Abstract
Background
The pharyngoesophageal segment commonly referred to as the upper esophageal sphincter (UES) generates a high-pressure zone (HPZ) between the pharynx and the esophagus. However, the exact anatomical components of the UES-HPZ remain incompletely determined.
Objective
To systematically define the US signature of various components of the pharyngoesophageal junction and to determine how these structures contribute to the development of the UES-HPZ.
Design
Prospective, experimental study.
Setting
Tertiary Academic Medical Center.
Patients
This study involved 18 healthy volunteers.
Intervention
We studied 5 participants by using a high-frequency US miniprobe (US-MP) and concurrent fluoroscopy and another 13 participants by using the US-MP and concurrent manometry.
Main Outcome Measurements
Relative contribution of various muscles in the UES-HPZ.
Results
Manometrically, the UES-HPZ had a median length of 4.0 cm (range 3.0–4.5 cm). A C-shaped muscle, believed to represent the cricopharyngeus muscle, was observed for a median length of 3.5 cm (range 2.0–4.0 cm). The oval configuration representing the esophageal contribution to the UES was seen in 10 of 13 participants (77%) at the distal HPZ (esophagus to UES transition zone). The flat configuration of the inferior constrictor muscle was noted in 7 of 13 participants (54%) at the proximal HPZ (UES to pharynx transition zone). There were 4 to 5 wall layers versus 3 layers in the distal and proximal HPZ, respectively. The mean (± SD) muscle thickness was relatively constant along the length of the UES-HPZ.
Limitations
Air artifacts in the UES-HPZ.
Conclusion
The configuration and layers of the UES-HPZ vary along its length. The upper esophagus is a significant contributor to the distal UES-HPZ.
The pharyngoesophageal segment, commonly referred to as the upper esophageal sphincter (UES), generates a high-pressure zone (HPZ) between the pharynx and the esophagus. This HPZ spans the uppermost portions of the esophagus and cricopharyngeus muscle and the most distal part of the inferior pharyngeal constrictors. In animal studies,1 each of these components either individually or collectively participates in various functions of the UES, such as maintaining tone, relaxation during deglutition, and reflexive contraction during various types of stimulation.2
There are relatively few studies concurrently evaluating the anatomic and physiologic characteristics of the UES, in contrast to the large number of studies regarding the body of the esophagus and the lower esophageal sphincter muscle. There is also disagreement on which muscles contribute to the UES and the degree to which each muscle is distributed in this region.
In clinical practice, the criterion standard for identifying the UES is manometric localization by using manometry catheters. With the advent of the high-frequency-US mini-probe (US-MP), compared with standard echoendoscopes, it is now possible to study the esophageal wall in greater detail.1,3–5 In vitro studies have shown that this modality can measure the esophageal wall with an accuracy of 0.01 mm.6 These miniprobes can therefore be used to characterize the components of the UES-HPZ. Indeed, recent studies7 have demonstrated that it is feasible to use a miniature high-resolution intraluminal US probe to anatomically visualize the UES. The objective of this study was to systematically define the US signature of various components of the pharyngoesophageal segment and to determine how these visualized structures correlate with the manometric aspects of the UES-HPZ.
METHODS
US-MP was performed by using the UM-DP20-25R, 20-MHz miniprobe (Olympus America, Inc, Melville, NY), which was passed through the nostril. The image patterns seen were correlated with the position of the probe in the pharyngoesophageal segment as determined with (1) concurrent fluoroscopy in 5 participants and (2) concurrent manometry in 13 additional participants. All participants were in the sitting-upright position while being studied. The acquired real-time images (US-MP and fluoroscopy) and manometric tracings were digitally recorded concurrently for subsequent analysis. All volunteers underwent prior transnasal, unsedated EGD8 to document the absence of associated upper GI lesions.
This study was approved by the Human Research and Review Committee of the Medical College of Wisconsin, and written informed consent was obtained from each participant.
