Abstract
Osteoprotegerin (OPG) is involved in the regulation of bone turnover, but little is known about this protein during pregnancy or among neonates. We undertook a prospective longitudinal study to identify relationships between OPG, markers of bone turnover and birth outcomes in 155 pregnant adolescents (13–18 years) and their newborns. Maternal blood samples were collected at mid-gestation and at delivery. Cord blood was obtained at delivery. Serum OPG, estradiol and markers of bone formation (osteocalcin) and resorption (N-telopeptide) were assessed in all samples. Placental OPG expression was assessed in placental tissue obtained at delivery. Bone markers and OPG increased significantly from mid-gestation (26.0 ± 3.4 weeks) to delivery (39.3 ± 2.6 weeks). Neonatal OPG was significantly lower, but bone turnover markers were significantly higher than maternal values at mid-gestation and at parturition (P < 0.001). African-American adolescents had higher concentrations of OPG than Caucasian adolescents at mid-gestation (P = 0.01) and delivery (P = 0.04). Gestational age and estradiol were also predictors of maternal OPG at mid-gestation and delivery. OPG concentrations in cord blood were correlated with maternal OPG concentrations and were negatively associated with infant birth weight z-score (P = 0.02) and ponderal index (P = 0.02). In conclusion, maternal OPG concentrations increased across gestation and were significantly higher than neonatal OPG concentrations. Maternal and neonatal OPG concentrations were not associated with markers of bone turnover or placental OPG expression, but neonatal OPG was inversely associated with neonatal anthropometric measures. Additional research is needed to identify roles of OPG during pregnancy.
Keywords: adolescent pregnancy, birth weight, bone, osteoprotegerin
Introduction
Adolescent pregnancy is a significant public health problem in the United States. Approximately 410,000 adolescents gave birth in 2009.1 When childbearing occurs before attainment of peak skeletal mass, there is a potential competition for nutrients between the adolescent and her developing fetus. Pregnant adolescents also have a greater risk for adverse birth outcomes including premature birth, low birth weight and higher neonatal mortality when compared with adult women.2
Pregnancy is a state of high bone turnover as maternal calcium physiology responds to the calcium demands required to mineralize the fetal skeleton. Several studies have noted that markers of bone turnover increase throughout gestation.3–6 Osteocalcin (OC) is the major non-collagenous protein found in bone and is commonly used as an index of bone formation. N-telopeptide (NTX) is a collagen cross-linking domain that is released into the circulation when bone is resorbed. Although overall bone metabolic activity increases during pregnancy, many reports have documented a net loss of maternal trabecular bone following gestation.3,4,7 This pregnancy-induced bone loss is greater in pregnant adolescents than among pregnant adults.8,9
In 1997, the Receptor Activator of Nuclear factor Kappa-β (RANK)/RANK-ligand (RANK-L)/osteoprotegerin (OPG) system was identified as a major regulator of the coordinated activity of osteoclasts and osteoblasts.10 Interactions between RANK and RANK-L stimulate osteoclastogenesis and bone resorption. OPG is a soluble decoy receptor for RANK-L and may protect bone mass by limiting RANK/RANK-L interactions.11 At present, few studies have investigated OPG concentrations during pregnancy. Existing data are limited, and comprise a few relatively small, cross-sectional studies,12–14 that suggest that OPG concentrations increase from early pregnancy (15–18 weeks) to term.12–14 It is known that the in utero environment plays a role in programming offspring’s risk for chronic disease in adult life.15,16 Several studies have linked infant size at birth with adult bone mineral content (BMC) and risk of osteoporosis.17,18 Currently, the possible impact of variation in circulating OPG during pregnancy and in the fetus on bone turnover markers or other maternal or neonatal characteristics remains unexplored.
The objective of this research was to characterize the longitudinal changes and determinants of circulating OPG concentrations across gestation in a large cohort of pregnant adolescents and their neonates. We aimed to determine whether OPG concentrations in neonatal and maternal circulation were significantly related, and whether they were associated with other variables including bone turnover markers and placental OPG expression. We also investigated whether maternal or neonatal OPG concentrations were associated with infant birth weight z-score and ponderal index.
Methods
Study participants
A cohort of 155 pregnant adolescents (≤18 years) was recruited to participate in a prospective, longitudinal study of calcium and bone homeostasis. Adolescents were recruited from the Rochester Adolescent Maternity Program (RAMP) in Rochester, NY. Pregnant adolescents were between 12 and 30 weeks of gestation at entry into the study, were otherwise healthy and were carrying a single fetus. Exclusion criteria included known medical complications or diseases such as HIV infection, diabetes, gestational hypertension and diagnosed eating disorders or malabsorption diseases. Informed written consent was obtained from all participants, and study procedures were approved by the Institutional Review Boards of the University of Rochester and Cornell University. Maternal race, ethnicity (Hispanic or non-Hispanic) and smoking history were self-reported. Infant race was classified as African American if the mother reported both herself and the father as African American; as Caucasian if the mother reported both herself and the father as Caucasian; or as biracial if the infant’s maternal and paternal reported race differed. Infant ethnicity was classified similarly as either Hispanic, non-Hispanic or multi-ethnic. Age at menarche was self-reported, and gynecological age was calculated as the number of years elapsed from menarche to conception. At birth, infant weight and length were recorded by clinical staff using standard procedures, and neonatal ponderal index (kg/m3) was calculated using the length and weight measures. Birth weight z-scores were generated using published curves specific to infant sex and gestational age at birth.19 Upon delivery, the placenta was collected and weighed to the nearest 0.1 g.
Blood sample collection and analyses
A maternal blood sample (10 ml) was collected at mid-gestation by clinical staff during a routine prenatal visit. Because most visits occurred mid-day, participants were not counseled to fast before blood collection. At delivery, a second, non-fasting maternal blood sample and a 10ml cord blood sample were collected. All samples were allowed to clot at room temperature before serum was separated and stored at −80°C until analysis. All biochemical analyses were obtained using these serum samples.
