Abstract
Vibrio choleraeis a Gram-negative enteric pathogen. This unit includes protocols for the growth and maintenance of V. cholerae in the laboratory.
Keywords: Vibrio cholerae, cholera, growth, laboratory
INTRODUCTION
The causative agent of the diarrheal disease cholera is the Gram-negative bacterium Vibrio cholerae. This enteric pathogen naturally inhabits an aquatic environment. Although most outbreaks occur in areas where water quality and sanitation are poor, V. cholerae can also be found in the waters all around the world. Cholera is endemic to Asia, Africa and South America. Additionally, outbreaks have emerged after conflict and natural disasters, such as in Iraq and Zimbabwe. Of the >200 serogroups, only O1 and O139 are known to cause epidemic disease due to the presence of the two major virulence factors, toxin co-regulated pilus (TCP) and cholera toxin (CT). The O1 serogroup is further divided into classical and El Tor biotypes. The classical biotype was responsible for the first six cholera pandemics, while the El Tor biotype is responsible for the current pandemic. The distinction between the classical and El Tor biotypes is based on their biochemical characteristics. For example, the El Tor biotype is naturally resistant to the antibiotic polymyxin B (see Table 2).
Table 2. Antibiotic stock solutions for use with V. cholerae cultures.
| Antibiotic | Solvent | Stock concentration | Working concentration | Storage |
|---|---|---|---|---|
| Ampicillin | Water | 100 mg/ml | 100 μg/ml | 4°C |
| Chloramphenicol | Ethanol | 34 mg/ml | 34 μg/ml | 4°C |
| Kanamycin | Water | 45 mg/ml | 45 μg/ml | 4°C |
| Polymyxin B | Water | 50,000 U/ml | 50 U/ ml | 4°C |
| Streptomycin | Water | 100 mg/ml | 100 μg/ml | 4°C |
| Tetracycline | Methanol | 15 mg/ml | 15 μg/ml | -20°C |
NOTE: All antibiotics should be filter sterilized by passage through a 0.22 μm filter.
NOTE: Dilute antibiotic 1:1,000 into media. For example, when working with a 5 ml broth culture, add 5 μl of the antibiotic stock solution.
NOTE: When making agar plates, autoclave media and let cool to 50°C before adding antibiotics.
Reports of non-O1 and non-O139 serogroups have recently emerged in the US. For example, O75 and O141 have surfaced in the Gulf Coast waters in recent years. The factors that contribute to virulence are not well understood in these strains. While most non-O1 and non-O139 serogroupss do not carry the tcp and ctx genes, cases of non-O1 and non-O139 serogroups causing severe cholera-like gastroenteritis have been reported (3, 4, 9).
This unit describes basic techniques to grow and maintain Vibrio cholerae in the laboratory. A relatively vigorous organism, V. cholerae can be grown in a variety of media formulations over a range of temperatures. These protocols should provide the reader with the ability to maintain and grow V. cholerae for use in a variety of assays.
CAUTION: Vibrio cholerae is a Biosafety Level 2 (BSL-2) pathogen. Follow protocols for the handling of BSL-2 organisms outlined by your institution. For general biosafety information see UNIT 1A.1 .
STRATEGIC PLANNING
Strain Selection
O395, N16961 and C6706 are commonly used laboratory strains of the O1 serogroup. The V. cholerae strain O395 is of the classical biotype, while the N16961 and C6706 strains are of the El Tor biotype. Various clinical and environmental strains have been isolated, several of which have been sequenced (see Table 1). Strain selection is an important aspect of designing your experiments. For example, El Tor strains are best for the study of biofilms, whereas classical strains demonstrate a more robust autoagglutination phenotype than El Tor strains. Of note, the O1 El Tor biotype is responsible for the most recent epidemics of cholera. Thus, many investigators are using the corresponding strains for their research. Since 2004, there have been clinical reports of El Tor variant strains that may exhibit properties of both classical and El Tor biotype strains, suggesting that studies of both biotypes are critical (1, 7, 8).
