Abstract
Occult hepatitis B virus (HBV) infection is of concern in human immunodeficiency virus (HIV)–infected persons. We observed that 2% of 400 HIV-infected women with antibodies to hepatitis B core antigen alone had occult HBV infection (i.e., detectable HBV DNA in the absence of HBV surface antigen). CD4 cell counts of <200 cells/mm3 were more common among occult HBV-infected women than among those without occult HBV infection. Aminotransferase levels did not appear to be associated with being positive for HBV DNA.
Occult hepatitis B virus (HBV) infection, defined as detectable HBV DNA in the absence of hepatitis B surface antigen (HBsAg), is of concern in HIV-infected persons. The presence of antibodies to hepatitis B core antigen (anti-HBc) alone is most commonly reported within this definition, but various serologic patterns can exist [1]. An early study found that 30% of 57 HIV-infected persons positive for anti-HBc alone had persistent HBV DNA in serum, and 89% were viremic at least once during follow-up [2]. The presence of anti-HBc alone is reportedly more common in HIV-infected women than HIV-infected men [3], but few, if any, studies have determined the prevalence or outcome of occult HBV infection in HIV-infected women. Using a large cohort of women positive for anti-HBc alone with or at risk for HIV infection, we examined the baseline prevalence and clinical outcomes of occult HBV infection, assessing HBV DNA levels in relation to serum aminotransferase levels and HIV-related factors.
Methods
The Women’s Interagency HIV Study is a prospective cohort of 2791 HIV-infected and 975 HIV-uninfected women enrolled at 6 sites (Bronx and Brooklyn, New York; Chicago, IL; Los Angeles and San Francisco, CA; and Washington, D.C.) either from October 1994 through November 1995 or from October 2001 through September 2002. Informed consent was obtained from all participants in accordance with the US Department of Health and Human Services guidelines and the institutional review boards of participating institutions. Details of recruitment and baseline cohort characteristics have been described previously [4, 5]. Every 6 months, participants are examined and complete questionnaires that include data on demographic characteristics, disease characteristics, and medication use. CD4 cell counts and HIV RNA levels are determined every 6 months for HIV-infected women, and aminotransferase levels are determined annually. Hepatitis C virus (HCV) antibody testing was performed at baseline, with HCV RNA testing for those who had HCV antibodies.
Tests for hepatitis B surface antibody (anti-HBs), anti-HBc, and HBsAg were performed at baseline (study entry) for 2132 of the 3766 women, using the Ausab EIA, Corzyme EIA, and Auszyme Microparticle EIA, respectively (Abbott Laboratories). Of the remaining 1634 subjects, 1620 were tested for anti-HBc and HBsAg at baseline but not for anti-HBs; 553 of these 1620 subjects tested positive for anti-HBc and negative for HBsAg. Among these, 501 had stored serum samples that were obtained at or within 18 months of their baseline visit; the samples were tested for anti-HBs using Vitros ECi (Ortho Diagnostics). Therefore, of the 3700 women with serum specimens available to distinguish a pattern of positivity for anti-HBc alone, 490 (13.2%) had anti-HBc alone. Of these, 452 had serum specimens available for HBV DNA quantification, which was determined using the COBAS Amplicor Monitor test (Roche Diagnostics; lower limit of detection, 200 copies/mL).
We measured the prevalence of occult HBV infection, constructing 95% CIs assuming a binomial distribution. The characteristics of women with and without detectable HBV DNA were compared using Fisher’s exact test for proportions and Student’s t test for means of continuous variables. For women who tested positive for HBV DNA, additional HBV DNA testing was performed at follow-up visits when any of the following were true: (1) either the serum alanine aminotransferase or aspartate aminotransferase level was >2 times that measured on the prior visit; (2) antiretroviral therapy (ART) was started or changed, or the visit was the visit prior to the start of ART; (3) a change in CD4 cell count of ≥ 100 cells/mm3 or a change in HIV RNA level of ≥ 1 log was observed in the absence of any report of ART; or (4) this was the last visit with any aminotransferase, ART, CD4 cell, and HIV RNA data. Stata software, version 8 (StataCorp), was used for analyses.
