Abstract
The use of focused ultrasonic waves to modulate neural structures has gained recent interest due to its potential in treating neurological disorders non-invasively. While several papers have focused on the use of ultrasound neuromodulation on peripheral nerves, none of these studies have been performed on the vagus nerve. We present preliminary observations on the effects of focused pulsed ultrasound (FPUS) on the conduction of the left cervical vagus nerve of a Long Evans rat. Ultrasound energy was applied at a frequency of 1.1 MHz, and at spatial-peak, temporal average intensities that ranged from 13.6 to 93.4 W/cm2. Vagus nerve inhibition was observed in most cases. Results of this preliminary study suggested that there is a proportional relationship between acoustic intensity and the level of nerve inhibition.
Keywords: acoustic neuromodulation, vagus nerve, focused ultrasound, nerve modulation
Introduction
Acoustic neuromodulation, the use of low intensity focused ultrasound to stimulate or inhibit neural structures, has gained recent interest due to its potential to non-invasively treat neurological disorders. The field of acoustic neuromodulation could be divided into two major categories: 1) one that focuses on the central nervous system (CNS) and 2) another that focuses on the peripheral nervous system (PNS).
Two recent articles provide a comprehensive review on the use of low intensity pulsed ultrasound (LIPUS) for neuromodulation. The first, published by Bystritsky and colleagues (Bystritsky, Korb et al. 2011) summarizes early and current studies on acoustic neuromodulation, providing perspectives on safety issues, current challenges and future applications. The second review article, published by Gavrilov and Tsirulnikov (Gavrilov and Tsirulnikov 2012), shows how to use LIPUS to input sensory information to humans.
Most of the recent publications on acoustic neuromodulation focus on the excitation or inhibition of CNS structures. At least three groups have observed motor activity in response to transcranial ultrasound (Tufail, Matyushov et al. 2010, Kim, Taghados et al. 2012, King, Brown et al. 2013). Others studies (Tyler, Tufail et al. 2008, Tufail, Matyushov et al. 2010, Min, Yang et al. 2011) report the direct activation of brain structures using ultrasound, while one study reports suppressing of epileptic signal bursts in a rat model (Min, Bystritsky et al. 2011).
Among the papers that focus on the PNS, Tsui et.al (Tsui, Wang et al. 2005) observed that, when using continuous ultrasound signals at 3.5MHz, low intensity US seemed to excite excised bullfrog sciatic nerves, while higher intensity pulses seemed to cause inhibition. In this study, nerve conduction suppression was attributed to ultrasound-induced thermal effects, while increases in compound action potentials were attributed to mechanical effects. In a more recent study, also performed on sciatic nerves of bullfrogs, Colucci and colleagues (Colucci, Strichartz et al. 2009) reported decreases in the amplitude of electrically evoked compound action potentials due to thermal effects induced by ultrasound at frequencies of 0.66 and 1.99 MHz.
The vagus nerve and electrical vagus nerve stimulation (VNS) treatment
The vagus nerve, also known as the “wandering nerve”, is the tenth cranial nerve. It consists of both somatic and visceral afferent and efferent fibers. In the cervical portion of the vagus nerve, about 80% of the fibers are afferents, which carry visceral and somatic sensory information to the CNS. Afferent fibers end in the nucleus of the tractus solitarius (NTS). From the NTS, vagal impulses project into a variety of structures within the posterior fossa, including all of the other nuclei of the dorsal medullary complex (Henry 2002).
The remaining 20% of fibers are efferents that provide parasympathetic innervation to organs such as the lungs, heart and the gastro-intestinal tract (Henry 2002, Aalbers, Vles et al. 2011). Smaller diameter, unmyelinated C-fibers, predominate over faster myelinated B and A fibers. The left vagus nerve carries parasympathetic fibers that less densely innervate the ventricles, while the right vagus nerve carries parasympathetic fibers that densely innervate the cardiac atria.
Electrical vagus nerve stimulation (VNS) consists of applying electrical stimuli to the vagus nerve via implanted cuff electrodes. Multiple studies have shown that electrical VNS effectively suppresses and prevents various types of epileptic seizures (Milby, Halpern et al. 2008). However, the anticonvulsive mechanism of electrical VNS is still not fully understood.
