Abstract
Background
Exposure to chronic stress produces negative effects on mood and hippocampus-dependent memory formation. Alterations in signaling cascades and histone acetylation present a mechanism of modulation of transcription that may underlie stress-dependent processes in the hippocampus critical to learning and memory and development of depressive behaviors.
Methods
The rat model of chronic variable stress (CVS) was used to investigate the role of changes in protein acetylation and other molecular components of hippocampus-dependent memory formation and anhedonic behavior in response to CVS.
Results
Chronic variable stress treatment decreased both extracellular signal-regulated protein kinases 1 and 2 activation and Bcl-2 expression in all three regions of the hippocampus that corresponded behaviorally with a decrease in memory for the novel object location task and increased anhedonia. Extracellular signal-regulated protein kinases 1 and 2 activation was not significantly affected in the amygdala and increased in the medial prefrontal cortex by CVS. Chronic variable stress had no significant effect on activation of Akt in the hippocampus. We investigated molecular and behavioral effects of infusion of the sirtuin inhibitor, sirtinol, into the dentate gyrus (DG). Sirtinol infusion into the DG prevented the CVS-mediated decrease in extracellular signal-regulated protein kinases 1 and 2 activity and Bcl-2 expression, as well as histone acetylation in the DG previously observed following CVS. This corresponded to enhanced performance on the novel object location memory task, as well as reduced anhedonic behavior.
Conclusions
These results suggest that changes in sirtuin activity contribute to changes in molecular cascades and histone acetylation within the hippocampus observed following CVS and may represent a novel therapeutic target for stress-induced depression.
Keywords: Anhedonia, Bcl-2, epigenetics, hippocampus, histone acetylation, spatial memory
While many studies have shown that chronic stress has the ability to exert negative influences on hippocampus function (1–4), the molecular mechanisms responsible for this effect are poorly understood. We previously demonstrated that the nicotinamide adenine dinucleotide-dependent deacetylase, SIRT1, is activated in the hippocampus during chronic stress (5). SIRT1 is part of the sirtuin family of class III histone deacetylases (SIRT1-7) that is highly expressed throughout the hippocampus (6) and is localized to both the cytoplasm and the cell nucleus, where it can deacetylate histones as well as stress response factors (7). The function of this increased SIRT1 activity in chronic stress is unknown.
Sirtuins play a role in cognitive function and mood regulation, but their role in memory formation is not well understood. Recent studies suggest that SIRT1 expression is essential for hippocampus-dependent memory formation (8,9), but another study showed that overexpression of SIRT1 inhibits memory formation in young and old animals (10). In humans, variations in the SIRT1 gene are associated with anxiety (11), and SIRT1 expression is correlated with prevalence of depression (12). Brain-specific Sirt1 knockout mice showed reduced anxiety, while Sirt1 overexpressing mice exhibited enhanced anxiety. Furthermore, socially defeated wild-type mice demonstrated reduced sucrose preference, while brain-specific Sirt1 knockout mice did not (11), suggesting SIRT1 function mediates a susceptibility to stress-induced anhedonia. Additionally, SIRT1 in the nucleus accumbens is associated with chronic cocaine use in rodent models (13). This contrasting evidence suggests that sirtuin activity is important for both mood regulation and memory formation and its activity must be tightly regulated for proper hippocampus function. We hypothesized that hyperactivation of sirtuins during chronic stress modulates molecular and behavioral effects of chronic stress. We specifically investigated molecular cascades in the hippocampus, hippocampus-dependent memory, and anhedonic behavior.
Methods and Materials
Rats
Male Wistar rats (42 days of age; Harlan Inc., Indianapolis, Indiana) were housed in pairs on a 12:12 light/dark cycle (lights on at 0700 hours) and received food and water ad libitum. Rats were given 14 days to acclimate before experimental manipulation. All experimental procedures were conducted during the light cycle and were approved by the Tulane University Institutional Animal Care and Use Committee.
Chronic Variable Stress
Rats (56 days of age) were randomly assigned to control and chronic variable stress (CVS) groups. Chronic variable stress was conducted using a modified method previously reported (5,14) (Supplemental Methods and Materials in Supplement 1) and consisted of twice-daily exposure to randomly assigned stressors applied over 14 days. Sirtinol infusion techniques are described in Supplemental Methods & Materials in Supplement 1. Physiological markers of stress are reported in Supplemental Results in Supplement 1.
Sucrose Preference Test
A subset of rats was tested on day 14 of CVS protocol for sucrose preference. Rats were given 12 hours (0700 to 1900 hours) to habituate to a free choice between two bottles, both containing tap water. At 1900 hours, one bottle was replaced with 3% sucrose. The other bottle remained as tap water. Rats were given free choice between sucrose and tap water for 12 hours (1900 to 0700 hours). To prevent possible effects of side preference, the position of the sucrose bottle was randomly distributed among the cages. Consumption was calculated as the percentage of sucrose fluid volume consumed to total fluid volume consumed.
Corticosterone Assay
Trunk blood was allowed to coagulate at room temperature for 90 minutes. Samples were centrifuged at 2000 g for 15 minutes, serum was collected, and samples were stored at −20°C. Samples were sent to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for corticosterone measurements by radioimmunoassay.
SIRT1 Activity Assay
SIRT1 activity was assessed in extracts from hippocampal preparations via a commercially available kit (Sirtuin 1 Activity Assay Kit; Calbiochem, San Diego, California) (Supplemental Methods and Materials in Supplement 1).
Tissue Processing
Molecular analysis was performed on a separate group of rats that did not receive memory or sucrose preference testing to avoid the effects of behavioral testing on molecular changes as previously described (5) (Supplemental Methods and Materials in Supplement 1).
Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) assays were performed as previously described (15).
Messenger RNA Extraction and Reverse Transcriptase Polymerase Chain Reaction
Samples were processed using the RNAeasy Mini Kit (Qiagen, Alameda, California) according to the manufacturer’s instructions. The RNA was purified with RNAeasy microcolumns and reverse-transcribed using iScript cDNA Synthesis Kit (Biorad, Hercules, California). Complementary DNA was quantified by quantitative polymerase chain reaction using iQ SYBR Green (BioRad). Each reaction was run in triplicate and analyzed using the ΔΔCt method. Real-time polymerase chain reaction assays were tested to determine and compare the efficiencies of the target and control gene amplifications to ensure high (90% to 100%) and similar efficiency. A single peak on the melt curve confirmed the presence of specific amplification products. The primer sequences used for the reverse transcriptase polymerase chain reactions are described in Supplement 1.