Results
Of the 452 women who tested positive for anti-HBc alone (400 HIV-infected and 52 HIV-uninfected women), 8 tested positive for HBV DNA, and all of these 8 were HIV infected. The prevalence of occult HBV infection was 1.8% (95% CI, 0.8%–3.5%) among all women included in the study and 2.0% (95% CI, 0.9%–3.9%) among HIV-infected women. None of the HBV DNA-positive women and 14 of the HBV DNA-negative women reported receiving ART with anti-HBV activity (lamivudine, 13 women; tenofovir, 1 woman) at baseline. The characteristics of HBV DNA-positive and -negative women were similar (table 1). Among the HIV-infected patients, HBV DNA-positive women were more likely than HBV DNA-negative women to have a CD4 cell count of <200 cells/mm3 and had a trend toward a higher HIV RNA level.
Table 1.
Characteristics of women with a serological pattern of antibody to hepatitis B virus (HBV) core, according to the presence or absence of HBV DNA
| HBV DNA status | |||
|---|---|---|---|
| Characteristic | Negative (n = 444) |
Positive (n = 8) |
pa |
| HIV infected | 392 (88) | 8 (100) | .4 |
| Age, mean years ± SD | 39 ± 7 | 37 ± 6 | .45 |
| Nonwhite race | 356 (80) | 7 (88) | .51 |
| HIV risk category | .71 | ||
| Injectiond rug use | 275 (62) | 5 (63) | |
| Heterosexual sex | 107 (24) | 3 (38) | |
| Transfusion | 10 (2) | 0 | |
| Unidentified | 50 (11) | 0 | |
| Heavy alcohol useb | 63 (14) | 2 (25) | .32 |
| Current smoking | 319 (72) | 6 (75) | .61 |
| Current crack use | 130 (29) | 2 (25) | .79 |
| Current injection drug use | 82 (18) | 0 | .57 |
| Mean body mass indexc ± SD | 27 ± 7 | 24 ± 6 | .15 |
| Abnormal AST leveld | 163 (42) | 2 (25) | .28 |
| Abnormal ALT leveld | 132 (34) | 2 (25) | .45 |
| HCV antibody positive | 345 (78) | 5 (63) | .25 |
| CD4 cell count of <200 cells/mm3e | 102 (27) | 6 (75) | <.01 |
| Log HIV RNA level, mean ± SDe | 4.2 ± 1.0 | 4.9 ± 0.8 | .06 |
NOTE. Data are no. (%) unless indicated otherwise. ALT, alanine aminotransferase; AST, aspartate aminotransferase; HCV, hepatitis C virus.
Determined by Fisher’s exact test for proportions and Student’s t test for means.
Defined as consumption of ≥ 14 drinks/week.
Calculated as weight in kilograms divided by the square of height in meters.
Defined as an ALT or AST level >40 IU/L; the denominator is 388 because of missing ALT and AST data.
HIV-positive subjects only (n = 400); for CD4 cell count, the denominator is 378 because of missing CD4 cell count data.
Table 2 presents HBV DNA levels for women with occult HBV infection in relation to aminotransferase levels, CD4 cell counts, and ART. Patients 1–5 had low HBV DNA levels at baseline. HBV DNA was undetectable at follow-up for patients 1–3. Patients 4 and 5 died of AIDS-related illnesses. Patients 2–5 also had chronic HCV infection. Patients 6–8 had higher initial HBV DNA levels and even higher follow-up levels, although aminotransferase levels remained in a normal range, except for patient 6. Patient 6 died of an undetermined cause, and patient 7 died of an AIDS-related illness.
Table 2.
Clinical and laboratory follow-up data for the 8 patients with occult hepatitis B virus (HBV) infection.
