Abstract
This study examined whether individual differences in social motivation affect the extent of processing of social versus nonsocial information. Event-related potentials were recorded in 13 children with autism spectrum disorder and 11 typically developing children during passive viewing of unfamiliar faces and houses. One image in each category was presented repeatedly, the rest were shown once. Analyses indicated no group differences in the early perceptual responses. Only typical children evidenced larger P600 for the repeated faces. These results were replicated during a retest session. Individual differences in memory for the repeated faces correlated with standardized behavioral assessments of social skills.
Keywords: ASD, ERP, face, memory, repetition, social motivation
Stimuli that are inherently interesting and relevant to motivational needs tend to attract attention. In humans, social stimuli such as faces have a unique motivational value and may attract attention even when a person is actively engaged in another task (e.g., Vuilleumier, 2000). Deficits in social motivation have been identified as one of the core features of autism spectrum disorder (ASD) (Chevallier, Kohls, Troiani, Brodkin & Schultz, 2012a; Dawson, Webb & McPartland, 2005). However, assessing social motivation is challenging, especially in younger and nonverbal individuals, due to the reliance on overt behavioral, most often verbal, responses. Recent studies of social motivation utilized self-report questionnaires regarding anhedonia, the lack of pleasure in social and nonsocial situations (Chevallier, Grèzes, Molesworth, Berthoz & Happé, 2012b), or examined changes in explicit evaluations of others depending on specific social contexts (Chevallier, Molesworth & Happe, 2012c).
Studies examining social cognition, which may depend on social motivation (Chevallier at al., 2012b), have identified a number of differences between individuals with ASD and their typically developing peers. A prominent area of research is the examination of face processing, a fundamental component of social perception and effective social interaction. Individuals with ASD exhibit significant impairment in face processing (Corbett et al., 2009; Dawson, Webb & McPartland, 2005) compared to typical peers. Persons with ASD often show reduced interest in faces (Klin, Sparrow, de Bildt, Cicchetti, Cohen & Volkmar, 1999; Riby & Hancock, 2009), and individual differences in social competence have been correlated with face processing ability in persons with ASD (Deruelle, Rondan, Gepner & Tardif, 2004; Klin et al., 1999). However, most of these studies utilized tasks that relied primarily on basic perceptual processes (e.g., face matching), explicitly directed attention to the stimuli for the purpose of comparison, identification, or description, and/or included a significant verbal component (e.g., verbal labeling of facial expressions; see Jemel, Mottron & Dawson, 2006 for review). Thus, their results might have overestimated or underestimated the actual ability of individuals with ASD to spontaneously detect, attend to, and comprehend complex social information conveyed by faces in daily social interactions.
Measures of brain activity, such as event-related potentials (ERP), allow investigators to examine information processing in great detail, often without the requirement of overt behavioral responses, thus reducing participants’ cognitive load and motivation-related confounds (e.g., differences in the cooperative engagement with the task). Multiple ERP studies in children and adults with ASD examined perceptual mechanisms supporting face processing and subsequently identified delays in occipito-temporal N170 brain responses (McPartland, Dawson, Webb, Panagiotides, & Carver, 2004; O’Connor, Hamm & Kirk, 2007; Hileman, Henderson, Mundy, Newell & Jaime, 2011), interpreted as reduced attention to faces. Recently, similar perceptual delays in face processing have been reported in 10-month-old infants at high risk for ASD due to family history (Elsabbagh et al., 2009).
Yet, detecting and orienting to social information (e.g., faces) is just one aspect of social motivation, which also includes seeking social interactions and maintaining social bonds (Chevallier et al., 2012b). The latter would also necessitate forming representations of the social stimuli (Dawson, Webb, & McPartland, 2005). ERPs provide an opportunity to examine multiple stages of stimulus processing, from orienting and basic perceptual analysis to attention and memory, all of which could be affected by the motivational salience of the stimuli (for review see Bromberg-Martin et al., 2010). The ability to remember and recognize faces is important for successful social functioning (e.g., Ellis & Young, 1998), and emerges very early in development. Typical newborns learn quickly to recognize their mother’s face (Pascalis, de Schonen, Morton, Deruelle & Fabre-Grenet, 1995), and 9-month-old infants can discriminate between familiar and novel faces even when they differ only in a single feature (i.e., eyes or mouth; Key, Stone & Williams, 2009).
Conversely, behavioral and ERP studies in persons with ASD noted difficulties in remembering faces as evidenced by reduced sensitivity to facial familiarity (Dawson, Carver, Meltzoff, Panagiotides, McPartland & Webb, 2002), slower habituation to novel faces (Webb et al., 2010b), and poor recognition of familiar faces (Boucher, Lewis & Collis, 1998; Dawson et al., 2002; Klin et al., 1999; but see Webb et al. 2010a for a report of intact face recognition in adults with ASD). These deficits appear to be more pronounced in younger children with autism (Kuusikko-Gauffin et al., 2011; but see O’Hearn, Schroer, Minshew, & Luna, 2010 for opposing findings) and may be already present in the first year of life. Key & Stone (2012) reported that despite the overall ability to distinguish their mother’s face from a stranger, 9-month-old infant siblings of children with ASD compared to typical infants showed reduced featural processing of the stranger’s face beyond the initial perceptual analysis. Developmental delays in face recognition (mother vs. stranger) have been also identified in 12-month-olds at high-risk for ASD (Luyster, Wagner, Vogel-Farley, Tager-Flusberg, & Nelson, 2011) and in 18–30-month-olds with ASD (Webb et al., 2011).
