Abstract
Neuronal gap junction (GJ) channels composed of connexin36 (Cx36) play an important role in neuronal synchronization and network dynamics. Here we show that Cx36-containing electrical synapses between inhibitory neurons of the thalamic reticular nucleus are bi-directionally modulated by changes in intracellular free magnesium concentration ([Mg2+]i). Chimeragenesis demonstrates that the first extracellular loop of Cx36 contains a Mg2+-sensitive domain, and site-directed mutagenesis shows that the pore-lining residue D47 is critical in determining high Mg2+-sensitivity. Single channel analysis of Mg2+-sensitive chimeras and mutants reveals that [Mg2+]i controls the strength of electrical coupling mostly via gating mechanisms. In addition, asymmetric transjunctional [Mg2+]i induces strong instantaneous rectification, providing a novel mechanism for electrical rectification in homotypic Cx36 GJs. We suggest that Mg2+-dependent synaptic plasticity of Cx36-containing electrical synapses could underlie neuronal circuit reconfiguration via changes in brain energy metabolism that affects neuronal levels of intracellular ATP and [Mg2+]i.
Introduction
Magnesium is the second most abundant intracellular cation after potassium, and is a critical cofactor in many enzymatic reactions involving energy metabolism. Magnesium is highly concentrated in cellular organelles, such as mitochondria, nucleus and endoplasmic reticulum, and it binds to several ionic cytoplasmic constituents. Importantly, phosphonucleotides, such as ATP, bind magnesium ions (MgATP2−) and the enzymatic hydrolysis of their phosphate groups depends on this interaction1. Therefore, the intracellular concentration of free magnesium ([Mg2+]i) is closely related to cell bioenergetics and is expected to vary according to the state of cellular metabolism and levels of intracellular ATP2. Resting [Mg2+]i is less than 10% of total cellular magnesium, and it ranges from 0.2 to 3.5 mM in neurons depending on cell type and species3,4,5. Under physiological conditions, depolarization triggers an increase of [Mg2+]i in sensory neurons6, while glutamate exposure induces a [Mg2+]i surge in forebrain and hippocampal neurons7,8. More recently, it was shown that activation of a nitric oxide signaling pathway can also trigger an increase of [Mg2+]i in hippocampal neurons9. Moreover, enhanced [Mg2+]i can be expected with a reduction in the levels of ATP during periods of waking and hyperactivity10. Conversely, reduction in [Mg2+]i can be expected with an increase in ATP levels during glucose or lactate exposure11 and during the first hours of sleep10. In pathological conditions, early onset of ischemic cell death is mainly due to the inability of mitochondria to produce ATP, resulting in the failure to regulate transmembrane ion gradients12, which impacts [Mg2+]i. Long-lasting elevation in brain [Mg2+]i occurs in some acute and chronic brain pathologies such as hypoxia/ischemia13,14 and in patients with schizophrenia15. In contrast, [Mg2+]i is reduced after traumatic brain injury16,17 and in patients with Parkinson18, Alzheimer19, multiple sclerosis20, amyotrophic lateral sclerosis21, chronic migraine22 and mitochondrial diseases23.
Electrical synapses are specialized intercellular junctions formed by clusters of gap junction (GJ) channels that allow bidirectional electrotonic signaling between neurons. Many roles for electrical synapses have been documented, such as synchronization and coordination of neuronal networks24, memory formation25 and lateral excitation in olfactory glomeruli26. GJ channels are formed by the connexin (Cx) and innexin gene families in vertebrates and invertebrates, respectively. Six Cx (or innexin) proteins oligomerize into a pore-forming hemichannel (HC), and the docking of two HCs contributed by adjacent cells forms a GJ channel. The docking of HCs from apposing cells containing the same Cx type results in homotypic GJs, while the docking of HCs containing different Cxs results in heterotypic GJs. Sensitivity of junctional conductance (gj) to transjunctional voltage (Vj) is a common property of all GJs. Each apposed/junctional HC (aHC) has two distinct Vj-sensitive gates that are responsible for the steady-state gj–Vj relationship (gj,ss–Vj). This relationship is typically symmetric for either polarity of Vj in homotypic junctions27, but asymmetric in heterotypic junctions where aHCs have Vj-sensitivity and/or single channel conductance differences, which leads to an asymmetry in electrical signal transfer and metabolic communication28,29. An instantaneous gj–Vj relationship (gj,inst–Vj), however, is more relevant with respect to electrical synapses since neuronal membrane potential fluctuates in the ms time scale during action potentials. Many electrical synapses rectify instantaneously30,31,32,33; i.e. electrical signals are preferentially transmitted anterogradely or retrogradely. Electrical synapses between neurons in the mammalian CNS are typically formed by Cx3634, which is commonly expressed throughout the CNS34,35. Modulation of electrical synapses can occur by different factors such as phosphorylation36,37, changes in pH38 and exposure to lipophilic molecules39. Interestingly, Cx36-containing electrical synapses can undergo activity-dependent long-term depression40, or CaMKII- and PKA-dependent long-term potentiation41,42.
We recently reported a novel Mg2+-dependent form of electrical synaptic plasticity between neurons of the trigeminal mesencephalic nucleus (MesV) and in heterologous expression systems transfected with Cx3643. We showed that the strength of electrical synaptic transmission is augmented or reduced by low or high [Mg2+]i, respectively. The gj of GJs formed of Cxs 26, 30.2, 32, 36, 43, 45, 47, and 57 expressed in HeLa cells was reduced by increasing [Mg2+]i, whereas lowering [Mg2+]i increased gj only in Cx36 expressing cells, indicating that Cx36 GJs are strongly inhibited by normal/resting [Mg2+]i. We also demonstrated that Mg2+ ions are permeable to Cx36 GJs and an effect of Mg2+ on gj is fully reversible43.
Here, we show that electrical synapses formed by Cx36 in the thalamic reticular nucleus (TRN) are also bi-directionally modulated by changes in [Mg2+]i and that an altered Mg-ATP equilibrium can trigger Mg2+-dependent plasticity of neuronal electrical coupling. We sought to locate the molecular domains of Cx36 GJ channels that contribute to such unusually high sensitivity to [Mg2+]i using chimeragenesis and site-directed mutagenesis. Our data show that a negatively-charged aspartate (D47), located in the first extracellular loop (E1), is responsible for high Mg2+-sensitivity. Single channel analysis of chimeras reveals that changes in [Mg2+]i affect the voltage-dependent gating of channels without changing the single channel conductance. We also found that [Mg2+]i modulates the gj,inst–Vj dependence of Cx36 GJs by producing a hyperbolic gj,inst–Vj relationship that is unique to Cx36 GJs. Previously, we showed that asymmetry in the transjunctional [Mg2+]i results in an asymmetry of steady-state gj (gj,ss) dependence on Vj.43 We now demonstrate that asymmetry in the transjunctional [Mg2+]i results in an asymmetric gj,inst–Vj relationship of homotypic Cx36 GJ channels. Hence, the intercellular gradient of divalent cations, such as Mg2+, is a novel mechanism that can generate instantaneous rectification in homotypic Cx36 GJs. In addition, we show that the second extracellular loop (E2) is an important molecular component that contributes to the incompatibility between neuronal Cx36 and astrocytic Cx43 HCs to dock and form functional heterotypic GJs.
Results
Electrical synapses in the TRN are modulated by [Mg2+]i
To test whether native electrical synapses expressing Cx36 are sensitive to changes in [Mg2+]i, we used a BAC transgenic mouse line (Tg(Gjd2-EGFP)JM16Gsat/Mmucd)44, in which expression of the EGFP reporter gene is driven by the promoter of Cx36 and expression of the endogenous Cx36 protein is left intact. In Gjd2-EGFP mice, one can easily identify EGFP-positive neurons, facilitating the selection of adjacent pairs of electrically-coupled neurons for electrophysiological analysis. The TRN was chosen for examination due to its relatively high incidence of electrical coupling45. It is a diencephalic layer of GABAergic interneurons that forms a capsule around the ventrobasal (VB) complex of the thalamus, and plays an important role in switching states of arousal and consciousness46. Acute horizontal slices of mouse thalamus were used for confocal fluorescence imaging of the TRN (Fig. 1a,b) and for measuring gj using a dual whole-cell patch clamp (Fig. 1c) in pairs of neurons displaying EGFP fluorescence (Fig. 1d). From a total of 57 neuronal pairs recorded, 18 pairs were electrically coupled (31.6%). The intrinsic firing properties (Fig. 1e) and attenuated evoked responses (Supplementary Fig. 1) of electrically coupled EGFP-expressing neurons were similar to those previously reported45. To reduce or increase [Mg2+]i, we used pipette solutions with K2ATP or MgATP, respectively, as previously shown2,43. Pipette solutions with K2ATP (7 mM) showed a ~40% increase in gj after 25 min of recording (Fig. 1f,g). Conversely, solutions with MgATP (7 mM) showed a ~50% decrease in gj after 25 min of recording (Fig. 1f,g). Therefore, inhibitory interneurons from the TRN showed a significant bi-directional Mg2+-dependent modulation of gj, in a similar manner as reported for excitatory neurons from the MesV43.
Figure 1. Magnesium dependent plasticity of electrical synaptic transmission between TRN neurons.
