocytes were recovered and sorted on an LSRII flow cytometer (BD Biosciences). FlowJo software (Tree Star, San Carlos, CA, USA) was used to determine percentages of CFSE-stained cells. % Specific killing = 1 – ((% CFSEHigh/% CFSELow)immunized/(% CFSEHigh/% CFSELow)non-immunized.
Tumor immunotherapy
For tumor treatment studies, C57BL/6 mice, 8–10 weeks old, were implanted with 106 MC-38-cea2 cells subcutaneously (SQ) in the flank. The MC-38-cea2 cell line was kindly provided by Dr J Schlom, National Institutes of Health (NIH)-NCI, Bethesda, MD, USA via Dr HK Lyerly, Duke University Medical Center, Durham, NC, USA. This cell line is derived from the mouse adenocarcinoma, MC-38, which has been transduced with a retrovirus construct expressing a complementary DNA-encoding human CEA.44
Fluorescent immunostaining
Tumors were collected, fixed in 4% formalin for 48 h and then kept in 70% EtOH until paraffin embedding. Sections of 7 μm were obtained from paraffin-embedded blocks using a manual rotary microtome (Leica Microsystems, Bannockburn, IL, USA). Tissue was deparaffinized over three incubations in xylene (5 min each) and rehydrated in a series of EtOH dilutions, two steps each of 100, 95 and 70% EtOH, each lasting 3 min. Finally, a 5-min water incubation was undertaken to fully hydrate the tissue. To reverse formalin-induced cross-linking, slides were then treated with sodium citrate buffer for 30 min, bringing the solution to sub-boiling temperature. Triton X-100 (0.5%) was used to make the tissue permeable for ensuing antibody administration and penetration. The tissues were incubated with blocking solution (10% normal goat serum/1% BSA/PBS) for 1 h before antibody treatment. Primary antibodies were also placed in blocking solution to enrich for specific antibody–antigen interaction. A 1:250 concentration (antibody: blocking solution) of rabbit polyclonal anti-activated caspase-3 (ab13847 from Abcam, Cambridge, MA, USA) and a 1:250 of rabbit polyclonal anti-granzyme B (ab4059 from Abcam) were used. The antibody was then incubated with the tissue overnight at 4 °C. In order to remove unbound primary antibody, the slides were rinsed vigorously four times in 1 × PBS, each wash lasting 12 min. The sections were than incubated with secondary antibodies conjugated to Alexa Fluorophore 488 or 555 (Molecular Probes, Grand Island NY, USA). Four additional washes in 1 × PBS followed. Nuclear counterstaining was done for 10 min using 4’,6-diamidino-2-phenylindole (DAPI) in RO H2O (1:10 000; Molecular Probes). Finally, we mounted the slides in ProLong GOLD Antifade reagent to suppress photobleaching (Molecular Probes). The images were taken using the Nikon Eclipse 90i microscope (Melville, NY, USA).
Hematoxylin and eosin staining
Tissue and slide preparation was performed exactly as indicated in the fluorescent immunostainind method section, up to treatment with sodium citrate buffer. After a 5-min water hydration step, Gill's no. 3 hematoxylin (GHS316, Sigma-Aldrich) was applied to stain nuclei for 1.5 min, followed by a quick rinse. Eosin stock solution was prepared with 1 g of eosin in 20 ml of water and 80 ml of 95% EtOH. A working solution was prepared with a 1:3 dilution of the stock using 80% EtOH. Glacial acetic acid was added to the working solution and eosin staining also lasted 1.5 min. Dehydration of the tissue was complete using two steps each of 95 and 100% EtOH, each step lasting 2 min. Two additional xylene steps were applied, each lasting 3 min. Finally, Permount, SP15-100 Toluene Solution UN1294, (Fisher Scientific, Bridgewater, NJ, USA) was applied with the help of a Pasteur pipette to the tissue, and no. 1 microscope coverslip (Globe Scientific, Paramus, NJ, USA) was placed before imaging the tissue using Nikon Eclipse 90i microscope.
Statistical analysis
Statistically significant differences in toxicities associated with innate immune responses were determined using two-way analysis of variance (ANOVA) with a Student–Newman–Keuls post-hoc test (P value<0.05). For ELISPOT analysis, a two-way ANOVA was used followed by Bonferroni post-hoc test (P<0. 05). For flow cytometry, a one-way ANOVA with a Student– Newman–Keuls post-hoc test was used. For in vivo cytotoxic T lymphocyte (CTL) assay, a one-way ANOVA with a Student–Newman–Keuls post-hoc test was used. Statistically significant differences in the total tumor volume between groups of animals were determined by the Student's t-test. All graphs in this paper are presented as mean±s.d. Statistical analyses were performed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA).
RESULTS
EAT-2-expressing Ad vectors enhance Ad vector-induced innate immune responses in vivo
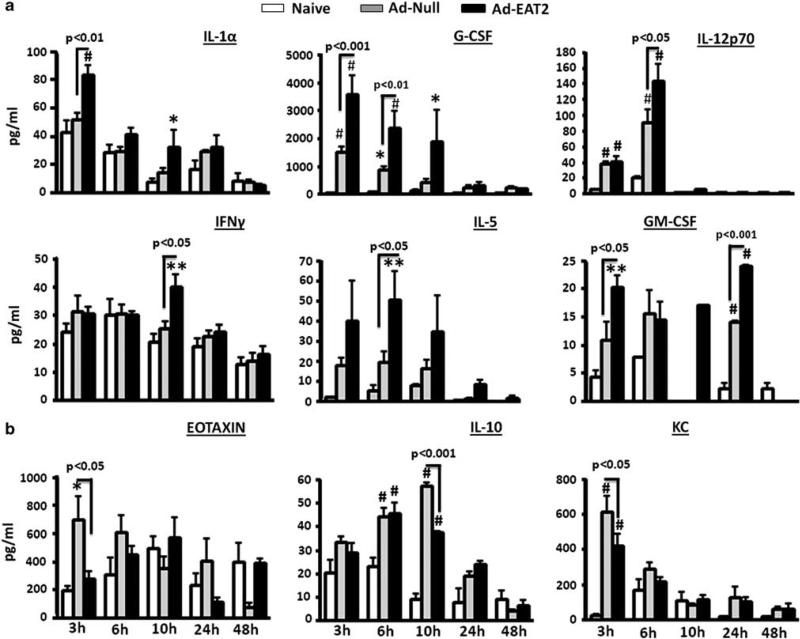
We previously confirmed that EAT-2 overexpression significantly induces proinflammatory cytokine and chemokine responses in vivo measured by a multiplex-based assay.20 To study the kinetics of proinflammatory cytokines production and to broadly analyze the maximal impact that Ad-mediated expression of EAT-2 might have upon innate immune cytokine and chemokine responses, we administered rAd5-EAT2 or the rAd5-Null control vaccine into C57BL/6 mice and measured the induction levels of several cytokine and chemokines, utilizing a 23-plex multiplex-based assay. Plasma samples were collected at 3, 6, 10, 24 and 48 h post injection (hpi), and production of proinflammatory cytokine and chemokines was evaluated. Administration of the rAd5-EAT2 vector into C57BL/6 mice resulted in induction of significantly higher plasma levels of IL-1α (at 3 hpi (P<0.01)), G-CSF (at 3 hpi (P<0.001), 6 hpi (P<0.01) and 10 hpi (P<0.05)), IL-5 (at 6 hpi (P<0.05)), IL-12p70 (at 6 hpi (P<0.05)), IFNg (10 hpi (P<0.05)) and GM-CSF (at 3 hpi (P<0.05) and 24 hpi(P<0.001)), as directly compared with mice identically treated with the rAd5-Null control (Figure 1a). Interestingly, the levels of EOTAXIN (at 3 hpi (P<0.05)), IL-10 (at 10 hpi (P<0.001)) and KC (at 3 hpi (P<0.05)) were significantly reduced in rAd5-EAT2 injected mice, as compared with rAd5-Null-injected controls (Figure 1b). The levels of IL-1β, IL-12p40, IL-6, IL-9, MCP-1, MIP-1α, MIP-1β and RANTES were also significantly (P<0.001) induced by rAd5-EAT2 vectors; however, these levels were not statistically different between rAd5-EAT2 and rAd5-Null-injected controls (Supplementary Figure 1). We also measured the levels of IL-3 and IL-4; however, no significant inductions were observed in mice injected with both rAd5-EAT2 and rAd5-Null vectors (data not shown).