Results
Impaired Object Location Following Chronic Variable Stress
We first investigated the performance of control and CVS-treated rats in the object location task. On the day of training and testing (morning of day 15), rats received an initial sample trial and were tested for object location memory 30 minutes later (Figure 1A). No differences in total object investigation independent of location were detected between control rats and rats exposed to chronic variable stress during the sample trial (t10 = ± .86, p >.1) or during the retention trial (t10 =±.83, p > .1). A significant difference in object location memory between the groups was revealed (t10 = ±3.32, p < .01), in which rats subjected to chronic variable stress spent significantly less time investigating the object in the novel location during the retention trial than control rats (Figure 1B). Furthermore, control rats (t5 = ±4.60, p < .01), but not rats exposed to chronic variable stress (t5 =±.06, p > .1), investigated the object in the novel location at a level greater than chance during the retention trial (Figure 1B). These results show that CVS has a significant negative effect on object location memory in adult male rats.
Figure 1.
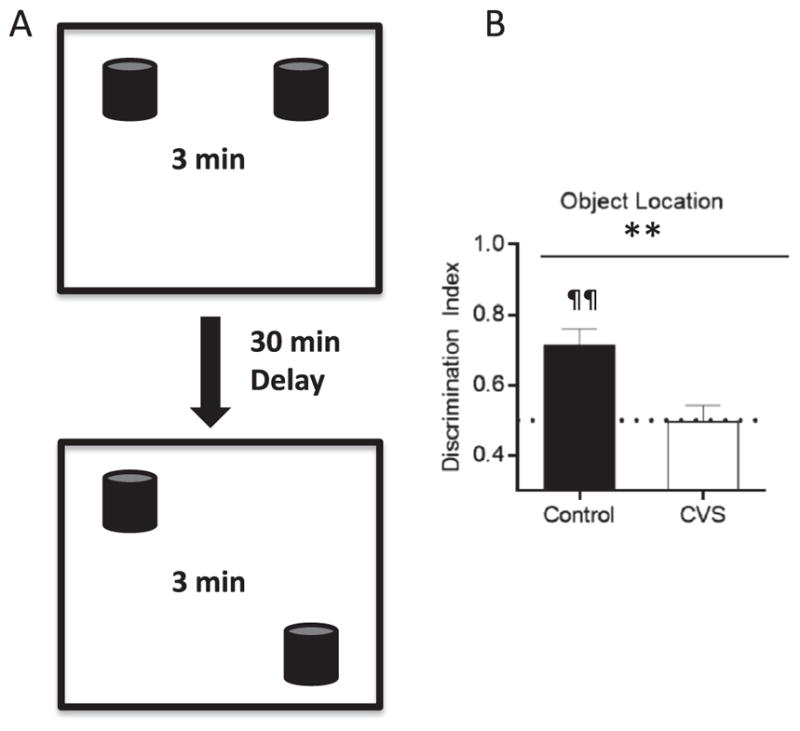
Chronic variable stress (CVS) decreases memory formation in the hippocampal-dependent task, novel object location. (A) Schematic representation of the behavioral protocol used for novel object location in a black Plexiglas open arena. During the sample phase, rats were exposed to two identical objects and allowed to freely explore for 3 minutes. Following a 30-minute delay period in their home cages, rats were returned to the open arena for the 3-minute retention trial in which one of the objects had been moved to a novel location. The discrimination index was defined as the ratio of time spent investigating the object in the novel location relative to total time investigating the objects during the first minute of the retention trial. (B) Chronic variable stress treated animals spent significantly less time investigating the object in the novel location than control rats. In addition, control rats only investigated the object in the novel location at a level greater than chance. The dotted line represents chance level (.5). Data are shown as means ± SE; **p< .01 versus control, ¶¶p < .01 versus chance (.5); (n = 6 animals per group).
Regulation of Extracellular Signal-Regulated Protein Kinases 1 and 2 and the Downstream Neuroprotective Target Bcl-2 in Chronic Variable Stress
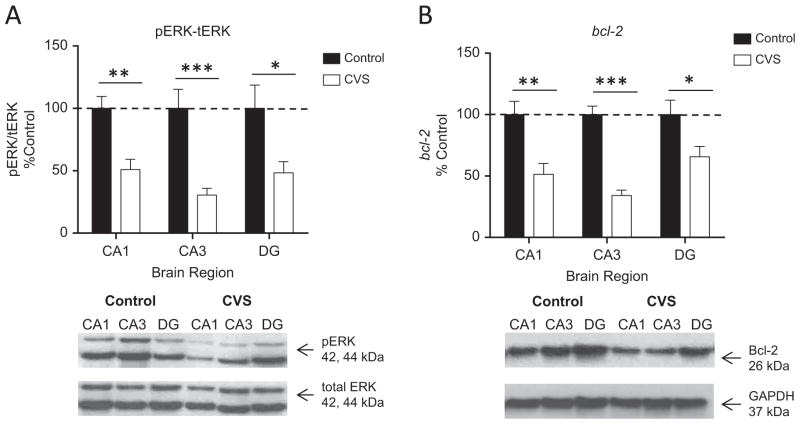
Given the relationship between the extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) pathway and memory formation, ERK1/2 activation in the subregions of the hippocampus were investigated by Western blot in control and CVS-treated rats. Two-way analysis of variance (ANOVA) revealed a significant effect of CVS condition on ERK1/2 activation (F1,25 = 35.04, p < .0001; Figure 2A). Subsequent pairwise comparisons showed a significant reduction in immunoreactivity of the phospho-ERK1/2 antibody relative to total ERK1/2 across all three regions of the hippocampus of CVS rats (cornu ammonis 1 [CA1] [51 ± 8%, n = 5 compared with control rats at 100 ± 10%, n = 6, p < .05]; cornu ammonis 3 [CA3] [31 ± 5%, n = 5 compared with control rats at 100 ± 15%, n = 6, p < .001]; and dentate gyrus [DG] [48 ± 9%, n = 5 compared with control rats at 100 ± 19%, n = 4, p < .05]). These results show that CVS significantly reduced ERK1/2 activation in three subregions of the hippocampus.
Figure 2.
Activation of extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) and expression of its downstream neuroprotective target protein, Bcl-2, in the subregions of the hippocampus of chronic variable stress (CVS) animals. (A) Quantification and representative Western blots of phosphorylated ERK1/2 relative to total ERK1/2 in the hippocampus subregions of control and CVS animals. A significant decrease in activated ERK1/2 relative to total ERK1/2 was observed in the cornu ammonis 1 (CA1), cornu ammonis 3 (CA3), and dentate gyrus (DG) regions of the hippocampus of CVS animals compared with control animals. (B) Quantification and representative Western blots of Bcl-2 protein expression relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein from the hippocampus of control and CVS animals. Chronic variable stress resulted in a significant decrease in Bcl-2 protein relative to GAPDH protein in all three regions of the hippocampus compared with control animals. Data are shown as mean ± SE; *p < .05 vs. control; **p < .01 vs. control; ***p < .0001 vs. control; (n = 4–6 animals per group). pERK, phosphorylated extracellular signal-regulated kinase; tERK, total extracellular signal-regulated kinase.