(a,b) Confocal fluorescence images of horizontal brain slices from a Gjd2-EGFP transgenic mouse showing thalamic reticular nucleus (TRN) interneurons expressing EGFP driven by Cx36 promoter. VB, ventrobasal nucleus of the Thalamus; IC, internal capsule. Scale bars correspond to 100 (a) and 10 (b) µm. (c,d) IR-DIC (c) and fluorescence (d) images of an electrically coupled pair of TRN neurons during dual whole-cell patch clamp. Scale bar correspond to 20 µm. (e) Current-clamp recordings from a TRN neuron showing typical spiking and rebound burst behavior; voltage traces were recorded during 0.5 s current steps of −100 (grey) or 200 (black) pA. (f) Voltage-clamp recordings showing averaged transjunctional current traces (10–15 averaged traces) obtained soon after patch openings (initial; left) and after ~25 min of recording (right) for low Mg2+ (K2ATP, top) and high Mg2+ (MgATP, bottom) conditions; current traces were recorded during 0.5 s transjunctional voltage steps of −40 mV. (g) Mean percentage changes of junctional conductance (gj) from initial values after ~25 min from patch openings with pipette solutions containing 7 mM of K2ATP (grey) or MgATP (black). Numbers of cell pairs are indicated within columns, and error bars correspond to s.e.m.
E1 contains a pore-lining Mg2+-sensitive domain
To locate the position of putative Mg2+-sensitive domain/s in Cx36, we performed structure-function studies by assessing gj in response to [Mg2+]i in pairs of RIN cells expressing Cx36/Cx43 chimeras and mutants with single amino acid substitutions. We selected Cx43 because it shows a higher single channel conductance (γopen) (~110 pS47), a higher Vj–gating sensitivity and a lower sensitivity to changes in [Mg2+]i43, relative to Cx36. Chimeras (CH) were generated by sequential exchange of corresponding domains of Cx36 and Cx43 using a modified version of the “sticky feet” protocol48 (See Methods and Supplementary Figs. 2 and 3). We swapped selected domains at the expected interface between membrane and extracellular domains, and generated a total of sixteen chimeras from which eight formed junctional plaques (all chimeras were tagged with EGFP at the C-terminus (CT) and expressed in RIN cells) and only four (CH1-CH4) formed functional channels exhibiting electrical cell-cell coupling (Fig. 2). We studied sensitivity to [Mg2+]i by measuring gj at the beginning of the recording (gj,initial) and the ratio of gj,final/gj,initial, where gj,final is the gj value at the steady state level (after ~25 min), using pipette solutions with low or high free Mg2+ concentrations; [Mg2+]p = 0.01 or 5 mM. Cell pairs with approximately the same size of junctional plaques were used to study wild type and chimeric GJs. The Mg2+-sensitivity of homotypic GJs formed by CH1 (see Supplementary Fig. 4 for amino acid sequence of functional chimeras), in which the NT and first transmembrane domain (M1) of Cx36 was replaced by those of Cx43, was similar to the Mg2+-sensitivity of Cx36 (Fig. 3). Homotypic GJs formed by CH2 or CH3, in which E1 of Cx43 was replaced by E1 of Cx36, showed Mg2+-sensitivity similar to that of Cx36 GJs (Fig. 3). GJs formed by CH4, in which only E2 of Cx43 was replaced by E2 of Cx36, showed no changes in sensitivity to Mg2+ and was similar to that of Cx43 GJs (Fig. 3). Altogether, results from Cx36/Cx43 chimeras indicate that E1 contains a Mg2+-sensitive domain that can be transferred between Cxs, and that NT, M1 and E2 are not involved in Mg2+-sensitivity.
Figure 2. All generated Cx36/Cx43 chimeras.
Chimeras were generated from Cx36 (green) and Cx43 (red). Chimeras that form functional channels and junctional plaques (JPs) are shown in yellow background. Functional chimeras are named from CH1 to CH4. Chimeras that form JPs but do not exhibit electrical cell-cell coupling are shown in blue background. Chimeras that do not form JPs or exhibit electrical cell-cell coupling are shown in grey background.
Figure 3. Differences in sensitivity to [Mg2+]i between Cx36/Cx43 chimeras and mutants.
Experiments were performed in pairs of RIN cells expressing Cx36 (green), Cx43 (red), Cx36/Cx43 chimeras (CH1-CH4), and amino acid substitutions (D47G or G46D). (a) Mean transjunctional conductance measured soon after patch opening (gj,initial). (b) Mean gj (normalized to initial gj value) measured after ~25 min using pipette solutions containing 0.01 mM (grey) or 5 mM (black) free Mg2+ ([Mg2+]p). The dotted line marks the value of normalized gj equal to 1. Total numbers of cell pairs are indicated within columns, and error bars correspond to s.e.m.
D47 is critical for determining high sensitivity to [Mg2+]i
To locate region/s in E1 that may be responsible for the difference in Mg2+-sensitivity between Cx36 and Cx43, we generated single amino acid substitutions in non-conserved charged residues of Cx36 and Cx43 (Supplementary Fig. 5). Mutation M52K and V54D in Cx36, and E62N in Cx43 had no effect on Mg2+-sensitivity. In contrast, GJs formed of Cx36*D47G lost sensitivity to resting/initial [Mg2+]i, while GJs formed of Cx43*G46D gained sensitivity to resting/initial [Mg2+]i (Fig. 3). Position D47 in Cx36 corresponds to position G46 in Cx43. Moreover, GJs formed of CH3*D47G lost sensitivity to resting/initial [Mg2+]i (Fig. 3). In summary, these data demonstrate that E1 contains a Mg2+-sensitive domain in which the D47 residue is critical to determine the uniquely high sensitivity of Cx36 GJ channels to Mg2+, and that insertion of this single residue in Cx43 confers high sensitivity to Mg2+.
The γopen of Cx36/Cx43 chimeras is not affected by [Mg2+]i
The γopen of Cx36 GJ channels remains uncertain due to its very low conductance49,50. For similar reasons, we were unable to examine with sufficient resolution the effects of [Mg2+]i on single Cx36 GJ channels. However, the effect of [Mg2+]i at the single channel level was amenable to analysis in Cx43-based chimeras and mutants, which exhibited γopens similar to that of Cx43 GJs. We found that γopen of CH3 remained unchanged when [Mg2+]p = 0.01 and 5 mM (Fig. 4a,e). These results are in agreement with our hypothesis that [Mg2+]i controls electrical transmission mostly via gating mechanisms, as we previously suggested using a stochastic 16-state of GJ channels43. Furthermore, γopen of homotypic CH3*D47G and CH4 GJs, both with low sensitivity to Mg2+ compared to that of CH3 GJs, was also similar to γopen of Cx43 and remained unchanged in [Mg2+]p = 0.01 and 5 mM (Fig. 4b–c,e and Supplementary Fig. 6). In addition, γopen of Cx43*G46D GJs remained close to that of Cx43 at high and low [Mg2+]p (Fig. 4d–e). Homotypic GJs formed by CH3, CH3*D47G, CH4, and Cx43*G46D did not show Ij rectification at the single channel level. Thus, Mg2+-dependent changes in gj for these three chimeras and Cx43 most likely are defined by differences in Mg2+ binding affinity and its effects on gating, but not by changes in γopen.
Figure 4. Effect of [Mg2+]i on single channel conductance from homotypic GJs formed by CH3, CH3*D47G, CH4, or Cx43*G46D.
(a–d) Transjunctional current (Ij) recordings of single channel events obtained at indicated Vjs (top) and using [Mg2+]p = 0.01 mM. Numbers attached to arrows show single channel conductances at the open state (γopen). (e) Averaged γopen for homotypic GJs formed by CH3, CH3*D47G, CH4, and Cx43*G46D using [Mg2+]p = 0.01 (gray) or 5 (black) mM. Total numbers of cell pairs are indicated within columns, and error bars correspond to s.e.m.
[Mg2+]i affects gj via gating mechanisms
CH3 channels possess the Mg2+-sensitive E1 domain of Cx36 (Figs. 2 and 3) and the high γopen is similar to that of Cx43 (Fig. 4), which allows for analysis of Mg2+-dependent plasticity at the single channel level. We studied gj and its dependence on Vj in pairs of weakly-coupled RIN cells expressing homotypic CH3 GJs. We measured gj,ss–Vj relationships using Vj ramps from 0 to +90 and −90 mV in amplitude and 30 s in duration (Fig. 5a, top trace). Under high [Mg2+]p, there was a relatively fast run-down of gj in CH3 GJs, thus gj–Vj plots from four consecutive measurements show different gjs at the beginning of each ramp (Fig. 5a–c). The initial gj was ~3.8 nS, corresponding to ~33 open CH3 GJ channels (Fig. 5a,c). Three minutes later, only one GJ channel was open during the fourth Vj ramp (red traces in Fig. 5a,c). To study the effects of Mg2+ occupancy inside the pore on Vj-gating, a transjunctional gradient of [Mg2+]i was created by having different [Mg2+]p (Fig. 5d); under these conditions relative positivity or negativity on the side with higher [Mg2+]i should increase or reduce Mg2+ occupancy, respectively. The transjunctional asymmetry in [Mg2+]i resulted in strong asymmetric gj,ss–Vj dependence measured using Vj ramps (Fig. 5e). At the single channel level, negative Vj steps applied in the cell with lower [Mg2+]i facilitated closing events, while positive Vj steps facilitated opening events (Fig. 5f). The γopen of CH3 GJ channels remained at ~115 pS regardless of the Vj polarity and Mg2+ occupancy (Fig. 5g–h). These results indicate that an increase in Mg2+ concentration inside the pore tends to close Vj-sensitive gates.