In a recent review of 90 studies on face memory in ASD, Weigelt, Koldewyn, & Kanwisher (2012) concluded that although persons with ASD may engage face-specific perceptual mechanisms, they are less successful in face recognition, especially in tasks involving a delay. Yet, there is a paucity of data concerning the process of forming memories for social information.
Previous studies compared already familiar faces (family members, friends, celebrities) to novel stimuli or included extensive familiarization protocols prior to examining behavioral or brain evidence of face recognition. Recently, Webb et al. (2010a) included repeated presentations of unfamiliar faces in addition to familiar and novel faces in their oddball paradigm examining face processing in adults with ASD and typical development. The authors reported no group differences in the amplitude of the occipito-temporal P1, N170, P2, N250, and face-N400 responses between the adults with ASD and typical controls for the familiar and repeated vs. novel faces, although the groups differed occasionally in the hemisphere distribution of the stimulus-related effects. The analyzed ERP markers could be interpreted to reflect primarily perceptual processing (with the possible exception of the face-N400), while ERP responses previously identified in memory studies as indexing stimulus recognition (frontal N400) and recall (parietal P600) have not been examined. The authors attributed the lack of group differences in the timing of the P1 and N170 responses to increased heterogeneity associated with a significantly larger sample of participants with ASD, and possible procedural details, such as the use of attention-directing fixation points (Webb et al., 2010a).
In typical participants, ERP studies requiring active memorization and recall or recognition of the studied items among novel distractors consistently demonstrate that compared to novel (not previously learned) stimuli, repeated items elicit more positive amplitudes of the frontal N400 (FN400) and posterior P600 (Curran & Cleary, 2003; Duarte, Ranganath, Winward, Hayward & Knight, 2004). These responses have been elicited by a variety of stimuli, including spoken and written words and pictures. The functional significance of these memory indices varies. The FN400 effect onsets at 300–400 ms post-stimulus and reflects general stimulus familiarity (e.g., Friedman & Johnson, 2000). The parietal P600 effect is present within 400–900 ms after stimulus onset and is thought to reflect recall of information (Wilding, 2000). The size of the P600 response can be modulated by depth of cognitive processing and by the amount of retrieved episodic information (see Friedman & Johnson, 2000, for a review). When faces were used as the stimuli, memory-related modulations of this posterior positivity have also been reported in two distinct temporal windows, such as 255–650 ms (Nelson, Thomas, de Haan & Wewerka, 1998) and 500–800ms (Curran & Hancock, 2007).
Although very informative, tasks requiring active memorization and recall of information may be difficult to administer to participants who may be unmotivated or unable to follow the instructions or provide verbal responses due to a developmental or intellectual disability. To address this challenge, Jessen and colleagues (2002) developed a passive viewing paradigm where most of the stimuli (color photographs of complex indoor and outdoor scenes that did not include faces) were presented only once while a small subset of pictures was repeated several times throughout the test session. To ensure attention to the stimuli, participants were asked to respond with a button press every time a distinct probe stimulus was presented. Using fMRI, the authors demonstrated that compared to the images shown only once, the repeated stimuli were associated with reduced activation in the occipital, inferior temporal, and right parietal regions, as well as in the bilateral anterior hippocampus (Jessen, Manka, Scheef, Granath, Schild, & Heun, 2002), structures previously identified as involved in active novelty detection tasks (e.g., Menon et al., 2000).
Recently, we adapted this procedure for use with ERPs and observed that repeated nonsocial images were associated with increased parietal P600 amplitudes in young adults with Down syndrome (DS), reflecting a memory trace for the repeated stimuli, while ERPs of the older participants with DS did not show such amplitude modulation (Key & Dykens, 2014). Another important finding of that study was the fact that ERP indices of repetition detection were independent of participants’ IQ, suggesting that our ERP paradigm was likely measuring a basic process of memory trace formation for the repeated stimuli. This feature is particularly promising as an effective way to examine the effects of internal motivation on the extent of information processing.
Increased stimulus familiarity resulting from repeated exposure to identical stimuli reflects a basic form of learning and memory (Yonelinas, 2002). For any information to be remembered, even if only temporarily, it has to receive sufficient amount of attention. In the absence of explicit instructions directing a participant to memorize presented pictures, it can be assumed that in a stream of visually diverse stimuli, a person is likely to allocate more information processing resources to the images he or she personally finds to be more salient, interesting or motivating. In the context of social motivation, we hypothesized that compared to persons with ASD, typically developing individuals would be more likely to attend to human faces than to nonsocial stimuli (see also Chevallier et al. 2012b) and process them to the extent sufficient to develop a stronger memory trace.
