SUMMARY
The hepatitis B virus (HBV) replicates via an error-prone reverse transcriptase generating potential drug-resistant quasispecies. The degree of HBV variability in liver vs peripheral blood mononuclear cells (PBMC) in patients on long-term suppressive antivirals is unclear. We characterized HBV replication, drug resistance and molecular diversity in patients with plasma HBV DNA undetectable by clinical assays. Explant liver (n = 9), PBMC (n = 6) and plasma (n = 7) from nine such patients undergoing liver transplantation were evaluated for HBV genomes by sensitive PCR/nucleic acid hybridization assay. Cases with HBV DNA in liver and PBMC were tested for covalently closed circular DNA (HBV cccDNA). HBV polymerase (P) amplicons were cloned, sequenced and both P and overlapping surface (S) gene sequences were analysed. HBV DNA was detected in 43% (3/7) of plasma, 100% (9/9) of liver and 83% (5/6) of PBMC samples. HBV cccDNA was detected in all liver and one PBMC sample. Four patients had a clinical diagnosis of resistance. HBV P gene sequencing revealed 100% wild type (wt) in plasma (2/2), 83% wt in PBMC (5/6) but livers of 3/9 (33%) contained wt and 6/9 (66%) carried resistance to lamivudine and/or adefovir. The translated S gene revealed no changes affecting HBV antigenicity. Sequences from livers with antiviral resistant mutants revealed greater interpatient quasispecies diversity. Despite apparent HBV suppression, the liver continues to support HBV replication and extrahepatic HBV can be detected. PBMC may be a sanctuary for wt virus during antiviral therapy, while the liver harbours more drug-resistant viruses. Drug resistance correlates with intrahepatic viral diversity.
Keywords: antiviral resistance, diversity, HBV, peripheral blood mononuclear cells, quasispecies, reservoirs
INTRODUCTION
The hepatitis B virus (HBV) replicates via an error-prone viral reverse transcriptase (rt) [1] and exists within the host as a collection of genetically distinct, closely related variants known as quasispecies. These quasispecies are subject to the selective pressure of the host immune system or antivirals targeting the virus polymerase (P) gene [2], potentially leading to progressive selection of variants with reduced drug susceptibility. The goal of anti-HBV therapy is durable virological suppression and prevention of resistance, especially because virological flares in cirrhotic patients can precipitate hepatic decompensation [3]. The hepatitis B surface (S) gene is completely overlapped by the HBV P, and antiviral resistant mutations in the HBV P gene can affect the HBV envelope sequence, leading to altered hepatitis B surface (HBsAg) antigenicity [4,5]. This has been linked to hepatitis B immune globulin (HBIG) treatment failures in liver transplant (LT) recipients [6–10].
Compartmentalization of viral variants in different host tissues is common in many chronic viral infections [11–14]. Although hepatocytes are recognized as the main target, HBV and other members of the hepadnaviral family, such as woodchuck hepatitis virus (WHV), have significant lymphotropic properties [15,16]. Hepadnaviral infection of lymphoid cells is an important mechanism whereby the virus escapes immune recognition [15–19] and lymphoid reservoirs, particularly those harbouring drug-resistant HBV, may be key to the development of antiviral resistance. One study revealed the exclusive compartmentalization of a HBV variant with a potent G145R immune escape mutation in peripheral blood leucocytes [20]. In LT recipients, HBV in peripheral blood mononuclear cells (PBMC) has been implicated in graft reinfection with a specific lymphoid cell–derived viral variant [21–24]. In other viral infections, such as human immunodeficiency virus (HIV), viral diversity is linked to disease progression and development of drug resistance [25], but HBV genetic variation in liver vs extrahepatic compartments is not well recognized. An additional factor in the maintenance of chronic hepatitis B is the existence of the stable HBV replicative intermediate, covalently closed circular DNA (cccDNA) [26–28]. Current therapies do not eliminate cccDNA, which serves as a template for HBV replication, preventing virus eradication [26,29], especially because lymphoid cells can archive cccDNA [16,17,19].
The introduction of sensitive PCR assays has facilitated detection of low-level HBV infection [28]. The detection limits of the more sensitive nested PCR/nucleic acid hybridization (PCR/NAH) assays are below 10 virus genome copies or equivalents (vge) per mL (approximately 2–8 IU/mL) [30–32]. The most common method for detecting drug resistance is direct sequencing of HBV P gene amplicons, but the ability to detect low-frequency variants in the heterogeneous viral population is limited [33]. Therefore, analysis of clonally amplified HBV sequences increases the probability of detecting minority variants and is the basis for newer sequencing technologies [33,34].
This study aims to characterize HBV replication in liver and PBMC and determine the frequency of HBV S and P mutants associated with resistance. We hypothesized that HBV replication, increasing quasispecies diversity and drug-resistant mutants continue to occur in both liver and extrahepatic sites despite suppressive anti-HBV therapy and HBV DNA undetectable by clinical PCR assays.
MATERIALS AND METHODS
Patients
From February to May 2007, nine patients underwent liver transplantation for HBV-related hepatocellular carcinoma (HCC) and complications of cirrhosis at the University of California San Francisco. All patients provided written, informed consent to participate in this investigation, and the study received local institutional ethics review board approval according to the World Medical Association Declaration of Helsinki. There were eight men and one women enrolled (Table 1), all of Asian decent, with median age 59 years (range 38–67 years). All patients were on anti-HBV therapy for at least 6 months prior to evaluation (median duration 15 months, range 6–38) and had undetectable HBV DNA by clinical PCR-based assays (Table 2). A clinical diagnosis of antiviral resistance was based on a history of virological breakthrough on treatment, after confirmation of compliance. A history of lamivudine resistance (LMVr) was reported in 44% (4/9) of patients, including one patient with both LMVr and adefovir resistance (ADVr). One patient had a history of ‘slow response to adefovir (ADV) therapy’, but had undetectable HBV DNA in serum at the time of transplant after therapy was changed to combined ADV and lamivudine (LMV). One other patient (Case 7) had a history of coinfection with HCV, but HCV RNA was undetectable in serum according to clinical PCR-based assay (Cobas Amplicor HCV test v2.0; Roche Diagnostics, Branchburg, NJ, USA).
Table 1.