Figure 5. Mg2+ Effect on Vj-gating and single channel conductance of homotypic GJs formed of CH3.
(a) Changes of Ij (bottom trace) of CH3 GJs in response to repeated 30 s long Vj ramps from 0 to −90 mV and from 0 to +90 mV with intermediate small amplitude ramps (−10 mV) (top trace) using symmetric [Mg2+]p = 5 mM. (b,d) Diagrams illustrating [Mg2+]p in cell-1 and cell-2 and the stimulation site of the Vj protocol for experiments shown in a and c (b), or e (d). (c) gj–Vj relations obtained from experiment shown in (a). Colors match the Ij data shown in (a). (e) Asymmetric gj,ss–Vj dependence (normalized to gj value at Vj zero) was measured by applying Vj ramps from 0 to −70 and from 0 to +70 mV (30 s in duration) under transjunctional Mg2+ asymmetry shown in (d). Negative potentials applied on the side with low [Mg2+]i decreased gj presumably by increasing Mg2+ occupancy of the pore through ionophoresis (n=4). (f) gj trace (bottom) showing unitary gating events of the homotypic CH3 GJ channel obtained during Vj steps of ±55 mV (top trace) applied in cell-1 (as illustrated in d). Negative Vjs facilitated closing transitions, whereas positive Vjs facilitated opening transitions. (g-h) Count histograms for all gj data obtained at negative (g) or positive (h) Vjs shown in (f). Both histograms show peaks corresponding to the single channel conductance of ~115 pS.
Transjunctional asymmetry of [Mg2+]i induces rectification
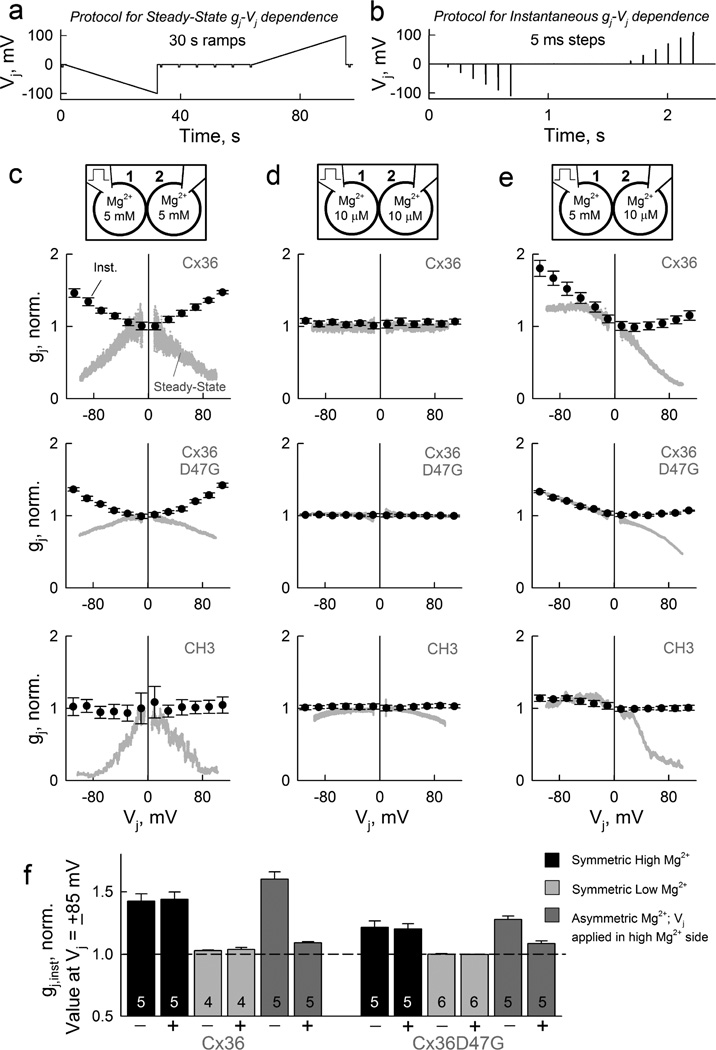
To determine whether [Mg2+]i affects γopen of Cx36 GJ channels in a Vj-dependent manner, we examined the gj,inst–Vj relationship at different [Mg2+]i. The gj,inst–Vj dependence is relevant to the behavior of electrical synapses, because Vj generated in neurons arises mostly from action potentials with fast (milliseconds) oscillatory changes in the membrane potential. Instantaneous macroscopic Ijs (Ij,inst) mainly reflect the dependence of γopen on Vj in the absence of Vj-dependent gating. Thus, we measured steady-state and instantaneous gj–Vj dependencies at different [Mg2+]i by using different Vj protocols (Fig. 6a–b). We found that under high [Mg2+]i, the gj,inst (normalized to gj value at zero Vj) of Cx36 GJs increased while the gj,ss decreased by increasing Vjs for both polarities (Fig. 6c, top panel). Low [Mg2+]i fully eliminated or strongly reduced instantaneous and steady-state gj dependencies on Vj (Fig. 6d, top panel). Moreover, transjunctional asymmetry in [Mg2+]i induced asymmetric steady-state and instantaneous gj–Vj dependencies (Fig. 6e, top panel). The effects of symmetric and asymmetric [Mg2+]i on steady-state and instantaneous gj–Vj dependencies were still present, albeit reduced, in GJs formed by Cx36*D47G (Fig. 6c–e, middle panels, & Fig. 6f), but absent for gj,inst–Vj dependence in GJs formed by CH3 (Fig. 6c–e, bottom panels). In addition, we found that the effects of [Mg2+]i were eliminated in CH1 only for gj,inst–Vj but not for gj,ss–Vj dependencies (Supplementary Fig. 7), suggesting that residues in NT or M1 of Cx36 are necessary for the peculiar hyperbola-like gj,inst–Vj rectification. Altogether, these results suggest that [Mg2+]i affects Cx36 GJ channels by: 1) gating through its binding in E1 and/or stabilizing a closed conformation of the channel; and 2) rectification of Ij,inst depending on Vj (see Discussion).
Figure 6. GJs formed by Cx36 show Mg2+-dependent rectification of both steady-state and instantaneous conductance-voltage relationships.
(a–b) Vj protocols used to obtain steady-state (a) and instantaneous (b) gj–Vj relationships. (c–e) Steady-state (grey) and instantaneous (black) gj–Vj relationships (normalized to gj value at Vj zero) were measured ~30 min after opening of patches under symmetric high (c), low (d), and asymmetric Mg2+ conditions (e) in homotypic GJs formed of Cx36 (top row), Cx36*D47G (middle row) and CH3 (bottom row). Each data point for instantaneous gj–Vj relationships was obtained by averaging data from ~10 consecutive Vj protocols shown in (b), and error bars correspond to s.e.m. Top diagrams in each column show [Mg2+]p and stimulation site. (f) Mean gj,inst values measured at Vjs equal to −85 and +85 mV (normalized to gj,inst value at Vj zero) at symmetric high (black) and low (light gray) [Mg2+]p, and asymmetric Mg2+ conditions (dark gray) for GJs formed of Cx36 (left) and Cx36*D47G (right). Total numbers of cell pairs are indicated within columns, and error bars correspond to s.e.m.