Since Bcl-2 is downstream of ERK1/2 activation, the effect of chronic stress on expression of Bcl-2 in the subregions of the hippocampus was also determined. Bcl-2 is a neuroprotective protein within the Bcl-2 family of antiapoptotic proteins and exerts neurotrophic effects when activated downstream of ERK1/2 (16,17). Two-way ANOVA revealed a significant effect of CVS condition (F1,25 = 40.40, p < .0001; Figure 2C). Further pairwise comparisons demonstrated that there was a significant reduction in immunoreactivity of the Bcl-2 antibody relative to glyceraldehyde 3-phosphate dehydrogenase antibody in the CA1 (51 ± 9%, n = 6), CA3 (34 ± 4%, n = 5), and DG (66 ± 8%, n = 5) regions of the hippocampus of CVS rats compared with control rats (CA1 [100 ± 11%, n = 6, p < .01]; CA3 [100 ± 7%, n = 4, p < .001]; and DG [100 ± 12%, n = 6, p < .05]). This suggests that ERK1/2 activation and Bcl-2 expression are decreased in parallel by CVS in each area of the hippocampus. We also investigated ERK1/2 activation in other areas of the brain known to be involved in the response to stress, specifically the medial prefrontal cortex and amygdala (Figure S1 in Supplement 1).
Transcriptional Regulation of Bcl-2 in the DG of Chronically Stressed Rats
We have previously shown decreased acetylation of H4(K12) in the DG of CVS-treated rats (5). To further investigate how changes in H4 acetylation in DG contribute to transcriptional regulation of genes important for learning and memory, ChIP on the DG of chronically stressed rats was performed. A comparison by t test showed a significant increase in acetylation of H4(K12) associated with the Bcl-2 promoter in chronically stressed rats (2624 ± 1069, n = 4; Figure 3A) compared with control rats (23 ± 9, n = 6, t10 = 3.1, p < .05).
Figure 3.
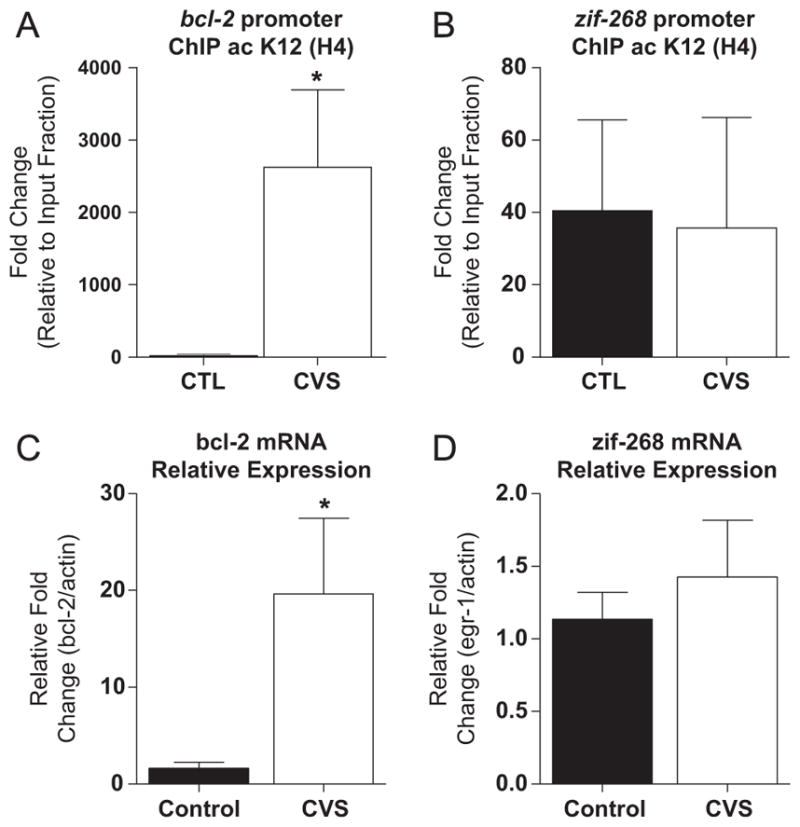
Regulation of Bcl-2 gene expression in the dentate gyrus (DG) of chronic variable stress (CVS) animals. (A) Chromatin immunoprecipitation (ChIP) for the Bcl-2 promoter associated with acetylation of lysine 12 on histone 4 (ac K12 [H4]). Chronic variable stress results in a significantly increased fold change relative to the input fraction compared with control (CTL) animals. (B) Chromatin immunoprecipitation for the zif-268 promoter associated with ac K12 (H4). No significant difference between control and CVS animals was seen in regulation of the zif-268 promoter associated with ac K12 (H4). (C) Quantification of relative fold change in Bcl-2 messenger RNA (mRNA) expression normalized to actin in the DG of both control and CVS animals. A significant increase in Bcl-2 mRNA expression was seen compared with control animals. (D) No significant difference in zif-268 mRNA expression was observed between control and CVS animals in the DG of the hippocampus. Data are shown as means ± SE; *p < .05 vs. control; (n = 4–6 animals per group for ChIP; n = 8 animals per group for mRNA).
We also investigated transcriptional regulation at the promoter of another gene involved in long-term memory formation and regulated by stress in the hippocampus, Zif-268. Chromatin immunoprecipitation from the DG revealed that there was no significant difference in transcriptional regulation of the Zif-268 promoter (t9 = .12, p = .95; Figure 3B) associated with observed alterations in H4(K12) for CVS rats. This suggests that increased acetylation of H4(K12) associated with the Bcl-2 promoter observed in CVS rats is specific and not a reflection of global transcriptional regulation.
We also investigated messenger RNA (mRNA) expression levels of both Bcl-2 and zif-268. A significant increase in Bcl-2 mRNA expression relative to actin in the DG of CVS animals was observed (20 ± 8%, n = 8; Figure 3C) compared with control animals (2 ± 1%, n = 8, t14 = 2.30, p < .05). In contrast, mRNA levels of zif-268 were unchanged between control and CVS animals (t14 = .67, p = .51; Figure 3D).
Effects of Sirtuins in the Hippocampus
Of the seven mammalian homologues of sirtuins, SIRT1, SIRT2, SIRT3, and SIRT5 have characterized deacetylase functions; however, SIRT3 and SIRT5 are functionally restricted to the mitochondria, limiting their role in transcriptional regulation (18–21). Therefore, we targeted SIRT1 and SIRT2 as a possible mechanism to regulate histone acetylation. SIRT1 is localized to both the nucleus (22,23) and the cytoplasm (24). SIRT1 is highly expressed in the brain, particularly within the structures of the hippocampus and hypothalamus, which are important for a role in the hypothalamic-pituitary-adrenal axis (6). In addition to its function as a histone deacetylase, SIRT1 deacetylates several nonhistone targets, including the hormone receptors (21); p300, a histone acetyltransferase and nuclear receptor co-activator (25); and several transcription factors including p53 and NFκB (26). SIRT2 is primarily a cytoplasmic enzyme responsible for the deacetylation of α-tubulin (27).