Detection of HBV DNA in plasma, liver and peripheral blood mononuclear cells and HBV cccDNA in HBV DNA-reactive liver and lymphoid cell samples in patients on suppressive anti-HBV therapy
| Pre-LT HBV DNA or HBV cccDNA by PCR/NAH (vge/mL or vge/µg total DNA) | |||||
|---|---|---|---|---|---|
| Age/sex/ethnicity | Plasma HBV DNA (detected HBV genes) |
Liver explant HBV DNA (detected HBV genes) |
Liver explant HBV cccDNA |
PBMC HBV DNA (detected HBV genes) |
PBMC HBV cccDNA |
| Case 1 | – | 102–103 (C, S, X, P) | ~103 | – | – |
| 59/M Chinese | |||||
| Case 2 | – | 102–103 (C, S, X, P) | ~103 | – | – |
| 66/M Chinese | |||||
| Case 3 | – | ~104 (C, S, X, P) | <102 | – | – |
| 50/M Chinese | |||||
| Case 4 | – | ~103 (C, S, X, P) | <102 | ~102 (X, P) | – |
| 50/M Polynesian | |||||
| Case 5 | <102 (C, S) | 102–103 (C, S, X, P) | <102 | ~102 (P) | – |
| 60/M Asian | |||||
| Case 6 | ~102 (P) | ~103 (C, S, X, P) | <102 | ~102 (C, S, X, P) | ~102 |
| 67/M Philippine | |||||
| Case 7* | ~102 (C, X, P) | ~103 (C, S, X, P) | ~103 | ~102 (C, S, P) | – |
| 63/F Asian | |||||
| Case 8 | – | 102–103 (C, S, X, P) | ~103 | – | – |
| 55/M Burmese | |||||
| Case 9 | – | 102–103 (C, S, P) | <102 | <102 (P) | – |
| 38/M Chinese | |||||
PCR/NAH, PCR/nucleic acid hybridization assay (sensitivity <10 virus genome equivalents, vge/mL); PBMC, peripheral blood mononuclear cells; HBV cccDNA, covalently closed circular DNA; C, HBV core gene; S, HBV surface gene; P, HBV polymerase gene; X, HBV X gene.
Case 7 was HCV antibody positive but HCV RNA was undetectable according to clinical PCR assay.
Table 2.
Summary of antiviral therapy, HBV polymerase and concomitant surface gene sequence data in liver, PBMC and plasma of patients on suppressive anti-HBV therapy
| Case | Current antiviral (prior therapy) duration (months)* |
Anti-HBV drug resistance (clinical) |
Plasma† % wt or drug-resistant and S gene alterations |
Liver† % wt or drug-resistant and S gene alterations |
PBMC† % wt or drug-resistant and S gene alterations |
|---|---|---|---|---|---|
| 1 | ETV 9 | LMV | – | wt, 100% | – |
| 2 | LMV, ADF 30 | LMV | – | LMV, 40% (rtM204I; sW743L) wt, 60% | – |
| 3 | LMV, TDF (ADV) 7 | LMV, ADF | – | LMV, 20% (rtM204V; sI741M) ADF 10% (rtA181V; sF674L) | – |
| 4 | TDF (ADV) 19 | No | – | ADF, 20% (rtA181S; sW673C) wt 80% | wt, 100% |
| 5 | LMV 38 | No | – | wt, 100% | wt, 100% |
| 6 | FTC, TDF (LMV, ADV) 6 | Slow response to ADF | wt, 100% | LMV, 30% (rtM204I; sW743stop) wt, 70% | LMV, 16% (rtM204I; sI741M) ETV, 11% (rtS184N; sV685M) wt, 73% |
| 7 | LMV 15 | No | wt, 100% | wt, 100% | wt, 100% |
| 8 | ADV (LMV) 8 | LMV | – | ‡LMV, 50% (rtM204V or rtL180M; sI741M) wt, 50% | – |
| 9 | LMV, TDF 36 | No | – | LMV, 5% (rtL180P; sS667P) wt, 95% | wt, 100% |
PBMC, peripheral blood mononuclear cells; ETV, entecavir; LMV; lamivudine; ADF, adefovir; TDF; tenofovir; FTC, emtricitabine; wt, wild-type; ADV, adefovir.
Median duration of antiviral therapy 15 months, range 6–38 months.
10–20 clones of HBV P gene sequence analysed in liver and 20 clones analysed in PBMC and plasma.
Case 8 Liver: 50% clones carried wt HBV P gene and 50% LMVr variants rtM204V or rtL180M.
Samples
Whole blood for isolation of plasma and PBMC was collected pre-operatively. PBMC were isolated by density gradient centrifugation on Ficoll-Histopaque plus (Pharmacia, Uppsala, Sweden) and stored at −80 °C, as reported [35]. The nontumour liver tissue was collected within 60 min after removal of the explant, divided into small fragments, snap frozen in liquid nitrogen and stored at −80 °C.
HBV DNA and covalently closed circular DNA detection
Total DNA was isolated from 200 µL of plasma, approximately 2 × 106 PBMC or about 50–100 mg of liver tissue using standard proteinase K digestion and phenol–chloroform extraction procedure [35,36]. HBV DNA was detected by direct and, if required, nested PCR applying four sets of oligonucleotide primers specific for nonoverlapping regions of the S, core (C), P and X genes. The C and P gene primer sequences and amplification conditions were reported previously [37,38]. The HBV S gene primers used were S direct-forward TCGTGTTACAGGCGGGGTTT; S direct-reverse CGAACCACTGAACAAATGGC; S nested-forward CAAGGTATGTTGCCCGTTTG; and S nested-reverse GGCACTAGTAAACTGAGCCA under the following conditions: denaturation at 95 °C for 5 min, then 30 cycles of 95 °C for 30 s, 52 °C for 30 s and 72 °C for 30 s, and then final extension at 72 °C for 15 min. Similarly, the X gene sequence was amplified with the direct and nested primer pairs: X direct-forward CCATACTGCGGAACTCCTAGC, X direct-reverse ACAGACCAATTTATGCCTACAGCC, X nested-forward, CTGGAGCAAACATTATCGGG and X nested-reverse CAAAGACCTTTAACCTGATCTCC. The following amplification conditions were used: denaturation at 95 °C for 5 min, then 30 cycles of 95 °C for 30 s, 52 °C for 30 s, 72 °C for 30 s and final extension at 72 °C for 15 min. The specificity of the virus sequence amplified and the validity of negative and positive controls were routinely verified by NAH analysis with a radiolabelled complete recombinant HBV DNA probe [37,38].