| Patient, visit no.a | HBV DNA level, copies/mL |
AST level, IU/L |
ALT level, IU/L |
HIV RNA level, copies/mL |
CD4 cell count, cells/mm3 |
Antiretroviral medications |
|---|---|---|---|---|---|---|
| Patient 1 | ||||||
| Visit 1 | 230 | 60 | 44 | 280,000 | 20 | None reported |
| Visit 5 | <200 | 231 | 243 | 62,000 | 37 | None reported |
| Visit 9 | <200 | 73 | 65 | 150,000 | 33 | None reported |
| Visit 11 | <200 | 35 | 17 | 2600 | 235 | IDV, ABC,3TC |
| Visit 13 | <200 | 112 | 107 | 910,000 | 26 | None reported |
| Visit 17 | <200 | 38 | 46 | 80 | 537 | d4T, 3TC, TDF, LPV/r |
| Visit 21 | <200 | 47 | 68 | 80 | 1139 | d4T, 3TC, TDF LPV/r |
| Patient 2 | ||||||
| Visit 1 | 250 | 24 | 15 | 110,000 | 116 | None reported |
| Visit 7 | <200 | 44 | 47 | 130,000 | 114 | None reported |
| Visit 9 | <200 | 31 | 39 | 3200 | 152 | d4T, 3TC, IDV |
| Visit 13 | <200 | 65 | 75 | 710 | 428 | NFV, SQV, NVP |
| Visit 17 | <200 | 57 | 46 | 80 | 439 | NFV, SQV, TDF |
| Visit 21 | <200 | 26 | 19 | 434 | Missing | NFV, SQV, TDF |
| Patient 3 | ||||||
| Visit 1 | 206 | 20 | 18 | 2200 | 194 | None reported |
| Visit 9 | <200 | 49 | 52 | 51,000 | 363 | None reported |
| Visit 17 | <200 | 41 | 45 | 13,000 | 940 | None reported |
| Visit 21 | <200 | 57 | 65 | 22,000 | 277 | None reported |
| Patient 4: visit 1b | 376 | 36 | 13 | 210,000 | 23 | None reported |
| Patient 5: visit 1b | 611 | 61 | 53 | 330,000 | 0 | None reported |
| Patient 6 | ||||||
| Visit 1 | 17,600 | 35 | 27 | 1,110,000 | 415 | None reported |
| Visit 9 | 17,000,000 | 56 | 28 | 8300 | 126 | None reported |
| Visit 11c | <200 | 65 | 46 | 13,000 | 198 | 3TC, ddl, NFV |
| Patient 7 | ||||||
| Visit 1 | 800,000 | 32 | 24 | 400,000 | 25 | None reported |
| Visit 3d | 13,000,000 | 26 | 9 | 200,000 | 11 | None reported |
| Patient 8 | ||||||
| Visit 1 | 9190 | 15 | 7 | 11,000 | 613 | None reported |
| Visit 13e | 18,500,000 | 34 | 30 | 57,000 | 283 | None reported |
NOTE. ABC, abacavir; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ddl, didanosine; d4t, stavudine; IDV, indinavir; LPV/r, lopinavir/ritonavir; NFV, nelfinavir; NVP, nevirapine; SQV, saquinavir; TDF, tenofovir; 3TC, lamivudine.
Women’s Interagency HIV Study semiannual visits are numbered consecutively, with visit 1 corresponding to the initial Women’s Interagency HIV Study visit that occurred from October 1994 through March 1995 and visit 21 corresponding to the most recent visit included in the study from October 2004 through March 2005. AST and ALT data were collected at odd-numbered visits.
Patient died after the first visit.
Patient died after the 11th visit.
Patient died after the third visit.
Patient was lost to follow-up after the 13th visit.
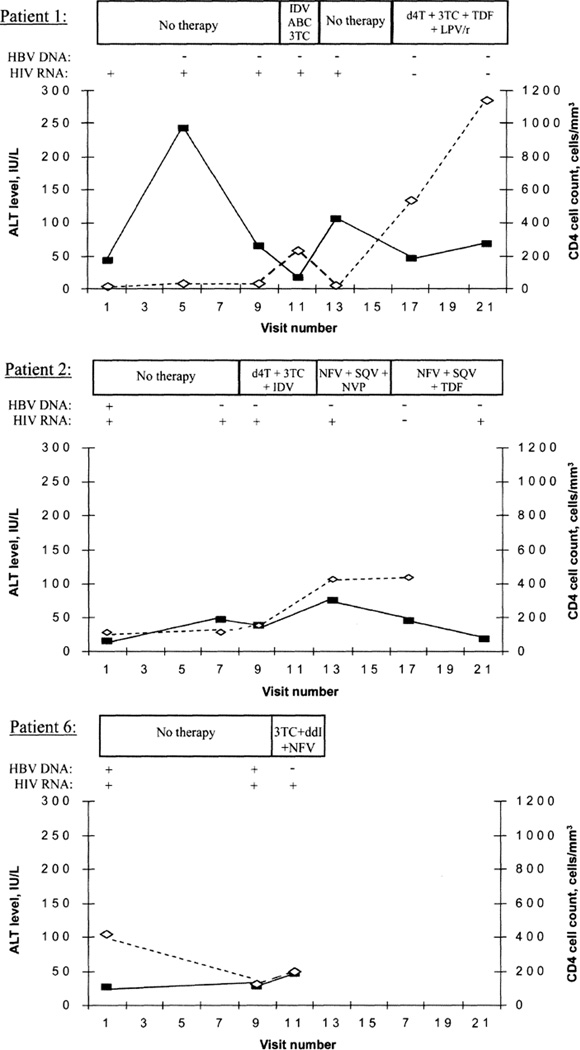
Alanine aminotransferase and CD4 cell levels, in relation to HBV DNA level, for patients 1, 2, and 6 (who initiated ART) are shown in figure 1. For patient 1, the alanine aminotransferase level was highest when the CD4 cell count was <50 cells/mm3. Upon recovery of the CD4 cell count, the alanine aminotransferase level was lowest when treatment with abacavir, lamivudine, and indinavir was reported and higher when treatment with stavudine, lamivudine, tenofovir, and lopinavir plus ritonavir was reported. For patient 2, the alanine aminotransferase level was highest when treatment with nevirapine, saquinavir, and nelfinavir was reported. Patient 6’s alanine aminotransferase levels increased after initiation of ART, whereas HBV DNA levels became undetectable. Aspartate aminotransferase levels followed a similar pattern.