Figure 1.
Kinetics of proinflammatory cytokine and chemokine production following recombinant Ad5 vector expressing EAT-2 (rAd5-EAT2) administration. Wild-type (WT) C57BL/6 mice (n = 8) were either mock injected or intravenously injected with 7.5 × 1010 vps of either rAd5-EAT2 or rAd5-Null control vectors. Plasma was collected at 3, 6, 10, 24 and 48 h after virus injection. Cytokine induction was evaluated using a 23-plex multiplexed bead array-based quantitative system. Increases (a) or decreases (b) in cytokines and chemokines responses following EAT-2 overexpression are shown. The bars represent mean±s.d. Statistical analysis was completed using a two-way analysis of variance (ANOVA) with a Student–Newman–Keuls post-hoc test, P<0.05 was deemed a statistically significant difference. * Denotes P<0.05, ** denotes P<0. 01, # denotes P<0.001, statistically significant difference from mock-injected animals.
Ad vector-expressing EAT-2 enhances T-cell responses to the CEA Simultaneous administration of antigens with adjuvants can stimulate the innate immune system to significantly improve the adaptive immune responses to the antigenic target.45,46
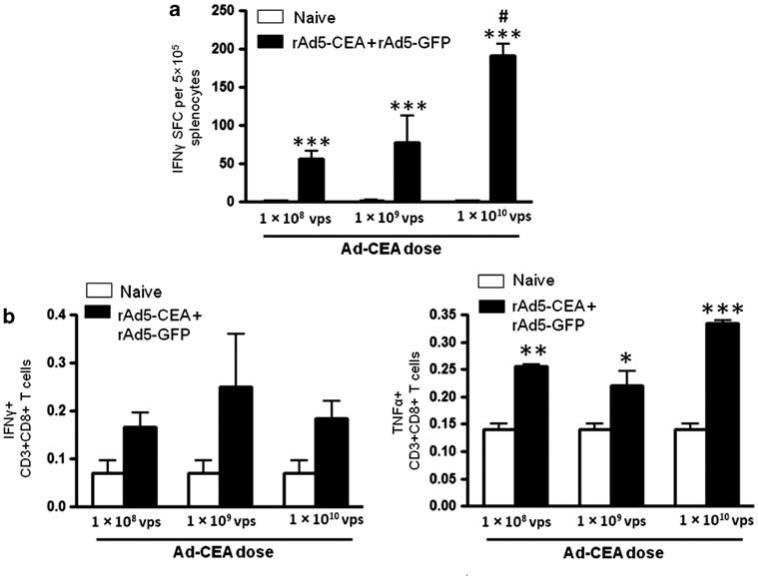
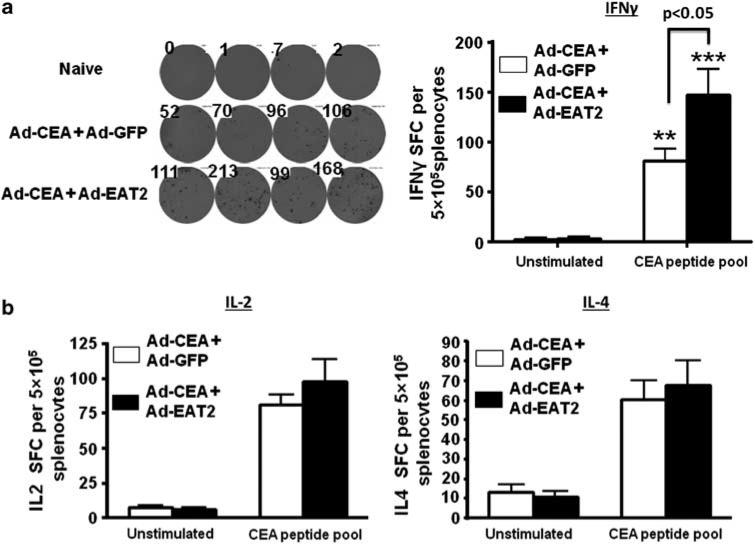
We previously confirmed that Ad vectors expressing EAT-2 significantly improved induction of adaptive immune responses to pathogen-derived foreign antigens.20,33,34 To investigate whether the enhanced innate immune responses promoted by Ad-mediated expression of EAT-2 could also influence the adaptive immune responses to a non-pathogen-derived self-antigen such as CEA; we co-immunized C57BL/6 mice with rAd5-based vectors expressing CEA alongside rAd5 vectors expressing-EAT2, or as, a control, rAd5 vectors expressing GFP (rAd5-GFP). We performed initial dose curve studies to identify the appropriate dose of rAd5-CEA that can be used in our studies in the C57BL/6 mice (Figure 2). Splenocytes derived from vaccinated mice were ex vivo-stimulated with the human CEA-specific peptide pools (15 mers with 11 aa overlap covering full-length CEA), as previously described.47 Based on ELISPOT and cytokines intracellular staining data, we identified 5 × 108 vps of rAd5-CEA as the most relevant experimental doses for these studies (Figure 2). Mice were then co-immunized intramuscularly with rAd5-CEA and rAd5-EAT2 or rAd5-CEA and rAd5-GFP, with a total dose per animal of 1 × 109 vps. At 14 dpi (days post immunization), splenocytes derived from mice co-immunized with rAd5-CEA and rAd5-EAT2 were stimulated ex vivo with immunodominant CEA-specific peptides. Mice that were immunized with the rAd5-CEA and rAd5-EAT2 formulation, contained significantly (P<0. 05) increased numbers of CEA-specific, IFN-γ-secreting T cells as compared with mice injected with the control rAd5-CEA formulation (Figure 3). The levels of IL-2 and IL-4 secreting CEA-specific T cells derived from mice co-immunized with rAd5-CEA and rAd5-EAT2 were also trended to increase, however, these did not reach statistical significance (Figure 3). Splenocytes derived from naive or the two sets of co-vaccinated animals were also stimulated with the GFP immunodominant peptide (DTLVNRIEL; Genscript, Piscataway, NJ, USA) and IFNγ ELISPOT analysis was performed. As expected, we observed significant (P<0.001) GFP-specific responses only in rAd5-CEA and rAd5-GFP co-injected control mice, as compared with both naives and rAd5-CEA + rAd5-EAT2 co-injected mice (Supplementary Figure 2a). We also evaluated T-cell immune responses to adenovirus-derived antigens in splenocytes derived from co-vaccinated mice. We observed significant induction of Ad5-specific T-cell responses in rAd5-CEA + rAd5-EAT2 and control vectors; however, no significant differences (P>0.05) were observed between these two groups (Supplementary Figure 2b).
Figure 2.
Dose curve analysis for carcinoembryonic antigen (CEA)-specific CD8+ T cells induced by recombinant Ad5 vector (rAd5)-CEA vaccine. Wild-type (WT) C57BL/6 mice (n = 3) were co-immunized intramuscularly in the tibialis anterior with escalating viral particles (VPs) of rAd5-CEA mixed with rAd5-GFP (total of 1 × 108, 1 × 109 and 1 × 1010 vps mixed before injection). At 14 dpi, splenocytes were collected and stimulated ex vivo with the CEA-specific peptide mix, followed by interferon (IFN)-γ ELISPOT (a) or FACS intracellular staining analysis for IFNγ and TNFα (b), performed as described in Materials and methods. Spot-forming cells (SFCs) were quantified using an ELISPOT reader. Data are presented as mean±s.d. Statistical analysis was completed using a one-way analysis of variance (ANOVA) with a Student–Newman–Keuls post-hoc test, P<0.05 was deemed as a statistically significant difference. * Denotes P<0.05, ** denotes P<0. 01, *** denotes P<0.001, statistically significant difference from naive animals. # denotes P<0.05, statistically significant difference from animals vaccinated with 1 × 108 and 1 × 109 of rAd5-CEA + rAd5-GFP vaccines.