Maternal and cord blood NTX concentrations were measured in serum using enzyme-linked immunosorbent assay (ELISA; Ostex International, Seattle, WA, USA) at the University of Rochester CLIA-certified laboratory. OC was measured at Yale University in the laboratory of Dr Caren Gundberg using a radioimmunoassay that recognizes both the intact and major proteolytic product, as previously published20 [intra-assay and inter-assay coefficient of variation (CV) were 3.8% and 7.1%, respectively]. Serum OPG was measured using a commercially available ELISA (DY805: R&D Systems, Minneapolis, MN, USA; inter- and intra-assay CV were 5.5% and 6.5%, respectively) and estradiol was analyzed using a commercially available ELISA from Alpco Diagnostics (Salem, NH, USA; inter- and intra-assay CV were 4.2% and 10.1%, respectively). Leptin was measured using a commercially available ELISA from Millipore (Billerica, MA, USA; inter- and intra-assay CV were 4.5% and 8.3%, respectively).
Placental collection and Western blot
Five, one-square-inch placental tissue samples were collected from various locations throughout the entire placenta in order to obtain a sample as representative as possible. Maternal and fetal membranes were removed, and tissue samples were pooled, homogenized and frozen at −80°C until analysis. Protein lysates were generated using a Polytron PT3100 tissue homogenizer (Kinematica Inc., Lucerne, Switzerland) at 5000 rpm in a hypertonic lysis buffer containing protease inhibitor. Homogenized tissue was centrifuged at 13,000 rpm for 20 min at 4°C. Protein concentrations in the supernatant were determined with the Bio-Rad assay (Bio-Rad, Hercules, CA, USA). Lysates were frozen at −80°C in SDS sample buffer until analysis.
Relative placental OPG expression was determined via Western blot. Lysates were separated on a 10% SDS-PAGE gel and transferred to a PVDF membrane (Millipore, Billerica, MA, USA). Membranes were blocked in Odyssey Blocking Buffer (Li-Cor BioSciences, Lincoln, NB, USA) for 1 h at room temperature and then probed with Goat-anti-OPG (AF805: R&D Systems, Minneapolis, MN, USA) at a concentration of 0.3 µg/ml and Rabbit-anti-β-actin (AbCam, Cambridge, MA, USA) at 6.7 ng/ml overnight at 4°C. Fluorescent secondary antibodies were used: Donkey-anti-Goat 800 at a concentration of 0.17 µg/ml (Li-Cor BioSciences) and Chicken-anti-Rabbit 647 at 0.13 µg/ml (Invitrogen, Carlsbad, CA, USA). All antibodies were prepared in a solution of Odyssey blocking buffer diluted 1:1 in a phosphate buffered saline solution. The OPG and β-actin bands were quantified using the Odyssey infrared imaging system (Li-Cor Biosciences), and the ratio of OPG to β-actin was calculated. Intra-membrane variation was controlled for by normalizing the OPG:β-actin ratio of individual placentas to a standard control placental sample that was run on every gel. This standard placental control lysate was prepared as described above using tissue collected from a healthy adult woman delivering at term.
Statistical analyses
Statistical analyses were undertaken using SAS 9.2 and JMP 8.0 (SAS Institute Inc., Cary, NC). Results are reported as mean ± S.D. unless otherwise noted. Paired t-tests or nonparametric tests were used to assess changes in biochemical markers within subjects from mid-gestation to delivery. Independent t-tests or ANOVA were used to determine whether normally distributed variables differed by race, and the Wilcoxon rank sum test was utilized for nonparametric data. The Shapiro–Wilk test was used to assess normality of variables. Simple and multiple linear regression were used to assess statistical determinants of OPG at mid-gestation and delivery. Non-normally distributed variables were log-transformed as necessary to ensure normality of models’ residuals. In multiple linear regressions, interaction terms were evaluated, but none were significant and thus are not reported. When modeling determinants of mid-gestation OPG concentrations, one participant’s data were excluded because her OPG value was an outlier (over 4 S.D. from the mean). Study findings were not impacted if this data point was included or excluded. Simple and multiple linear regression analyses were used to assess the relationships between OPG and infant outcomes. Sample size calculations were undertaken to determine the sample size necessary to appropriately characterize mean OPG concentrations and yield a minimal detectable difference equivalent to 25% of the previously reported S.D. in OPG concentrations in pregnant women at term. Using these parameters, we required a sample size of 120 to yield a power of 80% at an αof 0.05.14 The sample size we achieved in this study also provided us with 80% power (α = 0.05) to detect a minimal detectable difference of 0.24 in birth weight z-score and 0.10 kg/m3 in ponderal index.19,21 Data were considered significant if P-values were <0.05. P-values between 0.05 and 0.10 were considered trends.
Results
Subject characteristics
Characteristics of the study population as a whole, and among the group when stratified by race, are presented in Table 1. Of those enrolled, 65% were African American and 35% were Caucasian. Two teens identified their race as American Indian. In order to classify maternal race as a bivariate category and avoid loss of data from the two American-Indian adolescents, these two adolescents were included within the African-American cohort. None of the race-specific analyses differed if data were analyzed with these two adolescents combined with either the African-American or Caucasian cohorts, and all study results remained significant if these two subjects were excluded. Maternal age at enrollment into the study ranged from 13.6 to 18.7 years and birth weight z-score ranged from −2.58 to 2.09. Approximately 8.6% of teens delivered prematurely (<37 weeks); 12.0% delivered a low-birth-weight (LBW) infant (<2500 g); and 6.7% delivered a large-for-gestational-age (LGA) infant (>4500 g). A significantly higher percentage of Caucasian participants self-identified as Hispanic (55.6%) than did African-American participants (6.9%; P < 0.01). There were no differences in the length of gestation, birth weight, birth weight z-score, ponderal index, rate of preterm, LBW or LGA by infant sex, or maternal race (Table 1).