For PCR detection of HBV cccDNA, total DNA was digested with mung bean nuclease (Invitrogen Canada Inc., Burlington, ON, Canada) to eliminate single-stranded DNA sequences and then amplified by PCR using primers spanning the nick region of the HBV genome, as described [37]. For nested PCR, 10 µL of the direct PCR product was used.
As contamination controls, mock DNA isolation was carried out where all reagents except the DNA sample replaced with water were used. PCR contamination controls consisted of water added to the direct or nested PCR cocktail instead of nucleic acid. Positive controls included recombinant HBV DNA or HBV DNA extracted from serum, PBMC or liver tissue of a serum HBsAg-positive chronic HBV carrier.
The sensitivity of PCR/NAH was determined by amplification of serial dilutions of recombinant HBV DNA and phosphorimage densitometer analysis of the resulting hybridization signals. The assays detection sensitivities were <10 vge/mL or <10 vge/µg total DNA for HBV DNA and µ102 copies per µg for HBV cccDNA [18,19]. The results were reported in vge/mL or vge/µg total DNA.
HBV polymerase and overlapping surface gene sequence analysis
Amplified fragments of HBV P gene from all nine liver explants and five PBMC samples were cloned using the TOPO-TA cloning system (Invitrogen Canada Inc.). Ten to 20 clones per sample were sequenced bidirectionally by universal priming using 3730 XL sequencing instrument (Applied Biosystems, Foster City, CA, USA). HBV P gene sequences and the translated overlapping HBV surface region sequence data were analysed with help of Sequencher software V4.7 (Gene Codes Co., Ann Arbor, MI, USA). Phylogenetic and evolutionary changes within the HBV rt region and quasispecies complexity were examined using the MEGA v4.0 software package [39] (The Biodesign Institute, Temple, AZ, USA).
RESULTS
Detection of HBV and its replication in liver explants
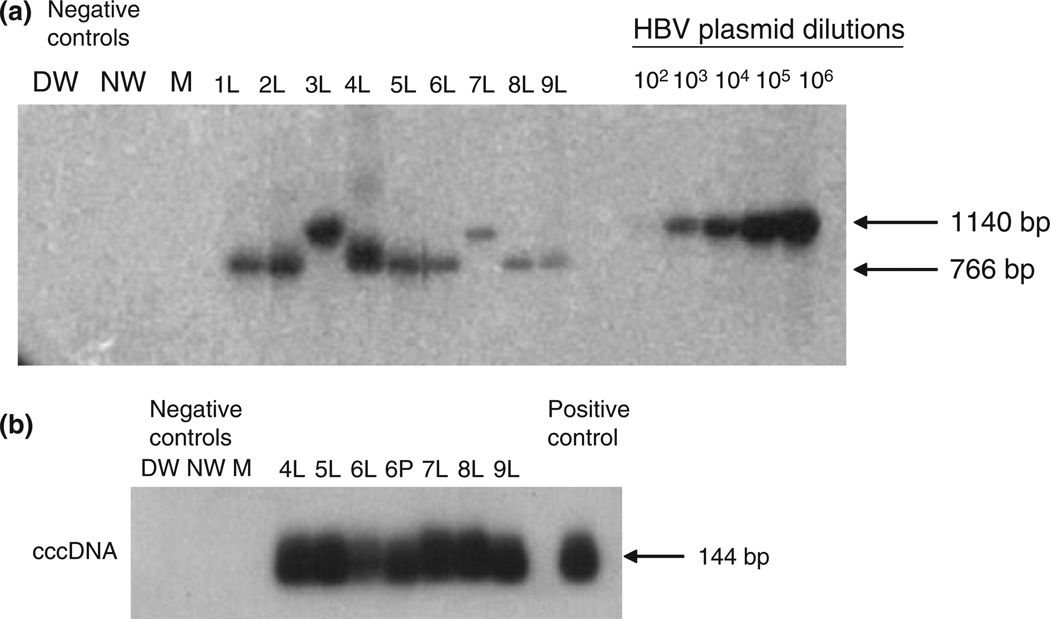
All patients investigated were serum HBsAg positive and had been treated with HBV antiviral therapy for at least 6 months prior to sample collection. Despite undetectable HBV DNA in plasma by clinical PCR-based assays, liver explants from all nine patients were HBV DNA reactive by PCR/NAH at estimated levels between 102 and 104 vge/µg of total DNA. In eight explants, HBV DNA was detected using all four HBV gene-specific primers (C, S, X and P), while in one explant (Case 9) the sequences of all genes except that of the X gene were detected (Table 1 and Fig. 1a). Moreover, all liver explants were HBV cccDNA reactive at levels ranging from 102 to 103 vge/µg of total liver DNA (Table 1 and Fig. 1b).
Fig. 1.
HBV genome detection in liver and peripheral blood mononuclear cells (PBMC) collected from patients on suppressive anti-HBV therapy. (a) Detection of HBV P gene in explant liver (L) from nine patients (Cases 1–9). (b) Detection of HBV covalently closed circular DNA cccDNA in explant liver (L) and PBMC (P) from six patients (Cases 4–9). HBV DNA and cccDNA were detected using direct and nested PCR/nucleic acid hybridization (except in Cases 3 and 7 in which HBV DNA could be detected by a single round PCR). Serial dilutions of recombinant HBV DNA, used as a positive control, (102–106 virus genome equivalents, vge/mL) were simultaneously tested to determine approximate HBV viral load in liver samples by densitometry analysis. Negative controls included direct PCR water (DW), nested PCR water (NW), and a mock (M) extracted sample. The size of the expected direct (1140 bp) or nested (766 bp) DNA and cccDNA amplicons (144 bp) is indicated to the left of the figure.