Mg2+-sensitive heterotypic GJs show asymmetric gj- Vj relation
Heterotypic GJs formed by Cxs with highly different properties, such as Cx36 and Cx43, present a valuable tool for a high resolution analysis of the individual aHC properties. Our studies revealed that Cx36 does not form either JPs or functional coupling with Cx43, consistent with reports that neurons and astrocytes do not form Cx36/Cx43 heterotypic GJs51. We found that Cx36 or Cx43 were able to form functional heterotypic channels with chimeras that contain E2 of Cx36 or Cx43, respectively (Fig. 7). Thus, E2 determines incompatibility between Cx36 and Cx43. For heterotypic pairings, we used Cx36 and Cx43 tagged with CFP, while all chimeras were tagged with EGFP. This allowed us to detect junctional plaques with heterotypic GJs visible in two colors28. In Cx43/CH3 heterotypic GJs, both aHCs have a similar unitary conductance (γopen,H), but differential sensitivity to [Mg2+]i (Figs. 3 and 4). Thus, this heterotypic configuration allows the study of Mg2+-sensitivity in CH3 aHCs, and any detected asymmetry can be attributed to the difference in Mg2+-sensitivity of aHCs but not γopen,H. Indeed, heterotypic Cx43/CH3 GJs show marked asymmetric gj,ss–Vj dependence under symmetric high [Mg2+]i (Fig. 8a). At [Mg2+]p = 5 mM, positive Vj ramps applied on the Cx43 side induced strong gating of the CH3 aHC, suggesting that CH3 aHCs have a negative gating polarity, as has been proposed for Cx4352. However, Vj-dependent gating of CH3 aHC at negative Vjs almost disappears under symmetric low [Mg2+]i (Fig. 8b). At the single channel level, heterotypic Cx43/CH3 GJs showed asymmetric gating behavior (Fig. 8c). Negative Vj steps applied on the Cx43 side induced fast flickering of channels, while positive Vj steps induced channel closing (Fig. 8c). Moreover, the asymmetric gating behavior of homotypic CH3 GJ channels under asymmetric [Mg2+]i (Fig. 5c) can be replicated in heterotypic Cx43/CH3 channels under symmetric [Mg2+]i (Fig. 8d). As expected from γopens of Cx43 and CH3 homotypic GJs, the γopen of heterotypic Cx43/CH3 GJs is ~110 pS, and does not change under high or low [Mg2+]i. Thus, the macroscopic asymmetric gj,ss–Vj dependence shown in Fig. 8a can be explained by a Mg2+-dependent modulation of gating mechanisms, in which negative potentials induce the transition of gates to a closed state, while positive potentials tend to reopen the gates. At low [Mg2+]i, most of the Vj-dependent gating is lost (Fig. 8b), suggesting that Mg2+ is necessary for Vj-sensitive gating. Furthermore, we studied gj,ss–Vj dependence of Cx43/CH3 GJs under asymmetric [Mg2+]i (Fig. 8e,f). These experiments revealed that the direction of the Mg2+ gradient is important; the gj,ss–Vj asymmetry is strengthened when the Cx43 side has higher [Mg2+]i (Fig. 8e) or reduced when the Cx43 side has lower [Mg2+]i (Fig. 8f). These results strongly support the hypothesis that the site of Mg2+ interaction in CH3 aHC is located within the pore, and that high [Mg2+] inside the pore increases Vj-sensitive gating.
Figure 7. Compatibility between Cx36 and Cx43 GJ channels with functional chimeras.
Among all examined heterotypic combinations between wild type Cx36 (green) or Cx43 (red) with CH1, CH2, CH3, or CH4, only those shown in yellow background formed junctional plaques and exhibit electrical cell-cell coupling, while those shown in grey background did not form junctional plaques or exhibit electrical cell-cell coupling.
Figure 8. Mg2+-dependent asymmetry in steady-state gj–Vj relationship and single channel conductance in Cx43/CH3 heterotypic GJs.
(a–b) Asymmetric gjss–Vj relationships (normalized to gj,ss value at Vj =0, and obtained using the same Vj protocol shown in Fig. 6a) with pipette solutions containing symmetric high (a) or low (b) [Mg2+]p in heterotypic Cx43/CH3 GJs (n=5). Top diagram in each plot shows [Mg2+]p used in cell-1 and cell-2, stimulation sites and expressed Cxs. (c) Ij records of single channel events at symmetric [Mg2+]p obtained during Vj steps of ±85 mV (top trace) applied in cell-1 expressing Cx43. (d) Ij records (bottom trace) under symmetric high [Mg2+]p showing unitary gating events of Cx43/CH3 GJ channel obtained during Vj steps of ±60 mV (top trace) applied in cell-1 expressing Cx43. Positive Vjs facilitated closing transitions, while negative Vjs facilitated opening transitions. (e,f) Asymmetric gjss–Vj relationships (normalized to gj,ss value at Vj =0) obtained under asymmetric Mg2+ conditions (see diagrams) in heterotypic Cx43/CH3 GJs (n=5).
We studied gj,ss–Vj and gj,inst–Vj dependencies and sensitivity to [Mg2+]i of Cx36 aHC in Cx36/CH4 heterotypic GJs. This heterotypic configuration allows for a higher resolution analysis of Mg2+-sensitivity and of Vj-gating of Cx36 aHCs; in Cx36/CH4 GJs almost all Vj drops across the Cx36 aHCs due to a ~15 fold lower γopen,H than in CH453, making CH4 aHC virtually insensitive to Vj. We found that under high symmetric [Mg2+]p (5 mM), gj,inst (normalized to gj value at zero Vj) of heterotypic Cx36/CH4 GJs increased for both polarities of Vj (Fig. 9a, bottom), while gj,ss–Vj showed a marked asymmetric dependence (Fig. 9a, top). Interestingly, gj,inst–Vj dependence of heterotypic Cx36/CH4 GJs becomes less symmetric at low [Mg2+]p (Fig. 9b, bottom), in which gj,inst increased only at relative negativity on the Cx36 side. The asymmetric gj,ss–Vj dependence almost disappears under low [Mg2+]p (0.01 mM, Fig. 9b, top) due to a reduction in Vj-sensitivity, indicating that most of the asymmetry is due to the Mg2+-sensitivity of Cx36 aHC. In order to study the mechanism of Ij,inst–Vj rectification of the Cx36 aHC in more detail, we simulated gj,inst–Vj dependence curves that fit our experimental data using a stochastic four state model (S4SM) of GJ channels54. gj,inst–Vj relationships of the Cx36 homotypic and Cx36/CH4 heterotypic GJs were simulated using a hyperbolic equation describing the Cx36 aHC conductance: γopen,H = γopen,H,0 *(e(VH/rH) + e(-VH/rMg))/2, where γopen,H,0 is γopen,H at VH = 0, VH is voltage across aHC, rH and rMg are Mg2+-independent and Mg2+-dependent rectification coefficients of aHC, respectively. The CH4 aHC conductance was described using a single exponential equation: γopen,H = γopen,H,0 *e(VH/rH). The simulated gj,inst–Vj curves for Cx36 (pink) and CH4 (purple) aHCs produced curves with good fit (grey) for steady-state and instantaneous gj–Vj dependence of experimental data from heterotypic Cx36/CH4 GJs at low and high symmetric [Mg2+]i (Fig. 9a–b). The same hyperbolic equation describing γopen,H of Cx36 and similar rectification values used in simulation of heterotypic Cx36/CH4 GJs were also used to simulate experimental data for homotypic Cx36 GJs at high and low [Mg2+]i (Fig. 9c–d). All values of rectification and gating parameters are presented in Supplementary Table 1.
Figure 9. Mg2+-dependent asymmetry in steady-state and instantaneous gj–Vj relationship in Cx36/CH4 heterotypic GJs.
(a–b) Asymmetric steady-state (top) and instantaneous (bottom) gj–Vj relationships shown in black (normalized to gj value at Vj =0, and obtained using the same Vj protocol shown in Figs. 6a and 6b, respectively) obtained under symmetric high (a) and low (b) [Mg2+]p in heterotypic Cx36/CH4 GJs (n=5). The top diagram in each plot shows [Mg2+]p used in cell-1 and cell-2, stimulation sites and expressed Cxs. Each data point for gj,inst–Vj relationships was obtained by averaging data from ~10 consecutive Vj protocols, and error bars correspond to s.e.m. Simulated curves shown in grey for steady-state (top) and instantaneous (bottom) gj–Vj relationships were obtained using the S4SM. Dotted lines show gj,inst–Vj relationships for CH4 (purple) and Cx36 (pink) aHCs used in simulation of steady-state and instantaneous gj–Vj relationships. (c-d) Symmetric steady-state (top) and instantaneous (bottom) gj–Vj relationships for homotypic Cx36 GJs are shown in black (data from Fig. 6c,d). Simulated curves shown in grey were obtained using the S4SM and similar rectification parameters were obtained from Cx36 aHCs shown in (a) and (b). All parameters for simulation are reported in Supplementary Table 1.
Discussion
Electrical synapses are known to function throughout the mammalian CNS, and Cx36 expression is necessary to produce robust neuronal coupling in many brain areas35. We recently showed Mg2+-dependent modulation of signal transfer at electrical synapses between excitatory MesV neurons in the midbrain and that this [Mg2+]i effect was similar to that observed in heterologous expression systems43.
Here, we demonstrated that electrical synapses formed by Cx36 GJs between GABAergic interneurons in the TRN also show Mg2+-dependent synaptic plasticity, and that the ratio between the total intracellular ATP and Mg2+ contributes to regulation of electrical coupling (Fig. 1). Although the magnitude of changes in gj between TRN (Fig. 1) and MesV43 neurons were significant (~30–40%), they were smaller than those observed in RIN cells expressing Cx36. This distinction may be explained by differences in the initial [Mg2+]i and other divalent cations as well as the concentration of ATP and phosphocreatine, that exert a Mg2+ buffering capacity, and the location of JPs with respect to patch pipette attachment at the soma. Despite these differences, the magnitude of gj changes is comparable to that of previous reports on long-term depression or potentiation of neuronal coupling40,42. Taken together, these results support the hypothesis that Mg2+-dependent synaptic plasticity of Cx36-containing electrical synapses is neuronal-type independent and is a common mechanism that affects the strength of neuronal electrical coupling in the CNS.