Potential application of ultrasound vagus nerve modulation
One question that arises now is, can vagus nerve acoustic neuromodulation be used as a non-invasive alternative to electrical VNS? In order to address this broader question, several basic questions must be answered. The first of these questions: Does FPUS modulate vagus nerve activity?
We present preliminary observations on the effects of FPUS on the conduction of the left cervical vagus nerve of a rat. Although several studies involving acoustic neuromodulation on peripheral nerves have been published, none of these studies have been performed on the vagus nerve.
Materials and Methods
Animal preparation
Surgeries and experimental procedures were performed using Long Evans rats, at the Center for Implantable Devices (CID) of the Weldon School of Biomedical Engineering at Purdue University. All experimental protocols were reviewed and approved by the Purdue Animal Care and Use Committee (PACUC). Prior to surgery, the animals were given ad-libitum access to food and water. After induction of anesthesia (isoflurane 1-4% in Oxygen, 1-3 L/min flow rate), the fur covering the neck was shaved and cleaned with alternating scrubs of betadine and ethanol. Ophthalmic ointment was placed on the eyes of the subject to prevent them from drying during the procedure. Butorphanol tartrate, an analgesic, was administered pre-operatively (1.5 mg/kg, SC).
The skin and soft tissue were retracted to expose the underlying sternohyoid muscle, which sits atop the carotid bifurcation. Surgical retractors were then carefully placed to hold open the incision site. Using a blunt dissection technique, the soft and connective tissues ∼1-2 mm to the left of midline (to the right of midline from the surgeon's perspective) were gently separated in a longitudinal direction (parallel to the initial incision) until the trachea and carotid artery could be seen with 1-2 cm of access (to enable implantation cuff electrodes). The left cervical vagus nerve sits adjacent and runs parallel to the carotid artery. The carotid sheath was carefully dissected to expose a 1-2 cm segment of the left cervical vagus nerve. Stimulating (distal to brain) and recording (proximal to brain) cuff electrodes (∼0.5 cm in length with a 0.5 mm inner diameter) were wrapped around the vagus nerve.
Electrical and ultrasonic stimulation
The vagus nerve was electrically stimulated with an electrical stimulator (S-48, Grass Technologies, Rhode Island, USA) using 0.5 ms monophasic, constant voltage pulses at a repetition rate of 1 Hz. The amplitude of the stimulating pulse was incrementally increased to find the maximum response voltage (maximal activation) and then decreased until 50% of the maximum amplitude of the compound action potential (CAP) was recorded.
A focused ultrasound transducer (H-101, Sonic Concepts, Washington, USA) was used to deliver acoustic energy to the vagus nerve. An RF amplifier was used to drive the transducer at a frequency of 1.1 MHz. A coupling cone filled with de-gassed water was used to couple the transducer to the nerve. The tip of the coupling cone was placed such that the acoustic focus was located on the nerve and between the electrodes. Figure 1 illustrates the placement of the FUS transducer relative to the stimulating and recording cuff electrodes. Pulsed focused ultrasound was utilized to insonify the nerve.
Figure 1.
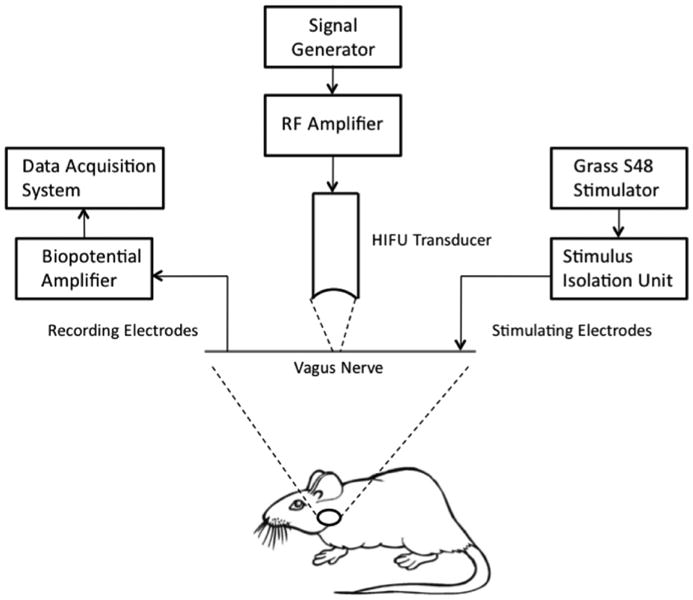
Experimental setup, illustrating the placement of the FUS transducer relative to the stimulating and recording cuff electrodes.