Figure 1.
Alanine aminotransferase (ALT; boxes) and CD4 cell levels (diamonds) and presence or absence of hepatitis B virus (HBV) DNA for patients 1, 2, and 6 in relation to antiretroviral therapy. ABC, abacavir; ddl, didanosine; d4t, stavudine; IDV, indinavir; LPV/r, lopinavir/ritonavir; NFV, nelfinavir; NVP, nevirapine; SQV, saquinavir; TDF, tenofovir; 3TC, lamivudine.
Discussion
In this large cohort of women positive for anti-HBc alone, we found a 2% prevalence of occult HBV infection among those who were HIV infected. HIV-infected women with occult HBV infection were more likely to have a CD4 cell count of <200 cells/mm3 than were those without occult HBV infection. Aminotransferase levels did not appear to predict the presence or absence of HBV DNA at follow-up visits among women with occult HBV infection.
The prevalence of occult HBV infection we detected is lower than the 30% prevalence reported in an early study of HIV-infected men with anti-HBc alone [2]. The HBV DNA testing methodology used in that study differed from ours, which highlights one of the difficulties in comparing prevalence across studies. That study used nested PCR with targeting of different genes for amplification. This technique has high sensitivity but lower specificity because of false-positive results associated with contamination or amplification of non–HBV DNA targets. More recent studies have used commercial assays, such as the one we used, to quantify HBV DNA. Although less sensitive, these more standardized assays allow for comparisons between studies.
Studies using these commercial assays report a prevalence that varies between 0% and 33% in HIV-infected persons. In one study that reported a 0.6% prevalence among 160 subjects with anti-HBc alone, most were receiving ART with anti-HBV activity [6]. Among the studies with few, if any, patients undergoing ART with anti-HBV activity, there were no cases of occult HBV infection among 85 subjects with anti-HBc alone in one [7], whereas another found detectable HBV DNA in 10% of 38 subjects with anti-HBc alone [8]. In contrast, a 33% prevalence of occult HBV infection was observed in an African study that identified 20 HIV-infected subjects with anti-HBc alone [8, 9]. Thus, even in studies that use the same definitions for occult HBV infection and the same HBV DNA assays, other factors may contribute to differences in prevalence.
Among the 8 women with occult HBV infection in our study, most had low initial HBV DNA levels. Among the 6 with follow-up data available, one-half had undetectable HBV DNA levels at later visits, despite occasional mild elevations in serum alanine aminotransferase and/or serum aspartate aminotransferase levels. HCV coinfection, other liver disease, or drug toxicity could explain the aminotransferase elevations. The 3 women with higher baseline HBV DNA levels had even higher levels subsequently. Regardless of HBV DNA level, aminotransferase levels were not markedly elevated with occult HBV infection, as is seen in HIV-infected patients with nonoccult HBV infection [10].
In contrast, a study of HIV-infected patients with occult HBV infection (examined among all patients without detectable HBsAg) found that hepatic flares were more common among patients with detectable HBV DNA [11]. Low HBV DNA levels (<3500 copies/mL) were observed, and none of the patients were persistently HBV DNA positive. However, in some patients, elevations in alanine aminotransferase levels of >4 times the normal value were observed in association with detectable HBV DNA levels. Most patients appeared to have recently started receiving ART or changed their ART regimens. Therefore, ART-related toxicity or immune reconstitution could have contributed to these flares.