Table 1. Selected V. cholerae strains for which the genomic sequence is publically available.
| Strain | Source | TCP/CT | Serogroup/Biotype |
|---|---|---|---|
| N16961 | Human | +/+ | O1/El Tor |
| O395 | Human | +/+ | O1/classical |
| 1587 | Human | -/- | O12 |
| 2740-80 | Environmental | +/- | O1/El Tor |
| 623-39 | Human | -/- | non-O1/O139 |
| AM-19226 | Human | -/- | O39 |
| B33 | Human | +/+ | O1/El Tor |
| MO10 | Human | +/+ | O139 |
| MAK757 | Human | +/+ | O1/El Tor |
| MZO-2 | Human | -/- | O14 |
| MZO-3 | Human | -/- | O37 |
| RC385 | Environmental | -/- | O135 |
| V51 | Human | +/+ | O141 |
| V52 | Human | +/+ | O37 |
Growth Conditions
Vibrio cholerae grows well under standard laboratory conditions (LB at 37°C). Vibrio cholerae is able to grow between 20°C and 45°C. Unlike other bacteria, V. cholerae is unable to survive at 4°C for extended periods. Store plates at room temperature. V. cholerae is able to grow in a wide pH range. We suggest using neutral pH (7.0) for maintenance and growth of this strain. Broth cultures grow best with aeration, therefore a test tube roller or flask shaker are required for optimal growth and yield. In rich medium, such as LB, the generation time is approximately 40 minutes during exponential growth. An over night culture reaches densities of >109 CFU/ml.
Media
V. cholerae is able to grow in a variety of different media. In the following protocols we suggest growth in Luria Broth (LB). V. cholerae can also be grown in several defined minimal mediums (see APPENDIX 4A) that are amendable to the study of particular nutrient requirements. Special media, such as, plasmid broth can be used for isolation of plasmid DNA from broth cultures (see recipe). TCBS and TTGA agars are commonly used to isolate and identify V. cholerae from clinical specimens or environmental samples (see UNIT 6A.5.6). UNIT 1.1 and APPENDIX 4A describe several protocols for preparing commonly used liquid and solid media.
Basic protocol 1: GROWTH OF V. CHOLERAE FROM A FROZEN STOCK
V. cholerae should be preserved in frozen stocks in 30% glycerol. These are typically stored at -80°C. Growth from a freezer stock is an essential starting point for any experiment. It is important to streak from a freezer stock onto an agar plate. Placing V. cholerae directly into liquid media from a freezer stock is not recommended. It is good laboratory practice to initiate experiments from a single colony, in order to start with a clonal population.
Materials
V. cholerae frozen stock (see Basic Protocol 3)
LB agar plates (see APPENDIX 4A)
Wooden applicator stick, toothpick or inoculating loop, sterile
37°C incubator
- From a frozen stock, remove a small amount of slightly thawed bacteria.Do not thaw the frozen stock, as it will decrease the stock viability over time, instead remove a small chip of ice.
Streak heavily onto agar for isolated colonies (see UNIT 1.3.2).
Incubate at 37°C for 16-24 hr.
Basic protocol 2: GROWTH OF V. CHOLERAE IN LIQUID MEDIUM
Broth cultures are commonly used for a variety of experiments such as, growth curves, DNA extraction, protein analyses, and for the preparation of electrocompetent cells for transformations. For general laboratory purposes, LB broth is commonly used.
Materials
V. cholerae grown on agar (see Basic Protocol 1)
LB broth (see APPENDIX 4A)
Antibiotics if needed (see Table 1)
Wooden applicator sticks or inoculating loop, sterile
Capped test tubes, sterile
37°C incubator with a mechanism for rotating or shaking cultures
Using aseptic technique, add LB to sterile test tube or flask (For example, 5 ml to a 15 X 125 mm tube).
Using a sterile wooden stick or inoculation loop, pick a single colony from the prepared streak plate.