Figure 3.
Carcinoembryonic antigen (CEA)-specific T-cell immune responses elicited by recombinant Ad5 vector (rAd5)-CEA and rAd5-Ewing's sarcoma's-activated transcript 2 (EAT2) co-immunization. Wild-type (WT) C57BL/6 mice (n = 4) were co-immunized intramuscularly in the tibialis anterior with equivalent viral particles (VPs) of rAd5-CEA mixed with either rAd5-GFP=or rAd5-EAT2 (total of 1 × 109 vps mixed before injection). At 14 dpi, splenocytes were collected and stimulated ex vivo with the CEA-specific peptide mix, followed by interferon (IFN)-γ (a) and interleukin (IL)-2 or IL-4 (b) ELISPOT performed as described in Materials and methods. Spot-forming cells (SFCs) were quantified using an ELISPOT reader. Data are presented as mean±s.d. Data are representative of two independent experiments with similar results. Statistical analysis was completed using two-way analysis of variance (ANOVA) with a Bonferroni post-hoc test, P<0.05 was deemed as a statistically significant difference. Representative data from two independent experiments are shown.
Assessment of CEA-specific T-cell cytokine responses by multiparameter flow cytometry
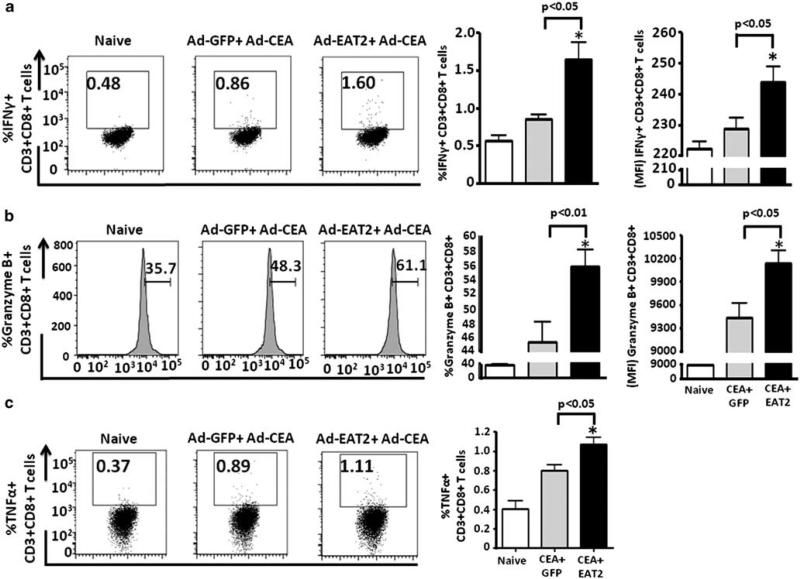
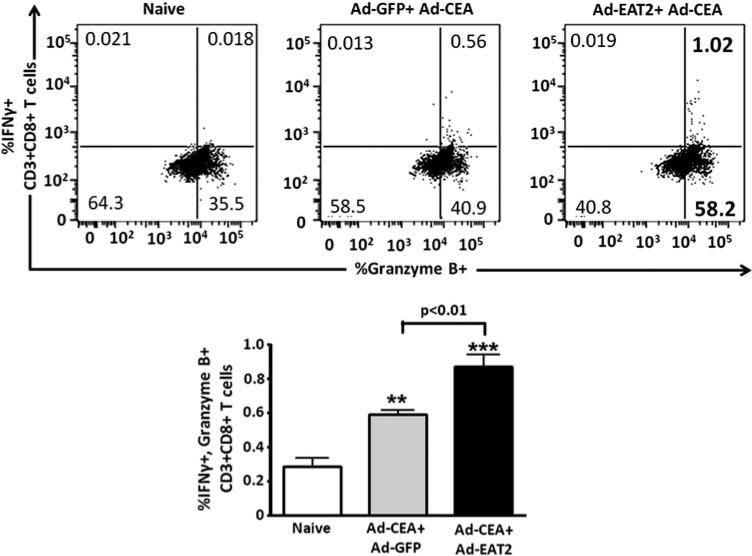
Improvements in vaccine-induced protective immunity and the induction of long-lived memory responses to specific antigens are generally correlated with the improved induction of increased numbers of antigen-specific T cells that express several cytokines.48,49 To evaluate the expression of cytokines in CD8+ T cells generated after rAd5-CEA and rAd5-EAT2 co-vaccination, seven-color flow cytometry was used to enumerate the frequency of IFNγ-, TNFα-, granzyme B-, Perforin- and/or IL-2-producing CD8+ T cells after ex vivo stimulation with the CEA-specific peptides. We observed significantly increased frequencies of CEA-specific IFNγ- (P<0.05), TNFα- (P<0.05) and granzyme B-producing (P<0.01) CD8+ T cells derived from rAd5-CEA and rAd5-EAT2 co-immunized mice, as compared with mice vaccinated with the control vaccines (Figure 4). No significant expression of Perforin and IL-2 were observed in CD8+ T cells derived from either the experimental or control groups of vaccinated animals (data not shown). We also evaluated IFNg and granzyme B double-positive CD8+ T cells. Our analysis revealed statistically higher (P<0. 01) numbers of CEA-specific IFNγ/granzyme B-expressing CD8+ Tcells derived from EAT2-augmented rAd5-CEA vaccine-immunized mice, as compared with mice vaccinated with control vaccines (Figure 5). The frequency of CEA-specific IFNγ/TNFα double-positive CD8+ T cells were also trended to increase in rAd5-EAT2 vaccinated mice; however, no statistically significant differences were observed between the experimental and control groups (data not shown).
Figure 4.
Inclusion of Ewing's sarcoma's-activated transcript 2 (EAT-2) adaptor in the vaccine formulation enhances carcinoembryonic antigen (CEA)-specific CD8+ T cells. C57BL/6 (n = 4) mice were co-immunized with equivalent viral particles (VPs) of Ad-CEA mixed with either Ad5- GFP or Ad5-EAT2 (1 × 109 total vps). At 14 dpi, the mice were killed and lymphocytes were isolated from spleen. Multiparameter flow cytometry was used to enumerate the frequency of cytokine-producing CD8+ T cells. Gate were set based on negative control (naive) and placed consistently across samples. The total frequency of CD8+ T cells derived from C57BL/6 mice expressing interferon (IFN)-γ (a), granzyme B (b) or TNFα (c) is shown. The bars represent mean±s.d. Data are representative of two independent experiments with similar results. Statistical analysis was completed using one-way analysis of variance (ANOVA) with a Student–Newman–Keuls post-hoc test, P<0.05 was deemed as a statistically significant difference. * Denotes P<0.05, statistically different from naive animals.
Figure 5.
Inclusion of Ewing's sarcoma's-activated transcript 2 (EAT-2) adaptor in the vaccine formulation enhances carcinoembryonic antigen (CEA)-specific CD8+ T cells. C57BL/6 (n = 4) mice were co-immunized with equivalent viral particles (VPs) of Ad-CEA mixed with either Ad5-GFP or Ad5-EAT2 (1 × 109 total vps). At 14 dpi, the mice were killed and lymphocytes were isolated from spleen. Multiparameter flow cytometry was used to enumerate the frequency of cytokine-producing CD8+ T cells. The total frequency of CD8+ T cells derived from C57BL/6 mice expressing interferon (IFN)-γ and granzyme B is shown. The bars represent mean±s.d. Data are representative of two independent experiments with similar results. Statistical analysis was completed using one-way analysis of variance (ANOVA) with a Student– Newman–Keuls post-hoc test, P<0.05 was deemed a statistically significant difference. * Denotes P<0. 05, ** denotes P<0.01, statistically different from naive animals.
