Abstract
Rationale
Results from clinical studies have shown that topiramate effectively reduces alcohol consumption in a population of heavy-drinking alcohol-dependent humans.
Objectives
We undertook this preclinical study in order to establish topiramate's efficacy in a rodent model and to determine whether topiramate's efficacy may vary with level of drinking and/or genetic background.
Methods
The effects of acutely administered topiramate (0, 5, and 10 mg/kg) on ethanol consumption were examined in a large group of ethanol-preferring (P) rats (N=20) in order to assess the relationship between level of consumption and treatment effect using a two-bottle free-choice paradigm (10% ethanol versus water). We also evaluated the effects of topiramate in two groups of Wistar rats that were given access to ethanol under either the standard two-bottle free-choice paradigm or under conditions that are known to produce higher levels of daily ethanol consumption (i.e. three-bottle free choice).
Results
Topiramate treatment produced a modest, but persistent (average of 5 days), reduction in ethanol consumption in P rats, and this effect did not vary with level of consumption. Topiramate did not affect ethanol consumption in either group of Wistar rats.
Conclusions
The results from this study establish in a rodent model that topiramate effectively and persistently reduces ethanol consumption and suggests that its efficacy may depend on genetic vulnerability but not level of drinking.
Keywords: Alcohol, Consumption, Ethanol, P rats, Wistar rats, Topiramate
Introduction
Based on the assumption that topiramate would produce a widespread suppression of DA through interactions with glutamate and GABA, even in the absence of preclinical data, our group tested topiramate's efficacy for the treatment of alcohol dependence in a placebo-controlled randomized clinical trial (Johnson et al. 2003). This study showed that topiramate effectively reduced alcohol consumption in a heavy-drinking population of alcohol-dependent humans. These results were confirmed by a subsequent clinical trial and in a recent multi-site, double-blind, randomized, placebo-controlled clinical trial (Johnson et al. 2007, 2008). Unlike most potential pharmacotherapies for alcohol dependence, the development of topiramate proceeded from a theoretical neurobiological framework rather than from empirical preclinical studies. As such, it is important to establish the efficacy of topiramate to reduce ethanol consumption in an animal model so that we can begin to investigate the mechanisms for its efficacy. Such studies may also help to identify target populations that would be most benefit from topiramate treatment.
An initial study done by Gabriel and Cunningham (2005) in C57BL/6J mice showed that high doses of topiramate (i.e. 25 and 50 mg/kg), but not low doses (1–10 mg/kg), reduced ethanol consumption although saccharin and water consumption also was affected at the high topiramate doses. In contrast, Nguyen et al. (2007) showed in C57BL/6 (B6) mice that topiramate dose-dependently (10–90 mg/kg) reduced ethanol consumption without affecting food or water consumption and without reducing motor activity. In studies with Wistar rats, topiramate treatment has been shown to selectively reduce the consumption of a sweetened ethanol solution (10% ethanol/5% sucrose; Knapp et al. 2007) and to modestly reduce the progressive ratio responding for beer (Hargreaves and McGregor 2007). However, Hargreaves and McGregor (2007) also showed that topiramate did not reduce beer consumption under a 24-h-access choice procedure.
In this study, we examined the effects of topiramate on ethanol consumption in rats bred for a preference for ethanol (i.e., alcohol-preferring (P) rats). The use of P rats allowed us to determine the effects of topiramate in animals that consume physiologically relevant levels of ethanol when presented with an unsweetened ethanol solution under a 24-h two-bottle free-choice paradigm. A large group of P rats (N=20) was tested in order to determine whether there was a relationship between level of consumption and topiramate's efficacy at reducing consumption. To further evaluate the relationship between level of drinking and treatment response, we also examined the effect of topiramate in their background control strain (Wistar rats, which drink less ethanol daily as compared to P rats) under two different access conditions, the standard two-bottle choice paradigm and using the three-bottle choice paradigm which is known to produce higher levels of consumption (Hölter et al. 1998; Wolffgramm and Heyne 1995). The comparison of P rats and Wistar rats also allowed for a preliminary look at efficacy of topiramate as a function of genetic background since this factor is known to be involved not only in the development of alcohol dependence (Lê et al. 2001; McBride et al. 1997) but also in treatment (Rodd et al. 2004).