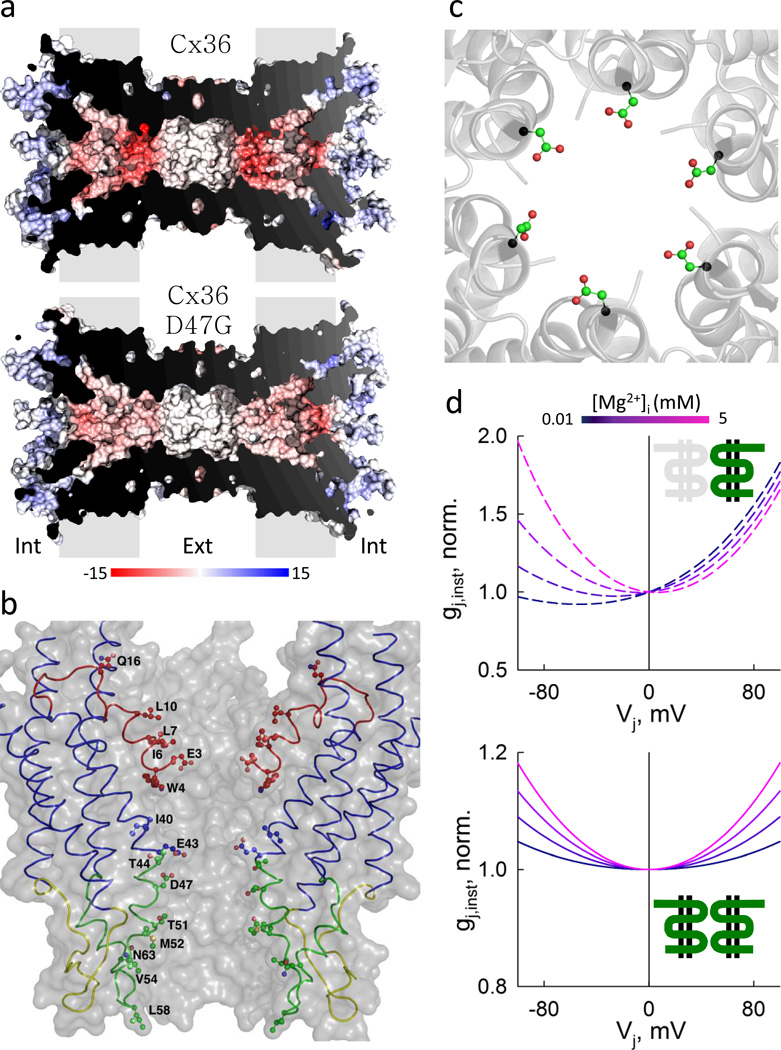
We recently suggested that Mg2+ exerts its effects on gj of Cx36 GJs via interaction with a domain in the channel lumen43. This interaction may affect Vj-sensitive gates by modulating their sensitivity to voltage and stabilizing a closed state conformation43. Previously, we showed that sensitivity to high [Mg2+]i is similar in wild type Cx36 and Cx43, and tagged with fluorescent proteins43. Here, using color variants of GFP tagged to Cx36/Cx43 chimeras and mutants, we demonstrate that E1 of Cx36 contains a Mg2+-sensitive domain and that it can be transferred to Cx43 (Fig. 3). In addition, single amino acid substitutions targeted to E1 of Cx36 and Cx43 revealed that residues in E1 are indeed responsible for the sensitivity to Mg2+ and that particularly D47 is critical for high Mg2+-sensitivity in Cx36 GJs (Fig. 3). Interestingly, the G46D mutation in Cx43 (corresponding location of D47 in Cx36) was sufficient to significantly increase sensitivity to Mg2+ in Cx43 (Fig. 3). Furthermore, as predicted from the crystal structure of Cx2655, the side chains of the residue D47 in GJs formed by Cx36 face the pore, form a negatively-charged hexameric ring and contribute significantly to the negative surface potential of the pore (Fig. 10a–c), supporting the view that Mg2+ interacts with a pore-lining domain located in E1 and that residue D47 provides strong electro negative surface potential, which may increase Mg2+ occupancy. It is noteworthy that recent quantum chemistry studies in Cx26 structure have proposed that Ca2+ may directly interact with E47 (E49 in Cx36) to induce closure of the channel by a gating mechanism56. Other intracellular cations, such as spermine, have been shown to affect Vj-dependent gating mechanisms by interacting with charged residues located in the N-terminus57. In addition, spermine can influence the Mg-ATP binding affinity2, and therefore modulate its action on gj and gating.
Figure 10. Homology models of Cx36 and Cx36*D47G GJ channel structure and Mg2+-dependent modulation of instantaneous rectification.
(a) Cross-sections of GJ channels formed by Cx36 (top) or Cx36*D47G (bottom) generated by sequence homology to Cx26 crystal structure. Electrostatic surface potential is displayed for both channels and was estimated with DELPHI (see Methods). The color bar at the bottom represents electrostatic surface potentials ranging from −15 (red) to 15 (blue) kTe−1. (b) Side view of a Cx36 aHC indicating the pore-lining residues of only two Cx subunits represented with thin ribbon style (principal chain). Main domains of Cx subunits are depicted with different colors and the side chains of pore-lining residues are represented with sticks and spheres. (c) View of the pore from the center of the channel towards the cytoplasmic mouth. The six D47 residues are displayed with sticks and spheres to illustrate the orientation and position of their side chains with respect to the lumen of the channel. (d) Normalized gj,inst—Vj plots for aHCs (top) and GJ channels (bottom) formed by Cx36 generated using different values for Mg2+-dependent coefficient (rMg) with the S4SM of GJ channels gating. Color bar at the top represents different [Mg2+]i from 0.01 to 5 mM.
Single channel analysis of Cx43-based chimeras and mutants (CH2, CH3, CH4, and Cx43*G46D) showed that γopen is not affected by [Mg2+]i (Fig. 4), and a long-lived residual state is absent, indicating that the fast gating mechanism is inhibited most likely due to C-terminus tagging by fluorescent proteins58. The latter can reduce gj,ss dependence on Vj in Cx43/CH3 heterotypic GJs (Fig. 8), but should not influence gj,ss-Vj dependence of Cx36/CH4 GJs (Fig. 9) due to a significant difference in γopen,H of Cx36 and CH4 aHCs, making CH4 aHC unlikely to be gated by Vj. In addition, γopen and Vj-sensitive gating records under transjunctional asymmetry in [Mg2+]i allowed us to conclude that changes in [Mg2+] inside the pore are necessary for the observed asymmetry in the gj,ss–Vj relationship of CH3 GJs (Figs. 5d–f and 8e–f).
Cx36 is not compatible to dock with Cx43, but is compatible with CH2 and CH4. Cx43 is compatible with CH1 and CH3 but not with CH2 and CH4 (Fig. 7). These data suggest that E2 is an important structural determinant for the incompatibility between Cx36 and Cx43, consistent with reports showing the key role E2 plays in determining compatibility between different Cxs59,60. Cx43/CH3 heterotypic GJs showed a marked asymmetry in the gj–Vj relationship, and this asymmetry was dependent on [Mg2+]i (Fig. 8a–b). CH3 GJs exhibited similar sensitivity to Mg2+ compared to that of Cx36 (Fig. 3), while its γopen is >15 fold higher than that of Cx36. Macroscopic and single channel recordings of Cx43/CH3 GJs (Fig. 8a,d) under high [Mg2+]i show significant Vj-gating asymmetry with pronounced sensitivity to Vj at relative negativity on the CH3 side. The dependence of Vj-gating asymmetry on the Mg2+ gradient in Cx43/CH3 heterotypic GJs (Fig. 8e–f) demonstrates that asymmetric gating is determined not only by [Mg2+]i concentration inside the pore, but also by its influence on Vj-sensitivity. When [Mg2+]i is higher on the Cx43 side, the gj,ss–Vj asymmetry is enhanced compared to that under high symmetric [Mg2+]i. These effects are presumably due to increased Vj-gating and Mg2+ occupancy during positive Vjs applied on the Cx43 side, and decreased Vj-gating and Mg2+ occupancy during negative Vjs applied on the Cx43 side (Fig. 8e). Conversely, when [Mg2+]i is lower on the Cx43 side, the gj–Vj asymmetry is reduced and opposite compared to the one at high symmetric [Mg2+]i (Fig. 8f). Consistent with our data, a three-state model of Mg2+-dependent gating of Cx37 HCs, also suggests the stabilization of a closed state by Mg2+ binding61.