Group A: concurrent US-MP endoscopy and fluoroscopy
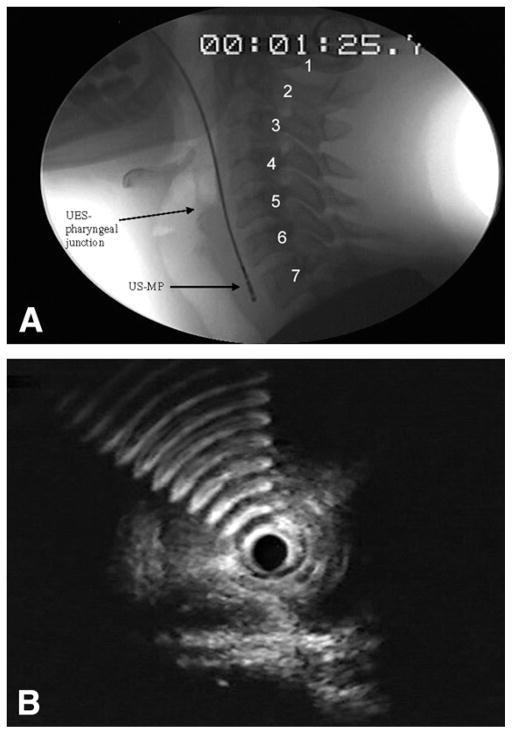
We studied 5 healthy participants with a mean (± standard deviation [SD]) age of 43 years ± 11 years (3 men) who underwent transnasal US-MP endoscopy to map a span of 7 cm, ranging from 2 cm above to 5 cm below the pharyngo-UES junction as visualized fluoroscopically. By using fluoroscopic still images, we designated the pharyngo-UES junction (Fig. 1A) as the segment between the hypopharynx and the UES and used this as our reference point. However, because the pharyngo-UES junction is not stationary, cervical vertebrae were used as stationary landmarks (Fig. 1A). The corresponding US images of the pharyngo-UES segment (Fig. 1B) showed hyperechoic artifacts, representing an air-tissue interface.
Figure 1.
A, Representative fluoroscopic image of cervical vertebrae (1–7) used as a stationary point of reference for placing 0.5-cm intervals above and below the UES-pharyngeal junction. UES, upper esophageal sphincter; US-MP, US miniprobe. B, Hyperechoic artifacts abruptly appear when the transducer of the US-MP is positioned at the level of the UES-pharyngeal junction during station pull through. This image demonstrates the air-tissue interface where air artifacts are first noted anterior to the flat-shaped muscle.
Before the US-MP was passed, the nostril was anesthetized by local application of viscous lidocaine. By using fluoroscopy to confirm the probe position, we were able to verify the sonographic cross-sectional images of the muscle wall, according to the axial distance between the level of the pharyngo-UES junction and the tip of the US-MP. Both the US-MP station pull-through images and the fluoroscopic video pictures were simultaneously recorded and were digitally edited into still images. With the axial length of the US-MP transducer known, we corrected for fluoroscopic magnification. The corresponding synchronized sonographic image for each 0.5-cm interval was descriptively analyzed according to muscle shape, thickness, and number of layers by two endosonographers (L.V.H. and K.D.) in a blinded fashion. The study was repeated 3 times in each participant.
Group B: concurrent US-MP endoscopy and manometry
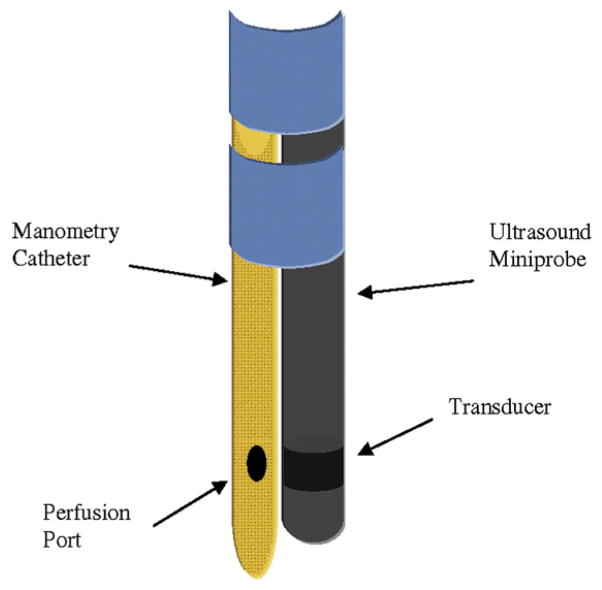
We studied an additional 13 healthy volunteers with a mean (± SD) age of 36 ± 12 years (10 men) by using the US-MP with concurrent esophageal manometry to define the HPZ of the pharyngoesophageal segment, which is otherwise unidentifiable by using fluoroscopy or endosonography. A technique was developed in which a specially designed catheter with two perfusion ports located at the same level was used. The US-MP catheter (outer diameter, 2.5 mm) was securely taped to the manometry catheter (outer diameter, 3 mm) with its transducer positioned at the same level of (and lying between) the perfusion ports (Fig. 2). With this assembly, it was possible to simultaneously record the luminal pressure and US images of the muscle wall at the same level within the pharyngo-UES segment. The water-perfused plastic catheter did not cause any significant interference in the US images obtained. Swallowing was monitored by using submental surface electromyography.