Methods
Animals
P rats (n=20) and Wistar rats (n=20) were obtained from the Indiana Alcohol Research Center's Animal Production Core and Charles River Laboratory, respectively. The rats weighed between 350 and 400 g at the start of the study. The P rats, which were originally developed by mass selection from a Wistar foundation stock, had been selectively bred for high ethanol consumption (as determined under 24-h free-choice conditions) for more than 55 generations. Furthermore, these rats have been characterized by numerous studies as a valid animal model of excessive ethanol-drinking behavior (Lê and Shaham 2002; Rodd et al. 2004). Rats were maintained on a 12:12 light: dark cycle, with lights on at 7:00 a.m. The animals were single-housed in clear, polycarbonate cages with unlimited access to food and water, which, along with ethanol, were measured daily. Fresh ethanol and water were presented multiple times a week. During the sucrose fading procedure, the positions of the water and ethanol bottles were switched daily and during the consumption component the bottle positions were switched at random intervals to ensure the animals were tracking the ethanol rather than bottle position. Animals were weighed three times per week. These animals were part of an ongoing study to investigate pharmacotherapies for ethanol dependence and had an average of approximately 3 months with free access to unsweetened ethanol prior to treatment. All protocols were approved by the Institutional Animal Care and Use Committee and are in accordance with the guidelines set forth by the National Institutes of Health.
Procedure
Free-choice access
A sucrose-fading procedure (Samson et al. 1988) was used to induce drinking in P rats (n=20) and Wistar rats (n=10). Although P rats will readily consume ethanol without the use of a sucrose-fading procedure, this procedure was used in both groups to control for training. Initially, the rats had access to a 10% sucrose solution and water for 2 days. Then, ethanol was gradually introduced in 2% increments following 2 days of ≥70% preference for the sucrose/ethanol solution (i.e. animals were maintained on each concentration until they showed ≥70% preference for 2 days). Once the ethanol was at a 10% v/v concentration, the sucrose was gradually faded out in 2% increments following 2 days of ≥60% preference. Following the end of the sucrose-fading procedure, preference was measured daily, but no further measures were used to maintain 60% preference. In order to have a group of higher-drinking Wistar rats, a second group of Wistar rats (n=10) were given access to 5% and 10% ethanol along with water under a three-bottle free-choice paradigm; the use of multiple alcohol concentrations allows for higher drinking levels without sucrose fading.
Effect of topiramate on ethanol consumption
The effect of topiramate on ethanol (sucrose-free) consumption under the two-bottle (P rats and Wistar rats) and three-bottle (Wistar rats only) free-choice paradigm were examined on a stable baseline (stability was defined as no increasing or decreasing trend in ethanol consumption, with a variation of ≤1 g/kg over three consecutive days) using a within-subject, Latin square design. On test days, a single treatment of either topiramate (5 or 10 mg/kg intraperitoneally) or an equal volume of saline was administered during the daily weigh sessions that were conducted between 12:00 p.m. and 3:00 p.m., with water and ethanol consumption measured 24 h after each injection. The treatments were given between these times because previous work in humans has shown that peak levels are observed 2–4 h after a single injection (Easterling et al. 1988) and based on its half-life which is between 19 and 23 h (Easterling et al. 1988). As such, we expected to have a maximal effect during the dark phase when the most drinking occurs. Doses were selected based on previous research in rats (Cagetti et al. 2004; Hargreaves and McGregor 2007), with the order of administration counterbalanced between subjects. All treatments were given once, except for vehicle which was given twice, with the average used for analysis. A minimum of 3 days of stable consumption at baseline levels separated each test session.
Drugs
Sucrose (Fisher Scientific, Pittsburgh, PA, USA) was dissolved in tap water, and diluted in 95% ethyl alcohol (Fisher Scientific) and tap water. Topiramate HCl was purchased from Sigma-Aldrich (St. Louis, MO, USA). It was dissolved in 0.9% sodium chloride and administered it at a volume of 1 ml/kg.
Data analysis
The effect of topiramate (0, 5, and 10 mg/kg) on ethanol consumption was examined in P rats and both groups of Wistar rats by comparing percent change from baseline consumption (calculated from the day preceding each treatment) on the day of treatment and the 3 days following treatment using separate, repeated-measures analysis of variance (ANOVAs). We also examined the effects of topiramate on ethanol consumption as a function of strain/group (P rats, two-bottle and three-bottle choice Wistar rats) on percent change from baseline on the day of treatment using a two-factor analysis of variance. To examine the persistent changes within the 10 mg/kg topiramate condition, we used the paired t test comparing baseline consumption with consumption on the four sessions following treatment. Such analysis was not appropriate under the vehicle and 5 mg/kg topiramate conditions because there were no significant changes from baseline consumption and generally treatments had resumed. Pairwise comparisons of topiramate and vehicle were conducted using the Dunnett t test. The relationship between consumption and the effect of topiramate treatment was assessed in P rats by calculating the Pearson correlation co-efficient. Similar analyses were used to examine the preference for ethanol over water and for food and water intake. Ethanol consumption was compared between the two groups of Wistar rats by calculating baseline consumption (three sessions preceding each treatment) and analyzed using a repeated-measures analysis of variance. For comparison between strains a focused analysis of the effect of topiramate was conducted on the day of treatment using repeated-measures ANOVA. A p value of <0.05 was considered to be statistically significant.