Detection of HBV genomes in plasma vs PBMC compartment
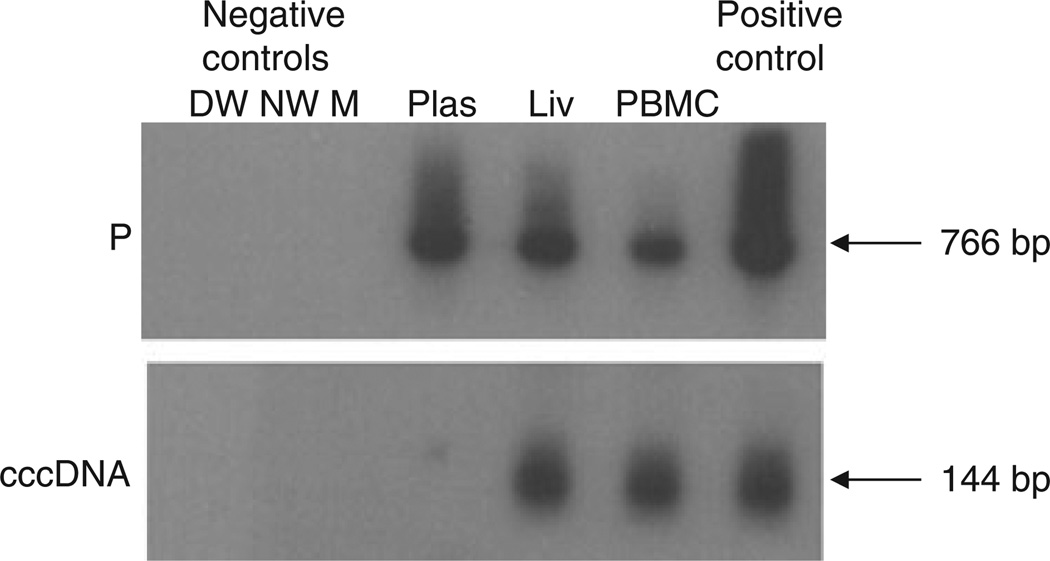
Plasma samples from three cases of seven (43%; Cases 5, 6, 7) were found HBV DNA reactive at <102 vge/mL when tested by PCR/NAH (Table 1, Figs 2 & 3). Plasma from Case 5 tested positive with both HBV C and S gene-specific primers (Table 1), while that from Case 6 was reactive with the P gene-specific primers only (Fig. 3). Plasma from Case 7 was positive using primers specific for HBV C, X and P genes (Table 1 and Fig. 2). HBV DNA was detectable in PBMC from five of six (83%) patients at levels approximately 102 vge/µg total DNA and one of the samples (Case 6) also was HBV cccDNA reactive (Table 1, Figs 2 & 3). In three of the PBMC samples, HBV DNA was identified using two or three primer pairs specific for C, S and P genes or X and P genes (Table 1 and Fig. 2). In PBMC from the remaining two cases, only HBV P gene amplicons were detected (Table 1). PBMC from the only patient (Case 6), in whom HBV cccDNA could be detected (Table 1 and Fig. 3), were also reactive for HBV DNA when tested with primers specific for all four virus genes.
Fig. 2.
Detection of HBV P gene in plasma and PBMC of six patients (Cases 4–9) and HBV C gene in plasma of Case 5 by PCR/nucleic acid hybridization. All patients were on long-term suppressive antiviral therapy, and HBV DNA was undetectable in serum by clinical PCR assays. All reactions were run in parallel with strict contamination controls as described in the legend to Fig. 1. The positive control was recombinant HBV DNA. The molecular sizes of the expected HBV C (403 bp) and P (766 bp) gene fragments are indicated to the left of each panel.
Fig. 3.
Detection of HBV P gene and HBV covalently closed circular DNA (cccDNA) amplicons in different compartments of HBV occurrence in a patient (Case 6) treated with emtricitabine (FTC) and tenofovir (TDF). The detection of HBV DNA in plasma (Plas), liver (Liv) and peripheral blood mononuclear cells (PBMC) by PCR/nucleic acid hybridization is illustrated, although HBV DNA was undetectable in serum by standard clinical assays. All reactions were run in parallel with strict contamination controls, as described in legend to Fig. 1. The size of the expected direct HBV P gene amplicons (766 bp) and the HBV cccDNA amplicons (144 bp) is indicated to the left of each panel.
Detection of antiviral therapy resistant HBV mutants in explanted livers
Four patients (Cases 1, 2, 3 and 8) had clinically diagnosed HBV antiviral drug resistance and were on therapy for LMVr (Table 2). One patient received entecavir (ETV) monotherapy (Case 1); one was treated with LMV and ADV (Case 2), and one with ADV monotherapy (Case 8). Moreover, Case 3 was treated with LMV and tenofovir (TDF) because of presumed antiviral resistance to ADV and LMV. Clonal sequence analysis of HBV P gene derived from the nine explants revealed that three (Cases 1, 5 and 7) carried solely (100% of clones) wild-type (wt) sequence (Table 2). Liver explants from three other patients (Cases 2, 6 and 8) carried HBV genomes with LMVr mutations at the position rtM204I/rtL180M (rt; 40% of clones in Case 2, 30% of clones in Case 6 and 100% of clones in Case 8), while liver from Case 9 displayed HBV P gene mutation indicative of ADVr (5% of clones, rtL180P) and Case 3 displayed both LMVr (20%, rtM204I) and ADVr (100%, rtA181V) mutant virus. Overall, the sequencing data matched the clinical impression of antiviral resistance to LMV in three of four cases (Cases 2, 3 and 8). Case 1 had a history of LMVr that had been treated with ETV. However, clonal sequencing revealed only the presence of wt virus, suggesting this case may represent a suboptimal response to LMV or unrecognized nonadherence. Five patients (Cases 4, 5, 6, 7 and 9) had no known prior clinical history of antiviral drug resistance. Sequencing in two cases (Cases 5 and 7) revealed the presence of wt virus only (100% clones). These two patients had been successfully treated with long-term LMV prophylaxis. Case 7 also had a history of prior HCV exposure and had comparable levels of HBV DNA in liver, plasma and PBMC. Two other patients (Cases 4 and 9) carried HBV ADVr mutants. In Case 4, this represented 20% of clones, but was only 5% of clones in Case 9. Interestingly, although Case 4 had a history of ADV exposure, Case 9 had no known prior exposure. Finally, sequencing of HBV P gene fragment from explant of Case 6 revealed that 30% of clones carried LMVr mutation (rtM204I). This patient had been exposed to multiple HBV drugs, including LMV, ADV, emtricitabine (FTC) and TDF, but had no known clinical history of LMVr.