One limitation of our study is that HBV DNA testing was not repeated for women with anti-HBc alone who were not HBV DNA positive at baseline, nor was it performed for HBsAg-negative women with serologic patterns indicative of hepatitis B other than anti-HBc alone. Therefore, it is possible that we missed some patients with occult HBV infection who had intermittent viremia or a serologic pattern other than anti-HBc alone. In HBV DNA–positive subjects, we may have missed hepatic flares associated with detectable increases in the HBV DNA level, because aminotransferase testing was only performed annually. Finally, the small number of cases of occult HBV infection and limited follow-up restricts conclusions on the long-term outcome of occult HBV infection in this cohort.
In summary, we found that 2% of HIV-infected women with anti-HBc alone had occult HBV infection. Those with occult HBV infection were more likely to have a CD4 cell count of <200 cells/mm3. Elevations in aminotransferase levels did not appear to be associated with detectable HBV DNA. The possibility of occult HBV infection should be considered in HIV-infected patients with anti-HBc alone, particularly in those with severe immunosuppression. HBV DNA testing is important before HAART is initiated, so that drugs with anti-HBV activity may be included.
Acknowledgments
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group, with centers at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, New York (Howard Minkoff); Washington, DC, Metropolitan Consortium (Mary Young); the Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); and the Data Coordinating Center (Stephen Gange).
Financial support. The WIHS is funded by the National Institutes of Health (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, UO1-AI-42590, UO1-HD-32632, MO1-RR-00071, MO1-RR-00079, and MO1-RR-00083). National Institutes of Health support was provided to P.C.T. (K23 AI 66943-01) and J.I.T. (K12 RR024140). This work was made possible in part by core services provided by the UCSF Liver Center (National Institutes of Health P30 DK26743) and by Bristol-Myers Squibb (which played no role in analyzing the data or in writing the paper).
P.C.T. received recent research funding from Gilead for statistical analysis not related to this study (no salary support).
Footnotes
Potential conflicts of interest. All other authors: no conflicts.
References
- 1.Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis. 2002;2:479–486. doi: 10.1016/s1473-3099(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 2.Hofer M, Joller-Jemelka HI, Grob PJ, Luthy R, Opravil M. Frequent chronic hepatitis B virus infection in HIV-infected patients positive for antibody to hepatitis B core antigen only. Swiss HIV Cohort Study. Eur J Clin Microbiol Infect Dis. 1998;17:6–13. doi: 10.1007/BF01584356. [DOI] [PubMed] [Google Scholar]
- 3.Neau D, Winnock M, Galperine T, et al. Isolated antibodies against the core antigen of hepatitis B virus in HIV-infected patients. HIV Med. 2004;5:171–173. doi: 10.1111/j.1468-1293.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- 4.Bacon MC, von Wyl V, Alden C, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 6.Neau D, Winnock M, Jouvencel AC, et al. Occult hepatitis B virus infection in HIV-infected patients with isolated antibodies to hepatitis B core antigen: Aquitaine cohort, 2002–2003. Clin Infect Dis. 2005;40:750–753. doi: 10.1086/427882. [DOI] [PubMed] [Google Scholar]
- 7.Nunez M, Rios P, Perez-Olmeda M, Soriano V. Lack of ‘occult’ hepatitis B virus infection in HIV-infected patients. AIDS. 2002;16:2099–2101. doi: 10.1097/00002030-200210180-00024. [DOI] [PubMed] [Google Scholar]
- 8.Shire NJ, Rouster SD, Rajicic N, Sherman KE. Occult hepatitis B in HIV-infected patients. J Acquir Immune Defic Syndr. 2004;36:869–875. doi: 10.1097/00126334-200407010-00015. [DOI] [PubMed] [Google Scholar]
- 9.Mphahlele MJ, Lukhwareni A, Burnett RJ, Moropeng LM, Ngobeni JM. High risk of occult hepatitis B virus infection in HIV-positive patients from South Africa. J Clin Virol. 2006;35:14–20. doi: 10.1016/j.jcv.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Peters MG, Andersen J, Lynch P, et al. Randomized controlled study of tenofovir and adefovir in chronic hepatitis B virus and HIV infection: ACTG A5127. Hepatology. 2006;44:1110–1116. doi: 10.1002/hep.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filippini P, Coppola N, Pisapia R, et al. Impact of occult hepatitis B virus infection in HIV patients naive for antiretroviral therapy. AIDS. 2006;20:1253–1260. doi: 10.1097/01.aids.0000232232.41877.2a. [DOI] [PubMed] [Google Scholar]