A calibrated needle hydrophone (HNR-1000, Onda Corp., California, USA) was used to determine the acoustic intensity generated by the transducer for a given input voltage. This method enables the accurate measurement of the amount of acoustic energy delivered to the nerve.
Data acquisition
A total of 9 trials were performed on a single rat. Each trial consisted of 5 seconds of initial recording of evoked potentials without ultrasound, 15 seconds with ultrasound exposure, and 10 seconds of recovery. Table 1 shows the ultrasound parameters used for each trial. Compound action potentials (CAPs) were recorded using a data acquisition board (USB-6353, National Instruments, Texas, USA) and custom LabVIEW software. Electrical recording were processed offline using custom software developed in MATLAB (MathWorks, Massachusetts, USA).
Table 1.
Ultrasound parameters used for each experimental trial, and increases in acoustic gel temperatures after ultrasound exposure.
| Trial # | Intensity (W/cm2) |
PRF (kHz) |
Duty Cycle (%) | ISPTA (W/cm2) |
Mechanical Index | ΔT (°C) mean ± std |
|---|---|---|---|---|---|---|
| 1 | 74.8 | 1 | 90.9 | 68.0 | 1.43 | 0.02 ± 0.02 |
| 2 | 89.0 | 1 | 90.9 | 80.9 | 1.56 | 0.15 ± 0.08 |
| 3 | 102.8 | 1 | 90.9 | 93.4 | 1.67 | 0.13 ± 0.06 |
| 4 | 74.8 | 0.33 | 30.3 | 22.7 | 1.43 | 0.11 ± 0.14 |
| 5 | 89.0 | 0.33 | 30.3 | 27.0 | 1.56 | 0.17 ± 0.06 |
| 6 | 102.8 | 0.33 | 30.3 | 31.1 | 1.67 | 0.27 ± 0.06 |
| 7 | 74.8 | 0.2 | 18.2 | 13.6 | 1.43 | 0.60 ± 0.13 |
| 8 | 89.0 | 0.2 | 18.2 | 16.2 | 1.56 | 0.92 ± 0.13 |
| 9 | 102.8 | 0.2 | 18.2 | 18.7 | 1.67 | 0.93 ± 0.12 |
PRF-Pulse repetition frequency
Temperature measurements at the acoustic focus
A small thermocouple was placed in close proximity to the nerve to record nerve temperature during ultrasound exposure. However, this method resulted in unreliable temperature measurements since it was difficult to consistently and precisely place the thermocouple at the acoustic focus during the experimental trials. To improve accuracy in measuring the temperature at the acoustic focus, additional temperature measurements were performed. Approximately 2 cm3 of acoustic gel was placed over the coupling cone such that the acoustic focused lied within the gel. A small type K thermocouple was carefully placed at the transducer's acoustic focus. Ultrasound was applied to the gel for at least 15 seconds using the same ultrasound parameters employed during the experimental trials. Three temperature measurements were performed for each acoustic condition. Temperature measurements consisted of the increase in temperature after 15 seconds of ultrasound exposure, with respect to room temperature (24°C). The measured temperatures are shown in Table 1.
Results and Discussion
Figure 2a shows vagus nerve CAPs recorded before, and five and fifteen seconds after onset of ultrasound. A more detailed representation of the peaks of interest is shown in Figure 2b. The amplitudes and locations of these peaks varied as ultrasound was applied. More specifically, the CAP peak amplitude decreased and peak latency increased during the 15s period of ultrasound exposure.