Clonal sequence analysis of HBV genomes in plasma, PBMC and liver
Sequence analysis of HBV P genes in plasma from two patients (Case 6 and 7) showed that both patients carried wt virus in 100% of clones sequenced. Similarly, PBMC samples of five patients demonstrated that four of them carried wt virus (Table 2). One patient (Case 6) carried LMVr (rtM204I; 16% of clones) and entecavir resistance (ETVr) (11% of clones; rtS181N) HBV variants within PBMC. In comparison with the other patients, Case 6 had been treated with multiple antiviral drugs, including FTC, TDF and LMV, but there was no known exposure to ETV. In contrast, sequencing analysis of HBV DNA from the liver explants revealed that only 33% (three of nine) of patients carried solely wt virus, whereas the six other cases demonstrated either LMVr and/or ADVr mutants in the liver. The overlapping HBV surface region was translated and analysed in all cases that showed antiviral resistant mutants within the P gene (i.e., liver from Case 2, 3, 4, 8 and 9 and liver and PBMC from case 6). Although all cases had concomitant alterations in HBV S gene sequence, none were known to affect key anti-HBs antigenic binding site (Table 2). However, lamivudine-resistant HBV (rtM204I) detected in liver of one patient was associated with a change to a termination codon in the overlapping HBV S gene region (Case 6, Table 2).
Comparison of HBV quasispecies sequence diversity in liver and PBMC
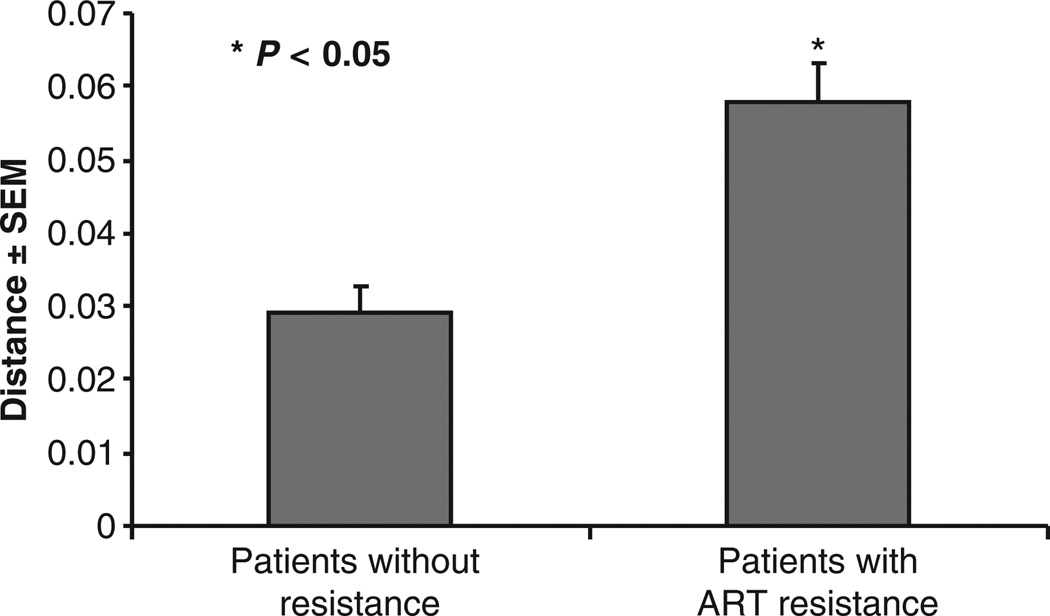
Analysis of multiple replicates in matched PBMC and liver from each patient revealed no significant difference in quasispecies diversity between these compartments. However, comparing patients (n = 6; Cases 2, 3, 4, 6, 8 and 9) with one or more antiviral resistant HBV mutants in liver to patients showing solely wt virus in liver (n = 3; Cases 1, 5 and 7) revealed a significantly higher interpatient quasispecies diversity rate in the former group (P < 0.05) (Fig. 4).
Fig. 4.
Sequence and phylogenetic analysis of HBV P gene in liver shows solely wild-type virus in Cases 1, 5, 7 and antiviral resistant (ART) mutations in Cases 2, 3, 4, 6, 8 and 9. There was a significant trend (P < 0.05) towards increased HBV quasispecies diversity in patients with antiviral resistant HBV quasispecies in liver compared to those with wild-type sequences. For each patient 10–20 HBV P gene clones were analysed.
DISCUSSION
To our knowledge, this study is the first detailed molecular evaluation of HBV genomes residing in liver, plasma and lymphoid cells in patients treated with suppressive oral anti-HBV drug treatment undergoing liver transplantation. Despite undetectable HBV DNA in plasma by clinical PCR assays, we found that 43% of patients tested had detectable HBV genomes in plasma, and P gene sequencing in two of these cases, showed that 100% of clones were wt virus. In addition, we found that 83% of patients tested had detectable HBV DNA in PBMC, including one case also HBV cccDNA reactive, and 80% of the virus detected within PBMC had predominantly wt sequence. In comparison, all cases were positive for HBV DNA and cccDNA in liver yet only 33% of liver explants carried wt HBV sequence. It is interesting that Case 6 in particular showed significant differences between the HBV quasispecies detected in three compartments (i.e., 100% wt in plasma vs 73% wt, 16% LMVr, 11% ETVr in PBMC vs 70% wt, 30% LMVr in liver). Advanced phylogenetic analysis of multiple replicates in matched PBMC and liver from each patient revealed no significant difference in quasispecies diversity. Not surprisingly, comparison of cases with antiviral resistant mutations in the liver with cases showing solely wt virus in the liver revealed significantly higher interpatient quasispecies diversity in the former group, indicating that drug resistance correlates with increased quasispecies variability.