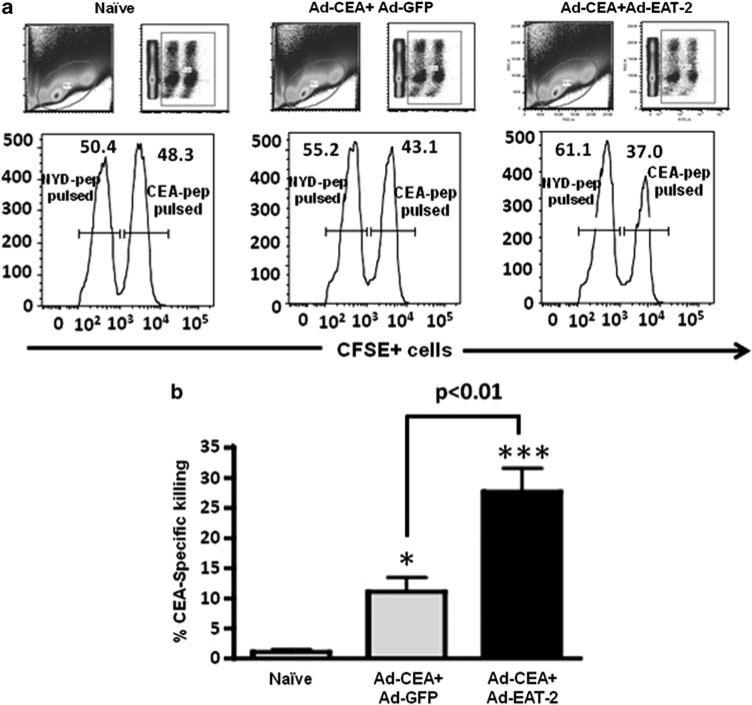
Cytotoxic CD8+ T cells from rAd5-EAT2 co-immunized mice exhibit improved CEA-specific cytotoxicity in vivo
The direct measurement of in vivo functionality of CD8+ T lymphocytes (CTL) to specifically kill target APCs provides a critical assessment as to their overall functional capacity. To evaluate the in vivo cytolytic activity of the CEA-specific CD8+ T lymphocytes generated after rAd5-CEA and rAd5-EAT2 co-immunization, mice were co-immunized with rAd5-CEA + rAd5-EAT2 or rAd5-CEA + rAd5-GFP. Fourteen days after vaccination, the two groups of mice were then injected with CFSE-labeled syngeneic splenocytes pulsed with either nonspecific or CEA-derived peptides, as described in the Materials and methods section and also previously.20,33,34 The elimination of the peptide-pulsed splenocytes (CFSEhigh) in the control or experimental animals was then examined by a flow cytometry-based CTL assay. CEA-specific CTL activities induced in mice co-immunized with rAd5-CEA and rAd5-EAT2 were significantly (P<0.01) higher, as compared with mice injected with the rAd5-CEA and rAd5-GFP controls (Figure 6).
Figure 6.
Increased in vivo cytolytic activity of the carcinoembryonic antigen (CEA)-specific T cells in recombinant Ad5 vector (rAd5)-CEA and rAd5-Ewing's sarcoma's-activated transcript 2 (EAT2) co-immunized mice. C57BL/6 (n = 6) mice were co-immunized with equivalent viral particles (VPs) of rAd5-CEA mixed with either rAd5-EAT2 or rAd5-GFP (1 × 109 total vps). At 14 dpi, syngeneic splenocytes were pulsed with either an irrelevant peptide (NYD-pep) and stained with 1 μm CFSELow or with CEA-specific peptides, and labeled with 10 μm (CFSEHigh). Twenty hours after adoptive transfer into either naive or co-immunized mice, splenocytes were collected using a LSRII flow cytometer. (a) Representative figures of the CTL analysis from naive or vaccinated mice are shown. (b) Analysis for percent CEA-specific killing is shown. % carboxyfluorescein succinimidyl ester (CFSE)-positive cells were quantified using FlowJo software. % specific killing = 1 – ((% CFSEHigh /% CFSELow)immunized/(%CFSEHigh /% CFSELow)non-immunized). * Denotes P<0.05, *** denotes P<0.001 statistically different from naive animals. Representative figure of two independent experiments is shown.
Improved immune recognition of existing CEA-expressing tumors in mice immunized with rAd5-CEA vaccines augmented with EAT2
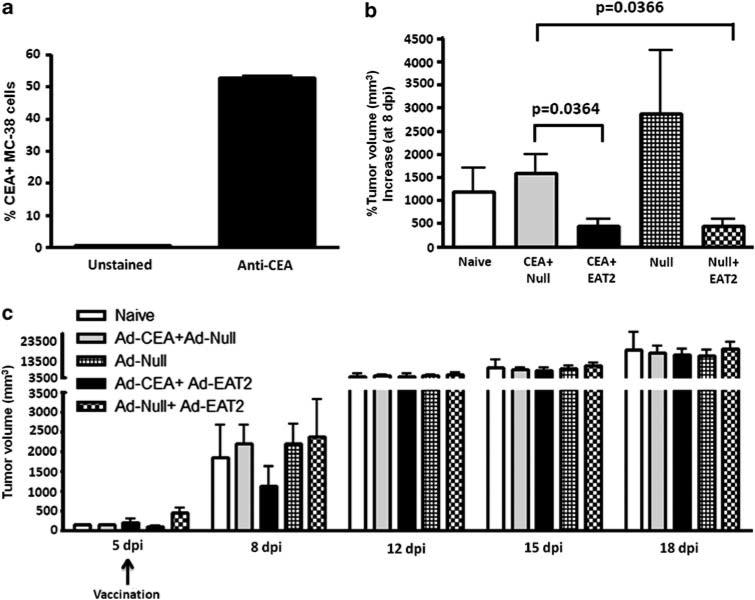
We next assessed whether the enhanced ability of Ad-EAT2-augmented Ad-CEA vaccines to induce functionally superior numbers of CEA-specific CTLs would have significant impact when assessed in a CEA-specific in vivo tumor model. MC-38-CEA-expressing colon carcinoma cells were used in our analysis. Before tumor implantation, we initially evaluated the expression of CEA protein on the MC-38 cells by flow cytometry. We observed that ~50% of the cultured MC-38 cells used in this study demonstrated significant levels of CEA expression on their surface just before implantation (Figure 7a). MC-38 cells (1 × 106) were SQ implanted into the right flank of WT C57BL/6 mice. Tumors were readily palpable 4–5 days after implantation using this experimental design strategy. On day 5, all implanted mice were randomly divided into five groups (n = 16 per group). One group of these mice were not vaccinated (mock), whereas the remaining four groups of mice were co-vaccinated intramuscularly with Ad vaccine cocktails containing a total of 1 × 1010 vps per mouse of rAd5-CEA + rAd5-EAT2, rAd5-CEA + rAd5-Null, rAd5-Null or rAd5- Null + rAd5-EAT2. All mice were monitored for tumor growth and tumor volumes were calculated. Interestingly, at 3 dpi, we observed significantly (P<0.05) reduced tumor growth (% tumor size increase between days 5 and 8) in rAd5-EAT2 and rAd5-CEA co-injected mice, as compared with rAd5-CEA and rAd5-Null-injected controls (Figure 7b). We also observed significantly (P<0.05) reduced tumor growth at 3 dpi in rAd5-EAT2 + rAd5-Null, as compared with rAd5-Null-injected mice (Figure 7b), suggesting a direct role of EAT-2-mediated innate immunity in controlling early tumor growth. However, at later time points, no inhibition of tumor growth was observed by any of the vaccination schemes (Figure 7c), suggesting a role for CEA non-expressing MC-38 cells in tumor growth mediation. These results suggest that inclusion of EAT-2 expression in therapeutic CEA-targeting vaccines can slow down the rate of growth of pre-established, CEA-expressing tumors.