Results
Effect of topiramate on ethanol consumption
In P rats, topiramate treatment produced a modest but persistent decrease in ethanol consumption as compared to baseline levels (Fig. 1a). An analysis of percent change from baseline on the treatment day revealed a significant overall effect of group (F2,57=3.786, p<0.05). Post hoc comparison of topiramate versus vehicle revealed that this effect was significant at the 10 mg/kg dose (T38= 14.37, p<0.05), but not at the 5 mg/kg dose (p>0.05). Further analysis within the 10 mg/kg topiramate condition revealed that consumption remained significantly decreased from baseline 4 days post treatment (i.e., mean consumption on post day 4 was 3.85±0.35; post 1, t19=4.72, p< 0.01; post 2, t19=3.57, p<0.01; post 3, t19=2.90, p<0.01; post 4 t18=2.91, p<0.01), but returned to baseline levels 5 days following 10 mg/kg topiramate treatment (i.e., mean consumption on post day 5 was 4.29±0.42; p>0.05). An analysis of the effects of 10 mg/kg topiramate on ethanol consumption as a function of baseline levels of drinking revealed a non-significant relationship (See Fig. 1b; r=0.17; p>0.05). Similar to its effects on consumption, topiramate treatment tended to produce a modest but persistent reduction in ethanol preference in the P rats (Fig. 1c; F2,57=2.80, p=0.069). Notably, there was no significant effect of topiramate on food or water consumption on the day of treatment (Table 1; p>0.05) or on any of the subsequent three sessions (p>0.05).
Fig. 1.
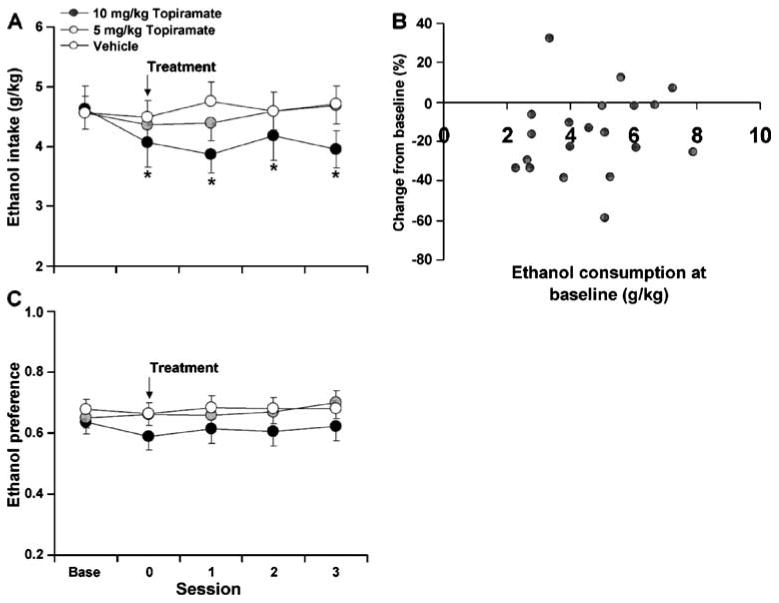
a The effect of acute topiramate treatment on mean (±SE) ethanol consumption at baseline (Base), on the day of treatment (0), and the three sessions following treatment (1, 2, 3) under the 24-h-access two-bottle choice procedure, *p<0.01 (significant difference as compared to vehicle and low topiramate treatment, n=20). b A correlation comparison between level of consumption at baseline and percent change from baseline level of intake on the day of 10 mg/kg topiramate treatment (n=20). c The effect of acute topiramate treatment on mean (±SE) preference in P rats at baseline, on the day of treatment, and the three sessions following treatment under the 24-h-access two-bottle choice procedure (n=20)
Table 1. No effect of topiramate on the day of treatment on food and water intake in P rats under the two-bottle choice procedure and Wistar rats tested under the two- and three-bottle choice procedure.