We found a unique gj,inst–Vj relationship dependence on [Mg2+]i of Cx36 GJ channels. Reported and preliminary data show that all examined Cxs with the exception of Cx36 demonstrate no or minimal decay of gj,inst dependence on Vj for both Vj polarities52. The symmetric increase in gj,inst at high Vjs for Cx36 was previously reported in the oocyte expression system at normal/resting [Mg2+]i62. Here, we show that gj,insts at high [Mg2+]i increases ~1.4 fold at Vj = ±100 mV, and that this increase disappears under low [Mg2+]i (Fig. 6c–d). All our attempts to replicate the observed gj,inst–Vj dependence at high [Mg2+]i using a one-dimensional Poisson-Nernst-Plank (PNP) model63 were unsuccessful. Studies of heterotypic CH4/Cx36 GJs show that gj,inst increased for both polarities of Vj at high [Mg2+]i (Fig. 9a) and only at relative negativity of Vj at low [Mg2+]i on the Cx36 side (Fig. 9b). Thus, the gj,inst–Vj relationship of Cx36 aHC transforms from hyperbola-like to exponential-like when [Mg2+]p decreases from 5 to 0.01 mM (Fig. 9a–b). An approximately 15-fold difference in γopen,H between Cx36 and CH4 aHCs allows us to assume that measured gj,inst-Vj rectification in Cx36/CH4 GJs can be attributed solely to the Cx36 aHC. These data suggest that gj,inst-Vj rectification of Cx36 aHC contains two exponential-like components in opposite orientation with respect to Vj polarity, defined by: 1) asymmetry of fixed charges inside the Cx36 aHC pore, as described by PNP equations63; and 2) [Mg2+]i. Fig. 10d (top) shows a family of simulated gj,inst-Vj plots for Cx36 aHCs using S4SM (details in the Results section), in which the rectification coefficient, rH, was constant and equal to 90 mV, and the Mg2+-dependent rectification coefficient, rMg, changed from ~200 to 80 mV when [Mg2+]i increased from ~0.01 to 5 mM. Fig. 10d (bottom) shows simulated gj,inst-Vj plots of homotypic Cx36 GJs using the same parameters as for Cx36 aHCs. Thus, Mg2+-dependent rectification can explain the transformation of gj,inst-Vj dependence observed in heterotypic Cx36/CH4 (Fig. 9a–b) and homotypic Cx36 (Fig. 9c–d) GJs. Hyperbola-like conductance-voltage rectification has also been shown in a solid back-to-back p-n junction64, but applicability of such junctions to GJ channels remains unclear. The gj,inst–Vj rectification was not observed under high or low [Mg2+]i in CH1 GJs (Supplementary Fig. 7), suggesting that residues in the NT-M1 region of the Cx36 protein are necessary for instantaneous rectification.
To our knowledge, molecular mechanisms of electrical rectification in GJs have been examined only in heterotypic GJs. In this regard, two mechanisms have been proposed: differences in fast Vj-dependent gating and gating polarity of aHCs of heterotypic GJs65,66; and/or rectification of the single channel conductance resulting from an asymmetry in the number and position of charged residues inside the channel pore of heterotypic GJs67. Thus, we propose that transjunctional asymmetry in [Mg2+]i can serve as a novel mechanism for electrical rectification in homotypic GJs (Fig. 6e). It is important to note that the degree of rectification in electrical synapses has been proposed to affect the dynamic output of neuronal networks68, and therefore this novel instantaneous Mg2+-dependent rectification could be important to explain the phenomenon of switching between firing states and changes in the output of neuronal networks during different metabolic states where [Mg2+]i is affected. Taken together, these findings suggest that changes in [Mg2+]i may be sufficient to induce plasticity of Cx36-based electrical synaptic transmission.
Methods
Generation of chimeras and mutants
All chimeras were generated using a modified version of the “sticky feet”–directed mutagenesis protocol48. Briefly, long PCR oligonucleotide primers that share a complementary sequence were used as forward or reverse primers to isolate fragments with complementary ends of two different genes (1st PCR step). Subsequently, these long DNA fragments were used as primer DNAs to produce chimeric fragments from two different genes (2nd PCR step). This protocol is illustrated in Supplementary Fig. 2. A total of 22 different DNA fragments with complementary ends (FX-1 and FX-2) were generated in order to produce 14 different chimeric fragments (FX) (Supplementary Fig. 3). Two additional chimeric fragments (F12 and F16) were generated by restriction enzyme subcloning (Supplementary Fig. 3). A list of all primer sequences and restriction enzymes used in the generation of each DNA fragment is provided in Supplementary Table 2. Design of primers was assisted by Clone Manager Professional 9 (Sci-Ed software, NC, USA). Platinum PCR SuperMix High Fidelity (Life Technologies, NY, USA) were used for all PCRs. PCR products were separated by acrylamide gel electrophoresis and isolated with a gel extraction kit (Quiagen). All restriction enzymes were purchased from New England Biolabs. Amino acid substitutions in Cx36 and Cx43 were introduced using the Quickchange Multi Site-directed Mutagenesis Kit (Agilent, TX, USA) or ordered from Genscript (New Jersey, USA) using the site-directed mutagenesis service. Chimeras and mutant fragments were subcloned into pEGFP-N1 (Clontech, CA, USA). All plasmid transfections were performed with Lipofectamine 2000 (Life Technologies, NY USA).
Cell lines and culture conditions
Experiments were performed in RIN cells (rat beta-cell insulinoma, ATCC CRL-2057) transfected with Cx36, Cx43, chimeras or mutants fused with colour variants of green fluorescent proteins (EGFP or CFP) attached to the CT. All experiments were performed with stable cell lines to minimize variability. All cell cultures were grown in RPMI 1640, with L-glutamine, supplemented with 8% fetal calf serum, 100 µg per ml streptomycin and 100 units per ml penicillin, and maintained in a CO2 incubator (37 °C and 5% CO2).
In vitro electrophysiology
Electrophysiological recordings were performed in cell cultures grown on glass coverslips and submerged on an experimental chamber mounted on the stage of an inverted IX70 microscope (Olympus) equipped with a fluorescence imaging system. Extracellular solution contained (in mM): 140 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 2 CsCl, 1 BaCl2, 5 glucose, 2 pyruvate, and 5 HEPES (pH 7.4 adjusted with NaOH). Standard pipette solution contained (in mM): 130 CsCl, 10 NaAsp, 1 MgCl2, 0.26 CaCl2, 2 EGTA, and 5 HEPES (pH 7.2 adjusted with CsOH). Resistance of recording pipettes was in the order of 3–5 MΩ. We used either EDTA or MgCl2 and the web-based Maxchelator software to adjust and calculate free Mg2+ concentration in the pipette solutions. Junctional conductance (gj) was measured using two EPC-8 patch clamp amplifiers (HEKA); briefly, a transjunctional voltage (Vj) was generated by modifying voltage in cell-1 (V1) and keeping the voltage in cell-2 (V2) constant (Vj=ΔV1). Application of Vj induced a transjunctional current (Ij) of opposite polarity to Vj (Ij = -ΔI2, and gj = Ij/Vj). Signals were digitized using an A/D converter (Axon instruments) and data were acquired and analyzed using custom-made software.
Brain-slice preparation and electrophysiology
A minimal number of animals were sacrificed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, and with the provisions of the Institutional Animal Care and Use Committee of the Marine Biological Laboratory. Horizontal brain slices (300-µm thick) were prepared from the BAC transgenic mouse line Tg(Gjd2-EGFP)JM16Gsat/Mmucd44, in which the expression of EGFP reporter gene is driven by the activity of the Cx36 promoter. Male or female mice age between P5 and P15 were used. Brain slices were obtained using a chilled VT1200 blade vibrating microtome (Leica Biosystems, IL, USA) and sliced in cold sucrose solution containing (in mM): 238 sucrose, 2.7 KCl, 1.25 KH2PO4, 26 NaHCO3, 11 Glucose, 2 CaCl2, and 2 MgSO4. Brain slices were transferred to an incubation chamber with extracellular recording solution and incubated for 20 min at 37 °C. The extracellular recording solution contained (in mM): 124 NaCl, 2.7 KCl, 1.25 KH2PO4, 26 NaHCO3, 10 Glucose, 2 CaCl2, and 2 MgSO4. The incubation chamber was then kept at room temperature for 30–40 min prior to electrophysiology. Brain slices were then transferred to a low-noise RC-27LD recording chamber (Warner Instruments, Hamden, CT) mounted on an Axio Examiner A1 microscope (Zeiss, Thornwood, NY) equipped with an Orca-R2 digital camera (Hamamatsu, Bridgewater, NJ) for infrared differential interference contrast (IR-DIC) and fluorescence imaging. Extracellular recording solution was continuously exchanged (~2 ml per min) at room temperature in the chamber by a gravity feed perfusion system. All sucrose and extracellular solutions were constantly bubbled and saturated with carbogen (95% oxygen/5% CO2) throughout the slice procedure and electrophysiology experiments. TRN neurons were identified based on characteristic location, cell shape and electrophysiological properties45. A standard pipette solution contained (in mM): 120 K-Gluconate, 20 KCl, 2 MgCl2, 0.2 EGTA, and 10 HEPES (pH 7.2 adjusted with KOH). Resistance of recording pipettes was on the order of 6–10 MΩ. We used K2ATP or MgATP to decrease or increase, respectively, free Mg2+ concentration in the pipette solutions2,43. Changes in membrane voltage and current were measured using two separate Axopatch 200B amplifiers, digitized using a Digidata 1440A, and acquired and analyzed using pClamp 10 software (Molecular Devices, Sunnyvale, CA). The gj was measured and calculated as explained for the in vitro electrophysiology (see above).
Confocal microscopy and fluorescence imaging
Fluorescence signals from EGFP expression in acute TRN brain slices were acquired using a LSM-780 Quasar confocal system configured on an inverted Observer Z1 microscope. Imaging during electrophysiology studies was conducted using an Axio Examiner A1 microscope (Zeiss, Oberkochen, Germany) equipped with an Orca-R2 digital camera (Hamamatsu Corp., Bridgewater, NJ). Image acquisition and processing were performed using ZEN software (Zeiss, Oberkochen, Germany). For in vitro studies, fluorescence signals from EGFP or CFP were acquired using an IX70 microscope (Olympus, USA) equipped with an ORCA-R2 digital camera (Hamamatsu Corp., Bridgewater, NJ). Image acquisition and processing were performed using UltraVIEW software (Perkin Elmer Life Sciences, Boston, MA).