Figure 2.
The specially designed manometry catheter with two perfusion ports, securely fastened (blue bands) to a US-MP. Note that the manometry catheter perfusion ports and US transducer were positioned at the same level to ensure that the same muscle is being simultaneously recorded by the manometry catheter and the US-MP. US-MP, US miniprobe.
After the nostril was anesthetized with viscous lidocaine, the complete recording assembly, consisting of the US-MP and manometry catheter, was advanced transnasally (both could easily pass through one nostril) into the proximal esophagus. Water was perfused at 0.5 mL/minute through each port. The US-MP/manometry catheter assembly was withdrawn by 0.5-cm increments until the pharynx was manometrically identified. The US video was digitally recorded (Sony digital capture unit UPA-P100MD/OEM; Sony Corp, Tokyo, Japan) and edited into still images at each 0.5-cm interval of the pull through. Each participant underwent 3 pull throughs. During the pull through, participants were asked to refrain from swallowing and hold their breathing. Between pull throughs, they were asked to swallow to clear the throat and the esophagus of any residual water.
During US-MP examination, the muscle configuration of the UES was assumed to be C-shaped, based on a previous study that used cadavers.7 We measured the mean maximal muscle thickness (MMMT) via computer software (Image J; http://rsb.info.nih.gov/ij/, NIH, Bethesda, MD) by obtaining the mean thickness (mm) for each quadrant. The endosonographic length of the UES was defined as the mean distance between the air-tissue interface and the point of manometric transformation from the UES-HPZ to the esophageal pressure. We used Medical Measurement System software (Enschede, The Netherlands) to analyze US cine-loops with corresponding manometric pressure readings.
RESULTS
The US-MP was successfully inserted into the esophagus in all 18 participants with minimal gag reflex. The mean (± SD) procedure time was 5.5 ± 2.5 minutes for 3 pull throughs. There were no complications.
