Abstract
Cortico‐striato‐thalamo‐cortical (CSTC) loops project from the cortex to the striatum, then from the striatum to the thalamus via the globus pallidus, and finally from the thalamus back to the cortex again. These loops have been implicated in Obsessive‐Compulsive Disorder (OCD) with particular focus on the limbic CSTC loop, which encompasses the orbitofrontal and anterior cingulate cortices, as well as the ventral striatum. Resting state functional‐connectivity MRI (rs‐fcMRI) studies, which examine temporal correlations in neural activity across brain regions at rest, have examined CSTC loop connectivity in patients with OCD and suggest hyperconnectivity within these loops in medicated adults with OCD. We used rs‐fcMRI to examine functional connectivity within CSTC loops in unmedicated adults with OCD (n = 23) versus healthy controls (HCs) (n = 20). Contrary to prior rs‐fcMRI studies in OCD patients on medications that report hyperconnectivity in the limbic CSTC loop, we found that compared with HCs, unmedicated OCD participants had reduced connectivity within the limbic CSTC loop. Exploratory analyses revealed that reduced connectivity within the limbic CSTC loop correlated with OCD symptom severity in the OCD group. Our finding of limbic loop hypoconnectivity in unmedicted OCD patients highlights the potential confounding effects of antidepressants on connectivity measures and the value of future examinations of the effects of pharmacological and/or behavioral treatments on limbic CSTC loop connectivity. Hum Brain Mapp 35:2852–2860, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: functional connectivity, obsessive‐compulsive disorder, striatum, orbitofrontal cortex
INTRODUCTION
Obsessive‐compulsive disorder (OCD) has a lifetime prevalence of 2%–3% [Robins et al., 1984; Kessler et al., 2005], is characterized by intrusive and repetitive thoughts or behaviors and is associated with significant occupational and social impairment [Koran et al., 1996; Huppert et al., 2009]. Animal and human studies suggest that OCD symptoms might be due in part to dysfunction in cortico‐striato‐thalamo‐cortical (CSTC) loops [Graybiel and Rauch, 2000; Maia et al., 2008]. Neuroimaging studies of OCD have focused largely on the function, size/shape, and metabolic content of the brain regions that comprise the CSTC loops [Atmaca et al., 2007a, 2007b; Baxter et al., 1992; Maia et al., 2008; Rosenberg et al., 2004]; less is known about the connectivity between these brain regions. Given that the CSTC loops function as integrated neural circuits, assessment of abnormalities in the connectivity between the brain regions that comprise CSTC loops is necessary.
The CSTC loops project from the cortex to the striatum, then from the striatum to the thalamus via the globus pallidus, and finally from the thalamus back to the cortex [Alexander and Crutcher, 1990; Alexander et al., 1986; Maia et al., 2008]. There are several CSTC loops that involve different cortical regions and run in parallel through the basal ganglia and thalamus. Each of these loops is thought to be involved in a different neurocognitive domain. Although the precise number of parallel loops is subject to debate [Middleton and Strick, 2001], a common subdivision is into three main loops: sensorimotor, associative (or cognitive), and limbic (or affective/motivational), involving the sensorimotor cortices, dorsolateral prefrontal cortex, and orbitofrontal and anterior cingulate cortices (OFC and ACC), respectively [Di Martino et al., 2008; Lehéricy et al., 2004; Yin and Knowlton, 2006]. Multiple lines of evidence, including studies of non‐human primates, suggest that OCD involves functional and anatomical abnormalities in the limbic CSTC loop, including the OFC, ACC, and ventral striatrum (a portion of the striatum that receives projections from the OFC and ACC) [Alexander et al., 1986; Haber, 2003]. These structures are hyperactive at rest in patients with OCD relative to healthy controls (HCs); this hyperactivity increases with symptom provocation and decreases following successful treatment [Maia et al., 2008; Saxena and Rauch, 2000]. Volumetric and gray‐matter‐density abnormalities have also been reported in these structures in patients with OCD [Maia et al., 2008; Radua et al., 2010].
Resting state functional‐connectivity MRI (rs‐fcMRI) studies, which examine temporal correlations in neural activity across brain regions at rest [Fox and Raichle, 2007; Posner et al., in press], have examined CSTC loop connectivity in adults and children with OCD [Fitzgerald et al., 2011; Harrison et al., 2009, 2012]. These studies indicate that OCD is associated with increased functional connectivity in several CSTC loops including connectivity between the ventral striatum (which encompasses the nucleus accumbens) and the OFC [Harrison et al., 2009, 2012], and between the dorsal striatrum and the ventral medial frontal cortex [Fitzgerald et al., 2011]. Although the striatal regions implicated across these studies differ, the finding of corticostriatal hyperconnectivity in OCD is consistent with the aforementioned evidence implicating abnormalities in CSTC loops in OCD, and in particular the limbic loop, involving the OFC, ACC, and ventral striatum.