Figure 7.
Recombinant Ad5 vector (rAd5)-Ewing's sarcoma's-activated transcript 2 (EAT2) treatment of established carcinoembryonic antigen (CEA)-expressing tumors. C57BL/6 mice (n = 16 per group) were inoculated with 106 MC-38-CEA adenocarcinomas cells SQ into the left flank at day 0. Five days following tumor implant, mice were either unvaccinated or vaccinated with the indicated viral vectors. Tumors were measured by two opposing directions and volumes were calculated according to the formula: volume = ((a × b)2/2). (a) Expression of CEA on MC-38 cells prior to tumor implantation. (b) Percent tumor volume increase at day 8. (c) Kinetics of tumor growth in naive or vaccinated mice. Error bars represent the s.d. of the tumor volume. Data are representative of two independent experiments with similar results.
Histological and molecular characterization of CEA-expressing tumors in rAd5-CEA and rAd5-EAT2 co-immunized mice
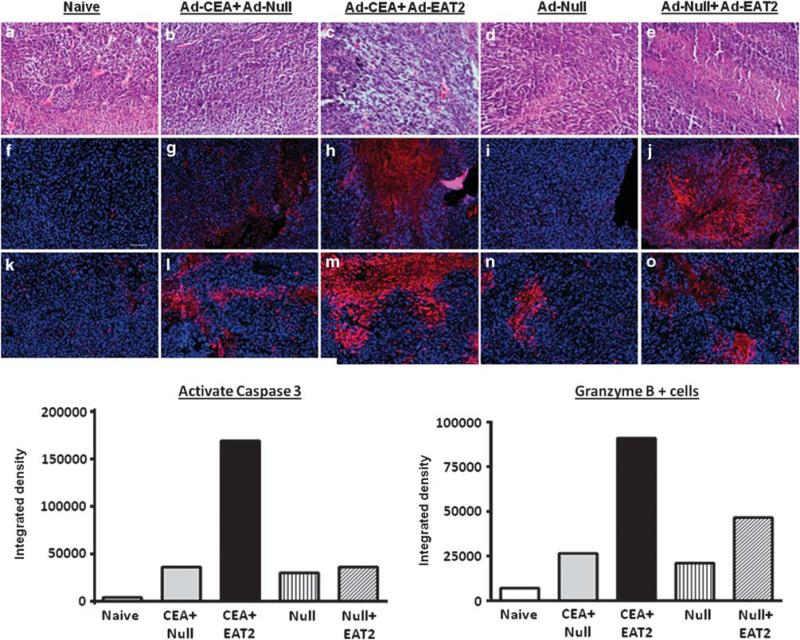
Changes in the innate and adaptive immune responses to CEA induced between experimental and control animals led us to ponder the histological composition of tumor tissues in the MC-38-treated mice. For example, EAT-2-augmented induction of CEA-specific CTLs should lead to more MC-38 cell death in vivo. Upon examination of MC-38 tumors derived from non-vaccinated animals, we found highly vascularized tumor beds with dense hyper-cellularity and few pyknotic cells (Figure 8a). Histologically, rAd5-CEA + rAd5-Null-injected animals had only few foci of cell death (Figure 8a). In contrast, rAd5-CEA rAd5-EAT2-immunized mice demonstrated histological changes+ consistent with tumor necrosis, including numerous pyknotic nuclei, cellular debris and disseminated loss of tumor integrity (Figure 8a). We also noted amorphous coagulums within the necrotic foci in the latter groups of mice (Figure 8a). Importantly, although histological necrosis appeared throughout the tumors isolated from these animals, vacuolated spaces also remained and could account for the nonsignificant change in overall tumor size, which we noted later in the time course of this experiment. To address the molecular mechanisms underlying these histological changes, we also tested for granzyme B expression in tumor-derived tissues from the previously vaccinated animals. We detected increased staining for granzyme B expression in tumor tissues-derived rAd5-CEA + rAd5-EAT2-immunized animals, as compared with other groups of animals (Figure 8 and Supplementary Figure 3). To correlate these findings with levels of cell death found within the tumors, we tested for activated caspase-3 expression. As indicated by histological analysis and granzyme B expression, we detected increased activation of caspase-3 in tumor tissues derived from rAd5-EAT2 co-injected mice, as compared with the controls (Figure 8 and Supplementary Figure 3). In total, these results suggest that the inclusion of the rAd5-EAT2 vaccine in Ad5-based vaccine formulations also expressing CEA improves the induction of innate and CEA-specific adaptive immune responses as measured by several phenotypic and functional assays, and can also lead to slowed CEA-expressing tumor growth that is positively correlated with beneficial histological and molecular changes, including evidence of enhanced disseminated tumor necrosis based upon elevated granzyme B expression levels and caspase-3 activation levels in the tumors derived from the rAd5-EAT2-treated animals.
Figure 8.
Tumor tissues derived from recombinant Ad5 vector expressing EAT-2 (rAd5-EAT2) and rAd5-EAT2-co-vaccinated mice have increased caspase-3 activation and granzyme B expression. Tumor tissues derived from rAd5-carcinoembryonic antigen (CEA)/rAd5-EAT2 co-injected animals (n = 6 per group) have numerous necrotic foci and pyknotic nuclei. (a-e) Hematoxylin and eosin (H&E). Immunohistochemistry for active caspase-3 (f–j) and granzyme B (k–o) (red) was performed on tumor tissue (18 dpi) shows limited cell death in naive and rAd5-CEA-immunized animals. In contrast, rAd5-CEA/rAd5-EAT2-co-injected mice have highly pyknotic nuclei and cellular debris marking more widely dispersed necrotic tissue. Immunohistochemistry for active caspase-3 (b) and granzyme (c) (red) was performed on tumor tissues at the end of the study (18 dpi). Tumor tissues derived from rAd5-EAT2 co-injected mice show a strong signal for active caspase-3 and granzyme B. Quantification was performed using the ImageJ software, (NIH, Bethesda, MD, USA).
DISCUSSION
In recent years, there has been accumulating evidence implicating a critical role for SLAM family of receptors and SAP family of adaptors in regulating both innate and adaptive immunity.29 We have shown previously that EAT-2 expression from Ad5-based vaccine platforms positively regulates effector and regulatory functions of innate immune cells inclusive of macrophage, DCs and NK cells.20,34 EAT-2 overexpression also enhances the production of several proinflammatory cytokines and chemokines immediately subsequent to vaccination with Ad vectors expressing EAT-2.20,34 Innate immune responses enhanced by EAT-2 delivery also resulted in improved induction of beneficial cellular immune responses to co-administered pathogen-derived antigens,20,33,34 and in this report, self-human antigens such as CEA. Specifically, our results confirmed that cellular immune responses to the TAA, CEA, were significantly improved by co-expression of the EAT-2 adaptor in CEA-specific Ad5-based vaccine formulations. These responses included increases in the frequency of IFNγ-, TNFα- and granzyme B-expressing CEA-specific CD8+ T lymphocytes, as well as improved in vivo functionality/cytotoxicity of CEA-specific CTLs. Necrotic tumor tissue in rAd5-EAT2-co-injected animals further confirms the role of functional CTLs. Such marked necrotic responses mark an important prognostic indicator in colorectal cancer.50
CEA is expressed at a high level in a number of human cancers, including colorectal, gastric and pancreatic carcinomas, and is therefore a potential TAA target for tumor immunotherapy. Although CEA, similar to many TAAs, is a weak immunogen in the tumor-bearing host and is weakly recognized by the immune system, previous vaccination studies indicate that immune responses toward CEA can be generated, and that CEA-specific T cells can lyse CEA-expressing carcinoma cells.9,51–53 Moreover, in a recent phase I/II human clinical trial, it has been shown that escalating doses of the novel Ad5-(E1-, E2b-)-CEA(6D) vaccine induce higher CEA-specific cell-mediated immune responses despite the presence of pre-existing Ad5 immunity in a majority (61.3%) of patients.47 In addition, minimal toxicity and overall patient survival (48% at 12 months) were similar regardless of pre-existing Ad5-neutralizing antibody titers.47 In the present study, we have shown that enhanced cellular immune responses can be improved when EAT-2 is included in an Ad5-based CEA-vaccine formulation. The improved responses afforded by the inclusion of EAT-2 in CEA-targeting vaccines might be beneficial in future, second generation cancer vaccines targeting CEA-overexpressing cancers.