Homology models and electrostatic surface potential
Structural homology models of Cx36 and Cx36*D47G were built using the known three-dimensional structure of Cx26 as a template55. The corresponding Cx26 cytoplasmic loop and C-terminus domains of Cx36 were deleted, and the target-template alignment was selected by hand, scoring a sequence identity of 47%. Based on this alignment, 200 models were generated by means of the MODELLER program and the best model was selected according to the DOPE score69. The electrostatic potential on the solvent accessible surface (surface potential) of the structural homology models was estimated using DELPHI, which provides finite difference solutions to the Poisson-Boltzmann equation70. We chose to display surface potentials according to the electrostatic potential found at the solvent accessible surface instead of the atomic surface charges used in the original Cx26 crystal structure article55. This method takes in consideration an average of all surrounding atomic surface charges and displays less extreme values of electrostatic surface potential. Default dielectric constants of 2.0 for interior (protein) and 80.0 for exterior (solvent) regions were used.
Data analysis
The analysis and statistics were performed using SigmaPlot v10 (Systat Software Inc, Chicago, IL) and pClamp 10 (Molecular Devices, Sunnyvale, CA). Averaged data are reported as the means ± s.e.m. Means for each group were compared using an unpaired student’s t-test.
Supplementary Material
Acknowledgments
We thank Michael V.L. Bennett, Vytautas K. Verselis and Thaddeus A. Bargiello for helpful comments and discussions. We thank Nerijus Paulauskas for assistance with S4SM, and Angele Bukauskiene and Alius Dicpinigaitis for excellent technical assistance. We thank Jim McIlvain and Elizabeth Dille from Zeiss for assistance with confocal imaging. Nicolás Palacios-Prado is a Howard Hughes Medical Institute International Student Research Fellow. This work was supported by the Grass Foundation with a Grass Fellowship to N.P-P., by a grant from the Canadian Institute of Health Research to J.I.N, and by the National Institute of Health grant R01NS 072238 to F.F.B.
Footnotes
Author contribution: N.P-P. and F.F.B. conceived and designed the experiments. N.P-P., S.C., J.F. and F.F.B. performed the experiments and analyzed the data. A.P. and J.I.N contributed reagents/materials/analysis tools and critically revised the paper. N.P-P and F.F.B coordinated the study and wrote the paper.
Additional Information
Supplementary Information accompanies this paper at…
Competing financial interests: The authors declare no competing financial interests.
References
- 1.Masuda T, Dobson GP, Veech RL. The Gibbs-Donnan near-equilibrium system of heart. J. Biol. Chem. 1990;265:20321–20334. [PubMed] [Google Scholar]
- 2.Luthi D, Gunzel D, McGuigan JA. Mg-ATP binding: its modification by spermine, the relevance to cytosolic Mg2+ buffering, changes in the intracellular ionized Mg2+ concentration and the estimation of Mg2+ by 31P–NMR. Exp. Physiol. 1999;84:231–252. [PubMed] [Google Scholar]
- 3.Taylor JS, et al. Free magnesium levels in normal human brain and brain tumors: 31P chemical-shift imaging measurements at 1.5 T. Proc. Natl. Acad. Sci. U. S. A. 1991;88:6810–6814. doi: 10.1073/pnas.88.15.6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C, Nakatani K, Koutalos Y. Free magnesium concentration in salamander photoreceptor outer segments. J. Physiol. 2003;553:125–135. doi: 10.1113/jphysiol.2003.053280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henrich M, Buckler KJ. Effects of anoxia, aglycemia, and acidosis on cytosolic Mg2+, ATP, and pH in rat sensory neurons. Am. J. Physiol. Cell. Physiol. 2008;294:280–294. doi: 10.1152/ajpcell.00345.2007. [DOI] [PubMed] [Google Scholar]
- 6.Kato H, Gotoh H, Kajikawa M, Suto K. Depolarization triggers intracellular magnesium surge in cultured dorsal root ganglion neurons. Brain Res. 1998;779:329–333. doi: 10.1016/s0006-8993(97)01232-8. [DOI] [PubMed] [Google Scholar]
- 7.Cheng C, Reynolds IJ. Subcellular localization of glutamate-stimulated intracellular magnesium concentration changes in cultured rat forebrain neurons using confocal microscopy. Neuroscience. 2000;95:973–979. doi: 10.1016/s0306-4522(99)00471-6. [DOI] [PubMed] [Google Scholar]
- 8.Shindo Y, Fujimoto A, Hotta K, Suzuki K, Oka K. Glutamate-induced calcium increase mediates magnesium release from mitochondria in rat hippocampal neurons. J. Neurosci. Res. 2010;88:3125–3132. doi: 10.1002/jnr.22467. [DOI] [PubMed] [Google Scholar]
- 9.Yamanaka R, Shindo Y, Hotta K, Suzuki K, Oka K. NO/cGMP/PKG signaling pathway induces magnesium release mediated by mitoKATP channel opening in rat hippocampal neurons. FEBS Lett. 2013;587:2643–2648. doi: 10.1016/j.febslet.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 10.Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R. Sleep and brain energy levels: ATP changes during sleep. J. Neurosci. 2010;30:9007–9016. doi: 10.1523/JNEUROSCI.1423-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ainscow EK, Mirshamsi S, Tang T, Ashford ML, Rutter GA. Dynamic imaging of free cytosolic ATP concentration during fuel sensing by rat hypothalamic neurones: evidence for ATP-independent control of ATP-sensitive K(+) channels. J. Physiol. 2002;544:429–445. doi: 10.1113/jphysiol.2002.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim. Biophys. Acta. 2010;1802:80–91. doi: 10.1016/j.bbadis.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Williams GD, Smith MB. Application of the accurate assessment of intracellular magnesium and pH from the 31P shifts of ATP to cerebral hypoxia-ischemia in neonatal rat. Mag. Res. Med. 1995;33:853–857. doi: 10.1002/mrm.1910330618. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, et al. Hypoxia induces an increase in intracellular magnesium via transient receptor potential melastatin 7 (TRPM7) channels in rat hippocampal neurons in vitro. J. Biol. Chem. 2011;286:20194–20207. doi: 10.1074/jbc.M110.148494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinsberger AD, et al. Magnetic resonance imaging volumetric and phosphorus 31 magnetic resonance spectroscopy measurements in schizophrenia. J. Psychiatry Neurosci. 1997;22:111–117. [PMC free article] [PubMed] [Google Scholar]
- 16.Cernak I, Radosevic P, Malicevic Z, Savic J. Experimental magnesium depletion in adult rabbits caused by blast overpressure. Magnes. Res. 1995;8:249–259. [PubMed] [Google Scholar]
- 17.Suzuki M, et al. Decrease in cerebral free magnesium concentration following closed head injury and effects of VA-045 in rats. Gen. Pharmacol. 1997;28:119–121. doi: 10.1016/s0306-3623(96)00148-6. [DOI] [PubMed] [Google Scholar]
- 18.Oyanagi K, et al. Magnesium deficiency over generations in rats with special references to the pathogenesis of the Parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Neuropathology. 2006;26:115–128. doi: 10.1111/j.1440-1789.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 19.Andrasi E, Igaz S, Molnar Z, Mako S. Disturbances of magnesium concentrations in various brain areas in Alzheimer's disease. Magnes. Res. 2000;13:189–196. [PubMed] [Google Scholar]
- 20.Stelmasiak Z, Solski J, Jakubowska B. Magnesium concentration in plasma and erythrocytes in MS. Acta Neurol. Scand. 1995;92:109–111. doi: 10.1111/j.1600-0404.1995.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 21.Yasui M, Yase Y, Kihira T, Adachi K, Suzuki Y. Magnesium and calcium contents in CNS tissues of amyotrophic lateral sclerosis patients from the Kii peninsula, Japan. Euro. Neurol. 1992;32:95–98. doi: 10.1159/000116800. [DOI] [PubMed] [Google Scholar]
- 22.Lodi R, et al. Deficit of brain and skeletal muscle bioenergetics and low brain magnesium in juvenile migraine: an in vivo 31P magnetic resonance spectroscopy interictal study. Pediatr. Res. 1997;42:866–871. doi: 10.1203/00006450-199712000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Barbiroli B, et al. Low brain intracellular free magnesium in mitochondrial cytopathies. J. Cereb. Blood. Flow. Metab. 1999;19:528–532. doi: 10.1097/00004647-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Bennett MV, Zukin RS. Electrical coupling and neuronal synchronization in the Mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- 25.Bissiere S, Zelikowsky M, Ponnusamy R, Jacobs NS, Blair HT, Fanselow MS. Electrical synapses control hippocampal contributions to fear learning and memory. Science. 2011;331:87–91. doi: 10.1126/science.1193785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaksi E, Wilson RI. Electrical coupling between olfactory glomeruli. Neuron. 2010;67:1034–1047. doi: 10.1016/j.neuron.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bukauskas FF, Weingart R. Voltage-dependent gating of single gap junction channels in an insect cell line. Biophys J. 1994;67:613–625. doi: 10.1016/S0006-3495(94)80521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palacios-Prado N, Bukauskas FF. Heterotypic gap junction channels as voltage-sensitive valves for intercellular signaling. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14855–14860. doi: 10.1073/pnas.0901923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palacios-Prado N, Bukauskas FF. Modulation of metabolic communication through gap junction channels by transjunctional voltage; synergistic and antagonistic effects of gating and ionophoresis. Biochim. Biophys. Acta. 2012;1818:1884–94. doi: 10.1016/j.bbamem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furshpan EJ, Potter DD. Transmission at the giant motor synapses of the crayfish. J. Physiol. 