Unlike adults, children with OCD demonstrate reduced connectivity in the CSTC loops with hypoconnectivity noted between the dorsal caudate and the rostral ACC, and between the thalamus and the dorsal ACC [Fitzgerald et al., 2011]. The divergent finding of CSTC loop hypo‐ vs. hyper‐connectivity in children vs. adults with OCD, respectively, suggests at least two potential hypotheses: First, the pathophysiology of OCD, at least in terms of CSTC loop connectivity, may be developmentally specific with different patterns of altered connectivity in children vs. adults with OCD. Alternatively, medication exposure may have an important confounding effect. That is, whereas studies of CSTC loop connectivity in adults with OCD have relied on samples in which most of the participants were medicated at the time of MRI scanning [Fitzgerald et al., 2011; Harrison et al., 2009, 2012], children with OCD who were studied were largely unmedicated [Fitzgerald et al., 2011]. Given that antidepressants—the most common medication in the treatment of OCD—can alter functional connectivity [Posner et al., 2013], it is important to examine CSTC loop connectivity without the potentially confounding influence of medication.
To examine this question, we used rs‐fcMRI to examine functional connectivity within CSTC loops in medication‐free adults with OCD versus HCs. We hypothesized that relative to HCs, unmedicated adults with OCD would demonstrate altered CSTC connectivity, particularly in the limbic loop, as previously described in medicated adults with OCD [Fitzgerald et al., 2011; Harrison et al., 2009, 2012]. We also explored group differences in connectivity within the sensorimotor or cognitive CTSC loops, and associations of CSTC connectivity with symptom severity in the OCD group.
METHODS
The Institutional Review Board of the New York State Psychiatric Institute (NYSPI) approved the study procedures. Participants provided written, informed consent.
Participants
Twenty‐three adults with OCD and 20 healthy adults, aged 19 to 56, were recruited. Participants were free of significant medical problems and current or past neurological disorders. No participants, including those with OCD, were taking psychotropic medications at the time of the MRI scan. In the OCD group, n = 22 participants were medication naïve; n = 11 had prior exposure to psychotropic medication. For OCD participants with prior medication exposure, the mean duration off medication before the MRI scan was 94 weeks (SD = 64; range: 30–182 weeks).
OCD participants fulfilled DSM‐IV criteria for OCD for at least 1 year. HCs had no current or past DSM‐IV Axis I disorder. HCs were group‐matched to the OCD patients by age, gender, socioeconomic status (SES), and IQ (Table 1).
Table 1.
Demographic and clinical characteristics of study participants
| OCD patients (n = 23) | Healthy controls (n = 20) | Test statistic | p value | |
|---|---|---|---|---|
| Age (years) | 30.9 (8.8) | 32.6 (10.0) | t(41) = 0.60 | 0.6 |
| Sex, M:F | 11:12 | 11:9 | Χ2(1) = 0.22 | 0.7 |
| Ethnicity | 16 Cau/4 His/3 AA | 14 Cau/3 His/2 AA/1 Un | Χ2(3) = 1.27 | 0.7 |
| FS‐IQ | 107.1 (27.3) | 118.2 (8.6) | t(41) = 1.9 | 0.1 |
| Age of OCD onset, years | 16.1 (7.5) | |||
| History of treatment with psychotropic | 11a | |||
| SRI | 9 | |||
| SNRI | 1 | |||
| Duration off psychotropic medication, before MRI Scan (in weeks) | 94 (64) | |||
| DSM‐IV Axis I comorbidity | ||||
| No current or past comorbidities | 17 | |||
| Specific phobia | 1 | |||
| Specific phobia, alcohol abuse, generalized anxiety disorder, and history of MDD | 1 | |||
| Binge eating disorder, social phobia, and history of MDD | 1 | |||
| History of MDD | 3 | |||
| Y‐BOCS | 25.9 (4.2) | |||
| HAM‐D | 4.6 (3.5) |
Note:AA, African American; Cau, Caucasian; His, Hispanic; OCD, Obsessive‐Compulsive Disorder; FS‐IQ, Full Scale Intelligence Quotient; MDD, Major Depressive Disorder; GAD, Generalized Anxiety Disorder; HAM‐D, Hamilton Depression Scale; Y‐BOCS, Yale‐Brown Obsessive Compulsive Scale; SRI, Serotonin reuptake inhibitor, SNRI, Serotonin norepinephrine reuptake inhibitor; Un, Unspecified. Values are mean (SD) unless specified.
One participant could not recall the specific psychotropic taken.
b
Diagnoses were made by clinical interview with a psychologist or psychiatrist and confirmed by a trained rater with the Structured Clinical Interview for DSM‐IV [Spitzer et al., 1995]. On the day of the MRI scan, OCD and depressive symptoms were assessed by a trained rater using the Yale–Brown Obsessive Compulsive Scale (Y‐BOCS) [Goodman et al., 1989a, 1989b] and the Hamilton Rating Scale for Depression (HAM‐D, 17‐item) [Hamilton, 1960], respectively.