Importantly, the fact that control-vaccinated animals did not eliminate the tumor suggests that in this mouse model, human CEA being expressed from the MC-38 cell line is not being significantly perceived as a foreign protein by the mouse immune system, so as to prompt cytotoxicity in the control mice. More importantly, the results suggest that the use of EAT-2 was potent enough to break this level of tolerance, as evidenced by decreased rates of growth of CEA-expressing tumors in EAT-2- and CEA-vaccinated mice. Future studies, beyond the scope of this manuscript, could further test this notion, for example, in mice transgenically expressing human CEA. Moreover, it has been shown previously that combining immunotherapeutic strategy, such as therapeutic cancer vaccines54,55 and targeted agents, that is, chemotherapy, functions to improve the clinical outcomes of the cancer vaccines.56 Including chemotherapeutic agents concomitant with activating SLAM signaling by vectors overexpressing EAT-2 adaptor protein might improve the outcome of cancer vaccines, in general, as well as cancer vaccines targeting CEA-expressing tumors. Future studies addressing these hypotheses in specific tumor mouse models will be required to verify these notions.
The molecular mechanisms leading to a potent memory T-cell responses from viral vaccine expression of the EAT-2 adaptor are not yet clear; however, several reports have shown that adaptive cellular immune responses are controlled primarily by the innate immune system.57 Specifically, it has been shown that proinflammatory cytokines and the abundance of costimulatory molecules on the surface of APCs (matured phenotype of APCs) can have a critical role in initiating and orchestrating the adaptive immune response toward antigens presented during vaccinations.27,28 Our present and previously published data have demonstrated that expression of EAT-2 during initial antigen presentation enhanced the production of proinflammatory cytokines and chemokines, as well as upregulated the expression of co-stimulatory molecules on the surface of APCs.34,58 In this study, we focused on the kinetics and amplitude of cytokine and chemokine production levels following rAd5-EAT2 administration in vivo. Significantly, EAT-2 overexpression enhances the soluble proinflammatory repertoire by enhancing the production of the Th1-type-skewing cytokines (IFNγ, IL-12 and IL-1α) while minimizing the induction of Th2-type-enhancing cytokines (IL-10) and chemokines (EOTAXIN and KC). Importantly, our previously published data34 revealed that EAT-2 overexpression at the time of vaccination directs the phenotype of antigen-specific CD8+ T cells toward effector memory (TEM)-biased cell population, a function that is primarily regulated by the IL-12 cytokine.59 Here, we have shown that administration of the EAT-2-expressing vaccine enhances early production of IL-12, suggesting a role of EAT-2-mediated IL-12 production in regulating the shift of memory T-cell responses toward the TEM phenotype. We also note that EAT-2 overexpression alters the expression of the pleiotropic cytokine, G-CSF, at higher levels early after vaccination. G-CSF is produced by macrophages, fibroblasts, bone marrow stromal cells and endothelial cells during acute inflammatory responses.60 G-CSF functions to expand and regulate the function of neutrophils, monocyte/macrophage and DCs following its binding to the G-CSF receptor.60 G-CSF also functions as a mediator of cytokine and chemokine production from these same innate immune cells.61 Importantly, G-CSF has been shown to exert immunomodulatory effects on T cells directly via G-CSF receptors on T cells62 or indirectly via innate immune cells such as neutrophils, macrophages and DCs.63
The regulatory role of DCs and macrophages in innate and adaptive immunity is widely described.57 We have shown that EAT-2 overexpression enhances the maturation and effector functions of DCs and macrophages both in vitro and in vivo.20,34 G-CSF is also known to have an important role in neutrophil expansion and survival. Neutrophils are known to have a critical role in bridging innate and adaptive immune responses.64,65 Neutrophils have been shown to produce several cytokines and chemokines (such as G-CSF, IL-1β, IL-6, IL-10, tumor growth factor (TGF)-b, IL-12, IFNγ and GM-CSF) that are crucial for regulating innate and adaptive immunity.64 For example, neutrophil-derived cytokines have been shown to induce DC maturation and to enhance IL-12 and TNFα production from DCs.66 Neutrophils have also been found to directly stimulate the production of IFNγ by human NK cells, thus influencing DC maturation and Th1-type immune responses.67 We speculate that EAT-2-mediated production of G-CSF, and thus DCs, macrophages, NK cells and neutrophils maturation/activation may be responsible for enhancing the cellular immune responses following rAd5-EAT2 vaccination. Furthermore, the SLAM family of receptors Ly108 has been shown to significantly control neutrophil functions,68 and Ad5 vectors have been shown to efficiently transduce neutrophils via the b2-integrin CD11b.69 It is possible that signaling downstream of Ly108 receptor in rAd5-EAT2-transduced neutrophils, combined with an enhanced induction of innate immune responses provided by rAd5-mediated transduction of EAT-2 into NK cells, DCs and macrophages,20,34 allows for induction of beneficial innate immune responses that result in higher magnitude cellular immune responses to co-expressed antigens. Although we believed that EAT-2 enhances innate and adaptive immune responses in part by interaction with phosphorylated immuno-receptor tyrosine-based switch motifs (ITSMs) of SLAM family of receptors, SAP adaptors have been shown to interact with receptors other that SLAM family members.70 Therefore, it remains a possibility that the enhanced innate immune profile prompted by EAT-2 adaptor expression may be mediated by SLAM-independent mechanisms as well.
Importantly, in a recent human HIV vaccine, study using system biology approach and using the Merck Ad5 HIV vaccine vectors (MRKAd5/HIV), it has been shown that EAT-2 gene was among one of the innate immune response genes that were associated with the enhanced immunogenicity of the MRKAd5/HIV.71 Transcriptional CD8+ T-cell signature analysis revealed significant induction of EAT-2 transcripts in human peripheral blood mononuclear cells (PBMCs) derived from groups of subjects that developed high-magnitude HIV/Gag-specific CD8+ T-cell responses, as compared with PBMCs derived from human subjects with low- and moderate-HIV/gag-specific CD8+ T-cell responses.71 This analysis is consistent with our current and previously published data,20,33,34 and suggests that increased expression of EAT-2 at the time of vaccination functions to magnify innate immune responses and thus, increase the magnitude of CD8+ T-cell responses to vaccine antigens.