| Strain/group | Treatment | Food (mean ± SE) | Water (mean ± SE) |
|---|---|---|---|
| P rats | Vehicle | 2.67±4.88 | 10.63±8.04 |
| 5 mg/kg | −4.54±5.55 | −2.91±7.81 | |
| 10 mg/kg | −4.02±6.88 | 15.15±9.38 | |
| Wistar rats (two-bottle choice) | Vehicle | −1.85±1.44 | 3.79±8.99 |
| 5 mg/kg | 0.13±1.13 | −3.93±5.81 | |
| 10 mg/kg | 0.95±1.85 | 4.88±9.71 | |
| Wistar rats (three-bottle choice) | Vehicle | 1.58%±2.09 | −9.94±6.08 |
| 5 mg/kg | −1.30%±1.30 | 4.30±4.98 | |
| 10 mg/kg | −0.42%±0.42 | −5.12±3.29 |
In contrast, in Wistar rats under the two-bottle choice paradigm, topiramate had no effect on ethanol consumption or preference (Fig. 2a and b; p>0.05). Similarly, no effect of topiramate on ethanol consumption or preference was observed in Wistar rats under the three-bottle choice paradigm (Fig. 2c and d; p>0.05) despite significantly higher levels of ethanol consumption in this group as compared to the two-bottle choice Wistar group (t18=3.59, p<0.05). Food and water consumption was not affected in either group of Wistar rats following topiramate treatment on the day of treatment (Table 1; p>0.05) or during any of the subsequent three sessions (p>0.05).
Fig. 2.
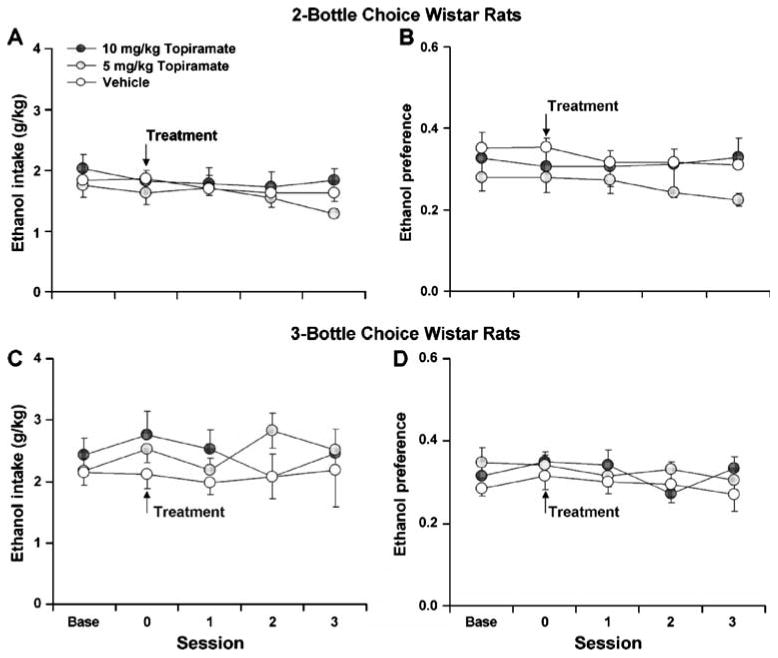
The effect of acute topiramate treatment on mean (±SE) ethanol consumption (a) and ethanol preference (b) in Wistar rats tested under the two-bottle choice procedure at baseline (Base), on the day of treatment (0), and the three sessions following treatment (1, 2, 3; n=10). The effect of acute topiramate treatment on mean (±SE) ethanol consumption (c) and ethanol preference (d) in Wistar rats tested under the three-bottle choice procedure at baseline, on the day of treatment, and the three sessions following treatment (n=10)
A summary of the effects topiramate on change from baseline on the day of treatment for P rats and Wistar rats under the two-bottle and three-bottle choice paradigm is shown in Fig. 3. An analysis of these data revealed a significant effect of strain/group (F2,37=9.31, p<0.01).
Fig. 3.