Imaging Procedures
MRI pulse sequences
Images were acquired at the New York State Psychiatric Institute on a GE Signa 3 T whole‐body scanner. Acquisition of T1‐weighted sagittal localizing images was followed by a 3D spoiled gradient recall (SPGR) image for coregistration with axial echoplanar images. Axial echoplanar images (TR = 2,200 msec, TE = 25 msec, 90° flip angle, single excitation per image, slice thickness 3.5 mm, 24 × 24 cm field of view, 64 × 64 matrix, no skip) were obtained to provide an effective resolution of 3.75 × 3.75 × 3.5 mm and whole‐brain coverage. For resting‐state image acquisition, participants were instructed to stay still, close their eyes, and let their minds wander freely. Two 5‐min resting‐state scans were obtained for each participant.
Statistical Analysis
Image preprocessing
We conducted standard image preprocessing using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) and the conn_toolbox (http://www.nitrc.org/projects/conn) for functional connectivity analysis. The functional images were motion‐ and slice‐time‐corrected. The images were then coregistered with a high‐resolution anatomical scan, normalized into the Montreal Neurological Institute (MNI) space, and resampled at 2 mm3. Finally, the images were smoothed with a Gaussian kernel of 6 mm3 FWHM [Friston et al., 1995]. Connectivity preprocessing procedures were implemented to reduce the influence of fMRI signal unrelated to neural activity and followed a previously described component‐based noise‐reduction approach [Behzadi et al., 2007; Chai et al., 2011]. This approach curtails the influence of potential confounds such as head motion, peripheral physiology, and other imaging artifacts (Supporting Information S1). Quantitative measurement of head motion did not differ by group, was minimal in both cases, and did not correlate with OCD symptom severity (our method address head motion Supporting Information S1). Because image acquisition of the cerebellum was incomplete, we excluded it from our analyses.
Seed‐based connectivity
Following preprocessing, we correlated the resting‐state BOLD time series voxel‐by‐voxel for each participant across the full length of the resting‐time series. Fisher z transformation was applied. We then generated connectivity maps, using as seeds six bilateral regions of interest (ROIs) in the striatum, for a total of 12 seeds. Specifically, we created spherical masks (radius = 4 mm) for each ROI, centered on the stereotactic coordinates published by Di Martino et al. [2008] (Supporting Information Table I), and we averaged the fMRI signal across all voxels in each sphere. These 12 striatal seeds were chosen because they have previously been shown to generate connectivity maps of the CSTC loops consistent with the canonical anatomical descriptions of these loops by Alexander et al. [1986], Di Martino et al. [2008]. A detailed description of the anatomy of the ROIs and their visual presentation is provided in the Supporting Information S2 and Figure S1.
Hypothesis Testing
Limbic loop
We generated seed‐based connectivity maps for each of the striatal ROIs for each participant. We first examined the connectivity maps to confirm that they were anatomically consistent with prior findings from the HC sample studied by Di Martino et al. [2008]. Next, to test our a priori hypothesis that limbic CSTC loop connectivity (based on seed regions in the inferior and superior VS striatum) would differ between OCD patients and HCs, we entered the seed‐based connectivity maps into second‐level, random‐effects factorial models with Group as the single factor with two levels (OCD and HCs). Given our hypothesis regarding CSTC loop connectivity and following standard imaging procedures [Guyer et al., 2008, Posner et al., 2013], we anatomically restricted these group comparisons to the limbic CSTC loop by masking the second‐level analyses with the mean connectivity map (based on the entire study sample) generated from the respective seed region.
Corrections for multiple statistical comparisons used the following criteria For any cluster to be considered statistically significant, the cluster had to contain at least 25 neighboring voxels, with each voxel in the cluster meeting an alpha of < 0.001. Using Monte Carlo simulations conducted with AlphaSim [Ward, 2000], we calculated that this dual requirement (i.e., cluster size ≥ 25 voxels and alpha ≤ 0.001) yields a corrected alpha of < 0.05. This correction includes the statistical tests necessary to examine the ROIs we used in our seed‐based connectivity analyses. The combined application of a voxel level statistical threshold and cluster filter minimizes the false‐positive identification of regions at any given threshold [Forman et al., 1995] because clustering can distinguish between true connectivity between regions, and noise that has less tendency to cluster.
Exploratory Analyses
Cognitive and sensorimotor loops
Following the same methods that we used for our a priori hypothesis testing, we examine connectivity within the cognitive loop (based on seeds in the dorsal caudate and ventral rostral putamen) and sensorimotor loop (based on seeds in the dorsal caudal putamen and the dorsal rostral putamen). This seed based approach to identifying these loops has been demonstrated elsewhere [Di Martino et al., 2008].
Symptom severity
We examined associations of CSTC loop connectivity with symptom severity in the OCD participants. We limited this analysis to the connections found to be atypical in OCD in our a priori hypothesis testing. We used the same striatal seed regions that we used in our a prior hypothesis testing and calculated Pearson's correlations between OCD symptom severity (as determined by the Y‐BOCS summary score) and functional connection strength.