Inoculate the test tube by suspending the colony in the broth.
Cover the test tube and vortex for 1-2 sec.
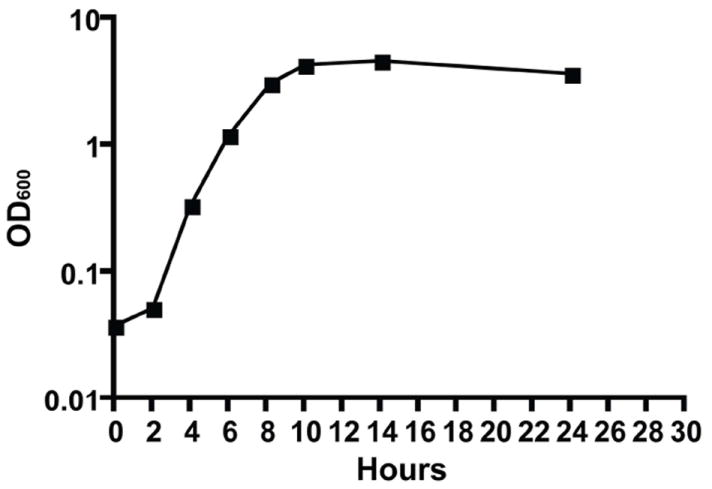
- Incubate the test tube on a roller for 12-16 hr at 37°C.See Figure 1 for a standard growth curve of V. cholerae to determine appropriate incubation time for a particular growth phase.
Figure 1. Growth curve of V. cholerae O1, classical strain O395, in LB broth.
NOTE: If you are preparing a large number of cultures, and all require the same antibiotics, then it is recommended that you prepare a stock solution of LB with the appropriate antibiotics. This reduces the number of manipulations.
NOTE: Capped flasks can be used when larger volumes are necessary. The growth of cultures should be in a flask that can hold the minimum of 5X the volume of broth. Incubate with shaking.
Basic protocol 3: PREPARATION OF V. CHOLERAE FROZEN STOCKS
V. cholerae should be stored for long-term usage in 30% glycerol at -80°C. We recommend that strains that are used frequently should be re-frozen after a single passage to have a back-up stock.
Materials
V. cholerae grown on LB agar containing antibiotics if appropriate (see Basic Protocol 1)
LB broth (see APPENDIX 4A)
50% glycerol (v/v), sterile
4 ml freezer vials, sterile (Wheaton, cat. No. 224882 or Cryovial®)
-80°C freezer
37°C incubator with a mechanism for rotating or shaking cultures
- Inoculate a single colony of V. cholerae into 5 ml liquid LB (see Basic Protocol 2).A larger volume can be used to grow cultures overnight. 5ml is a suggested starting volume.
Incubate at 37°C shaking for 15-16 hr.
Remove 1 ml of bacterial culture.
Add 1 ml of culture to 1.5 ml 50% glycerol solution.
Place suspension into a freezer vial.
Mix well by vortexing.
Immediately place into -80°C freezer.
Basic protocol 4: PRESERVATION OF V. CHOLERAE IN AGAR STABS
Stabs are useful for strain storage and are also convenient for shipping strains. V. cholerae can be stored for several years in agar stabs, but for long-term storage a frozen stock should be prepared (see Basic Protocol 3). See UNIT 1.3.4 for a detailed description on how to prepare and revive bacteria preserved in stab agar.
Materials
V. cholerae grown on agar containing antibiotics if appropriate
LB agar stab (see recipe)
Inoculating loop, sterile
37°C incubator
Using a sterile inoculating loop or flat toothpick, inoculate stab with a single colony (UNIT 1.3.4).
Incubate stab overnight at 37°C with the cap of the vial slightly loose.
Tightly seal the vial and store in a cool (15° to 22°C), dark place.
REAGENTS AND SOLUTIONS
LB broth
To 1L of deionized, distilled water, add 10 g Tryptone, 5 g Yeast Extract, 5 g NaCl and stir using a magnetic stir bar and plate at room temperature to dissolve. Aliquot appropriately. Autoclave.