We previously showed that CVS resulted in a decrease in acetylation of the SIRT1-specific nonhistone target, acetylated p53, and concomitant increase in SIRT1 activity in the CA3 and DG regions of the hippocampus with CVS (5). In this study, we examined differences in acetylation of α-tubulin, a SIRT2-specific nonhistone target (27) in the hippocampus of control and CVS animals (Figure 4). Western blotting revealed no significant difference in acetylation of α-tubulin between control and CVS animals (t = .3, df = 10; p = .8, n = 6 per group). These results show that the α-tubulin substrate for SIRT2 is not significantly differentially acetylated in response to CVS and suggest that SIRT2 may not be significantly activated in the hippocampus in the CVS-treated animals compared with control animals. It does not, however, rule out the possibility of SIRT2 deacetylation of other substrates.
Figure 4.
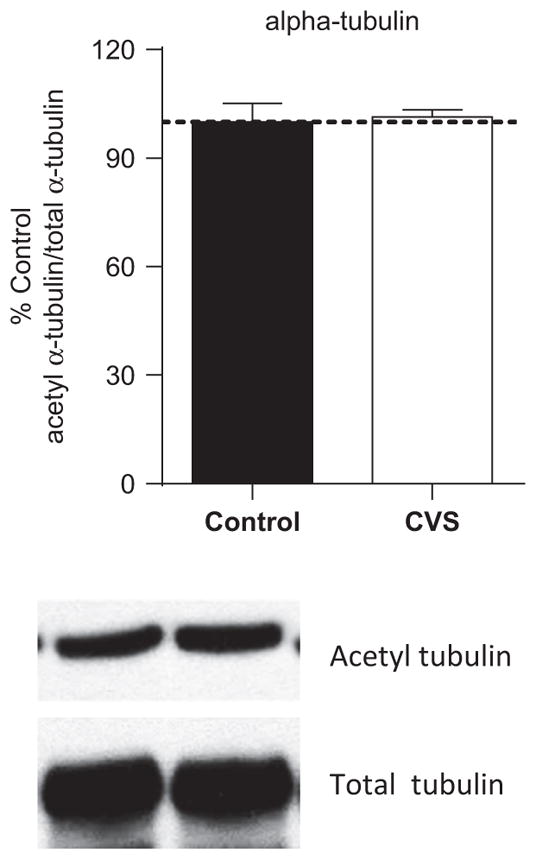
Alpha-tubulin is not significantly acetylated in chronic stress relative to control animals. Hippocampus tissue from control rats and chronic variable stress (CVS) treated rats was tested for levels of α-tubulin acetylation. Quantification (upper) and representative Western blots (lower) of acetylated α-tubulin relative to total α-tubulin from the cytosolic fraction of control and CVS animals. No significant difference of α-tubulin acetylation was observed between control and CVS-treated hippocampus (p = .8, n = 6 per group).
Sirtuin Inhibition in the DG Rescues the Effects of CVS on ERK1/2 and Bcl-2
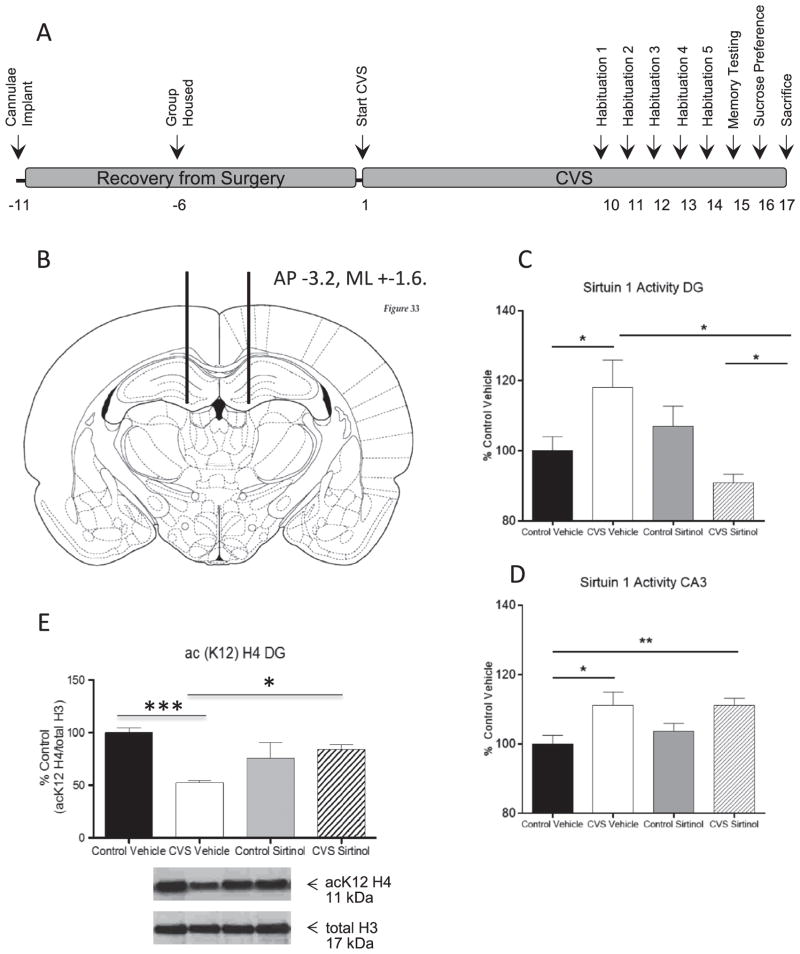
The physiological function of this increased SIRT activity in CVS was determined. Sirtinol, a SIRT1 and SIRT2 inhibitor, was infused in the DG according to methods (see Supplement 1). Figure 5A shows the timeline of treatments the animals received, and Figure 5B shows the site of the bilateral cannulation of the dorsal DG. Table S2 in Supplement 1 shows that chronic sirtinol infusion into the DG had no significant effect on physiological parameters associated with chronic stress exposure. Furthermore, no significant difference in corticosterone was observed between the CVS sham, CVS vehicle, or CVS sirtinol animals, suggesting that sirtinol does not affect baseline hypothalamic-pituitary-adrenal axis response to stress.
Figure 5.