Table 1.
Characteristics of the pregnant adolescents and their neonates at birtha
| Subject characteristics | All subjects | African-American adolescents | Caucasian adolescents |
|---|---|---|---|
| Total recruitment | 155 | 65.2% (101) | 34.8% (54) |
| Age at enrollment (years) | 17.1 ± 1.1 (155) | 17.1 ± 1.2 (101) | 17.2 ± 0.9 (54) |
| Weeks’ gestation | 21.7 ± 5.5 (155) | 22.0 ± 5.5 (101) | 21.1 ± 5.6 (54) |
| Hispanic (%) | 23.8 (37) | 6.9 (7)b | 55.6 (30) |
| Non-Hispanic (%) | 76.1 (118) | 93.1 (94) | 44.4 (24) |
| Pre-pregnancy BMI (kg/m2) | 24.7 ± 5.3 (153) | 24.5 ± 5.2 (99) | 25.1 ± 5.6 (54) |
| Multiparous (parity ≥ 1; %) | 9.0 (155) | 9.9 (101) | 7.4 (54) |
| Gestational age at mid-gestation blood collection (weeks) | 26.0 ± 3.4 (152) | 26.1 ± 3.3 (99) | 25.9 ± 3.5 (53) |
| Gestational age at delivery (weeks) | 39.3 ± 2.6 (149) | 39.1 ± 2.9 (97) | 39.6 ± 1.9 (52) |
| Birth weight (g) | 3202 ± 583 (147) | 3151 ± 588 (95) | 3294 ± 567 (52) |
| Infant sex (male) | 55.3% (83) | 56.1% (55) | 53.8% (28) |
| Birth weight z-score | −0.392 ± 0.904 (147) | −0.463 ± 0.884 (95) | −0.260 ± 0.933 (52) |
| Infant ponderal index (kg/m3) | 24.52 ± 2.10 (141) | 24.25 ± 3.00 (91) | 25.00 ± 3.26 (50) |
| OPG at mid-gestation (pg/ml) | 1949 ± 740 (138) | 2129 ± 764 (89)c | 1623 ± 571 (49) |
| OPG at delivery (pg/ml) | 2812 ± 1215 (123) | 2975 ± 1295 (78)d | 2530 ± 1016 (45) |
BMI, body mass index; OPG, osteoprotegerin.
Data are reported as the mean ± S.D., or percentage, with the sample size in parentheses.
Significantly different from Caucasians: P < 0.01.
Significantly different from Caucasians: P < 0.001.
Significantly different from Caucasians: P < 0.05.
Estradiol increased by 127% (3077 ± 1674 v. 5802 ± 2880 pg/ml) over the 13.1 ± 4.3-week interval that elapsed from the mid-gestation to delivery blood collection. The observed gestational increase in estradiol did not differ by race or maternal age across the 13- to 18-year age range studied.
Relationships between circulating maternal OPG and markers of bone turnover
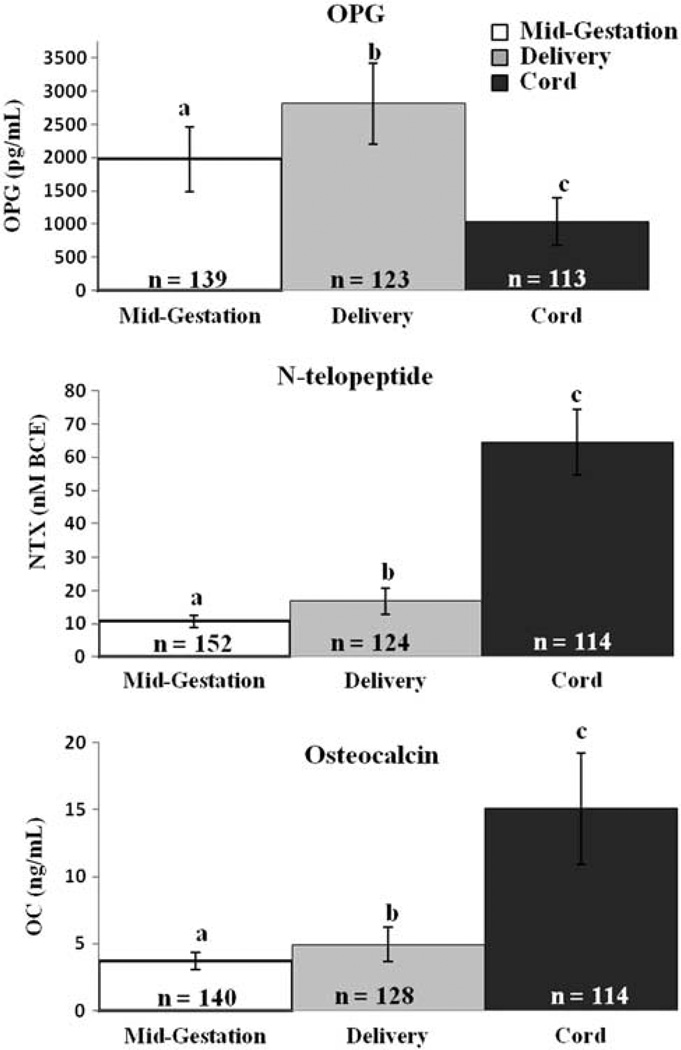
Mean maternal concentrations of OPG, NTX and OC all increased significantly from mid-gestation to delivery (P < 0.001; Fig. 1), and mid-gestation values were significantly correlated with those at delivery (P < 0.001 for all; n = 111, R2 = 0.55; n = 114, R2 = 0.10; n = 120, R2 = 0.27, respectively). Serum OC and NTX were highly correlated with each other at mid-gestation (26.0 ± 3.4 weeks: P < 0.001, n = 137, R2 = 0.08) and at delivery (39.3 ± 2.6 weeks: P < 0.001, n = 113, R2 = 0.26). Furthermore, the rate of increase in OC from mid-gestation to delivery (change in OC (ng/ml)/weeks elapsed) was highly correlated with the rate of increase in NTX (P = 0.002, n = 84, R2 = 0.11). Although correlated, the relative percent increase in NTX was significantly greater than the increase observed for OC (63% v. 38%; P < 0.001, n = 105). OPG was not significantly associated with NTX or OC at any time point measured. The relative increase in OPG (54%) did not significantly differ from that observed for NTX or OC. Neither maternal age (range: 13.6–18.7 years) nor gynecological age (range: 1.2–10.7 years) was associated with markers of bone turnover or OPG at any time point measured. OC was higher in Caucasian adolescents at delivery than in African-American adolescents (P = 0.023), although the difference between the observed means was minimal (0.7 ng/ml) and the relative increase in OC from mid-gestation to delivery did not differ as a function of race. In contrast, no significant differences in NTX were evident between racial groups when examined as either absolute values or as percent change across gestation.