1959;145:289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Auerbach AA, Bennett MVL. A rectifying electrotonic synapse in the central nervous system of a vertebrate. J. Gen. Physiol. 1969;53:211–237. doi: 10.1085/jgp.53.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phelan P, et al. Molecular mechanism of rectification at identified electrical synapses in the Drosophila giant fiber system. Curr. Biol. 2008;18:1955–1960. doi: 10.1016/j.cub.2008.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rash JE, et al. Molecular and functional asymmetry at a vertebrate electrical synapse. Neuron. 2013;79:957–969. doi: 10.1016/j.neuron.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sohl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat. Rev. Neurosci. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- 35.Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu. Rev. Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- 36.Ouyang X, Winbow VM, Patel LS, Burr GS, Mitchell CK, O'Brien J. Protein kinase A mediates regulation of gap junctions containing connexin35 through a complex pathway. Brain Res. Mol. Brain. Res. 2005;135:1–11. doi: 10.1016/j.molbrainres.2004.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alev C, et al. The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with glutamate receptors. Proc. Natl. Acad. Sci. U. S. A. 2008;105:20964–20969. doi: 10.1073/pnas.0805408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.González-Nieto D, et al. Regulation of neuronal connexin-36 channels by pH. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17169–17174. doi: 10.1073/pnas.0804189105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marandykina A, Palacios-Prado N, Rimkutė L, Skeberdis VA, Bukauskas FF. Regulation of Connexin-36 Gap Junction Channels by n-Alkanols and Arachidonic Acid. J. Physiol. 2013;591:2087–2101. doi: 10.1113/jphysiol.2013.250910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haas JS, Zavala B, Landisman CE. Activity-dependent long-term depression of electrical synapses. Science. 2011;334:389–393. doi: 10.1126/science.1207502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereda AE, et al. Ca2+/calmodulin-dependent kinase II mediates simultaneous enhancement of gap-junctional conductance and glutamatergic transmission. Proc. Natl. Acad. Sci. U. S. A. 1998;95:13272–13277. doi: 10.1073/pnas.95.22.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cachope R, Mackie K, Triller A, O'Brien J, Pereda AE. Potentiation of electrical and chemical synaptic transmission mediated by endocannabinoids. Neuron. 2007;56:1034–1047. doi: 10.1016/j.neuron.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palacios-Prado N, et al. Intracellular Magnesium-Dependent Modulation of Gap Junction Channels Formed by Neuronal connexin36. J. Neurosci. 2013;33:4741–4753. doi: 10.1523/JNEUROSCI.2825-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong S, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 45.Landisman CE, Long MA, Beierlein M, Deans MR, Paul DL, Connors BW. Electrical synapses in the thalamic reticular nucleus. J. Neurosci. 2002;22:1002–1009. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res. Brain. Res. Rev. 2004;46:1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Bukauskas FF, et al. Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2556–2561. doi: 10.1073/pnas.050588497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clackson T, Winter G. 'Sticky feet'-directed mutagenesis and its application to swapping antibody domains. Nucleic Acids Res. 1989;17:10163–10170. doi: 10.1093/nar/17.24.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srinivas M, et al. Functional properties of channels formed by the neuronal gap junction protein connexin36. J. Neurosci. 1999;19:9848–9855. doi: 10.1523/JNEUROSCI.19-22-09848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreno AP, Berthoud VM, Perez-Palacios G, Perez-Armendariz EM. Biophysical evidence that connexin-36 forms functional gap junction channels between pancreatic mouse beta-cells. Am. J. Physiol. Endocrinol. Metab. 2005;288:948–956. doi: 10.1152/ajpendo.00216.2004. [DOI] [PubMed] [Google Scholar]
- 51.Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J. Neurosci. 2001;21:1983–2000. doi: 10.1523/JNEUROSCI.21-06-01983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bukauskas FF, Verselis VK. Gap junction channel gating. Biochim. Biophys. Acta. 2004;1662:42–60. doi: 10.1016/j.bbamem.2004.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palacios-Prado N, Briggs SW, Skeberdis VA, Pranevicius M, Bennett MV, Bukauskas FF. pH-dependent modulation of voltage gating in connexin45 homotypic and connexin45/connexin43 heterotypic gap junctions. Proc. Natl. Acad. Sci. U. S. A. 2010;107:9897–9902. doi: 10.1073/pnas.1004552107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paulauskas N, Pranevicius M, Pranevicius H, Bukauskas FF. A stochastic four-state model of contingent gating of gap junction channels containing two "fast" gates sensitive to transjunctional voltage. Biophys. J. 2009;96:3936–3948. doi: 10.1016/j.bpj.2009.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maeda S, et al. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature. 2009;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- 56.Zonta F, Mammano F, Torsello M, Fortunati N, Orian L, Polimeno A. Role of gamma carboxylated Glu47 in connexin 26 hemichannel regulation by extracellular Ca: Insight from a local quantum chemistry study. Biochem. Biophys. Res. Commun. 2014;445:10–5. doi: 10.1016/j.bbrc.2014.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Musa H, Fenn E, Crye M, Gemel J, Beyer EC, Veenstra RD. Amino terminal glutamate residues confer spermine sensitivity and affect voltage gating and channel conductance of rat connexin40 gap junctions. J. Physiol. 2004;557:863–878. doi: 10.1113/jphysiol.2003.059386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bukauskas FF, Bukauskiene A, Bennett MVL, Verselis VK. Gating properties of gap junction channels assembled from connexin43 and connexin43 fused with green fluorescent protein. Biophys. J. 2001;81:137–152. doi: 10.1016/S0006-3495(01)75687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White TW, Bruzzone R, Wolfram S, Paul DL, Goodenough DA. Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: the second extracellular domain is a determinant of compatibility between connexins. J. Cell Biol. 1994;125:879–892. doi: 10.1083/jcb.125.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakagawa S, et al. Asparagine 175 of connexin32 is a critical residue for docking and forming functional heterotypic gap junction channels with connexin26. J. Biol. Chem. 2011;286:19672–19681. doi: 10.1074/jbc.M110.204958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramanan SV, Brink PR, Varadaraj K, Peterson E, Schirrmacher K, Banach K. A three-state model for connexin37 gating kinetics. Biophys. J. 1999;76:2520–2529. doi: 10.1016/S0006-3495(99)77406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teubner B, et al. Functional expression of the murine connexin 36 gene coding for a neuron-specific gap junctional protein. J. Membr. Biol. 2000;176:249–262. doi: 10.1007/s00232001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oh S, Rivkin S, Tang Q, Verselis VK, Bargiello TA. Determinants of gating polarity of a connexin 32 hemichannel. Biophys. J. 2004;87:912–928. doi: 10.1529/biophysj.103.038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiquito AJ, Amorim CA, Berengue OM, Araujo LS, Bernardo EP, Leite ER. Back-to-back Schottky diodes: the generalization of the diode theory in analysis and extraction of electrical parameters of nanodevices. J. Phys. Condens. Matter. 2012;24:225303. doi: 10.1088/0953-8984/24/22/225303. [DOI] [PubMed] [Google Scholar]
- 65.Jaslove SW, Brink PR. The mechanism of rectification at the electrotonic motor giant synapse of the crayfish. Nature. 1986;323:63–65. doi: 10.1038/323063a0. [DOI] [PubMed] [Google Scholar]
- 66.Verselis VK, Ginter CS, Bargiello TA. Opposite voltage gating polarities of two closely related connexins. Nature. 1994;368:348–351. doi: 10.1038/368348a0. [DOI] [PubMed] [Google Scholar]
- 67.Oh S, Rubin JB, Bennett MV, Verselis VK, Bargiello TA. Molecular determinants of electrical rectification of single channel conductance in gap junctions formed by connexins 26 and 32. J. Gen. Physiol. 1999;114:339–364. doi: 10.1085/jgp.114.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gutierrez GJ, Marder E. Rectifying electrical synapses can affect the influence of synaptic modulation on output pattern robustness. J. Neurosci. 2013;33:13238–13248. doi: 10.1523/JNEUROSCI.0937-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eswar N, Eramian D, Webb B, Shen MY, Sali A. Protein structure modeling with MODELLER. Methods. Mol. Biol. 2008;426:145–159. doi: 10.1007/978-1-60327-058-8_8. [DOI] [PubMed] [Google Scholar]
- 70.Rocchia W, Sridharan S, Nicholls A, Alexov E, Chiabrera A, Honig B. Rapid grid-based construction of the molecular surface and the use of induced surface charge to calculate reaction field energies: applications to the molecular systems and geometric objects. J. Comput. Chem. 2002;23:128–137. doi: 10.1002/jcc.1161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.