RESULTS
Hypothesis Testing
Limbic loop
In both OCD and HC subjects, the seed‐based connectivity maps generated from the inferior and superior VS showed connectivity with regions within the limbic CSTC loop including the medial OFC (BA 11), subgenual cingulate (BA 25), ventral ACC (BA 24/32), and the parahippocampal gyrus (Fig. 1 and Supporting Information Fig. S2a,b). In the OCD compared with the HC participants, there was significantly reduced connectivity of the left inferior VS with the left ACC (BA24/32, Fig. 1; Table 2). Reduced connectivity with the left medial OFC (BA 11) was detected at a slightly higher statistical threshold (P = 0.002). Compared with HCs, OCD participants also showed significantly reduced connectivity between the left superior VS and the body of the left caudate nucleus (Supporting Information Fig. S2b; Table 2). We detected no statistically significant group differences when using the right inferior or right superior VS as the seed region.
Figure 1.
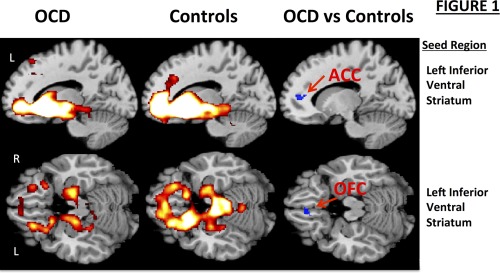
Resting‐state functional connectivity maps with the seed in the left inferior ventral striatum (VS). Compared with HCs (n = 20), OCD participants (n = 23) demonstrated significantly reduced connectivity of the left inferior VS with the left anterior cingulate cortex (ACC; x, y, z = −8, 36, 12; cluster size = 42 voxels; P cor < 0.05). Reduced connectivity with the left medial orbitofrontal cortex (OFC; x, y, z = −6, 34, −18; cluster size = 40 voxels) was also detected, though this finding did not meet statistical thresholding after correcting for multiple comparisons (P unc = 0.002). Sagittal (top row) and axial (bottom row) images are presented. L, left hemisphere; R, right hemisphere.
Table 2.
Regions demonstrating group differences in functional connectivity based on striatal seeds
| MNI coordinates | Cluster size | |||||||
|---|---|---|---|---|---|---|---|---|
| Seed region | x | y | z | Peak t | (voxels) | Brodmann area | Hemisphere | |
| Left inferior ventral striatum | Controls > OCD | |||||||
| Anterior cingulate cortex | −8 | 36 | 12 | 3.7 | 42 | 24/32 | L | |
| Orbitofrontal cortex | −6 | 34 | −18 | 3.7 | 40 | 11 | L | |
| OCD > Controls | ||||||||
| None | ||||||||
| Left superior ventral striatum | Controls > OCD | |||||||
| Caudate, body | −14 | 16 | 8 | 4.5 | 39 | N/A | L | |
| OCD > Controls | ||||||||
| None | ||||||||
| Right dorsal caudate | Controls > OCD | |||||||
| None | ||||||||
| OCD > Controls | ||||||||
| Anterior prefrontal cortex | −18 | 66 | 18 | 4.6 | 138 | 10 | L | |
| Inferior parietal lobule | 52 | −60 | 38 | 4.4 | 176 | 39 | R | |
| Left dorsal caudal putamen | Controls > OCD | |||||||
| Supplemental motor area | −56 | 4 | 12 | 3.9 | 35 | 6 | L | |
| OCD > Controls | ||||||||
| None | ||||||||
L, left; R, right; N/A, not applicable; MNI, Montreal Neurological Institute; One voxel = 2 cubic mm.
Exploratory Analyses
Cognitive CSTC loop
In both OCD and HC subjects, the seed maps generated from the dorsal caudate and ventral rostral putamen showed connectivity with regions within the cognitive CSTC loop including the dorsolateral and anterior prefrontal cortices (BA 47/10), frontal eye fields (BA 8), the inferior parietal lobule (BA 3), supramarginal gyrus (BA 40), and dorsal ACC (BA 24) (Supporting Information Figs. S3a and S3b). Compared with HCs, OCD subjects demonstrated increased connectivity of the right dorsal caudate with the anterior prefrontal cortex (BA 10) and with the inferior parietal lobule (BA 39) (Supporting Information Fig. S3a; Table II). No significant between‐group differences were detected with seeds in the left dorsal caudate or the ventral rostral putamen bilaterally.
Sensorimotor CSTC loop
In both OCD and HC subjects, seed maps generated from the dorsal caudal putamen and the dorsal rostral putamen showed connectivity with regions within the sensorimotor CSTC loop including the supplementary motor area (BA 6), primary motor cortex (BA 4), and mid‐cingulate motor areas (BA 24) (Supporting Information Figs. S4a and S4b). Compared with HCs, participants with OCD demonstrated reduced connectivity between the left dorsal caudal putamen and left supplementary motor area (BA 6) (Supporting Information Fig. S4a; Table II). No significant between‐group differences were detected with seeds in either the left or right dorsal rostral putamen or the right dorsal caudal putamen.