Fig. 1.
Serum concentrations of osteoprotegerin (OPG), N-telopeptide (NTX), and osteocalcin (OC) in pregnant adolescents and their neonates. Serum concentrations of OPG, NTX, and OC were measured in a group of 155 pregnant adolescents at mid-gestation (26.1 ± 3.3 weeks) and at delivery (39.3 ± 2.6 weeks), and in cord blood obtained from neonates at birth. Different superscripts indicate group means significantly differed from each other (P < 0.0001). In paired analyses, maternal concentrations of OPG (P < 0.0001), NTX (P < 0.0001), and OC (P < 0.0001) were significantly higher at delivery compared to mid-gestation. Both NTX (P < 0.0001) and OC (P < 0.0001) were significantly higher in the neonate at birth. In contrast OPG values in cord blood were significantly lower than those observed among pregnant adolescents at delivery (P < 0.0001).
Variables associated with circulating maternal OPG
At mid-gestation, none of the following variables were related to circulating OPG concentrations: maternal weight, weight gain, leptin, chronological age, gynecological age, maternal height, pre-pregnancy weight or body mass index (BMI). At mid-gestation, both estradiol (P = 0.001, n = 136, R2 = 0.08) and gestational age at sampling (P = 0.010, n = 139, R2 = 0.05) were significantly positively associated with OPG. Furthermore, OPG in African-American adolescents was on average 506 pg/ml higher than the mean observed in Caucasian adolescents (P = 0.007, n = 139). When NTX, estradiol, race and gestational age were all entered into a model of mid-gestation OPG, gestational age was no longer significant. The best model identified (using maternal mid-gestation NTX, estradiol and race as predictors) accounted for 20% of the variability (P < 0.001) in mid-gestation OPG.
At delivery, circulating OPG concentrations were not significantly related to maternal weight, weight gain, delivery leptin concentrations, height, pre-pregnancy weight, chronological age or gynecological age. Similar to observations evident at mid-gestation, delivery OPG concentrations were significantly and positively related to gestational age (P = 0.013, n = 123, R2 = 0.05) and were also positively associated with pre-pregnancy BMI (P = 0.036, n = 123, R2 = 0.04). The racial difference in OPG persisted, and was of similar magnitude (444 pg/ml, P = 0.044) to that observed at mid-gestation. The relationship between estradiol and OPG at delivery approached significance (P = 0.053, n = 122, R2 = 0.03), and was thus included as a covariate in the initial model of maternal OPG at delivery. The best model identified to predict OPG at delivery included estradiol, gestational age and race (P = 0.010), but this model predicted only 7% of the variation in maternal OPG.
Neonatal OPG, OC and NTX
NTX and OC were significantly higher in cord blood than in maternal blood at delivery (P < 0.001). In contrast, OPG was significantly lower in cord v. maternal blood at delivery (P < 0.001; Fig. 1). Cord blood OC tended to correlate with maternal OC at mid-gestation (P = 0.05, n = 100, R2 = 0.04), but not at delivery. NTX in cord blood was not associated with maternal levels at any time point or with cord OC or OPG concentrations. In contrast, cord OPG concentrations were highly correlated with maternal OPG concentrations at mid-gestation (P < 0.010, n = 99, R2 = 0.07) and at delivery (P = 0.001, n = 106, R2 = 0.06). Neonatal OPG was not associated with maternal or infant race, infant sex, estradiol, pre-pregnancy BMI, gestational age at delivery or any other study variable collected.