NOTE: If the media is to be autoclaved in a culture flask, always use a flask that holds a minimum of 2X the volume of media. For example, for 1 L of broth, use a 2 L flask.
NOTE: Add antibiotics after the media has cooled.
LB agar
To 1L of LB broth (see above recipe), add 15 g Agar. Heat to dissolve with constant mixing. Autoclave media.
Let sterile media cool to 50°C. Pour plates into sterile, disposable petri dishes. See APPENDIX 4A.
NOTE: When making agar plates containing antibiotics, autoclave media and let cool to 50°C prior to adding antibiotics.
Plasmid Broth
To 1 L of deionized, distilled water, add 12 g Tryptone, 24 g Yeast Extract and 5 ml of Glycerol. Mix thoroughly. Aliquot 90 ml of media into milk dilution (160 ml) bottles. Autoclave.
Before use, add 10 ml of sterile 1M potassium phosphate (KPO4-) buffer. Mix.
1M KPO4- buffer (pH 7.6)
To 300 ml of deionized, distilled water, add 69 g KH2PO4. Adjust pH to 7.6 with KOH. Increase volume to 500 ml with water. Aliquot buffer into milk dilution (160 ml) bottles. Autoclave. Add 10 ml of buffer to 90 ml of plasmid broth. Mix.
Agar stabs
To 500 ml of deionized, distilled water, add 3 g Agar, 5 g Nutrient Broth and 4 g NaCl. Heat to dissolve with constant mixing.
Use autoclavable glass vials with screw caps (Wheaton, Cat. No. 224882). Fill the vials ¾ full with media that has been cooled to 50°C. Loosely screw caps and autoclave for 15 min. Let cool and tighten caps.
NOTE: If the caps are not properly tightened, then the agar stabs will dry out over time.
COMMENTARY
Background Information
Vibrio cholerae O1 is the etiological agent of the diarrheal disease cholera. Although underreported, cholera cases are increasing in number and recent outbreaks have been reported as having case fatality rates as great as 40%. Cholera plagues many areas in Africa, Asia, South America and more recently in the Middle East. From the most recent WHO report,
“(cholera) remains a challenge in those countries where access to safe water and adequate sanitation cannot be guaranteed for all. Almost every developing country is facing either a cholera outbreak or the threat of an epidemic.”
–WHO 2005
V. cholerae has two major virulence factors, cholera toxin (CT) and the toxin co-regulated pilus (TCP). Cholera toxin is responsible for causing the secretory diarrhea of the disease cholera by initiating a signaling cascade in the small intestine. TCP is a type IV pilus that is required for V. cholerae to colonize the mammalian host. Additionally, motility and biofilm formation contribute to the fitness of V. cholerae in the environment as well as in the mammalian host.
Critical Parameters and Troubleshooting
If no growth is seen from the initial streaking from the frozen stock or subsequent re-streaks, it is possible that an insufficient amount of bacteria was selected. Start from the freezer stock using a larger ice chip or from the agar plate selecting the entire colony to streak upon the plate. Additionally, if the freezer stock is old or a frequently used stock, it is possible that the stock has lost viability and the back-up stock should be used. Often times, when no growth is observed, it is due to the use of an antibiotic that the strain is not resistant to.
Multiple passages of V. cholerae are not recommended, as mutations may accumulate, thus resulting in the lost of virulence traits.
Autoagglutination
Vibrio cholerae O1 classical biotype can autoagglutinate during growth in broth culture. Autoagglutination can be induced by growth at 30°C, starting pH 6.5. Occasionally, a strain will autoagglutinate under normal laboratory conditions (37°C, starting pH 7). Autoagglutination interferes with reading the optical density of the culture and may cause unwanted results in different assays. If autoagglutination occurs, then simply vortex the culture, making sure the bacterial suspension is homogeneous before use.