Correlations with Symptom Severity
Within the limbic loop, the connection strength between the left inferior VS and the left ACC (BA 32) correlated inversely with symptom severity (r = −0.51; P = 0.007). There was also a trend for an inverse association of OCD symptom severity with the connection strength between the left inferior VS and left medial OFC (r = −0.33; P = 0.06).
To further explore associations of OCD symptom severity with functional connectivity between (a) the left inferior VS and the left ACC (BA 32) and between (b) the left inferior VS and left medial OFC (BA11), we replaced the left inferior VS seed with seeds from the left ACC (BA 32) and left medial OFC (BA 11). Our goal was to test whether the correlations with symptom severity that we detected using the inferior VS seed could be replicated using ACC and OFC seeds, thus minimizing the possibility that the detected brain‐behavior correlations were dependent on seed selection. (Methods for this exploratory analysis are presented in the Supporting Information S3.) Using the ACC seed, the correlation of ACC/inferior VS connection strength with symptom severity was not significant (r = 0.23, P = 0.1). Using the medial OFC seed, we detected an inverse correlation of the connection strength between the left OFC and a large cluster (300 voxels) spanning the VS bilaterally with symptom severity (x, y, z = 8, 0, −8; r = −0.70; P ≤ 0.001; Fig. 2). Symptoms of depression, generalized anxiety, and head motion did not correlate with altered limbic loop connectivity, nor did we detect associations with altered connecitivity in the cognitive and sensorimotor loops.
Figure 2.
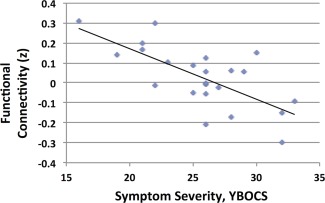
Connection strength between the left medial orbitofrontal cortex and the ventral striatum (x, y, z = 8, 0, −8; cluster size = 300 voxels) was significantly correlated with symptom severity (r = −0.70; P ≤ 0.001) in the OCD participants as determined by the Yale‐Brown Obsessive Compulsive Scale (Y‐BOCS) summary score. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Sensitivity analyses
First, to rule out the possibility that our findings were the result of unknown imaging artifacts, we conducted a sensitivity analysis by analyzing connectivity maps derived from a seed region in the primary visual cortex. We created this seed by averaging the fMRI signal across all voxels in visual cortex (BA17). We found no group differences in this sensitivity analysis (Supporting Information S4 and Fig. S5). Second, because of its proximity to the frontal and maxillary sinuses, the OFC can incur signal dropout during echoplanar imaging. Our tests revealed no significant group differences in the OFC's mean fMRI signal intensity; we therefore concluded that susceptibility artifacts were unlikely to confound the study results (Supporting Information S5). Third, some patients (n = 6) with OCD had current and/or prior comorbid Axis I disorders (Table 1). Excluding these subjects from our analyses of our a priori hypothesis did not meaningfully impact our findings. Fourth, even though all patients had been free from psychotropic medications for at least 30 weeks at the time of scanning, some patients (n = 11) had prior exposure to these medications. Covarying for prior exposure to psychotropic medications also did not meaningfully impact our findings.
DISCUSSION
We used rs‐fcMRI to examine functional connectivity within CSTC loops in unmedicated OCD compared with HC participants. Consistent with our hypothesis, connectivity within the limbic CSTC loop was altered in OCD. Compared with HCs, unmedicated OCD participants had reduced connectivity within this loop. Moreover, decreased connectivity within the limbic loop was associated with more severe OCD symptoms in the OCD group. We also found that unmedicated OCD participants had reduced connectivity within the sensorimotor and increased connectivity within the cognitive CSTC loops.
Consistent with prior findings of both functional [Saxena and Rauch, 2000] and structural [Atmaca et al., 2007a, 2007b] abnormalities within regions comprising the limbic CSTC loop in OCD, we detected reduced functional connectivity within this loop, specifically between the VS and the ACC (at our a priori threshold of cluster size ≥ 25 voxels and alpha ≤ 0.001), with a trend (P = 0.002) toward reduced connectivity between the VS and the medial OFC. Although prior rs‐fcMRI studies of patients with OCD have reported hyperconnectivity in the limbic CSTC loop [Harrison et al., 2009, 2012], we found that connectivity within this loop was reduced. A potential explanation for this discrepancy in findings is that our sample, unlike those from all but one prior rs‐fcMRI study of OCD [Beucke et al., in press], was unmedicated at the time of scanning. The only study that has examined rs‐fcMRI in an unmedicated sample compared medicated and unmedicated OCD participants to HCs (Beucke et al., in press). The study reported that unmedicated OCD participants had increased distant OFC connectivity (distant connectivity is a graph theory metric defined as the degree of connectivity of the OFC to all other brain regions > 12 mm distal to the OFC). Although we also examined rs‐fcMRI in unmedicated OCD participants, our findings differed (i.e., we found reduced connectivity between the OFC and VS in OCD.) It is important to note that there were critical differences in the methods (i.e., graph theory versus seed based) as well as the study samples. In our sample, 17 of 23 OCD participants had no comorbid disorder, whereas only 5 of 23 OCD participants in the Beucke et al. study were free of comorbid disorders. Likewise, 11 of 23 OCD participants in our study were medication naïve and the remaining were off medication for on average 94 weeks (SD = 64), whereas the unmedicated patients in the Beucke et al. study were off of medications “for at least 6 weeks.” This raises important questions not only about the best methods for establishing functional connectivity but also about the impact of comorbid conditions and recent medication exposure on connectivity findings.