Sirtinol infusion into the dentate gyrus (DG) blocks the chronic variable stress (CVS)-induced increase in SIRT1 activity. (A) Schematic showing the time line and experimental design. (B) Schematic of the rat brain showing the infusion coordinates (black lines) into the dorsal hippocampus. (C, D) Quantification of SIRT1 activity in the DG (C) and cornu ammonis 3 (CA3) (D) of the hippocampus of control and CVS animals following chronic DG infusion of either the SIRT1 inhibitor, sirtinol (50 μmol/L), or vehicle (5% hydroxypropyl β-cyclodextrin). (C) Chronic variable stress resulted in a significant increase in SIRT1 activity in the DG compared with control animals. Sirtinol attenuated this increase and resulted in a significant reduction in SIRT1 activity compared with vehicle-infused animals. (D) SIRT1 activity was significantly increased in the CA3 following CVS. Sirtinol infusion into the DG failed to rescue this effect in the CA3 region. (E) Immunoblotting of acetylated histone 4 lysine 12 (acH4[K12]) antibody from the DG of control and CVS animals following infusion of either sirtinol or vehicle. A significant decrease in acetylation of H4 (K12) was seen in the DG of CVS vehicle-treated animals compared with control vehicle-treated animals. No significant difference was observed in acetylation of H4 (K12) between control vehicle-treated, control sirtinol-treated, and CVS sirtinol-treated animals; however, CVS sirtinol-treated animals showed a significantly greater acetylation of H4 (K12) in the DG regions of the hippocampus compared with CVS vehicle-treated animals. Data are shown as means ± SE; *p < .05; **p < .01; ***p < .001; (n = 5–6 animals per group for sirtuin 1 activity; n = 6–8 animals per group for histone acetylation). (Coronal rat brain section reproduced with permission from Paxinos and Watson [45].) AP, anterior-posterior; ML, medial-lateral.
We next confirmed that sirtinol infusion into the DG of the hippocampus affected SIRT1 activity in the DG. One-way ANOVA revealed a significant effect of treatment condition on SIRT1 activity in the DG (F3,19 = 4.58, p = .02; Figure 5C). Subsequent pairwise comparisons confirmed our previous results, as there was a significant increase in SIRT1 activity in the DG of CVS vehicle-treated animals (118 ± 8%, n = 5) compared with control vehicle-treated animals (100 ± 4%, n = 5, p = .03). However, infusion of sirtinol into the DG of CVS animals resulted in a significant decrease in SIRT1 activity compared with CVS vehicle-treated animals (n = 5; p = .01). Surprisingly, levels of SIRT1 activity in CVS sirtinol-treated animals were also significantly less than in control sirtinol-treated animals (p = .03). Levels in CVS sirtinol-treated animals were not significantly different from control vehicle-treated animals, suggesting that sirtinol infusion was able to block the CVS-induced increase in SIRT1 in the DG. We also assessed SIRT1 activity in the nearby CA3 region. One-way ANOVA showed a significant effect of CVS on SIRT1 activity in the CA3 (F3,19 = 4.13, p = .02; Figure 5D). As we had previously observed, CVS resulted in an increase in SIRT1 activity in the CA3 region of the hippocampus of CVS-vehicle treated animals (111 ± 4%, n = 5) compared with control vehicle-treated animals (100 ± 3%, n = 5, p = .04). Sirtinol infusion did not reduce SIRT1 activity in the CA3 region, as CVS sirtinol-treated animals had significantly higher levels of SIRT1 activity than control vehicle-treated animals (111 ± 2%, n = 5, p = .01). These results demonstrate that the SIRT inhibition was localized to the DG in our study.
We also confirmed that infusion of sirtinol directly into the DG prevented the decrease in acetylation of H4(K12) we had previously seen. One-way ANOVA showed an overall effect of drug treatment on acetylation of H4(K12) in the DG (F3,26 = 7.43, p < .01; Figure 5E). Subsequent pairwise comparisons revealed CVS vehicle-treated animals showed a significant decrease in acetylation of H4(K12) (52 ± 3%, n = 7) compared with control vehicle-treated animals (100 ± 5%, n = 6, p < .001) in the DG. However, CVS sirtinol-treated animals had levels of H4(K12) acetylation that were significantly greater than CVS vehicle-treated animals (84 ± 4%, n = 8 compared with CVS vehicle-treated animals at 52 ± 3%, n = 7, p < .05), suggesting that chronic sirtinol infusion into the DG prevented decreased acetylation of H4(K12) seen as a result of CVS exposure. Furthermore, the level of H4(K12) acetylation in CVS sirtinol-treated animals was not significantly different than both control vehicle-treated and control sirtinol-treated animals.
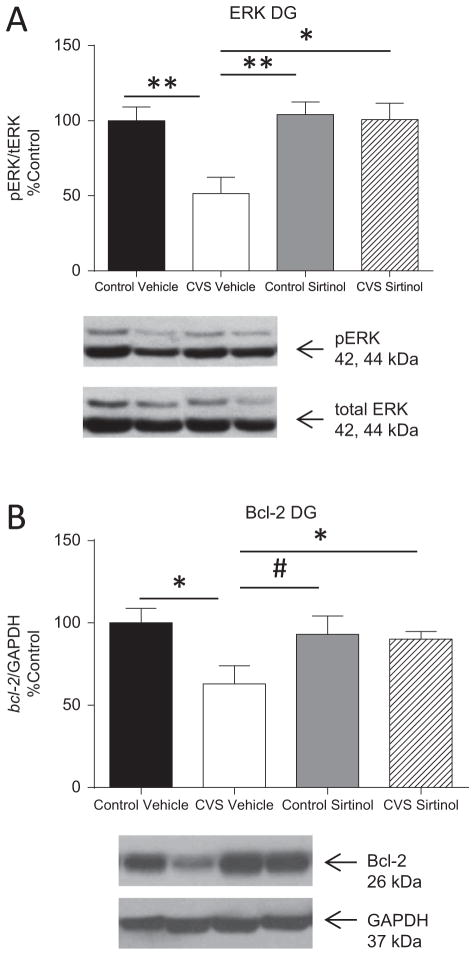
Next, effects of inhibition of SIRT activity in the DG of CVS animals on molecular changes in the DG were determined. Chronic variable stress resulted in a significant decrease in ERK1/2 activation in CVS vehicle-treated animals (52 ± 11%, n = 5; Figure 6A) compared with both control vehicle-treated animals (100 ± 9%, n = 5, p = .01) and control sirtinol-treated animals (104 ± 8%, n = 5, p = 0.005). Sirtinol infusion into the DG reversed the decrease in ERK1/2 activation in CVS sirtinol-treated animals (101 ± 11%, n = 6) compared with CVS vehicle-treated animals (p = .01). In fact, CVS sirtinol-treated animals had levels of ERK1/2 activation that were not significantly different from both control vehicle-treated or control sirtinol-treated animals, suggesting that blocking sirtuin activity was able to attenuate the effects of CVS on the ERK1/2 activation in the DG.
Figure 6.