Relationships between neonatal OPG concentrations and infant anthropometrics at birth
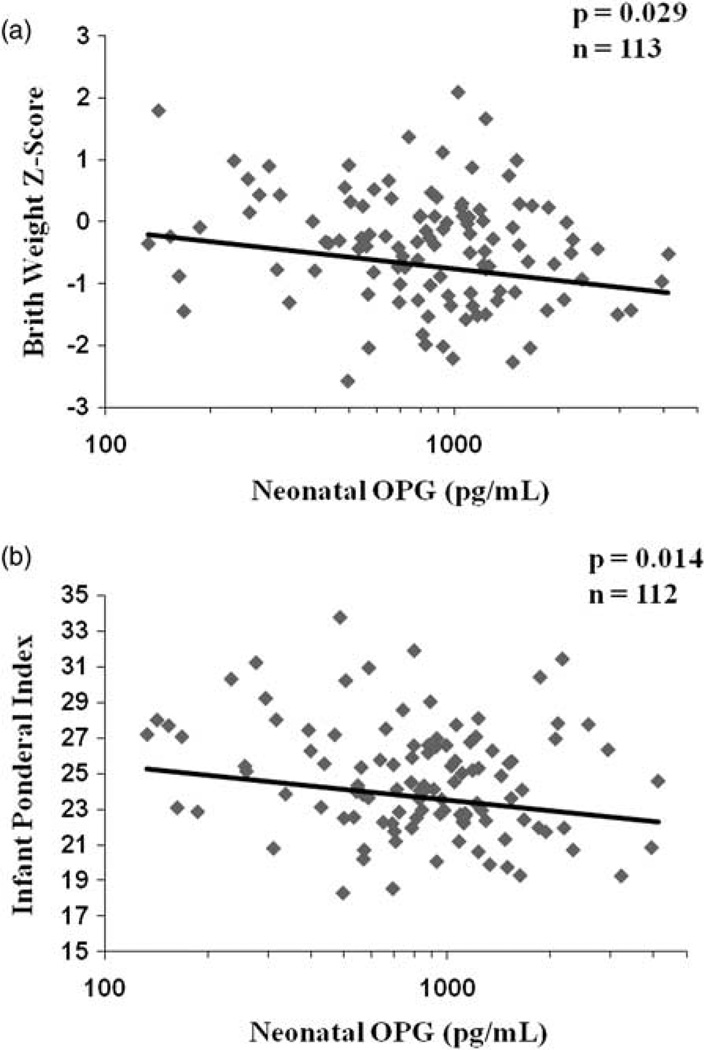
Neonatal OPG was significantly inversely correlated with both birth weight z-score (P = 0.029; Fig. 2a) and ponderal index (kg/m3; P = 0.014; Fig. 2b). As expected, gestational age explained 37% of the variation in birth weight. Neonatal OPG alone was not significantly associated with infant birth weight (P = 0.18). However, when neonatal OPG was added as a covariate along with gestational age, OPG became a significant predictor of birth weight (parameter estimate P = 0.020, n = 113), and explained an additional 3% of the variation in birth weight. Similarly, gestational age at delivery explained 6% of the variability in ponderal index at birth. When neonatal OPG was added as a covariate to the model for ponderal index, the parameter estimate for OPG remained significant (P = 0.006) and explained an additional 2% of the variation in infant ponderal index.
Fig. 2.
Neonatal OPG concentrations are inversely associated with birth weight z-score and infant ponderal index. Serum OPG concentrations were measured in cord blood collected at delivery from a group of 113 pregnant adolescents. Neonatal OPG was significantly inversely related to birth weight z-score (P = 0.029, n = 113, R2 = 0.05) and infant ponderal index, calculated as kg/m3 at delivery (P = 0.014, n = 112, R2 = 0.05). The average birth weight in these neonates was 3261 ± 481 g.
The difference in average birth weight z-score between the highest and lowest quartiles of cord blood OPG was 0.347. This difference represents a 192 g higher mean birth weight between the lowest and highest quartile of neonatal OPG. The difference in ponderal index between the lowest and highest quartiles of neonatal OPG was 1.72 kg/m3. Overall, the mean ponderal index of infants in the highest quartile of neonatal OPG was significantly lower (P < 0.001) than the average ponderal index previously published from a large cohort (n = 1040) of term infants.21 The observed relationships between OPG and ponderal index and between OPG and birth weight z-scores were independent of maternal and neonatal race.
Placental OPG
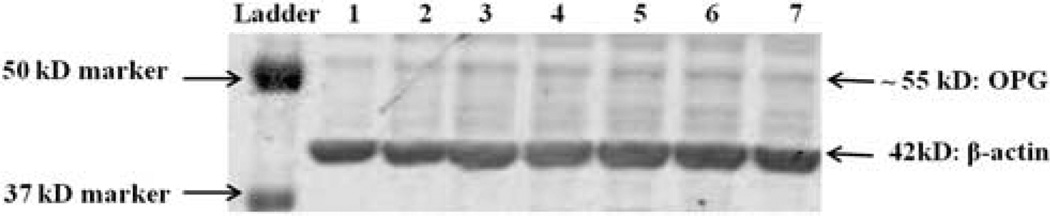
Placental OPG expression (via Western blot) was assessed in 68 placental samples; a representative blot is shown in Fig. 3. Neither placental weight nor placental expression of OPG was significantly associated with maternal OPG at mid-gestation or at delivery or with any of the other study measures obtained.
Fig. 3.
Placental expression of OPG in pregnant adolescents. Protein lysates were generated from homogenized placental tissue samples collected from 68 pregnant adolescents at delivery, and OPG expression was analyzed via western blot. Placental OPG expression was not related to maternal OPG at mid-gestation (P = 0.98, n = 59) or at delivery (P = 0.15, n = 62). Shown is a representative western blot with individual placental lysate samples in lanes 1–7.
Discussion
In this group of pregnant adolescents and their neonates, we found that maternal serum OPG concentrations across gestation and at birth were not significantly associated with maternal or neonatal bone turnover markers. Significant differences in maternal OPG concentration as a function of race were evident at both mid-gestation and at parturition. It is interesting to note that neonatal concentrations of OPG were significantly correlated with, but lower than, maternal concentrations and were negatively associated with neonatal anthropometric variables.
Expression of OPG has previously been characterized in the human placenta, amnion and choriodecidual tissue.10,13,22 Because maternal circulating OPG concentrations have been found to decrease by 38% within 4 days post partum,14 it has been postulated that the placenta contributes to circulating OPG during pregnancy.13 However, although we were able to detect placental expression of OPG, we found no significant associations between placental OPG expression and OPG concentrations in maternal circulation at mid-gestation or delivery, or in neonatal blood at term. In contrast, maternal and neonatal OPG concentrations were highly correlated. Because little is known about the transfer of this protein across the placenta, the relative contribution of maternal or placental OPG to fetal concentrations remains unknown.