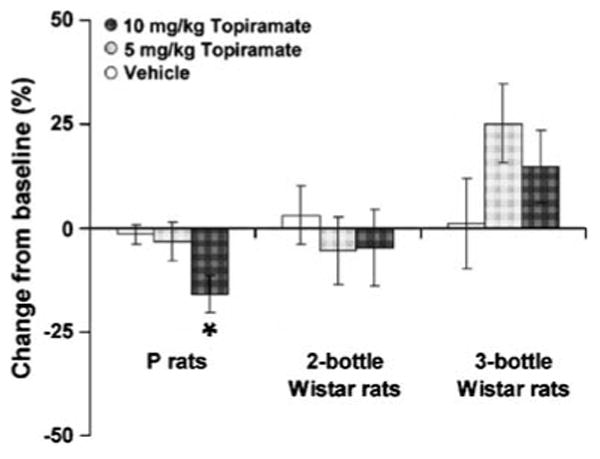
The effects of strain/group on percent change from baseline ethanol consumption (mean ± SE) in P rats and Wistar rats under the two- and three-bottle choice procedures following topiramate treatment (0, 5, and 10 mg/kg). *p<0.01 (n=20 (P rats)), 10 (two-bottle Wistar rats), and 10 (three-bottle Wistar rats)
Discussion
Topiramate's therapeutic effect to improve drinking outcomes has been most manifest among heavy-drinking, alcohol-dependent humans (Johnson et al. 2003, 2007, 2008). In light of the clinical findings, the goals of this study were to determine in an animal model of ethanol consumption whether topiramate would effectively reduce consumption and to determine whether the effect of topiramate was related to the level of consumption and/or genetic background. The results in P rats were consistent with the clinical data with topiramate producing a modest decrease in ethanol consumption. Surprisingly, in P rats, one administration of 10 mg/kg topiramate persistently decreased ethanol consumption in P rats for an average of 5 days. A similar trend was also observed for preference although the overall effect did not reach statistical significance. Lack of any changes in food and water consumption on the day of topiramate treatment or any of the following days demonstrates this effect is specific to ethanol consumption.
The effects of topiramate did not appear to be related to level of consumption in that there was no correlation between baseline consumption and treatment response (i.e., percent change from baseline on treatment days) within P rats. We also did not observe any effects of topiramate in either group of Wistar rats under the standard two-bottle choice conditions or under conditions that produced higher levels of consumption (i.e., three-bottle choice). However, it should be noted that while we did find significantly higher ethanol consumption in the three-bottle choice group of Wistar rats as compared to the other group of Wistar rats, it was still significantly less than the P rats although there was some overlap (i.e., some of the higher-drinking three-bottle choice Wistar rats did have equivalent levels of consumption with some of the lower-drinking P rats; data not shown). Even in light of higher levels of ethanol consumption in some of the Wistar animals, topiramate treatment did not reduce consumption in Wistar animals. Indeed, in some of the Wistar animals in the three-bottle choice group, topiramate treatment produced an increase in ethanol consumption although the overall group effect was not significant.
The data presented here suggests that genetic vulnerability, but not level of consumption, may be key to understanding topiramate's efficacy in reducing ethanol consumption. This finding is consistent with recent work in mice showing that topiramate's effects on acute intoxication varied by genetic strain (Chen and Holmes 2009). Differences between P rats and Wistar have been described for levels dopamine and GABA as well as signaling at these receptors that may explain the differential effects of topiramate on ethanol consumption by strain. For example, it is known that P rats have lower extracellular levels of dopamine, and higher densities of GABA axon terminals in the nucleus accumbens (see McBride et al. 1990 for review). Furthermore, in the VTA of P rats, dopamine neurons burst fire more frequently than those neurons in Wistar rats; this type of neuronal firing is known to enhance dopamine release (Morzorati 1998). Future studies are underway to understand how these known differences at baseline translate to a difference in the effects of topiramate on ethanol consumption by genetic strain.
In summary, these data show that topiramate modestly but persistently suppresses ethanol consumption in P rats, but not Wistar rats. Mechanistically, we propose that topiramate's efficacy to decrease ethanol consumption depends on genetic vulnerability but not level of drinking. Further research using chronic dosing procedures, like those used in human studies, and studies aimed at elucidating topiramate's underlying neurochemical mechanisms as a function of genetic strain will be necessary to further our understanding of its efficacy in humans.
Acknowledgments
Acknowledgment of funding and grants: This project was supported by funding from the Department of Psychiatry and Neurobehavioral Sciences at the University of Virginia in Charlottesville, Virginia. The P rats for this study were provided by the Indiana Alcohol Research Center, which is funded by grant 5P50AA007611-159005 from the National Institute on Alcohol Abuse and Alcoholism.
We thank Robert H. Cormier, Jr., BA, for his assistance with manuscript preparation, and Colin Bond for his technical assistance.
Footnotes
Declaration: The experiments described herein comply with the current laws of the United States of America.