We have previously demonstrated that pharmacotherapy with antidepressants can reverse baseline abnormalities in functional connectivity in the default mode network [Posner et al., 2013], and another study demonstrated that antidepressants can increase connectivity in the limbic CSTC loop [Heller et al., 2013]. Moreover, previous findings indicate that relative to age‐matched controls, children with OCD have corticostriatal hypoconnectivity [Fitzgerald et al., 2011]. This pediatric sample consisted of mostly unmedicated patients and is thus consistent with our finding of corticostriatal hypoconnectivity in unmedicated adults with OCD. A follow‐up, prospective study could directly test whether pharmacological interventions in OCD alter limbic CSTC loop connectivity, and more importantly, examine whether this change in connectivity affects the course of the illness.
Hypoconnectivity within the limbic CSTC loop was associated with OCD symptom severity, albeit the strength of relationship varied with seed selection. Specifically, reduced connectivity between the VS and ACC was associated with more severe OCD symptoms, but only when we used the VS as the seed region. Moreover, reduced connectivity between the VS and medial OFC was also associated with more severe OCD symptoms, although this association was stronger when we used left medial OFC rather than the VS as the seed region. Other studies have also found that the selection of seed regions can influence the findings from rs‐fcMRI [Fox and Greicius, 2010].
In addition to reduced connectivity within the limbic CSTC loop, participants with OCD had hypoconnectivity in the sensorimotor CSTC loop (i.e., between the left dorsal caudal putamen and the left supplementary motor area). A previous rs‐fcMRI study of medicated OCD subjects reported reduced connectivity in the sensorimotor CSTC loop in OCD subjects (i.e., between the dorsal putamen and the inferior prefrontal cortex, a region that extends into supplementary motor area), suggesting that medications may not alter the connectivity of this loop [Harrison et al., 2009]. Altered connectivity in sensorimotor loops is consistent with findings from behavioral studies suggesting that OCD is associated with abnormal sensorimotor gating, evidenced by deficits in prepulse inhibition [Ahmari et al., 2012; Swerdlow et al., 1993]. Abnormal functioning of the sensorimotor CSTC loop may, in part, contribute to the repetitive behaviors associated with OCD and indeed, repetitive transcranial magnetic stimulation to this loop (particularly to the supplementary motor area) has been shown to reduce compulsive behaviors [Mantovani et al., 2006].
In contrast to the hypoconnectivity detected in the limbic and sensorimotor CSTC loops, we detected hyperconnectivity within the cognitive CSTC loop (i.e., between the right dorsal caudate and the inferior parietal lobule), as well as hyperconnectivity between the dorsal caudate and a limbic‐associated region, the anterior prefrontal cortex. Analogous findings were previously reported in a sample of 60 participants with OCD, ranging from 8 to 50 years of age [Fitzgerald et al., 2011], with most of the participants on medication and using the same stereotactic coordinates for the right dorsal caudal seed region that we used in this study. Together, these findings suggest that antidepressants may not contribute to altered connectivity within the sensorimotor and cognitive CSTC loops, and may have more specific effects on connectivity within the limbic loop. We further speculate that hyperconnectivity between the cognitive CSTC loop and a limbic‐associated region (i.e., the anterior prefrontal cortex) may compensate for, and potentially suppress, maladaptive affective cues arising from the limbic CSTC loop. This interpretation is consistent with findings from non‐human primate research suggesting that cues from the limbic CSTC loop are propagated along the ventromedial‐dorsolateral axis from limbic to cognitive CSTC loops [Haber, 2003].
Several study limitations are worth noting. First, some OCD patients had comordid Axis I psychiatric disorders, including a history of major depressive disorder (n= 5, Table 1)—a disorder previously found to involve altered functional connectivity within limbic CSTC loops [Frodl et al., 2010; Seminowicz et al., 2004]. However, removing the OCD participants with current or prior comorbidities did not affect the findings from our hypothesis testing. Second, functional connectivity indirectly assesses neural connections by demonstrating a temporal coherence between two brain regions, but functional connections do not necessarily indicate that two brain regions are anatomically connected. Investigators have tried to bridge this divide by combining functional connectivity with tractography from diffusion imaging [Greicius et al., 2009; Herting et al., 2011], thereby assessing both functional and structural connectivity. A follow‐up study using diffusion imaging could determine whether altered structural connectivity is present in unmedicated patients with OCD within the same CSTC loops in which we found altered functional connectivity. Third, seed‐based rs‐fcMRI studies are potentially influenced by seed selection [Fox and Greicius, 2010]. We attempted to minimize any potential bias in seed selection by using previously defined striatal regions [Di Martino et al., 2008]. Follow‐up studies could further limit the impact of seed selection by combining task‐based fMRI and rs‐fcMRI. For example, rather than anatomically defining the VS based on a priori stereotactic coordinates (as we did in this study), future research could define this region based on findings from a reward‐processing fMRI task, such as the Monetary Incentive Delay task [Knutson et al., 2000], that reliably engages the VS in healthy individuals. Task‐related activations could then be used to define, on a subject‐by‐subject basis, the anatomy of the VS. Fourth, 11 of 23 (48%) participants in our OCD sample had prior exposure to psychotropics (Table 1). Our sensitivity analyses demonstrated that covarying for prior medication exposure did not have a substantial effect on our findings. Nevertheless, full elimination of the potentially confounding effects of prior medication would require inclusion of a 100% medication naïve sample. Finally, although ours is the largest unmedicated sample of OCD participants to be studied with rs‐fcMRI to date, our findings warrant replication in a larger sample.