Figure 2.
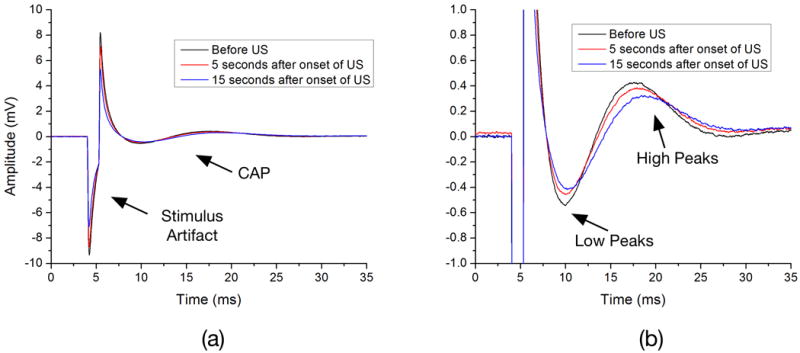
Vagus nerve compound action potentials recorded before, and five and fifteen seconds after onset of ultrasound (a). Detailed representation of the peaks of interest (b). The amplitudes and locations of these peaks varied as ultrasound was applied. More specifically, the compound action potentials (CAP) peaks were observed to decrease in amplitude and get delayed as time passed on during ultrasound exposure.
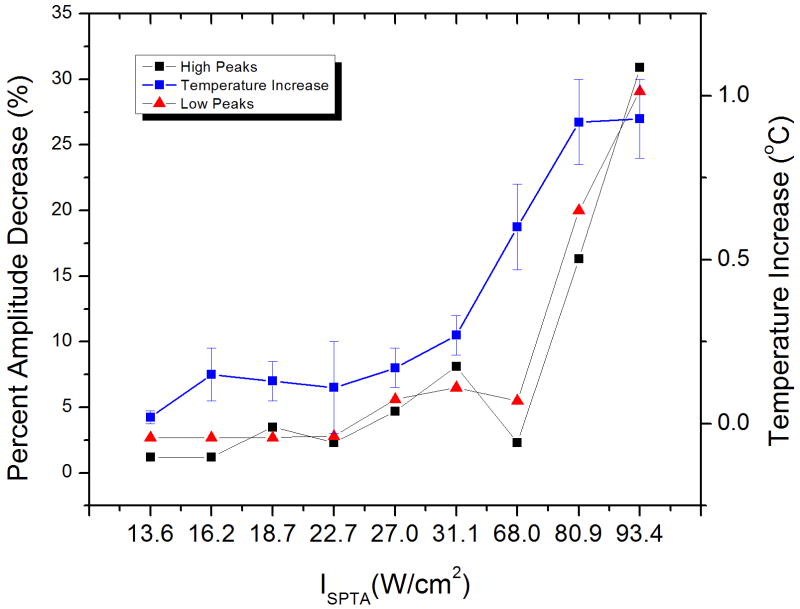
In order to quantify the ultrasound-induced changes in CAP morphology, the amplitudes of the first (low) and second (high) peaks were plotted as a function of time. The variations in amplitudes for the first and second peaks are depicted in Figure 3a-b. Trials 2, 3 and 6 show the largest response magnitude. The percent change in CAP amplitudes, as taken from the change in amplitude just before and after fifteen seconds of ultrasound exposure, were plotted as a function of Ispta. Figure 4 shows the percent amplitude change for both peaks, and the temperature increases of acoustic gel, as a function of Ispta. It can be noticed that the trend is to have increased changes in amplitude as the Ispta is increased. An exception to this trend was observed at an Ispta of 68.0 W/cm2, where the change in CAP amplitude was less than those observed at lower Ispta values. We attribute this lower than expected nerve inhibition to the experimental variation introduced by the fact that, on this preliminary study, only one trial was performed at each acoustic intensity level.
Figure 3.