The notable discrepancy in HBV genetic and molecular analysis between PBMC and liver suggests that virus residing within lymphoid cells might be, to a larger degree, protected from the effects of anti-HBV therapy. In addition, other studies have noted that some HBV variants identified in serum were not detected in liver or PBMC, suggesting that some HBV variants may display a particular tissue tropism or they are secreted into serum (or plasma) at disproportionally greater rates [21]. It is also highly likely that host immune selective pressure operating in vivo leads to selection and evolution of viral variants. This theory is supported by recent in vitro data using the woodchuck model for HBV in which repetitive passage of wild-type WHV in lymphocyte culture failed to distinguish a particular lymphotropic viral variant and random mutations that did occur immediately reverted to wt after reinfection of the woodchuck host [40]. However, this discrepancy could also be explained by the fact that resistance mutations may not have the opportunity to arise given the overall lower levels of HBV replication in the PBMC compartment compared to liver. Further, as HBV DNA is known to integrate into the intrahepatic host DNA, it is possible that PBMC also contain integrated HBV genomes that are largely resistant to antiviral therapy [17,26].
The results of HBV mutational (i.e., antiviral drug resistance) and genetic analysis from this study have several important clinical implications. First, HBV drug-resistant variants may be present in the liver, while they are absent in PBMC and plasma, hence assessing drug resistance in a single compartment may not be sufficient in predicting the long-term response to antiviral therapy. Secondly, it is intriguing that two cases (Case 6 and Case 9) in our study carried virus in the PBMC and liver with mutations determining resistance to two different anti-viral agents, namely ETV and ADV, respectively, even though the patients had never received those drugs. The presence of pre-existing HBV drug-resistant mutations have been reported [41–43] and their existence at baseline can potentially affect the efficacy of antiviral prophylaxis applied, especially if single drug therapy is utilized [44]. Thirdly, in patients with antiviral-resistant quasispecies found in liver or PBMC, analysis of the amino acid sequence encoded by the S gene did not reveal any changes known to affect HBsAg antigenicity. However, all cases with HBV P gene mutations had concomitant S gene amino acid changes, including one patient (Case 6) with lamivudine resistance (rtM204I) that was associated with generation of a termination codon (UAG) in the S gene sequence. Recent studies have demonstrated that although the major antigenic binding epitopes of HBsAg may not be affected, distant mutations can modulate the a-determinant by influencing epitope conformation [45]. Further studies are needed on how these changes associated with antiviral resistance can affect the topology and antigenicity of the HBsAg. This is of particular significance for LT recipients who receive HBIG prophylaxis because treatment failures have been known to occur as a result of specific surface gene mutations (i.e. sP120T and sG145R) [5].
As noted above, we found that despite apparent complete suppression of plasma HBV DNA, as evidenced by clinical laboratory assays, all nine patients carried HBV DNA and HBV cccDNA in the liver, 83% (5/6) of those tested carried HBV genomes in PBMC and 43% (3/7) carried HBV in plasma using sensitive PCR/NAH. These results are in accordance with data from Hussain et al. [46] showing persistence of intrahepatic HBV DNA in 94% and cccDNA in 89% of patients at the time of liver transplantation. However, only 56% of the patient cohort in that study had received antiviral therapy prior to transplant compared to 100% in our cohort, and PBMC were not evaluated as a HBV reservoir. In the current era of antiviral therapy, with the majority of patients on the waiting list having undetectable HBV DNA levels, HBV DNA levels in PBMC and liver may have relevance in predicting reinfection risk. Although 83% of patients were positive for HBV DNA in PBMC, only one case had detectable HBV cccDNA in this compartment. Previous studies in patients with low-level HBV infection have found that HBV replicates within PBMC [47] and in the woodchuck model of HBV infection, mitogen stimulation of infected PBMC can upregulate virus replication and increase detection of replicating virus [35]. In our study, the yield of PBMC was suboptimal to provide sufficient number of cells for culture and their examination after mitogen stimulation. In addition, detection of HBV genomes in PBMC most likely would be greatly enhanced by analysis of serial samples collected over long period of time, as was demonstrated in the woodchuck model of hepatitis B [19,35,48]. Nonetheless, we believe that the detection of HBV in PBMC using primers specific for multiple HBV genes already is consistent with the presence of replicating virus in the cases investigated, particularly because frequently HBV DNA was not identifiable in parallel plasma samples by using highly sensitive PCR/NAH assays.
In summary, this is the first study evaluating HBV compartmentalization in parallel plasma, PBMC and liver using ultrasensitive PCR/NAH together with genetic, mutational and phylogenetic analysis in patients on sterilizing antiviral therapy undergoing liver transplantation. We found that HBV can persist, albeit at low levels, in liver, circulating lymphoid cells and plasma. HBV polymerase gene sequence analysis in explant liver and PBMC showed that liver from these patients were less likely to carry wt virus compared to the PBMC compartment. These findings suggest that virus in lymphoid cells may be more resistant to antiviral therapy leading to tissue-specific compartmentalization of HBV viral populations, and there may be a differential rate of HBV variant secretion in serum or plasma. Furthermore, HBV quasispecies diversity in liver correlated with antiviral drug resistance and this may have implications for the emergence of dominant resistant HBV variants over time.
ACKNOWLEDGEMENTS
The authors thank Ms Christiane Abouzeid for assistance with patient sample collection.
GRANT SUPPORT
Dr Coffin was supported by the 2006 AASLD Advanced Hepatology Fellowship Award.