CONCLUSION
We demonstrated that relative to HCs, unmedicated participants with OCD have altered functional connectivity in specific CSTC loops, and reduced connectivity in the limbic CSTC loops was associated with more severe OCD symptoms. Future studies might examine the effects of pharmacological or behavioral treatments on limbic CSTC loop connectivity to explore whether baseline connectivity within this loop can predict treatment response and whether successful treatment leads to increased connectivity. If so, enhancing connectivity in the limbic CSTC loops could be a potential new treatment target for OCD.
ACKNOWLEDGMENTS
Dr. Posner is a principal investigator on an investigator‐initiated grant from Shire Pharmaceuticals. Dr. Simpson has served on a Scientific Advisory Board for Pfizer (for Lyrica, 2009–2010) and Jazz Pharmaceuticals (for Luvox CR, 2007–2008), consulted for Quintiles, Inc. (on therapeutic needs for OCD, 2012), and receives royalties from Cambridge University Press and UpToDate, Inc.
Supporting information
Supporting Information
REFERENCES
- Ahmari SE, Risbrough VB, Geyer MA, Simpson HB (2012): Impaired sensorimotor gating in unmedicated adults with obsessive–compulsive disorder. Neuropsychopharmacology 37:1216‐1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD (1990): Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends Neurosci 13:266‐271. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL (1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357‐381. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Yildirim H, Ozdemir H, Tezcan E, Kursad Poyraz A (2007a): Volumetric MRI study of key brain regions implicated in obsessive‐compulsive disorder. Progr Neuro‐Psychopharmacol Biol Psychiatr 31:46‐52. [DOI] [PubMed] [Google Scholar]
- Atmaca M, |Yildirim H, Ozdemir H, Tezcan E, Kursad Poyraz A (2007b): Volumetric MRI study of key brain regions implicated in obsessive–compulsive disorder. Progr Neuro‐Psychopharmacol Biol Psychiatr 31:46‐52. [DOI] [PubMed] [Google Scholar]
- Baxter LR Jr, Schwartz JM, Bergman KS, Szuba MP, Guze BH, Mazziotta JC, Alazraki A, Selin CE, Ferng HK, Munford P (1992): Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive‐compulsive disorder. Arch Gen Psychiatr 49:681. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT (2007): A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37:90‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beucke JC, Sepulcre J, Talukdar T, Linnman C, Zschenderlein K, Endrass T, Kaufmann C, Kathmann N (2013): Abnormally high degree connectivity of the orbitofrontal cortex in obsessive‐compulsive disorder. 70:619‐629. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield‐Gabrieli S (2011): Anticorrelations in resting state networks without global signal regression. Neuroimage 59:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies D, Kelly A, Uddin L, Shehzad Z, Biswal B, Walters J, Castellanos F, Milham M (2008): Functional connectivity of human striatum: A resting state FMRI study. Cereb Cortex 18:2735. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Stern ER, Angstadt M, Hanna GL, Abelson JL, Taylor SF (2011): Developmental alterations of frontal‐striatal‐thalamic connectivity in obsessive‐compulsive disorder. J Am Acad Child Adolesc Psychiatr 50:938‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33:636‐647. [DOI] [PubMed] [Google Scholar]
- Fox MD, Greicius M (2010): Clinical applications of resting state functional connectivity. Front Syst Neurosci 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700‐711. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner CD, Frith JB, Poline JB, Heather RS, Frackowiak RS (1995): Spatial registration and normalization of images. Hum Brain Mapp 3:165‐189. [Google Scholar]
- Frodl T, Bokde AL, Scheuerecker J, Lisiecka D, Schoepf V, Hampel H, Möller H‐J, Brückmann H, Wiesmann M, Meisenzahl E (2010): Functional connectivity bias of the orbitofrontal cortex in drug‐free patients with major depression. Biol Psychiatr 67:161‐167. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, Charney DS (1989a): The yale‐brown obsessive compulsive scale: II. Validity. Arch Gen Psychiatr 46:1012. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS (1989b): The yale‐brown obsessive compulsive scale: I. Development, use, and reliability. Arch Gen Psychiatr 46:1006. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL (2000): Toward a neurobiology review of obsessive compulsive disorder. Neuron 28:343. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF (2009): Resting‐state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure‐Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Chen G, Blair R, Leibenluft E, Fox NA (2008): Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatr 65:1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN (2003): The primate basal ganglia: Parallel and integrative networks. J Chem Neuroanat 26:317‐330. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960): A rating scale for depression. Br Med J 23:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Soriano‐Mas C, Pujol J, Ortiz H, Lopez‐Sola M, Hernandez‐Ribas R, Deus J, Alonso P, Yucel M, Pantelis C (2009): Altered corticostriatal functional connectivity in obsessive‐compulsive disorder. Arch Gen Psychiatr 66:1189‐1200. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Cardoner N, Deus J, Alonso P, López‐Solà M, Contreras‐Rodríguez O, Real E, Segalàs C, Blanco‐Hinojo L (2012): Brain corticostriatal systems and the major clinical symptom dimensions of obsessive‐compulsive disorder. Biol Psychiatr 73:321‐328. [DOI] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Light SN, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ (2013): Relationships between changes in sustained fronto‐striatal connectivity and positive affect in major depression resulting from antidepressant treatment. Am J Psychiatr 170:197‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Fair D, Nagel BJ (2011): Altered fronto‐cerebellar connectivity in alcohol‐naive youth with a family history of alcoholism. Neuroimage 54:2582‐2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert JD, Simpson HB, Nissenson KJ, Liebowitz MR, Foa EB (2009): Quality of life and functional impairment in obsessive compulsive disorder: A comparison of patients with and without comorbidity, patients in remission, and healthy controls. Depress Anxiety 26:39‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005): Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatr 62:593. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D (2000): FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage 12:20‐27. [DOI] [PubMed] [Google Scholar]
- Koran LM, Thienemann ML, Davenport R (1996): Quality of life for patients with obsessive‐compulsive disorder. Am J Psychiatr 153:783‐788. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Ducros M, Van De Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS (2004): Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol 55:522‐529. [DOI] [PubMed] [Google Scholar]
- Maia TV, Cooney RE, Peterson BS (2008): The neural bases of obsessive‐compulsive disorder in children and adults. Dev Psychopathol 20:1251‐1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Lisanby SH, Pieraccini F, Ulivelli M, Castrogiovanni P, Rossi S (2006): Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive‐compulsive disorder (OCD) and Tourette's syndrome (TS). Int J Neuro‐Psychopharmacol 9:95‐100. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (2001): A revised neuroanatomy of frontal‐subcortical circuits David G. Lichter and Jeffrey L. Cumming, eds., In: Frontal‐Subcortical Circuits in Psychiatric and Neurological Disorders. pp 44‐58. Guilford Press, New York, NY. [Google Scholar]
- Posner J, Hellerstein DJ, Gat I, Mechling A, Klahr K, Wang Z, McGrath PJ, Stewart JW, Peterson BS (2013): Antidepressants normalize the default mode network in patients with dysthymia. Race/Ethnicity 70:373‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Rauh V, Gruber A, Gat I, Wang Z, Peterson B: Dissociable attentional and affective circuits in medication‐naive children with attention‐deficit/hyperactivity disorder 213:24‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J, van den Heuvel OA, Surguladze S, Mataix‐Cols D (2010): Meta‐analytical comparison of voxel‐based morphometry studies in obsessive‐compulsive disorder vs other anxiety disorders. Arch Gen Psychiatr 67:701‐711. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Weissman MM, Orvaschel H, Gruenberg E, Burke JD Jr, Regier DA (1984): Lifetime prevalence of specific psychiatric disorders in three sites. Arch Gen Psychiatr 41:949. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee SP, Bhandari R, Rose M, Ivey J, Boyd C (2004): Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. J Am Acad Child Adolesc Psychiatr 43:1146‐1153. [DOI] [PubMed] [Google Scholar]
- Saxena S, Rauch SL (2000): Functional neuroimaging and the neuroanatomy of obsessive‐compulsive disorder. Psychiatric Clin N Am 23:563‐586. [DOI] [PubMed] [Google Scholar]
- Seminowicz D, Mayberg H, McIntosh A, Goldapple K, Kennedy S, Segal Z, Rafi‐Tari S (2004): Limbic‐frontal circuitry in major depression: A path modeling metanalysis. Neuroimage 22:409‐418. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Williams J, Gibbon M (1995): Structured Clinical Interview for DSM‐IV (SCID). New York: Biometrics Research. [Google Scholar]
- Swerdlow NR, Benbow C, Zisook S, Geyer MA (1993): A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biol Psychiatr 33:298‐301. [DOI] [PubMed] [Google Scholar]
- Ward B (2000): Simultaneous inference for fMRI data. [On‐line] Available: http://afni.nimh.nih.gov/pub/dist/doc/manual.AlphaSim.pdf. [Google Scholar]
- Yin HH, Knowlton BJ (2006): The role of the basal ganglia in habit |formation. Nat Rev Neurosci 7:464‐476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