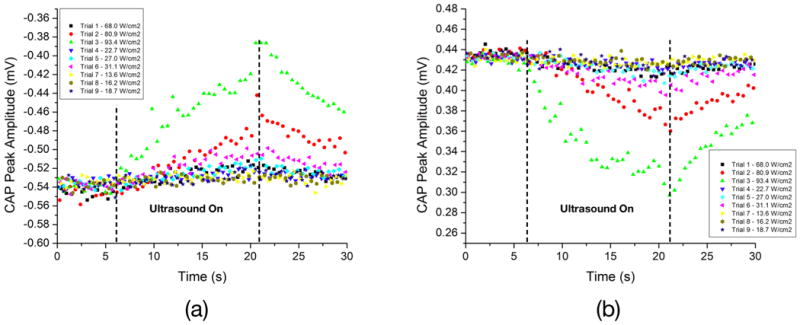
These plots show how the amplitudes of the first (a) and second (b) CAP peaks changed with time when ultrasound pulses were applied to the vagus nerve, for different acoustic intensities. Trials 1-3, 4-6, and 7-9 were performed at duty cycles of approximately 91%, 30% and 18%, respectively. These results indicate that, for 15 seconds of ultrasound exposure, reversible neuromodulation was achieved in all cases, most markedly seen in trials 2, 3, and 6, where the acoustic intensities were higher.
Figure 4.
Percent amplitude change for both CAP peaks as a function of Ispta. This plot also shows the observed acoustic gel temperature changes (calculated as the temperature difference before and after 15 seconds of ultrasound exposure) for each combination of acoustic parameters used in the experimental trials.
Temperature recordings at the acoustic gel indicated a moderate increase in temperature as a result of ultrasound exposure (see Table 1). The observation of these temperature increases would lead the reader to think that the inhibitory effects of ultrasound were due to thermal mechanisms. However, there is an observation that makes us wonder whether or not the observed responses were due exclusively to ultrasound induced heating. It is commonly known that the conduction velocity (CV) of peripheral nerves increases with temperature, while the amplitude of evoked potentials decrease as the nerve temperature increases (Dioszeghy and Stalberg 1992). Although we observed a decrease in the amplitude of evoked potential when ultrasound was applied, we also observed a decrease in conduction velocity (increase in peak latency) in all trials. This particular finding contradicts the observations made by Tsui et.al (Tsui, Wang et al. 2005), in which the conduction velocity of the sciatic nerve of a bullfrog increased with ultrasound intensity. It also contradicts the common knowledge that CV increases with temperature. Our results correlate with those reported by Wahab et al. (Wahab, Choi et al. 2012). In this study, performed on the giant axon of live earthworms, the authors investigate how pressure waves affect nerve conduction through ultrasound-induced radiation forces, while avoiding cavitational or thermal effects. They report changes in rate of decay of action potential amplitude and velocity that are proportional to radiation force.
Thus, it is our believe that although most of the observed neuromodulatory responses were most likely due to ultrasound-induced thermal effects, mechanical effects, more specifically radiation forces, might have also influenced the nerve responses.
Conclusions
Acoustic neuromodulation of the vagus nerve was observed in this preliminary study using focused pulsed ultrasound at a frequency of 1.1 MHz. Results of this preliminary study suggested that there is a proportional relationship between acoustic intensity and the level of inhibition. Additional studies will be needed to better understand the relationship of ultrasound parameters (pressure amplitude, carrier frequency, pulse repetition frequency and duty cycle) on vagus nerve neuromodulation. The goal of these future parametric studies should be to establish mathematical expressions that help predict nerve responses to different ultrasound parameters, such as in the case of electrical stimulation, where at least two forms of strength-duration curves are known. As of using vagus nerve acoustic neuromodulation to treat central nervous system diseases such as epilepsy and depression, studies should be conducted to determine if vagus nerve acoustic neuromodulation results in brain activation or inhibition.
Future work aims to isolate the influence of acoustic neuromodulation on the different fiber types that comprise the vagus nerve. Evidence suggests that acoustic neuromodulation reversibly inhibits vagal nerve conduction. Future work will quantify the degree of influence on each fiber type and determine the therapeutic potential of acoustic neuromodulation.