A significant negative relationship was noted between neonatal umbilical cord OPG concentrations and both birth weight z-score and ponderal index. This is the first time, to our knowledge, that such a relationship has been reported. Neither the observed relationships nor infant characteristics were significantly influenced by maternal or infant race. A study by Briana et al. found no significant association between cord OPG concentrations and birth weight, and no difference in OPG between appropriate-for-gestational-age (n = 20) and intrauterine growth restriction (n = 20) infants;23 however, their relatively small sample size may have limited their ability to detect associations. Our observed association between cord OPG concentrations and neonatal birth weight z-score may hint at a role for OPG in neonatal accrual of body stores of fat, bone or muscle. In a study of lean and obese children (aged 5–16 years), OPG was negatively associated with total and truncal body fat mass when correcting for pubertal stage and gender.24 However, in our study, OPG in cord blood was not related to cord blood or maternal leptin concentrations. The lack of a relationship between neonatal OPG and other indices of maternal nutritional status (BMI, weight gain, etc.) suggests that the association between neonatal OPG and neonatal size at birth is independent of maternal BMI during pregnancy.
The in utero environment (nutritional and environmental) is known to impact fetal skeletal development and mineralization,17,25–27 and changes evident at birth may be sustained into childhood and adulthood.28,29 Maternal under-nutrition or ‘constraint’ in utero may impact an offspring’s adult risk of osteoporosis, as studies have linked size at birth with adult bone mass17 and shown that the in utero environment mediates the impact of genetics on adult BMC and bone mineral density.30,31 We report a significant negative relationship between cord OPG and both birth weight z-score and ponderal index. Elevated OPG concentrations were observed among neonates that were smaller at birth. Because OPG plays a well-characterized role in the coordinated balance of osteoblast/osteoclast activity;10,11 the elevated neonatal OPG may potentially play a programming role in subsequent bone growth and mineralization. A limitation of this study was that postnatal neonatal Dual-energy X-ray Absorptiometry measures were not obtained. These were originally planned but the lack of compliance with the scheduled appointments following pregnancy precluded these measures.
Possible associations between OPG concentrations and concurrent changes in bone metabolic activity were explored. Whereas significant increases in bone turnover markers occurred across pregnancy, no significant relationships were evident between serum OPG and either OC or NTX in these adolescents. These findings are consistent with earlier data in pregnant adult women.13,14 The lack of association between OPG and markers of bone turnover may indicate that OPG is playing additional roles aside from regulation of bone turnover during pregnancy.
In this group of pregnant adolescents, maternal OPG concentrations were significantly associated with gestational age over the time interval in which the mid-gestation blood samples were obtained (20–37 weeks). In addition, in the cohort as a whole, and in paired analyses, OPG concentrations significantly increased from the mid-gestation measure to the measure obtained at delivery. Other studies have shown that OPG increases throughout pregnancy.13,14 Previous data from adult women have reported that OPG increases from 38% to 86% in the third trimester.4,14 Our finding of a 54.2% increase in OPG from 26.0 ± 3.4 weeks gestation to delivery (39.3 ± 2.6 weeks gestation) is consistent with these prior data. Although relative changes in OPG across gestation are variable, the absolute value we observed in these pregnant adolescents is significantly higher than previously reported for pregnant adults, in which values ranged from 863 pg/ml at 36 weeks,4 to between 13212 and 1400 pg/ml at delivery.14 Currently, no standard reference materials for OPG exist, and thus we cannot rule out methodological differences as the source of the elevated OPG concentrations evident in our adolescent population. Elevated OPG concentrations have previously been reported among other groups known to have alterations in bone turnover, namely osteoporotic and anorexic women.32–34 In these groups, the elevated OPG has been attributed to OPG’s compensatory bone sparing properties.32–34 Although the OPG concentrations in this adolescent cohort were higher than typically reported among adult women, OPG concentrations were not significantly related to either chronological or gynecological age of the adolescent.
Maternal race and circulating estradiol were significant predictors of maternal OPG at mid-gestation and at delivery. Previous data have shown that estradiol induces OPG expression in osteoblastic cells in vitro,35,36 but an earlier in vivo study failed to detect a relationship between estradiol and OPG in serum during pregnancy.4 The much larger sample size of our population and the increased bone turnover that occurs in pregnant adolescents may have influenced our ability to detect the observed associations. In this group of pregnant adolescents, African-American teens exhibited significantly higher OPG concentrations compared with the Caucasian adolescents. This is the first time to our knowledge that a racial difference in OPG has been reported in a pregnant or non-pregnant population. Significantly higher bone mass is known to be evident among African-American compared with non-Hispanic Caucasian women.37 Our observed differences in OPG are consistent with these racial differences in bone mass.
The role of OPG during pregnancy remains poorly understood. Despite the significant associations observed in our study, we were able to capture only a small percentage of the variability in OPG (20% at mid-gestation and 7% at delivery) using the available study measures. In addition to classically described roles, OPG also serves as a decoy tumor necrosis factor (TNF)-related receptor for the apoptosis-inducing ligand (TRAIL),38 and binds both TRAIL and RANK-L with similar affinity.39 During pregnancy, placental OPG expression may protect placental membranes from the actions of TRAIL to maintain membrane integrity until parturition.22 In addition, OPG may also play a role in modulating inflammation, as OPG expression has been found to be upregulated by inflammatory cytokines including Interleukin-1 and TNF-α,40 as well as by lipopolysaccharide in choriodecidua,22 but downregulated by TRAIL.41 OPG’s potential role in the inflammatory response to pregnancy may be a partial source of the racial differences in OPG detected in these adolescents. Differences in pro and anti-inflammatory cytokines have been found between African-American and Caucasian women during pregnancy and at parturition;42,43 however, we did not measure inflammatory cytokines in our study. These alternate and yet undiscovered roles of OPG may account for some of the unexplained variability in the OPG concentrations we observed.