In conclusion, our results establish that EAT-2 is an important immune modulatory protein that enhances vaccine-elicited effector memory CD8+ T-cell responses and can be harnessed in a manner that provides a new approach for enhancing the efficacy of Ad5-based (as well as other viral and non-viral-based vaccine vectors) CEA-expressing cancer vaccines specifically, as well as potentially other cancer vaccine strategies in general. Our findings also demonstrate that vaccine vectors that express EAT-2 during antigen vaccination can improve innate immune system responses and subsequently induce potent multiple antigen-specific cellular immune responses.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank the Michigan State University Laboratory Animal support facilities for their assistance in the humane care and maintenance of the animals used in this work. AA was supported by the NIH grants RO1DK-069884, P01CA078673 and the Michigan State University (MSU) Foundation, as well as the Osteopathic Heritage Foundation. YAA was supported by the King Abdullah bin Abdulaziz Scholarship grant, Ministry of Higher Education, Kingdom of Saudi Arabia. Financial support to BCS (no. DE13513) and YAK (no. 1F31DE022696-01) came from the NIH National Institute of Dental and Craniofacial Research.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on Cancer Gene Therapy website (http://www.nature.com/cgt)
REFERENCES
- 1.Melief CJ, Toes RE, Medema JP, van der Burg SH, Ossendorp F, Offringa R. Strategies for immunotherapy of cancer. Adv Immunol. 2000;75:235–282. doi: 10.1016/s0065-2776(00)75006-1. [DOI] [PubMed] [Google Scholar]
- 2.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 3.Hartman ZC, Wei J, Osada T, Glass O, Lei G, Yang XY, et al. An adenoviral vaccine encoding full-length inactivated human Her2 exhibits potent immunogenicty and enhanced therapeutic efficacy without oncogenicity. Clin Cancer Res. 2010;16:1466–1477. doi: 10.1158/1078-0432.CCR-09-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osada T, Woo CY, McKinney M, Yang XY, Lei G, Labreche HG, et al. Induction of Wilms’ tumor protein (WT1)-specific antitumor immunity using a truncated WT1-expressing adenovirus vaccine. Clin Cancer Res. 2009;15:2789–2796. doi: 10.1158/1078-0432.CCR-08-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Copier J, Bodman-Smith M, Dalgleish A. Current status and future applications of cellular therapies for cancer. Immunotherapy. 2011;3:507–516. doi: 10.2217/imt.11.18. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal C, Meropol NJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, et al. Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer. Ann Oncol. 2013;24:420–428. doi: 10.1093/annonc/mds336. [DOI] [PubMed] [Google Scholar]
- 7.Thompson JA, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991;5:344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- 8.Schlom J. Basic principles and applications of monoclonal antibodies in the management of carcinomas: the Richard and Hinda Rosenthal Foundation award lecture. Cancer Res. 1986;46:3225–3238. [PubMed] [Google Scholar]
- 9.Berinstein NL. Carcinoembryonic antigen as a target for therapeutic anticancer vaccines: a review. J Clin Oncol. 2002;20:2197–2207. doi: 10.1200/JCO.2002.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Rayani S, Marshall JL. Carcinoembryonic antigen as a vaccine target. Expert Rev Vaccines. 2008;7:987–993. doi: 10.1586/14760584.7.7.987. [DOI] [PubMed] [Google Scholar]
- 11.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabitzsch ES, Xu Y, Balint JP, Jr., Hartman ZC, Lyerly HK, Jones FR. Anti-tumor immunotherapy despite immunity to adenovirus using a novel adenoviral vector Ad5 [E1-, E2b-]-CEA. Cancer Immunol Immunother. 2010;59:1131–1135. doi: 10.1007/s00262-010-0847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mennuni C, Calvaruso F, Facciabene A, Aurisicchio L, Storto M, Scarselli E, et al. Efficient induction of T-cell responses to carcinoembryonic antigen by a heterologous prime-boost regimen using DNA and adenovirus vectors carrying a codon usage optimized cDNA. Int J Cancer. 2005;117:444–455. doi: 10.1002/ijc.21188. [DOI] [PubMed] [Google Scholar]
- 14.Marshall JL, Hoyer RJ, Toomey MA, Faraguna K, Chang P, Richmond E, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964–3973. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 15.Marshall JL, Hawkins MJ, Tsang KY, Richmond E, Pedicano JE, Zhu MZ, et al. Phase I study in cancer patients of a replication-defective avipox recombinant vaccine that expresses human carcinoembryonic antigen. J Clin Oncol. 1999;17:332–337. doi: 10.1200/JCO.1999.17.1.332. [DOI] [PubMed] [Google Scholar]
- 16.Tsang KY, Zaremba S, Nieroda CA, Zhu MZ, Hamilton JM, Schlom J. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995;87:982–990. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 17.Morse MA, Clay TM, Hobeika AC, Osada T, Khan S, Chui S, et al. Phase I study of immunization with dendritic cells modified with fowlpox encoding carcinoembryonic antigen and costimulatory molecules. Clin Cancer Res. 2005;11:3017–3024. doi: 10.1158/1078-0432.CCR-04-2172. [DOI] [PubMed] [Google Scholar]
- 18.Samanci A, Yi Q, Fagerberg J, Strigard K, Smith G, Ruden U, et al. Pharmacological administration of granulocyte/macrophage-colony-stimulating factor is of significant importance for the induction of a strong humoral and cellular response in patients immunized with recombinant carcinoembryonic antigen. Cancer Immunol Immunother. 1998;47:131–142. doi: 10.1007/s002620050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mincheff M, Altankova I, Zoubak S, Tchakarov S, Botev C, Petrov S, et al. In vivo transfection and/or cross-priming of dendritic cells following DNA and adenoviral immunizations for immunotherapy of cancer--changes in peripheral mononuclear subsets and intracellular IL-4 and IFN-gamma lymphokine profile. Crit Rev Oncol Hematol. 2001;39:125–132. doi: 10.1016/s1040-8428(01)00111-1. [DOI] [PubMed] [Google Scholar]
- 20.Aldhamen YA, Appledorn DM, Seregin SS, Liu CJ, Schuldt NJ, Godbehere S, et al. Expression of the SLAM family of receptors adapter EAT-2 as a novel strategy for enhancing beneficial immune responses to vaccine antigens. J Immunol. 2011;186:722–732. doi: 10.4049/jimmunol.1002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appledorn DM, Aldhamen YA, Depas W, Seregin SS, Liu CJ, Schuldt N, et al. A new adenovirus based vaccine vector expressing an Eimeria tenella derived TLR agonist improves cellular immune responses to an antigenic target. PLoS ONE. 2010;5:e9579. doi: 10.1371/journal.pone.0009579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke JM, Morse MA, Lyerly HK, Clay T, Osada T. Adenovirus vaccine immunotherapy targeting WT1-expressing tumors. Expert Opin Biol Ther. 2010;10:875–883. doi: 10.1517/14712591003798278. [DOI] [PubMed] [Google Scholar]
- 23.Tan WG, Jin HT, West EE, Penaloza-Macmaster P, Wieland A, Zilliox MJ, et al. Comparative analysis of SIV Gag specific effector and memory CD8 T cells induced by different adenovirus vectors. J Virol. 2012;87:1359–1372. doi: 10.1128/JVI.02055-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 25.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22:411–416. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, et al. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8 + T cell responses in nonhuman primates. Proc Natl Acad Sci USA. 2005;102:15190–15194. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 28.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Schwartzberg PL, Mueller KL, Qi H, Cannons JL. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nat Rev Immunol. 2009;9:39–46. doi: 10.1038/nri2456. [DOI] [PubMed] [Google Scholar]
- 30.Veillette A. SLAM-family receptors: immune regulators with or without SAP-family adaptors. Cold Spring Harb Perspect Biol. 2010;2:a002469. doi: 10.1101/cshperspect.a002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calpe S, Wang N, Romero X, Berger SB, Lanyi A, Engel P, et al. The SLAM and SAP gene families control innate and adaptive immune responses. Adv Immunol. 2008;97:177–250. doi: 10.1016/S0065-2776(08)00004-7. [DOI] [PubMed] [Google Scholar]
- 32.Calpe S, Wang N, Romero X, Berger SB, Lanyi A, Engel P, et al. The SLAM and SAP gene families control innate and adaptive immune responses. Adv Immunol. 2008;97:177–250. doi: 10.1016/S0065-2776(08)00004-7. [DOI] [PubMed] [Google Scholar]