SIRT1 inhibition is able to rescue the molecular effects of chronic variable stress (CVS) on extracellular signal-regulated kinase (ERK) and Bcl-2 protein expression in the dentate gyrus (DG). Quantification and representative Western blots of extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) and Bcl-2 protein expression in the DG of the hippocampus of control vehicle-treated, CVS vehicle-treated, control sirtinol-treated, and CVS sirtinol-treated animals. (A) Immunoblotting revealed that a significant reduction in phosphorylated ERK1/2 to total ERK1/2 protein expression was seen in the DG in CVS vehicle-treated animals. Sirtinol infusion into the DG significantly reversed this decrease to levels similar to both control vehicle-treated and control sirtinol-treated animals. (B) Bcl-2 protein expression was significantly decreased relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein expression in the DG following CVS exposure in the vehicle-treated group. Sirtinol significantly increased levels of Bcl-2 protein expression CVS exposure from the vehicle-treated CVS animals. Chronic variable stress sirtinol-treated animals had Bcl-2 protein levels that did not significantly differ from control vehicle-treated animals or control sirtinol-treated animals. Data are shown as means ± SE; *p < .05; **p <.01, #p = .09 (n = 5–6 animals per group). pERK, phosphorylated extracellular signal-regulated kinase; tERK, total extracellular signal-regulated kinase.
Chronic variable stress resulted in a significant decrease in Bcl-2 protein in CVS vehicle-treated animals (63 ± 11%, n = 5; Figure 6B) compared with control vehicle-treated animals (100 ± 9%, n = 5, p = .03) as previously observed, with a trend toward significant compared with control sirtinol-treated animals (93 ± 11%, n = 5, p = .09). Sirtinol attenuated the effect of CVS on Bcl-2 protein in the DG. Chronic variable stress sirtinol-treated animals (90 ± 5%, n = 6) had a significant increase in Bcl-2 protein expression in the DG compared with CVS vehicle-treated animals (p = .04). This suggests that increased sirtuin activity in the DG following CVS contributes to downregulation of ERK1/2 and Bcl-2. We also investigated the effects of CVS and sirtinol infusion in the DG on another important signal transduction pathway, the Akt pathway (Figure S2 in Supplement 1). The Akt pathway can be regulated in parallel with the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway in plasticity (28). In addition, Akt is responsible for direct inhibition of glycogen synthase kinase-3β, a kinase that has emerged as a significant target of mood-stabilizing agents like valproate and lithium and is regulated by some antidepressants (fluoxetine and imipramine) (29,30). The results suggest that Akt is not significantly affected by CVS or by sirtinol.
Sirtinol Treatment Prevents Stress-Induced Deficits in Spatial Memory
Sirtinol-infused rats were tested for object location memory similar to the protocol described above. There were no differences in total object investigation independent of location between groups during the sample trial (p = .8) or during the retention trial (p = .8; data not shown). Control animals, regardless of treatment, investigated the moved object at levels significantly greater than chance (control sham t13 = ±3.3, p < .05; control vehicle t8 = ±2.7, p < .01; control sirtinol t10 = ±4.8, p < .001). Furthermore, chronically stressed animals that received sirtinol infusion directly into the DG also investigated the novel location significantly greater than chance (t11 = ±4.7, p < .001). By contrast, untreated chronically stressed animals explored the novel location at chance levels (CVS sham t11 = ±.1, p > .05; CVS vehicle t8 = ±.9, p > .05). Two-way ANOVA revealed a significant main effect of drug on object location memory (F2,62 = 4.656, p = .02; Figure 7). No significant interaction (p = .2) or effect of stress (p = .2) was observed. These results suggest that while CVS has a negative effect on object location memory in adult male rats, chronic treatment with sirtinol into the dorsal DG attenuated this effect.
Figure 7.
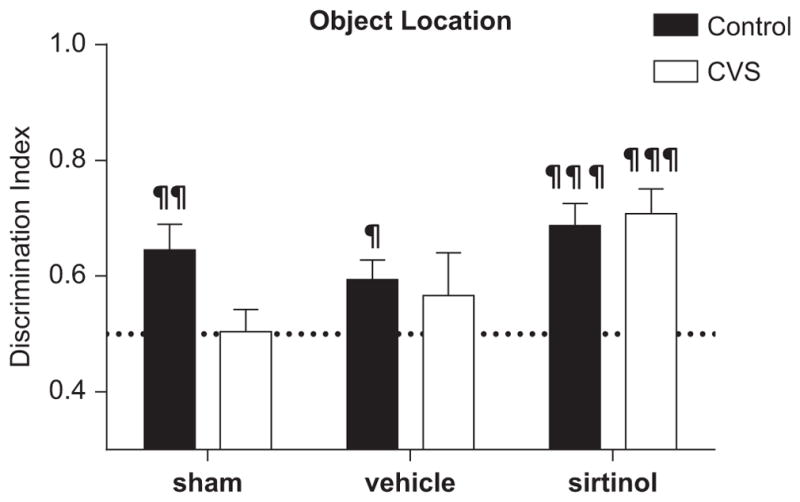
Sirtinol infusion into the dentate gyrus prevents memory impairment on the novel object location task. Chronic variable stress (CVS) animals that received chronic sirtinol infusion into the hippocampus performed significantly better than CVS sham and there was a trend toward significance compared with the CVS vehicle-treated animals on the novel object location task. Chronic variable stress sham and CVS vehicle-treated animals performed at the level of chance, while CVS sirtinol-treated, control sham, control vehicle-treated, and control sirtinol-treated animals all performed significantly better than chance. Data are shown as means ± SE, ¶p < .05, ¶¶p <.01, ¶¶¶p <.001 versus chance (.5). The dotted line represents chance level (.5). The results from animals shown in Figure 1 are included in graph.
Sirtinol Treatment Prevents the Development of Anhedonia in Chronically Stressed Animals
The effect of sirtinol infusion in the DG on sucrose preference as a measure of anhedonia was determined. Two-way ANOVA revealed a significant interaction of drug and stress condition on sucrose preference (F2,17 = 8.7 p <.01; Figure 8). Subsequent pairwise comparisons showed that CVS sham animals did not show preference for sucrose (50 ± 5%, n = 4) compared with control shams (83 ± 2%, n = 4, p < .01). Additionally, CVS vehicle-treated animals also demonstrated anhedonic behavior and did not show a preference for sucrose (48 ± 6%, n = 4, p <.01). However, CVS sirtinol-treated animals demonstrated a clear preference for sucrose (75 ±2%, n = 4) that was statistically greater than both CVS shams (p < .05) and CVS vehicle-treated animals (p < .05). There was no difference in sucrose preference between CVS sirtinol-treated animals and animals that were not chronically stressed regardless of drug treatment. This suggests that infusion of sirtinol into the DG of animals subjected to CVS prevented the deficit in sucrose preference.
Figure 8.