Group A: concurrent US-MP endoscopy and fluoroscopy
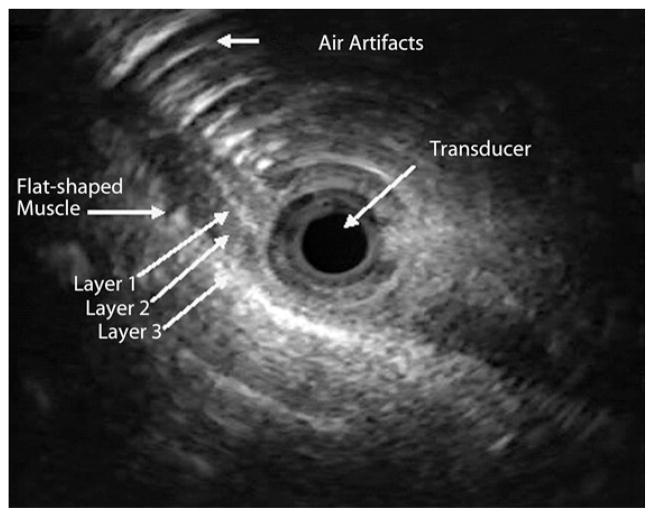
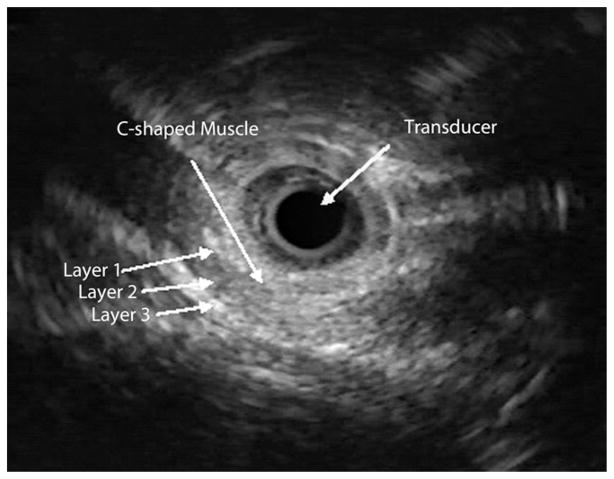
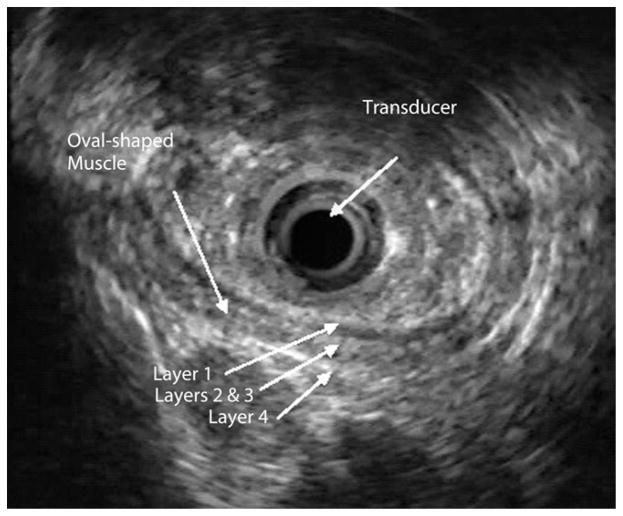
Proximal to the pharyngo-UES junction (hypopharynx), the muscle showed a flat configuration. At the air-tissue interface (upper level of the pharyngo-UES segment as seen with fluoroscopy), the muscle appeared flat with clear delineation to an area of hyperechoic bands, corresponding to acoustic artifacts from air (Fig. 3). Distal to the pharyngo-UES junction, a muscular structure with a “C” configuration was seen for a median length of 3.5 cm, range 2.0 to 4.0 cm (Fig. 4). As was shown in previous studies that used cadavers,7 this muscle represents the cricopharyngeus muscle. This C-shaped, hypoechoic structure was 3-layered and did not demonstrate the expected 4 to 5 layers typically seen in the proximal esophagus. Distal to the C configuration, an abrupt transformation of the muscle shape into an oval configuration characterized by 4 to 5 distinct wall layers was observed (Fig. 5). There was complete concordance between the two endosonographers with regard to determining cross-sectional muscle shape, number of layers, and MMMT.
Figure 3.
The US-MP image of a flat-shaped muscle at the level of the air-tissue interface. The characteristic air artifacts appear as series of semicircular, hyperechoic lines anterior to the muscle. Three sonographic layers were noted (layer 1, hyperechoic; layer 2, hypoechoic; and layer 3, hyperechoic). US-MP, US miniprobe.
Figure 4.
The US-MP image of a C-shaped muscle with 3 sonographic layers (layer 1, hyperechoic; layer 2, hypoechoic; and layer 3, hyperechoic). US-MP, US miniprobe.
Figure 5.
The US-MP image of an oval-shaped muscle with 4 sonographic layers (layer 1, mixed echoic; layers 2 and 3, hypoechoic; and layer 4, hyperechoic). US-MP, US miniprobe.