In conclusion, in pregnant adolescents OPG concentrations increased across gestation, but were unrelated to concurrent changes in markers of bone turnover. African-American adolescents had significantly higher circulating concentrations of OPG compared with Caucasian adolescents, in spite of similar concentrations of bone turnover markers. Neonatal OPG was lower than, but highly correlated with, maternal OPG concentrations, and was significantly inversely associated with birth weight z-score and ponderal index. Further research is needed to elucidate the mechanisms driving these relationships, and to fully clarify the roles of OPG in fetal physiology, as well as the possible associations with neonatal bone health and subsequent risk of osteoporosis.
Acknowledgments
This research was supported by the National Research Initiative of the USDA National Institute of Food and Agriculture, Award 2005-35200-1521 and by NIH Grant 5T32 HD007331-23, which includes funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the Office of Dietary Supplements (ODS). We thank Dr Caren Gundberg for performing the analyses of osteocalcin in our samples. We also thank Sonja Goedkoop for her assistance in the analyses of estradiol and leptin, and Tera Kent for laboratory support. We would also like to deeply thank the Strong Health Midwifery Group for their assistance in sample and data collection, and the adolescents who participated in this study.
References
- 1.Center for Disease Control and Prevention. Vital signs: teen pregnancy – United States, 1991–2009. Morb Mortal Wkly Rep. 2011;60:414–420. [PubMed] [Google Scholar]
- 2.Felice ME, Feinstein RA, Fisher MM, et al. Adolescent pregnancy – current trends and issues: 1998 American Academy of Pediatrics Committee on Adolescence, 1998–1999. Pediatrics. 1999;103:516–520. doi: 10.1542/peds.103.2.516. [DOI] [PubMed] [Google Scholar]
- 3.Black AJ, Topping J, Durham B, Farquharson RG, Fraser WD. A detailed assessment of alterations in bone turnover, calcium homeostasis, and bone density in normal pregnancy. J Bone Miner Res. 2000;15:557–563. doi: 10.1359/jbmr.2000.15.3.557. [DOI] [PubMed] [Google Scholar]
- 4.Naylor KE, Iqbal P, Fledelius C, Fraser RB, Eastell R. The effect of pregnancy on bone density and bone turnover. J Bone Miner Res. 2000;15:129–137. doi: 10.1359/jbmr.2000.15.1.129. [DOI] [PubMed] [Google Scholar]
- 5.Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. Am J Clin Nutr. 1995;61:514–523. doi: 10.1093/ajcn/61.3.514. [DOI] [PubMed] [Google Scholar]
- 6.Kovacs CS. Calcium and bone metabolism in pregnancy and lactation. J Clin Endocrinol Metab. 2001;86:2344–2348. doi: 10.1210/jcem.86.6.7575. [DOI] [PubMed] [Google Scholar]
- 7.Kolthoff N, Eiken P, Kristensen B, Nielsen SP. Bone mineral changes during pregnancy and lactation: a longitudinal cohort study. Clin Sci (Lond) 1998;94:405–412. doi: 10.1042/cs0940405. [DOI] [PubMed] [Google Scholar]
- 8.Sowers M, Wallace RB, Lemke JH. Correlates of forearm bone mass among women during maximal bone mineralization. Prev Med. 1985;14:585–596. doi: 10.1016/0091-7435(85)90079-9. [DOI] [PubMed] [Google Scholar]
- 9.Sowers MF, Scholl T, Harris L, Jannausch M. Bone loss in adolescent and adult pregnant women. Obstet Gynecol. 2000;96:189–193. doi: 10.1016/s0029-7844(00)00903-0. [DOI] [PubMed] [Google Scholar]
- 10.Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 11.Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001;79:243–253. doi: 10.1007/s001090100226. [DOI] [PubMed] [Google Scholar]
- 12.Hong JS, Santolaya-Forgas J, Romero R, et al. Maternal plasma osteoprotegerin concentration in normal pregnancy. Am J Obstet Gynecol. 2005;193:1011–1015. doi: 10.1016/j.ajog.2005.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naylor KE, Rogers A, Fraser RB, et al. Serum osteoprotegerin as a determinant of bone metabolism in a longitudinal study of human pregnancy and lactation. J Clin Endocrinol Metab. 2003;88:5361–5365. doi: 10.1210/jc.2003-030486. [DOI] [PubMed] [Google Scholar]
- 14.Uemura H, Yasui T, Kiyokawa M, et al. Serum osteoprotegerin/osteoclastogenesis-inhibitory factor during pregnancy and lactation and the relationship with calcium-regulating hormones and bone turnover markers. J Endocrinol. 2002;174:353–359. doi: 10.1677/joe.0.1740353. [DOI] [PubMed] [Google Scholar]
- 15.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 16.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper C, Westlake S, Harvey N, et al. Review: developmental origins of osteoporotic fracture. Osteoporos Int. 2006;17:337–347. doi: 10.1007/s00198-005-2039-5. [DOI] [PubMed] [Google Scholar]
- 18.Schlussel MM, Dos SV, Kac G. Birth weight and adult bone mass: a systematic literature review. Osteoporos Int. 2010;21:1981–1991. doi: 10.1007/s00198-010-1236-z. [DOI] [PubMed] [Google Scholar]
- 19.Kramer MS, Platt RW, Wen SW, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108:E35–E42. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- 20.Gundberg CM, Cole DE, Lian JB, Reade TM, Gallop PM. Serum osteocalcin in the treatment of inherited rickets with 1,25-dihydroxyvitamin D3. J Clin Endocrinol Metab. 1983;56:1063–1067. doi: 10.1210/jcem-56-5-1063. [DOI] [PubMed] [Google Scholar]
- 21.Andreasyan K, Ponsonby AL, Dwyer T, et al. Higher maternal dietary protein intake in late pregnancy is associated with a lower infant ponderal index at birth. Eur J Clin Nutr. 2007;61:498–508. doi: 10.1038/sj.ejcn.1602552. [DOI] [PubMed] [Google Scholar]