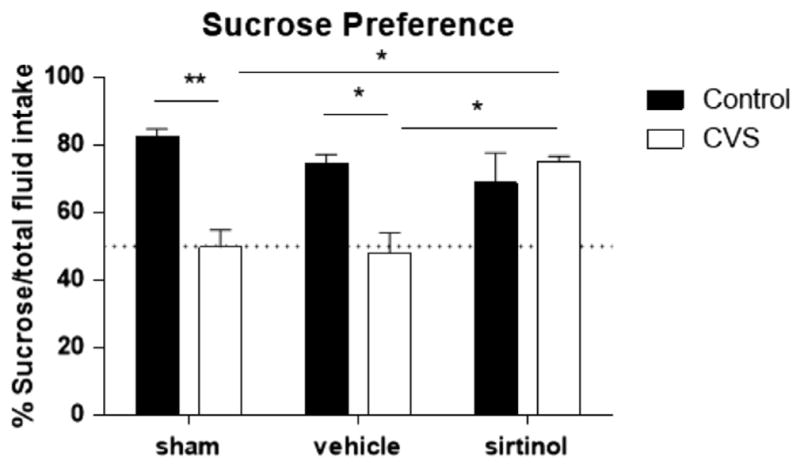
Sirtinol infusion prevents the development of depressive-like behavior in chronic variable stress (CVS) exposed rats. Sucrose preference was assessed on CVS and control animals subjected to either sham, vehicle, or sirtinol infusion into the dentate gyrus. Control animals regardless of condition demonstrated a clear preference for sucrose. Chronic variable stress sham and CVS vehicle-treated animals failed to show preference for sucrose. Chronic sirtinol infusion into the dentate gyrus prevented the development of this depressive-like behavior. The dotted line represents chance level (50%). Data are shown as means ± SE; *p < .05; **p < .01 (n = 8 animals per group).
Discussion
In this study, we showed that CVS increased depressive-like behavior as measured by decreased sucrose preference, a measure of anhedonic behavior, as well as decreased memory performance on the object location task. These behavioral changes corresponded to an overall decrease in H4(K12) acetylation, ERK1/2 phosphorylation, and Bcl-2 expression in the subregions of the hippocampus subsequent to chronic stress. We saw no significant effect of stress or sirtinol on Akt phosphorylation. This result is consistent with a previous study that showed sirtinol had no effect on the Akt pathway but reduced ERK1/2 signaling (31). Furthermore, we showed there is no effect of CVS on ERK1/2 phosphorylation in the amygdala, but ERK1/2 phosphorylation is increased in the medial prefrontal cortex and Bcl-2 levels are unchanged. This suggests that the effects of stress on ERK1/2 activation and subsequent pathways are region-specific. Importantly, we showed that the anhedonia and memory deficits, as well as the molecular effects, were reversed by sirtuin inhibition in the DG. Interestingly, sirtuin inhibition did not reverse the physiological effects of CVS, adrenal hypertrophy, reduced weight gain, and elevated corti-costerone levels. This suggests that sirtuin activity in the DG is not involved in regulation of these measures.
We found impaired object location memory in rats exposed to CVS. A limitation of this study is that we only performed one memory test. It is unknown whether sirtinol would reverse the effect of CVS on all tests of hippocampus-dependent memory. We did not investigate other tasks that involved swimming, such as Morris water maze, since the swim stress that CVS animals experience may confound the results. In addition, tests that require food deprivation, such as the radial arm maze, would introduce the stress of food deprivation into the control cohorts. Indeed, food deprivation increases corticosterone levels in mice (32). We conclude from our study that CVS impaired, and sirtinol reversed, object location memory. Consistent with previous reports that show a role for ERK1/2 in spatial memory (33–35), our results indicate that the integrity of ERK1/2 is essential for spatial learning and memory, as impaired object location memory corresponded to a decrease in ERK1/2 activation.
We also investigated whether CVS-induced changes in ERK1/2 phosphorylation are associated with reduced Bcl-2 transcriptional expression. Chronic stress can lead to depression, and Bcl-2 is implicated in depression, as Bcl-2 expression is increased in the hippocampus by chronic treatment with antidepressants (36) and valproate, a drug implicated as a histone deacetylase inhibitor (16). We previously showed that H4(K12) acetylation is decreased in the CA3 and DG regions of hippocampus in response to CVS (5). We saw a decrease in Bcl-2 protein expression in response to chronic stress. The ChIP and mRNA data suggest that this effect may not be due to a single epigenetic effect, as we observed an increase in H4(K12) acetylation at the Bcl-2 promoter and a corresponding increase in Bcl-2 mRNA in chronic stress. The opposing effects on the Bcl-2 mRNA may be a compensatory mechanism to transcriptionally upregulate Bcl-2 expression. The discrepancy between the protein regulation and transcript suggests posttranscriptional regulation of Bcl-2 by microRNAs or posttranslational degradation of the protein by chronic stress.
Investigation into the role of sirtuins in cognition and the effects of stress on the hippocampus is still in its early stages. While SIRT1 has been shown in some animal models to be essential for normal cognitive function and synaptic plasticity (8,9), others have shown deficits in learning and memory with SIRT1 overexpression (10). Nicotinamide, a sirtuin inhibitor, prevented memory impairment on the Morris water maze spatial task in a transgenic model for Alzheimer’s associated with cognitive decline, further implicating a role for sirtuins in hippocampus-dependent memory formation (37). Sirtinol infusion did not affect memory, anhedonic behavior, or SIRT1 activity levels in control animals. Previous results from our lab demonstrated a significant effect of sirtinol on histone acetylation in hippocampus slices from CVS-treated animals but not control animals. The effect of sirtinol in our experiments is consistent with recent publications that showed that variations in the SIRT1 gene are associated with anxiety in human populations (11), and SIRT1 expression is associated with prevalence of depression (12). The results from our study suggest that inhibition of excessive sirtuin activity in the DG during CVS may have beneficial effects on hippocampus-dependent memory formation and function. Furthermore, in chronically stressed animals, sirtuin inhibition contributed to improvement in memory formation and reduced anhedonia that was associated with an increase in ERK1/2 and Bcl-2 in the DG.
We did not investigate the specific mechanism by which sirtuin inhibition regulates the ERK1/2 cascade, but several possibilities exist. One possibility is that sirtinol, or SIRT1 activity, affects another pathway that impinges upon the ERK1/2 pathway. It is worth noting that CVS decreased SIRT1 activity in the presence of sirtinol (Figure 5C). This may have caused differential activation of another signaling cascade that modulated the ERK1/2 cascade. This suggests a possible interaction of SIRT1 and other sirtuins or a narrow range of optimal function of SIRT1 activity, such that excessive activity also inhibits the ERK1/2 cascade and ultimately hippocampus function.
Another possibility is that activation of SIRT1 suppresses proliferation of neuronal progenitors, whereas inhibition has the opposite effect by increasing differentiation (38). Since the DG is one of the areas of the brain where neurons continually regenerate into adulthood, stabilization of neurogenesis by sirtuin inhibition likely plays a role in the mechanism of the increased hippocampus function in these experiments. Indeed, reduced adult neurogenesis has been shown to occur in response to chronic stress exposure (39–44). Further studies will investigate the effects of SIRT1 inhibition on chronic stress-induced changes in neurogenesis specifically.