Group B: concurrent US-MP endoscopy and manometry
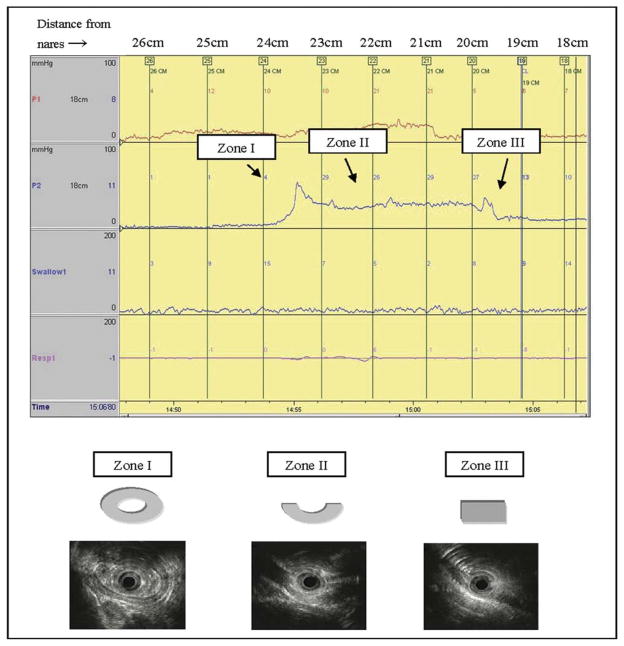
The US-MP cine-loops with corresponding manometric pressure readings were analyzed in this study. Manometrically, the UES-HPZ had a median length of 4.0 cm (range 3.0–4.5 cm). Sonographically, the UES-HPZ was characterized by 3 parameters: muscle configuration, number of muscle layers, and MMMT. These sonographic features were correlated with the manometric configuration of the HPZ. Three zones were identified: zone I (esophago-UES zone) corresponded to a manometrically observed onset of rise of pressure to peak pressure in the distal HPZ (esophagus to UES transition); zone II corresponded to the length of the peak pressure (UES), and zone III corresponded to the onset of a fall from peak pressure to baseline (UES to pharynx transition zone). The C-shaped muscle representing the cricopharyngeus muscle was seen in 6 of 13 participants (46%) in zone III, 12 of 13 participants (92%) in zone II, and 3 of 13 participants (23%) in zone I (Table 1). The oval configuration corresponding to the esophageal contribution to the UES was seen in 10 of 13 participants (77%) in zone I. Representative illustrations of the muscle configurations are shown in Figure 6. The number of muscle layers and the MMMT in each zone are shown in Table 2.
Table 1.
Number of participants in each upper esophageal sphincter high-pressure zone according to endosonographic muscle configuration
| Zone | No. of participants (%) per muscle shape | ||
|---|---|---|---|
| Oval | C | Flat | |
| I | 10 (77) | 3 (23) | 0 |
| II | 1 (8) | 12 (92) | 0 |
| III | 0 | 6 (46) | 7 (54) |
Figure 6.
The cross-sectional muscle shape for each of the 3 manometrically determined zones in the UES. Zone I (esophago-UES), predominantly oval-shaped; zone II (UES), predominantly C-shaped; and zone III (UES-pharyngeal), predominantly flat-shaped. The distance (cm) from the nares to the tip of the manometry catheter is shown at the top of the representative tracing. UES, upper esophageal sphincter.
Table 2.
Endosonographic characteristics of the upper esophageal sphincter high-pressure zone divided into 3 zones
| Zone* | I | II | III |
|---|---|---|---|
| No. of layers (% participants) | 4–5 (77) | 4–5 (8) | 3 (100) |
| 3 (23) | 3 (92) | ||
| MMMT (mm) | 2.9 | 2.9 | 2.4 |
MMMT, Mean maximal muscle thickness.
The number of muscle layers on cross-sectional imaging (percentage of participants with the number of muscle layers) and MMMT were tabulated according to muscle zones. Note that the proximal UES (zones II and III) demonstrates predominantly 3 muscle layers, whereas the distal UES (zone I) demonstrates 4 to 5 muscle layers, similar to the number of layers found at the body of the esophagus.
Zone I (esophago-UES); zone II (UES); and zone III (UES-pharyngeal).
DISCUSSION
Accurate information on the anatomy of the UES-HPZ is fundamental to a better understanding of its function. Unfortunately, the geometric and structural properties of the UES in humans during various functions have been difficult to evaluate. Our study used manometry for attributing anatomical components of the UES seen sonographically and yielded normative data from 18 participants.
Previous studies9–11 have shown that the physiologic length of the UES-HPZ of the UES is greater than the anatomic length as determined sonographically.12 This could be based on a presumption that the lower level of the UES ends at the level of change from C (cricopharyngeus) to oval (esophageal) configuration when evaluated sonographically. If we use this previous definition in our study, the anatomic length of the UES as determined by sonography will be shorter than the manometric length. We therefore propose that the sonographic UES-HPZ also includes the contribution from the oval-shaped upper esophageal muscles. With the use of this criteria, the sonographic length of the UES-HPZ should be the same as the manometric length.