Abbreviations
- ADV/ADVr
adefovir/adefovir resistance
- ART
antiviral therapy
- C
HBV core gene
- ccc-DNA
HBV covalently closed circular DNA
- ETV/ETVr
entecavir/entecavir resistance
- FTC
emtricitabine
- HBIG
hepatitis B immune globulin
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HIV
human immunodeficiency virus
- LMV/LMVr
lamivudine/lamivudine resistance
- LT
liver transplant
- P
HBV polymerase gene
- PBMC
peripheral blood mononuclear cells
- PCR/NAH
polymerase chain reaction/nucleic acid hybridization assay
- rt
reverse transcriptase
- S
HBV surface gene
- TDF/TDFr
tenofovir/tenofovir resistance
- vge
virus genome equivalents
- WHV
woodchuck hepatitis virus
- wt
wild-type
- X
HBV X gene
REFERENCES
- 1.Nassal M. Hepatitis B viruses: reverse transcription a different way. Virus Res. 2008;134(1–2):235–249. doi: 10.1016/j.virusres.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Stuyver LJ, Locarnini SA, Lok A, et al. Nomenclature for antiviral-resistant human hepatitis B virus mutations in the polymerase region. Hepatology. 2001;33(3):751–757. doi: 10.1053/jhep.2001.22166. [DOI] [PubMed] [Google Scholar]
- 3.Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125(6):1714–1722. doi: 10.1053/j.gastro.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Bartholomeusz A, Locarnini S. Hepatitis B virus mutations associated with antiviral therapy. J Med Virol. 2006;78(Suppl. 1):S52–S55. doi: 10.1002/jmv.20608. [DOI] [PubMed] [Google Scholar]
- 5.Sheldon J, Soriano V. Hepatitis B virus escape mutants induced by antiviral therapy. J Antimicrob Chemother. 2008;61(4):766–768. doi: 10.1093/jac/dkn014. [DOI] [PubMed] [Google Scholar]
- 6.Terrault N, Vyas G. Hepatitis B immune globulin preparations and use in liver transplantation. Clin Liver Dis. 2003;7(3):537–550. doi: 10.1016/s1089-3261(03)00045-x. [DOI] [PubMed] [Google Scholar]
- 7.Rosenau J, Bahr MJ, Tillmann HL, et al. Lamivudine and low-dose hepatitis B immune globulin for prophylaxis of hepatitis B reinfection after liver transplantation possible role of mutations in the YMDD motif prior to transplantation as a risk factor for reinfection. J Hepatol. 2001;34(6):895–902. doi: 10.1016/s0168-8278(01)00089-7. [DOI] [PubMed] [Google Scholar]
- 8.Protzer-Knolle U, Naumann U, Bartenschlager R, et al. Hepatitis B virus with antigenically altered hepatitis B surface antigen is selected by high-dose hepatitis B immune globulin after liver transplantation. Hepatology. 1998;27(1):254–263. doi: 10.1002/hep.510270138. [DOI] [PubMed] [Google Scholar]
- 9.Ghany MG, Ayola B, Villamil FG, et al. Hepatitis B virus S mutants in liver transplant recipients who were reinfected despite hepatitis B immune globulin prophylaxis. Hepatology. 1998;27(1):213–222. doi: 10.1002/hep.510270133. [DOI] [PubMed] [Google Scholar]
- 10.Terrault NA, Zhou S, Combs C, et al. Prophylaxis in liver transplant recipients using a fixed dosing schedule of hepatitis B immunoglobulin. Hepatology. 1996;24(6):1327–1333. doi: 10.1002/hep.510240601. [DOI] [PubMed] [Google Scholar]
- 11.Dahl V, Josefsson L, Palmer S. HIV reservoirs, latency, and reactivation: prospects for eradication. Antiviral Res. 2010;85(1):286–294. doi: 10.1016/j.antiviral.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Oldstone MB. Viral persistence: parameters, mechanisms and future predictions. Virology. 2006;344(1):111–118. doi: 10.1016/j.virol.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 13.van Marle G, Gill MJ, Kolodka D, McManus L, Grant T, Church DL. Compartmentalization of the gut viral reservoir in HIV-1 infected patients. Retrovirology. 2007;4:87. doi: 10.1186/1742-4690-4-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pomerantz RJ. Reservoirs, sanctuaries, and residual disease: the hiding spots of HIV-1. HIV Clin Trials. 2003;4(2):137–143. doi: 10.1310/80jh-148k-nadq-u927. [DOI] [PubMed] [Google Scholar]
- 15.Michalak TI. Occult persistence and lymphotropism of hepadnaviral infection: insights from the woodchuck viral hepatitis model. Immunol Rev. 2000;174:98–111. doi: 10.1034/j.1600-0528.2002.017406.x. [DOI] [PubMed] [Google Scholar]
- 16.Mason A, Wick M, White H, Perrillo R. Hepatitis B virus replication in diverse cell types during chronic hepatitis B virus infection. Hepatology. 1993;18(4):781–789. doi: 10.1002/hep.1840180406. [DOI] [PubMed] [Google Scholar]
- 17.Mason A, Yoffe B, Noonan C, et al. Hepatitis B virus DNA in peripheral-blood mononuclear cells in chronic hepatitis B after HBsAg clearance. Hepatology. 1992;16(1):36–41. doi: 10.1002/hep.1840160108. [DOI] [PubMed] [Google Scholar]
- 18.Lew Y-Y, Michalak TI. In vitro and in vivo infectivity and pathogenicity of the lymphoid-cell derived woodchuck hepatitis virus. J Virol. 2001;75:1770–1782. doi: 10.1128/JVI.75.4.1770-1782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michalak TI, Mulroney PM, Coffin CS. Low doses of hepadnavirus induce infection of the lymphatic system that does not engage the liver. J Virol. 2004;78:1730–1738. doi: 10.1128/JVI.78.4.1730-1738.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Datta S, Panigrahi R, Biswas A, et al. Genetic characterization of hepatitis B virus in peripheral blood leukocytes: evidence for selection and compartmentalization of viral variants with the immune escape G145R mutation. J Virol. 2009;83(19):9983–9992. doi: 10.1128/JVI.01905-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brind A, Jiang J, Samuel D, et al. Evidence for selection of hepatitis B mutants after liver transplantation through peripheral blood mononuclear cell infection. J Hepatol. 1997;26(2):228–235. doi: 10.1016/s0168-8278(97)80035-9. [DOI] [PubMed] [Google Scholar]
- 22.Feray C, Zignego AL, Samuel D, et al. Persistent hepatitis B virus infection of mononuclear blood cells without concomitant liver infection. The liver transplantation model. Transplantation. 1990;49(6):1155–1158. doi: 10.1097/00007890-199006000-00025. [DOI] [PubMed] [Google Scholar]
- 23.Petit MA, Buffello-Le Guillou D, Roche B, et al. Residual hepatitis B virus particles in liver transplant recipients receiving lamivudine: PCR quantitation of HBV DNA and ELISA of preS1 antigen. J Med Virol. 2001;65(3):493–504. [PubMed] [Google Scholar]