Conflicts of interest: The authors have no potential conflicts of interest pertaining to the subject of this report. In the past 4 years, Prof. Johnson has been a consultant for Ortho-McNeil Janssen Scientific Affairs LLC, Organon, and Transcept Pharmaceuticals Inc.
Ms. Breslin and Dr. Lynch have no financial disclosures to report.
References
- Cagetti E, Pinna G, Guidotti A, Baicy K, Olsen RW. Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompanied by altered behavioral responses to neurosteroids and memory function. Neuropharmacology. 2004;46:570–579. doi: 10.1016/j.neuropharm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Chen YC, Holmes A. Effects of topiramate and other anti-glutamatergic drugs on the acute intoxicating actions of ethanol in mice: modulation by genetic strain and stress. Neuropsychopharmacology. 2009;34:1454–1466. doi: 10.1038/npp.2008.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterling DE, Zakszewski T, Moyer MD, Margul BL, Marriott TB, Nayak RK. Plasma pharmacokinetics of topiramate, a new anticonvulsant, in humans. Epilepsia. 1988;29:662. [Google Scholar]
- Gabriel KI, Cunningham CL. Effects of topiramate on ethanol and saccharin consumption and preferences in C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:75–80. doi: 10.1097/01.alc.0000150014.79657.64. [DOI] [PubMed] [Google Scholar]
- Hargreaves GA, McGregor IS. Topiramate moderately reduces the motivation to consume alcohol and has a marked antidepressant effect in rats. Alcohol Clin Exp Res. 2007;31:1900–1907. doi: 10.1111/j.1530-0277.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- Hölter SM, Engelmann M, Kirschke C, Liebsch G, Landgraf R, Spanagel R. Long-term ethanol self-administration with repeated ethanol deprivation episodes changes ethanol drinking pattern and increases anxiety-related behaviour during ethanol deprivation in rats. Behav Pharmacol. 1998;9:41–48. [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Addolorato G, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O'Malley SS, Swift RM, Topiramate for Alcoholism Advisory Board. Topiramate for Alcoholism Study Group Improvement of physical health and quality of life of alcohol-dependent individuals with topiramate treatment. Arch Intern Med. 2008;168:1188–1199. doi: 10.1001/archinte.168.11.1188. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O'Malley SS, Swift RM, Topiramate for Alcoholism Advisory Board. Topiramate for Alcoholism Study Group Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Mercado M, Markley TL, Crosby S, Ciraulo DA, Kornetsky C. Zonisamide decreases ethanol intake in rats and mice. Pharmacol Biochem Behav. 2007;87:65–72. doi: 10.1016/j.pbb.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Lê AD, Kiianmaa K, Cunningham CL, Engel JA, Ericson M, Söderpalm B, Koob GF, Roberts AJ, Weiss F, Hyttiä P, Janhunen S, Mikkola J, Bäckström P, Ponomarev I, Crabbe JC. Neurobiological processes in alcohol addiction. Alcohol Clin Exp Res. 2001;25:144S–151S. doi: 10.1111/j.1530-0277.2001.tb02389.x. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Chernet E, Russell RN, Wong DT, Guan XM, Lumeng L, Li TK. Regional CNS densities of monoamine receptors in alcohol-naive alcohol-preferring P and -nonpreferring NP rats. Alcohol. 1997;14:141–148. doi: 10.1016/s0741-8329(96)00117-6. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Lumeng L, Li TK. Serotonin, dopamine and GABA involvement in alcohol drinking of selectively bred rats. Alcohol. 1990;7:199–205. doi: 10.1016/0741-8329(90)90005-w. [DOI] [PubMed] [Google Scholar]
- Morzorati SL. VTA dopamine neuron activity distinguishes alcohol-preferring (P) rats from Wistar rats. Alcohol Clin Exp Res. 1998;22:854–857. [PubMed] [Google Scholar]
- Nguyen SA, Malcolm R, Middaugh LD. Topiramate reduces ethanol consumption by C57BL/6 mice. Synapse. 2007;61:150–156. doi: 10.1002/syn.20350. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav. 2004;79:439–450. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Samson HH, Tolliver GA, Pfeffer AO, Sadeghi K, Haraguchi M. Relation of ethanol self-administration to feeding and drinking in a nonrestricted access situation in rats initiated to self-administer ethanol using the sucrose-fading technique. Alcohol. 1988;5:375–385. doi: 10.1016/0741-8329(88)90024-9. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat. Behav Brain Res. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]