The UES-HPZ encompasses 3 sonographically defined structures: circular muscles that represent the upper esophagus, C-shaped muscles that represent the cricopharyngeus muscle as shown by studies done on cadavers,7 and the flat component from the inferior pharyngeal region. From previous studies,13,14 it is not clear exactly which muscles contribute to the UES and to what degree the cricopharyngeus muscle is involved in the HPZ. Miller et al7 carefully evaluated the UES by using the US-MP (6.2F catheter) and manometry in 7 normal participants and 4 human cadavers and noted a C-shaped muscle at the HPZ that they identified as the cricopharyngeus muscle. Also, a recent study on 30 cadavers showed that upper longitudinal esophageal muscle fibers are anatomically continuous with the pharyngeal muscles, highlighting the importance of the contribution of the upper esophagus to UES function.15
Other studies16,17 have used fluoroscopy to characterize the UES in various disease states. Shapiro et al17 used video fluoroscopy among patients with isolated pharyngeal dysphagia and noted characteristic prominence of the cricopharyngeus muscle. We sought to characterize and define the UES-HPZ of healthy controls by using the US-MP that allows us to obtain high-resolution images of this region with concurrent manometry. As shown in our study, US-MP endoscopy has the advantage of providing reproducible quantitative measurements of the UES-HPZ, unlike fluoroscopy.
There are technical limitations that need to be considered in light of our findings. We were not able to keep the angle between the US-MP and esophageal lumen constant with each participant, thus distorting the image when the probe was not directly parallel to the wall of the UES and esophagus. Rhee et al18 have shown in vitro that off-center position and probe angles of <13 degrees do not affect the accuracy of measuring the cross-sectional area of the lumen. The presence of air artifact in the UES-HPZ was a major factor in explaining why we were not able to obtain high-resolution images in 20% of our participants. However, even in those cases judged to be of suboptimal quality, the C-shaped or flat muscle was still clearly distinguished from circular muscles on US. Another potential source of artifact is the manometry catheter that is attached to the US-MP. However, the water-perfused manometry catheter did not appear to impede high-frequency sound waves from travelling, because no major image interference was observed (Figs. 4 and 5). We also realize that several variables could have affected the measurement of the UES-HPZ in our study, such as participant sex and age, and so we plan to address this issue and potentially control for these variables in future studies.
In summary, transnasal US-MP endoscopy yields distinguishable descriptive analysis and quantitative measurement of different components of the UES-HPZ in healthy participants. Images can be validated by fluoroscopy and manometry, and muscle quantifications can be reproduced. We have shown that previous US definition underestimates the length of the UES-HPZ and that the upper esophagus is a significant contributor to the distal UES-HPZ. Future research should focus on how this method can be used to evaluate disorders of the UES-HPZ.
Take-home Message.
Little data exist on the anatomic properties of the upper esophageal sphincter (UES), and previous US definition underestimated the length of the UES high-pressure zone (UES-HPZ). The authors were able to provide normative data by using concurrent manometry and US miniprobe endoscopy. The UES is a significant contributor to the distal UES-HPZ. These findings can be used to study disorders of the upper esophagus and UES.
Abbreviations
- HPZ
high-pressure zone
- MMMT
mean maximal muscle thickness
- UES
upper esophageal sphincter
- US-MP
high-frequency US miniprobe
Footnotes
DISCLOSURE: Drs Shaker, Dua, and Surapaneni were supported by National Institutes of Health grants 5P01DK068051 and 5R01DK025731. All other authors disclosed no other financial relationships relevant to this publication.