- 24.Roche B, Feray C, Gigou M, et al. HBV DNA persistence 10 years after liver transplantation despite successful anti-HBS passive immunoprophylaxis. Hepatology. 2003;38(1):86–95. doi: 10.1053/jhep.2003.50294. [DOI] [PubMed] [Google Scholar]
- 25.Tan YJ, Lim SG, Hong W. Understanding human immunodeficiency virus type 1 and hepatitis C virus coinfection. Curr HIV Res. 2006;4(1):21–30. doi: 10.2174/157016206775197600. [DOI] [PubMed] [Google Scholar]
- 26.Mason AL, Xu L, Guo L, Kuhns M, Perrillo RP. Molecular basis for persistent hepatitis B virus infection in the liver after clearance of serum hepatitis B surface antigen. Hepatology. 1998;27(6):1736–1742. doi: 10.1002/hep.510270638. [DOI] [PubMed] [Google Scholar]
- 27.Mazet-Wagner AA, Baclet MC, Loustaud-Ratti V, Denis F, Alain S. Real-time PCR quantitation of hepatitis B virus total DNA and covalently closed circular DNA in peripheral blood mononuclear cells from hepatitis B virus-infected patients. J Virol Methods. 2006;138(1–2):70–79. doi: 10.1016/j.jviromet.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008;49(4):652–657. doi: 10.1016/j.jhep.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Zoulim F. New insight on hepatitis B virus persistence from the study of intrahepatic viral cccDNA. J Hepatol. 2005;42(3):302–308. doi: 10.1016/j.jhep.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Mulrooney-Cousins PM, Michalak TI. Persistent occult hepatitis B virus infection: experimental findings and clinical implications. World J Gastroenterol. 2007;13(43):5682–5686. doi: 10.3748/wjg.v13.i43.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bréchot C, Thiers V, Kremsdorf D, Nalpas B, Pol S, Paterlini-Bréchot P. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely “occult”? Hepatology. 2001;34:194–203. doi: 10.1053/jhep.2001.25172. [DOI] [PubMed] [Google Scholar]
- 32.Michalak TI, Pasquinelli C, Guilhot S, Chisari FV. Hepatitis B virus persistence after recovery from acute viral hepatitis. J Clin Invest. 1994;93:230–239. doi: 10.1172/JCI116950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pallier C, Castera L, Soulier A, et al. Dynamics of hepatitis B virus resistance to lamivudine. J Virol. 2006;80(2):643–653. doi: 10.1128/JVI.80.2.643-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solmone M, Vincenti D, Prosperi MC, Bruselles A, Ippolito G, Capobianchi MR. Use of massively parallel ultradeep pyrosequencing to characterize the genetic diversity of hepatitis B virus in drug-resistant and drug-naive patients and to detect minor variants in reverse transcriptase and hepatitis B S antigen. J Virol. 2009;83(4):1718–1726. doi: 10.1128/JVI.02011-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coffin CS, Michalak TI. Persistence of infectious hepadnavirus in the offspring of woodchuck mothers recovered from viral hepatitis. J Clin Invest. 1999;104:203–212. doi: 10.1172/JCI5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coffin CS, Pham TN, Mulrooney PM, Churchill ND, Michalak TI. Persistence of isolated antibodies to woodchuck hepatitis virus core antigen is indicative of occult infection. Hepatology. 2004;40(5):1053–1061. doi: 10.1002/hep.20419. [DOI] [PubMed] [Google Scholar]
- 37.Coffin CS, Mulrooney-Cousins PM, Lee SS, Michalak TI, Swain MG. Profound suppression of chronic hepatitis C following superinfection with hepatitis B virus. Liver Int. 2007;27(5):722–726. doi: 10.1111/j.1478-3231.2007.01477.x. [DOI] [PubMed] [Google Scholar]
- 38.Chang UI, Lee YC, Wie SH, et al. Evolution of viral load and changes of polymerase and precore/core promoter sequences in lamivudine-resistant hepatitis B virus during adefovir therapy. J Med Virol. 2007;79(7):902–910. doi: 10.1002/jmv.20819. [DOI] [PubMed] [Google Scholar]
- 39.Kumar S, Nei M, Dudley J, Tamura K. MEGA: a biologistcentric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform. 2008;9(4):299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mulrooney-Cousins PM, Michalak TI. Repeated passage of wild-type woodchuck hepatitis virus in lymphoid cells does not generate cell type-specific variants or alter virus infectivity. J Virol. 2008;82(15):7540–7550. doi: 10.1128/JVI.00405-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schildgen O, Sirma H, Funk A, et al. Variant of hepatitis B virus with primary resistance to adefovir. N Engl J Med. 2006;354(17):1807–1812. doi: 10.1056/NEJMoa051214. [DOI] [PubMed] [Google Scholar]
- 42.Locarnini S. Primary resistance, multidrug resistance, and cross-resistance pathways in HBV as a consequence of treatment failure. Hepatol Int. 2008;2(2):147–151. doi: 10.1007/s12072-008-9048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fung SK, Mazzulli T, El-Kashab M, Sherman M, Popovic V, Sablon E. Lamivudine- resistant mutation among treatment naive hepatitis B patients is common and may be associated with treatment failure. Hepatology. 2008;48(Suppl.):703A. [Google Scholar]
- 44.Coffin CS, Terrault NA. Management of hepatitis B in liver transplant recipients. J Viral Hepat. 2007;14(Suppl. 1):37–44. doi: 10.1111/j.1365-2893.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- 45.Beale MA, Ijaz S, Tedder RS. Genetic backbone modulates phenotype of Hepatitis B Surface Antigen “mutants”. J Gen Virol. 2010;91:68–73. doi: 10.1099/vir.0.013078-0. [DOI] [PubMed] [Google Scholar]
- 46.Hussain M, Soldevila-Pico C, Emre S, Luketic V, Lok AS. Presence of intrahepatic (total and ccc) HBV DNA is not predictive of HBV recurrence after liver transplantation. Liver Transpl. 2007;13(8):1137–1144. doi: 10.1002/lt.21179. [DOI] [PubMed] [Google Scholar]
- 47.Yoffe B, Noonan CA, Melnick JL, Hollinger FB. Hepatitis B virus DNA in mononuclear cells and analysis of cell subsets for the presence of replicative intermediates of viral DNA. J Infect Dis. 1986;153(3):471–477. doi: 10.1093/infdis/153.3.471. [DOI] [PubMed] [Google Scholar]
- 48.Michalak TI, Pardoe IU, Coffin CS, et al. Occult lifelong persistence of infectious hepadnavirus and residual liver inflammation in woodchucks convalescent from acute viral hepatitis. Hepatology. 1999;29(3):928–938. doi: 10.1002/hep.510290329. [DOI] [PubMed] [Google Scholar]