- 22.Lonergan M, Aponso D, Marvin KW, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), TRAIL receptors, and the soluble receptor osteoprotegerin in human gestational membranes and amniotic fluid during pregnancy and labor at term and preterm. J Clin Endocrinol Metab. 2003;88:3835–3844. doi: 10.1210/jc.2002-021905. [DOI] [PubMed] [Google Scholar]
- 23.Briana DD, Boutsikou M, Baka S, et al. Circulating osteoprotegerin and sRANKL concentrations in the perinatal period at term. The impact of intrauterine growth restriction. Neonatology. 2009;96:132–136. doi: 10.1159/000211666. [DOI] [PubMed] [Google Scholar]
- 24.Dimitri P, Wales JK, Bishop N. Adipokines, bone-derived factors and bone turnover in obese children; evidence for altered fat-bone signalling resulting in reduced bone mass. Bone. 2011;48:189–196. doi: 10.1016/j.bone.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 25.Specker B. Nutrition influences bone development from infancy through toddler years. J Nutr. 2004;134:691S–695S. doi: 10.1093/jn/134.3.691S. [DOI] [PubMed] [Google Scholar]
- 26.Namgung R, Tsang RC. Factors affecting newborn bone mineral content: in utero effects on newborn bone mineralization. Proc Nutr Soc. 2000;59:55–63. doi: 10.1017/s0029665100000070. [DOI] [PubMed] [Google Scholar]
- 27.Raman L, Rajalakshmi K, Krishnamachari KA, Sastry JG. Effect of calcium supplementation to undernourished mothers during pregnancy on the bone density of the bone density of the neonates. Am J Clin Nutr. 1978;31:466–469. doi: 10.1093/ajcn/31.3.466. [DOI] [PubMed] [Google Scholar]
- 28.Viljakainen HT, Korhonen T, Hytinantti T, et al. Maternal vitamin D status affects bone growth in early childhood – a prospective cohort study. Osteoporos Int. 2011;22:883–891. doi: 10.1007/s00198-010-1499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Javaid MK, Crozier SR, Harvey NC, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 30.Dennison EM, Arden NK, Keen RW, et al. Birthweight, vitamin D receptor genotype and the programming of osteoporosis. Paediatr Perinat Epidemiol. 2001;15:211–219. doi: 10.1046/j.1365-3016.2001.00350.x. [DOI] [PubMed] [Google Scholar]
- 31.Antoniades L, MacGregor AJ, Andrew T, Spector TD. Association of birth weight with osteoporosis and osteoarthritis in adult twins. Rheumatology (Oxford) 2003;42:791–796. doi: 10.1093/rheumatology/keg227. [DOI] [PubMed] [Google Scholar]
- 32.Dai Y, Shen L. Relationships between serum osteoprotegerin, matrix metalloproteinase-2 levels and bone metabolism in postmenopausal women. Chin Med J (Engl) 2007;120:2017–2021. [PubMed] [Google Scholar]
- 33.Jiang LS, Zhang ZM, Jiang SD, Chen WH, Dai LY. Differential bone metabolism between postmenopausal women with osteoarthritis and osteoporosis. J Bone Miner Res. 2008;23:475–483. doi: 10.1359/jbmr.071114. [DOI] [PubMed] [Google Scholar]
- 34.Ohwada R, Hotta M, Sato K, Shibasaki T, Takano K. The relationship between serum levels of estradiol and osteoprotegerin in patients with anorexia nervosa. Endocr J. 2007;54:953–959. doi: 10.1507/endocrj.k07-034. [DOI] [PubMed] [Google Scholar]
- 35.Hofbauer LC, Khosla S, Dunstan CR, et al. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140:4367–4370. doi: 10.1210/endo.140.9.7131. [DOI] [PubMed] [Google Scholar]
- 36.Li Q, Yu K, Tian X, et al. 17beta-estradiol overcomes human myeloma RPMI8226 cell suppression of growth, ALP activity, and mineralization in rat osteoblasts and improves RANKL/OPG balance in vitro. Leuk Res. 2009;33:1266–1271. doi: 10.1016/j.leukres.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Looker AC, Melton LJ, III, Harris T, et al. Age, gender, and race/ethnic differences in total body and subregional bone density. Osteoporos Int. 2009;20:1141–1149. doi: 10.1007/s00198-008-0809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emery JG, McDonnell P, Burke MB, et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 39.Vitovski S, Phillips JS, Sayers J, Croucher PI. Investigating the interaction between osteoprotegerin and receptor activator of NF-kappaB or tumor necrosis factor-related apoptosis-inducing ligand: evidence for a pivotal role for osteoprotegerin in regulating two distinct pathways. J Biol Chem. 2007;282:31601–31609. doi: 10.1074/jbc.M706078200. [DOI] [PubMed] [Google Scholar]
- 40.Collin-Osdoby P, Rothe L, Anderson F, et al. Receptor activator of NF-kappa B and osteoprotegerin expression by human microvascular endothelial cells, regulation by inflammatory cytokines, and role in human osteoclastogenesis. J Biol Chem. 2001;276:20659–20672. doi: 10.1074/jbc.M010153200. [DOI] [PubMed] [Google Scholar]
- 41.Corallini F, Celeghini C, Rimondi E, et al. TRAIL down-regulates the release of osteoprotegerin (OPG) by primary stromal cells. J Cell Physiol. 2011;226:2279–2286. doi: 10.1002/jcp.22564. [DOI] [PubMed] [Google Scholar]
- 42.Anum EA, Springel EH, Shriver MD, Strauss JF., III Genetic contributions to disparities in preterm birth. Pediatr Res. 2009;65:1–9. doi: 10.1203/PDR.0b013e31818912e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velez DR, Fortunato SJ, Morgan N, et al. Patterns of cytokine profiles differ with pregnancy outcome and ethnicity. Hum Reprod. 2008;23:1902–1909. doi: 10.1093/humrep/den170. [DOI] [PMC free article] [PubMed] [Google Scholar]