References
- 1.Liu JB, Miller LS, Goldberg BB, et al. Transnasal US of the esophagus: preliminary morphologic and function studies. Radiology. 1992;184:721–7. doi: 10.1148/radiology.184.3.1509056. [DOI] [PubMed] [Google Scholar]
- 2.Shaker R, Ren J, Xie P, et al. Characterization of the pharyngo-UES contractile reflex in humans. Am J Physiol. 1997;273:G854–8. doi: 10.1152/ajpgi.1997.273.4.G854. [DOI] [PubMed] [Google Scholar]
- 3.Mittal RK, Liu J, Puckett JL, et al. Sensory and motor function of the esophagus: lessons from ultrasound imaging. Gastroenterology. 2005;128:487–97. doi: 10.1053/j.gastro.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Chak A, Soweid A, Hoffman B, et al. Clinical implications of catheter probe-assisted endoluminal ultrasonography. Endoscopy. 1998;30:A169–72. doi: 10.1055/s-2007-1001509. [DOI] [PubMed] [Google Scholar]
- 5.Menzel J, Domschke W. Gastrointestinal miniprobe sonography: the current status. Am J Gastroenterol. 2000;95:605–16. doi: 10.1111/j.1572-0241.2000.01832.x. [DOI] [PubMed] [Google Scholar]
- 6.Miller LS, Liu J, Klenn PJ, et al. High-frequency endoluminal ultrasonography of the esophagus in human autopsy specimens. J Ultrasound Med. 1993;12:563–6. doi: 10.7863/jum.1993.12.10.563. [DOI] [PubMed] [Google Scholar]
- 7.Miller LS, Dai Q, Sweitzer BA, et al. Evaluation of the upper esophageal sphincter (UES) using simultaneous high-resolution endoluminal sonography (HRES) and manometry. Dig Dis Sci. 2004;49:703–9. doi: 10.1023/b:ddas.0000030077.15625.69. [DOI] [PubMed] [Google Scholar]
- 8.Shaker R, Saeian K. Unsedated transnasal laryngo-esophagogastroduo-denoscopy: an alternative to conventional endoscopy. Am J Med. 2001;111:153–6. doi: 10.1016/s0002-9343(01)00852-x. [DOI] [PubMed] [Google Scholar]
- 9.Palmer ED. Disorders of the cricopharyngeus muscle: a review. Gastroenterology. 1976;71:510–9. [PubMed] [Google Scholar]
- 10.Goyal RK. Disorders of the cricopharyngeus muscle. Otolaryngol Clin North Am. 1984;17:115–30. [PubMed] [Google Scholar]
- 11.Kahrilas PJ, Dodds WJ, Dent J, et al. Upper esophageal sphincter function during deglutition. Gastroenterology. 1988;95:52–62. doi: 10.1016/0016-5085(88)90290-9. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro J, Goyal RK. Disorders of the upper esophageal sphincter. In: Fried MP, editor. The larynx: a multidisciplinary approach. Boston: Little Brown and Company; 1988. pp. 293–317. [Google Scholar]
- 13.Goyal RK, Martin SB, Shapiro J, et al. The role of cricopharyngeus muscle in pharyngoesophageal disorders. Dysphagia. 1993;8:252–8. doi: 10.1007/BF01354547. [DOI] [PubMed] [Google Scholar]
- 14.Mu L, Sanders I. Neuromuscular compartments and fiber-type regionalization in the human inferior pharyngeal constrictor muscle. Anat Rec. 2001;264:367–77. doi: 10.1002/ar.10020. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Xu S, Tu L, et al. Anatomic continuity of longitudinal pharyngeal and esophageal muscles. Laryngoscope. 2007;117:282–7. doi: 10.1097/01.mlg.0000249935.81808.df. [DOI] [PubMed] [Google Scholar]
- 16.Kahrilas PJ, Lin S, Rademaker AW, et al. Impaired deglutitive airway protection: a videofluoroscopic analysis of severity and mechanism. Gastroenterology. 1997;113:1457–64. doi: 10.1053/gast.1997.v113.pm9352847. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro J, Martin S, DeGirolami U, et al. Inflammatory myopathy causing pharyngeal dysphagia: a new entity. Ann Otol Rhinol Laryngol. 1996;105:331–5. doi: 10.1177/000348949610500501. [DOI] [PubMed] [Google Scholar]
- 18.Rhee PL, Liu J, Puckett JL, et al. Measuring esophageal distension by high-frequency intraluminal ultrasound probe. Am J Physiol Gastrointest Liver Physiol. 2002;283:G886–92. doi: 10.1152/ajpgi.00107.2002. [DOI] [PubMed] [Google Scholar]