Anticipated Results
Basic Protocol 1 describes how to grow V. cholerae on solid media. After 16-20 hr of incubation, colonies will be large, semi-opaque, convex, smooth, round and off-white/tan in color. Basic Protocol 2 describes how to grow V. cholerae in liquid media. After 12-16 hrs, the cultures should be turbid. Basic Protocols 3 and 4 describe how to preserve V. cholerae either by freezing or in agar stabs. Regrowth from the preserved cultures should produce a large number of viable cells.
References
- 1.Ansaruzzaman M, Bhuiyan NA, Safa A, Sultana M, McUamule A, Mondlane C, Wang XY, Deen JL, von Seidlein L, Clemens JD, Lucas M, Sack DA, Balakrish Nair G. Genetic diversity of El Tor strains of Vibrio cholerae O1 with hybrid traits isolated from Bangladesh and Mozambique. Int J Med Microbiol. 2007;297:443–449. doi: 10.1016/j.ijmm.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Chun J, Grim CJ, Hasan NA, Lee JH, Choi SY, Haley BJ, Taviani E, Jeon YS, Kim DW, Lee JH, Brettin TS, Bruce DC, Challacombe JF, Detter JC, Han CS, Munk AC, Chertkov O, Meincke L, Saunders E, Walters RA, Huq A, Nair GB, Colwell RR. Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae. Proc Natl Acad Sci U S A. 2009;106:15442–15447. doi: 10.1073/pnas.0907787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crump JA, Bopp CA, Greene KD, Kubota KA, Middendorf RL, Wells JG, Mintz ED. Toxigenic Vibrio cholerae serogroup O141-associated cholera-like diarrhea and bloodstream infection in the United States. J Infect Dis. 2003;187:866–868. doi: 10.1086/368330. [DOI] [PubMed] [Google Scholar]
- 4.Dziejman M, Serruto D, Tam VC, Sturtevant D, Diraphat P, Faruque SM, Rahman MH, Heidelberg JF, Decker J, Li L, Montgomery KT, Grills G, Kucherlapati R, Mekalanos JJ. Genomic characterization of non-O1, non-O139 Vibrio cholerae reveals genes for a type III secretion system. Proc Natl Acad Sci U S A. 2005;102:3465–3470. doi: 10.1073/pnas.0409918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maloy SR, Stewart VJ, Taylor RK. Genetic analysis of pathogenic bacteria. Cold Spring Harbor Laboratory Press ; Cold Spring Harbor, NY: 1996. [Google Scholar]
- 6.Mohapatra SS, Ramachandran D, Mantri CK, Colwell RR, Singh DV. Determination of relationships among non-toxigenic Vibrio cholerae O1 biotype El Tor strains from housekeeping gene sequences and ribotype patterns. Res Microbiol. 2009;160:57–62. doi: 10.1016/j.resmic.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Nair GB, Qadri F, Holmgren J, Svennerholm AM, Safa A, Bhuiyan NA, Ahmad QS, Faruque SM, Faruque AS, Takeda Y, Sack DA. Cholera due to altered El Tor strains of Vibrio cholerae O1 in Bangladesh. J Clin Microbiol. 2006;44:4211–4213. doi: 10.1128/JCM.01304-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safa A, Sultana J, Dac Cam P, Mwansa JC, Kong RY. Vibrio cholerae O1 hybrid El Tor strains, Asia and Africa. Emerg Infect Dis. 2008;14:987–988. doi: 10.3201/eid1406.080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tobin-D’Angelo M, Smith AR, Bulens SN, Thomas S, Hodel M, Izumiya H, Arakawa E, Morita M, Watanabe H, Marin C, Parsons MB, Greene K, Cooper K, Haydel D, Bopp C, Yu P, Mintz E. Severe diarrhea caused by cholera toxin-producing Vibrio cholerae serogroup O75 infections acquired in the southeastern United States. Clin Infect Dis. 2008;47:1035–1040. doi: 10.1086/591973. [DOI] [PubMed] [Google Scholar]