Our study suggests that the DG is the primary locus within the hippocampus mediating aberrant regulation of these pathways in chronic stress, as sirtinol infusion directly into the DG prevented the molecular and behavioral changes we observed subsequent to chronic stress. These molecular effects ultimately influence memory formation and/or stress reactivity. These studies indicate sirtuin inhibition is a therapeutic target for cognitive effects of depression.
Supplementary Material
Acknowledgments
This work was supported by National Science Foundation Grant 1146853 and National Institutes of Health/Centers of Biomedical Research Excellence Grant P20RR016816: Mentoring Neuroscience in Louisiana and Tulane Research Enhancement funds to LAS.
We thank Paul Colombo and Brett East for assistance with infusions.
Footnotes
The authors report no biomedical financial interests, other than grants listed above, or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2013.07.029.
References
- 1.Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 2.Kleen JK, Sitomer MT, Killeen PR, Conrad CD. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behav Neurosci. 2006;120:842–851. doi: 10.1037/0735-7044.120.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S, Wang C, Wang W, Dong H, Hou P, Tang Y. Chronic mild stress impairs cognition in mice: From brain homeostasis to behavior. Life Sci. 2008;82:934–942. doi: 10.1016/j.lfs.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Song L, Che W, Min-Wei W, Murakami Y, Matsumoto K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacol Biochem Behav. 2006;83:186–193. doi: 10.1016/j.pbb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Ferland CL, Schrader LA. Regulation of histone acetylation in the hippocampus of chronically stressed rats: A potential role of sirtuins. Neuroscience. 2011;174:104–114. doi: 10.1016/j.neuroscience.2010.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, et al. Brain SIRT1: Anatomical distribution and regulation by energy availability. J Neurosci. 2008;28:9989–9996. doi: 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 8.Michan S, Li Y, Chou MM, Parrella E, Ge H, Long JM, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakefuda K, Fujita Y, Oyagi A, Hyakkoku K, Kojima T, Umemura K, et al. Sirtuin 1 overexpression mice show a reference memory deficit, but not neuroprotection. Biochem Biophys Res Commun. 2009;387:784–788. doi: 10.1016/j.bbrc.2009.07.119. [DOI] [PubMed] [Google Scholar]
- 11.Libert S, Pointer K, Bell EL, Das A, Cohen DE, Asara JM, et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell. 2011;147:1459–1472. doi: 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishi T, Yoshimura R, Kitajima T, Okochi T, Okumura T, Tsunoka T, et al. SIRT1 gene is associated with major depressive disorder in the Japanese population. J Affect Disord. 2010;126:167–173. doi: 10.1016/j.jad.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, 3rd, Maze I, et al. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62:335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, et al. Histone methylation regulates memory formation. J Neurosci. 2010;30:3589–3599. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao Y, Creson T, Zhang L, Li P, Du F, Yuan P, et al. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci. 2004;24:6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Creson TK, Yuan P, Manji HK, Chen G. Evidence for involvement of ERK, PI3K, and RSK in induction of Bcl-2 by valproate. J Mol Neurosci. 2009;37:123–134. doi: 10.1007/s12031-008-9122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwer B, North BJ, Frye RA, Ott M, Verdin E. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J Cell Biol. 2002;158:647–657. doi: 10.1083/jcb.200205057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci U S A. 2002;99:13653–13658. doi: 10.1073/pnas.222538099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whittle JR, Powell MJ, Popov VM, Shirley LA, Wang C, Pestell RG. Sirtuins, nuclear hormone receptor acetylation and transcriptional regulation. Trends Endocrinol Metab. 2007;18:356–364. doi: 10.1016/j.tem.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 23.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 24.Imai S, Guarente L. Ten years of NAD-dependent SIR2 family deacetylases: Implications for metabolic diseases. Trends Pharmacol Sci. 2010;31:212–220. doi: 10.1016/j.tips.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouras T, Fu M, Sauve AA, Wang F, Quong AA, Perkins ND, et al. SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J Biol Chem. 2005;280:10264–10276. doi: 10.1074/jbc.M408748200. [DOI] [PubMed] [Google Scholar]
- 26.Haigis MC, Guarente LP. Mammalian sirtuins–emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 27.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 28.Leal G, Comprido D, Duarte CB. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.04.005. published online ahead of print April 16. [DOI] [PubMed] [Google Scholar]
- 29.De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 30.Basta-Kaim A, Budziszewska B, Jaworska-Feil L, Tetich M, Kubera M, Leskiewicz M, Lasoń W. Inhibitory effect of imipramine on the human corticotropin-releasing-hormone gene promoter activity operates through a PI3-K/AKT mediated pathway. Neuropharmacology. 2005;49:156–164. doi: 10.1016/j.neuropharm.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guarnieri DJ, Brayton CE, Richards SM, Maldonado-Aviles J, Trinko JR, Nelson J, et al. Gene profiling reveals a role for stress hormones in the molecular and behavioral response to food restriction. Biol Psychiatry. 2012;71:358–365. doi: 10.1016/j.biopsych.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dash PK, Orsi SA, Moody M, Moore AN. A role for hippocampal Rho-ROCK pathway in long-term spatial memory. Biochem Biophys Res Commun. 2004;322:893–898. doi: 10.1016/j.bbrc.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30:4390–4400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray F, Hutson PH. Hippocampal Bcl-2 expression is selectively increased following chronic but not acute treatment with antidepressants, 5-HT(1A) or 5-HT(2C/2B) receptor antagonists. Eur J Pharmacol. 2007;569:41–47. doi: 10.1016/j.ejphar.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Green KN, Steffan JS, Martinez-Coria H, Sun X, Schreiber SS, Thompson LM, LaFerla FM. Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J Neurosci. 2008;28:11500–11510. doi: 10.1523/JNEUROSCI.3203-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schroter F, Ninnemann O, et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10:385–394. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- 39.Tanapat P, Galea LA, Gould E. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Int J Dev Neurosci. 1998;16:235–239. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- 40.Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joels M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, et al. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–231. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- 43.Lucassen PJ, Bosch OJ, Jousma E, Kromer SA, Andrew R, Seckl JR, Neumann ID. Prenatal stress reduces postnatal neurogenesis in rats selectively bred for high, but not low, anxiety: Possible key role of placental 11beta-hydroxysteroid dehydrogenase type 2. Eur J Neurosci. 2009;29:97–103. doi: 10.1111/j.1460-9568.2008.06543.x. [DOI] [PubMed] [Google Scholar]
- 44.Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci. 2004;19:131–144. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Amsterdam: Boston Academic Press/Elsevier; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.