- 33.Schuldt NJ, Aldhamen YA, Appledorn DM, Seregin SS, Kousa Y, Godbehere S, et al. Vaccine platforms combining circumsporozoite protein and potent immune modulators, rEA or EAT-2, paradoxically result in opposing immune responses. PLoS ONE. 2011;6:e24147. doi: 10.1371/journal.pone.0024147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aldhamen YA, Seregin SS, Schuldt NJ, Rastall DP, Liu CJ, Godbehere S, et al. Vaccines expressing the innate immune modulator EAT-2 elicit potent effector memory T lymphocyte responses despite pre-existing vaccine immunity. J Immunol. 2012;189:1349–1359. doi: 10.4049/jimmunol.1200736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Appledorn DM, McBride A, Seregin S, Scott JM, Schuldt N, Kiang A, et al. Complex interactions with several arms of the complement system dictate innate and humoral immunity to adenoviral vectors. Gene Ther. 2008;15:1606–1617. doi: 10.1038/gt.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng P, Graham FL. Construction of first-generation adenoviral vectors. Meth Mol Med. 2002;69:389–414. doi: 10.1385/1-59259-141-8:389. [DOI] [PubMed] [Google Scholar]
- 38.Morse MA, Hobeika AC, Osada T, Berglund P, Hubby B, Negri S, et al. An alphavirus vector overcomes the presence of neutralizing antibodies and elevated numbers of Tregs to induce immune responses in humans with advanced cancer. J Clin Invest. 2010;120:3234–3241. doi: 10.1172/JCI42672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amalfitano A, Begy CR, Chamberlain JS. Improved adenovirus packaging cell lines to support the growth of replication-defective gene-delivery vectors. Proc Natl Acad Sci USA. 1996;93:3352–3356. doi: 10.1073/pnas.93.8.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodges BL, Serra D, Hu H, Begy CA, Chamberlain JS, Amalfitano A. Multiply deleted [E1, polymerase-, and pTP-] adenovirus vector persists despite deletion of the preterminal protein. J Gene Med. 2000;2:250–259. doi: 10.1002/1521-2254(200007/08)2:4<250::AID-JGM113>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 41.Seregin SS, Appledorn DM, McBride AJ, Schuldt NJ, Aldhamen YA, Voss T, et al. Transient pretreatment with glucocorticoid ablates innate toxicity of systemically delivered adenoviral vectors without reducing efficacy. Mol Ther. 2009;17:685–696. doi: 10.1038/mt.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Appledorn DM, Patial S, McBride A, Godbehere S, Van Rooijen N, Parameswaran N, et al. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J Immunol. 2008;181:2134–2144. doi: 10.4049/jimmunol.181.3.2134. [DOI] [PubMed] [Google Scholar]
- 43.Perfetto SP, Chattopadhyay PK, Lamoreaux L, Nguyen R, Ambrozak D, Koup RA, et al. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J Immunol Meth. 2006;313:199–208. doi: 10.1016/j.jim.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Robbins PF, Kantor JA, Salgaller M, Hand PH, Fernsten PD, Schlom J. Transduction and expression of the human carcinoembryonic antigen gene in a murine colon carcinoma cell line. Cancer Res. 1991;51:3657–3662. [PubMed] [Google Scholar]
- 45.Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, et al. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195:617–624. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morse MA, Chaudhry A, Gabitzsch ES, Hobeika AC, Osada T, Clay TM, et al. Novel adenoviral vector induces T-cell responses despite anti-adenoviral neutralizing antibodies in colorectal cancer patients. Cancer Immunol Immunother. 2013;62:1293–1301. doi: 10.1007/s00262-013-1400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multi-functional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 49.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8 + T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pollheimer MJ, Kornprat P, Lindtner RA, Harbaum L, Schlemmer A, Rehak P, et al. Tumor necrosis is a new promising prognostic factor in colorectal cancer. Hum Pathol. 2010;41:1749–1757. doi: 10.1016/j.humpath.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 51.Zhu MZ, Marshall J, Cole D, Schlom J, Tsang KY. Specific cytolytic T-cell responses to human CEA from patients immunized with recombinant avipox-CEA vaccine. Clin Cancer Res. 2000;6:24–33. [PubMed] [Google Scholar]
- 52.Horig H, Medina FA, Conkright WA, Kaufman HL. Strategies for cancer therapy using carcinoembryonic antigen vaccines. Exp Rev Mol Med. 2000;2:1–24. doi: 10.1017/S146239940000168X. [DOI] [PubMed] [Google Scholar]
- 53.Foon KA, John WJ, Chakraborty M, Das R, Teitelbaum A, Garrison J, et al. Clinical and immune responses in resected colon cancer patients treated with anti-idio-type monoclonal antibody vaccine that mimics the carcinoembryonic antigen. J Clin Oncol. 1999;17:2889–5. doi: 10.1200/JCO.1999.17.9.2889. [DOI] [PubMed] [Google Scholar]
- 54.Marshall JL, Gulley JL, Arlen PM, Beetham PK, Tsang KY, Slack R, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–731. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 55.Madan RA, Bilusic M, Heery C, Schlom J, Gulley JL. Clinical evaluation of TRICOM vector therapeutic cancer vaccines. Semin Oncol. 2012;39:296–304. doi: 10.1053/j.seminoncol.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aldhamen YA, Seregin SS, Amalfitano A. Immune recognition of gene transfer vectors: focus on adenovirus as a paradigm. Front Immunol. 2011;2:40. doi: 10.3389/fimmu.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chowdhury FZ, Ramos HJ, Davis LS, Forman J, Farrar JD. IL-12 selectively programs effector pathways that are stably expressed in human CD8 + effector memory T cells in vivo. Blood. 2011;118:3890–3900. doi: 10.1182/blood-2011-05-357111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Franzke A. The role of G-CSF in adaptive immunity. Cytokine Growth Factor Rev. 2006;17:235–244. doi: 10.1016/j.cytogfr.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Xu S, Hoglund M, Hakansson L, Venge P. Granulocyte colony-stimulating factor (G-CSF) induces the production of cytokines in vivo. Br J Haematol. 2000;108:848–853. doi: 10.1046/j.1365-2141.2000.01943.x. [DOI] [PubMed] [Google Scholar]
- 62.Franzke A, Piao W, Lauber J, Gatzlaff P, Konecke C, Hansen W, et al. G-CSF as immune regulator in T cells expressing the G-CSF receptor: implications for transplantation and autoimmune diseases. Blood. 2003;102:734–739. doi: 10.1182/blood-2002-04-1200. [DOI] [PubMed] [Google Scholar]
- 63.Panopoulos AD, Watowich SS. Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and ’emergency’ hematopoiesis. Cytokine. 2008;42:277–288. doi: 10.1016/j.cyto.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 65.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 66.Bennouna S, Denkers EY. Microbial antigen triggers rapid mobilization of TNF-alpha to the surface of mouse neutrophils transforming them into inducers of high-level dendritic cell TNF-alpha production. J Immunol. 2005;174:4845–4851. doi: 10.4049/jimmunol.174.8.4845. [DOI] [PubMed] [Google Scholar]
- 67.Costantini C, Calzetti F, Perbellini O, Micheletti A, Scarponi C, Lonardi S, et al. Human neutrophils interact with both 6-sulfo LacNAc + DC and NK cells to amplify NK-derived IFN{gamma}: role of CD18, ICAM-1, and ICAM-3. Blood. 2011;117:1677–1686. doi: 10.1182/blood-2010-06-287243. [DOI] [PubMed] [Google Scholar]
- 68.Howie D, Laroux FS, Morra M, Satoskar AR, Rosas LE, Faubion WA, et al. Cutting edge: the SLAM family receptor Ly108 controls T cell and neutrophil functions. J Immunol. 2005;174:5931–5935. doi: 10.4049/jimmunol.174.10.5931. [DOI] [PubMed] [Google Scholar]
- 69.Cotter MJ, Zaiss AK, Muruve DA. Neutrophils interact with adenovirus vectors via Fc receptors and complement receptor 1. J Virol. 2005;79:14622–14631. doi: 10.1128/JVI.79.23.14622-14631.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ostrakhovitch EA, Wang Y, Li SS. SAP binds to CD22 and regulates B cell inhibitory signaling and calcium flux. Cell Signal. 2009;21:540–550. doi: 10.1016/j.cellsig.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 71.Zak DE, Andersen-Nissen E, Peterson ER, Sato A, Hamilton MK, Borgerding J, et al. Merck Ad5/HIV induces broad innate immune activation that predicts CD8 + T-cell responses but is attenuated by preexisting Ad5 immunity. Proc Natl Acad Sci USA. 2012;109:E3503–E3512. doi: 10.1073/pnas.1208972109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.