Acknowledgments
This work was supported primarily by RISE Program of the National Institutes of Health (NIH) under award number R25GM088023.
Grant sponsor: This work was supported primarily by the RISE Program of the National Institutes of Health (NIH) under award number R25GM088023.
References
- Aalbers M, Vles J, Klinkenberg S, Hoogland G, Majoie M, Rijkers K. Animal models for vagus nerve stimulation in epilepsy. Experimental Neurology. 2011;230(2):167–175. doi: 10.1016/j.expneurol.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Bystritsky A, Korb AS, Douglas PK, Cohen MS, Melega WP, Mulgaonkar AP, DeSalles A, Min BK, Yoof SS. A review of low-intensity focused ultrasound. Brain Stimulation. 2011;4(3):125–136. doi: 10.1016/j.brs.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Colucci V, Strichartz G, Jolesz F, Vykhodtseva N, Hynynen K. Focused Ultrasound Effects On Nerve Action Potential In Vitro. Ultrasound in Medicine and Biology. 2009;35(10):1737–1747. doi: 10.1016/j.ultrasmedbio.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dioszeghy P, Stalberg E. Changes In Motor And Sensory Nerve-Conduction Parameters With Temperature In Normal And Diseased Nerve. Electroencephalography and Clinical Neurophysiology. 1992;85(4):229–235. doi: 10.1016/0168-5597(92)90110-w. [DOI] [PubMed] [Google Scholar]
- Gavrilov LR, Tsirulnikov EM. Focused ultrasound as a tool to input sensory information to humans (Review) Acoustical Physics. 2012;58(1):1–21. [Google Scholar]
- Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59(6):S3–S14. doi: 10.1212/wnl.59.6_suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- Kim H, Taghados SJ, Fischer K, Maeng LS, Park S, Yoo SS. Noninvasive Transcranial Stimulation Of Rat Abducens Nerve By Focused Ultrasound. Ultrasound in Medicine and Biology. 2012;38(9):1568–1575. doi: 10.1016/j.ultrasmedbio.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RL, Brown JR, Newsome WT, Pauly KB. Effective Parameters For Ultrasound-Induced In Vivo Neurostimulation. Ultrasound in Medicine and Biology. 2013;39(2):312–331. doi: 10.1016/j.ultrasmedbio.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Milby AH, Halpern CH, Baltuch GH. Vagus nerve stimulation for epilepsy and depression. Neurotherapeutics. 2008;5(1):75–85. doi: 10.1016/j.nurt.2007.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min BK, Bystritsky A, Jung KI, Fischer K, Zhang Y, Maeng LS, Park SI, Chung YA, Jolesz FA, Yoo SS. Focused ultrasound-mediated suppression of chemically-induced acute epileptic EEG activity. Bmc Neuroscience. 2011;12 doi: 10.1186/1471-2202-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min BK, Yang PS, Bohlke M, Park S, Vago DR, Maher TJ, Yoo SS. Focused Ultrasound Modulates the Level of Cortical Neurotransmitters: Potential as a New Functional Brain Mapping Technique. International Journal of Imaging Systems and Technology. 2011;21(2):232–240. [Google Scholar]
- Tsui PH, Wang SH, Huang CC. In vitro effects of ultrasound with different energies on the conduction properties of neural tissue. Ultrasonics. 2005;43(7):560–565. doi: 10.1016/j.ultras.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, Tillery SIH, Tyler WJ. Transcranial Pulsed Ultrasound Stimulates Intact Brain Circuits. Neuron. 2010;66(5):681–694. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, Majestic C. Remote Excitation of Neuronal Circuits Using Low-Intensity, Low-Frequency Ultrasound. Plos One. 2008;3(10) doi: 10.1371/journal.pone.0003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab RA, Choi M, Liu YB, Krauthamer V, Zderic V, Myers MR. Mechanical bioeffects of pulsed high intensity focused ultrasound on a simple neural model. Medical Physics. 2012;39(7):4274–4283. doi: 10.1118/1.4729712. [DOI] [PubMed] [Google Scholar]