In the present study, we used the passive repetition detection ERP paradigm to examine incidental memory for repeated versus single presentations of social and nonsocial stimuli in children with ASD and typical development. Based on prior ERP studies of stimulus familiarity and recall (e.g., Curran & Hancock, 2007), we examined FN400 and posterior P600 indices of memory processes. We hypothesized that compared to responses elicited by images shown only once, repeated stimuli that participants find salient or interesting would be associated with smaller (less negative) FN400 and larger parietal P600 responses. Furthermore, we expected that compared to children with ASD, typical children would demonstrate better memory for the repeated social information, as indexed by greater differences in ERP amplitudes to repeated versus single presentation faces. Additionally, in light of the inconsistent findings with regard to the early perceptual responses (McPartland, et al., 2004; Hileman et al., 2011; Webb et al., 2010a), we examined occipito-temporal P1 and N170 to explore whether children with ASD differ from their typical peers in early perceptual processes and whether such differences may be associated with the more complex cognitive processes such as memory for social information.
To validate the assumption that ERP differences indexing memory for repeated vs. single faces reflect processes relevant to social cognition, salience and motivation, we examined correlations between participants’ ERP markers of memory for faces and houses and performance on standardized behavioral assessments of immediate and delayed memory for faces and caregiver reports of social functioning. Specifically, we hypothesized that greater differences between ERP responses to the repeated vs. single social stimuli would be associated with better behavioral performance on these measures.
Finally, given the novelty of the experimental paradigm, and to examine its utility for future use in treatment studies, we examined test-retest stability of the ERPs over a 3-week period.
Method
Participants
Twenty-four children (4 females; M age = 10.61, SD = 1.57 years, range 7–13 years) participated in the study (see Table 1 for detailed characteristics). Six participants were left-handed, the rest were right-handed (M laterality quotient = .51, SD =.71) as determined by Edinburgh Handedness Inventory (Oldfield, 1971). Participants comprised two diagnostic groups. One group included 11 individuals with typical development (2 females), while the other group had 13 participants with ASD (2 females). One additional typically developing participant was excluded from the study due to poor quality of EEG data. The two groups did not differ in age, but the typical group had higher verbal and composite IQ (see Table 1). The mean total IQ for the combined sample was 112.41 (SD = 15.71), with comparable performance in verbal versus nonverbal domains, M = 110.96, SD = 19.60 and M = 111.21, SD = 15.30, respectively.
Table 1.
Study sample demographics.
| ASD (n=13) | TD (n=11) | Group Difference | |||
|---|---|---|---|---|---|
| Mean (range) | SD | Mean (range) | SD | p-value | |
| Age at ERP (yrs) | 10.76 (8.60–13.67) | 1.50 | 10.43 (7.48–12.88) | 1.70 | .620 |
| Verbal IQ | 100.08 (74–130) | 17.13 | 123.82 (98–141) | 13.99 | .001 |
| Performance IQ | 106.23 (81–132) | 15.28 | 117.09 (93–137) | 13.71 | .083 |
| Full Scale IQ | 103.17 (85–122) | 13.72 | 123.50 (113–141) | 9.78 | .001 |
| Handedness | .14 (−.80–1.00) | .81 | .95 (.80–1.00) | .08 | .003 |
| ADOS research algorithm total | 12.92 (7–26) | 5.35 | |||
| SCQ total score | 19.82 (9–33) | 6.59 | 0.91 (0–4) | 1.45 | <.001 |
ADOS – Autism Diagnostic Observation Schedule; SCQ – Social Communication Questionnaire
ASD diagnosis was based on the Diagnostic and Statistical Manual (DSM-IV) criteria (APA, 2000) and established by all of the following: (1) a previous diagnosis by a psychologist, psychiatrist, or behavioral pediatrician with ASD expertise; (2) current clinical judgment (by B.A.C.) and (3) corroborated by the ADOS (Lord, 2000), administered by research-reliable personnel, with a total score at or above the ASD threshold for Module 3.
All participants had normal or corrected-to-normal vision. For inclusion in the study, all participants were required to be free of medication and have a medical history void of seizures, traumatic head injury, or serious medical condition confirmed by parental interview. Participants with ASD were recruited from the university clinic, parent support groups and school systems. Typical participants were recruited from the local community including area schools, research listserves and recreation centers. They did not have any known psychiatric, genetic or medical condition per parent report. Participants were matched based on age and gender, but not IQ. Social Communication Questionnaire (SCQ; Rutter, 2003) was used to rule out ASD in the typical sample. Parent report confirmed absence of family history of ASD or neurological disorders.
Parents/guardians of the participants provided written informed consent, and all participants provided their assent. The study was conducted with approval from the university Institutional Review Board.
The following diagnostic and psychological measures were administered, and results are presented in Table 1.
Diagnostic assessments
Autism Diagnostic Observation Schedule (ADOS)
The ADOS (Lord, 2000) is a semi-structured interview designed to assess behaviors characteristic of ASD. The ADOS assesses the participant’s engagement in a variety of play-based activities, conversations, demonstrations and responses to interview questions. The ADOS is considered the gold standard for supporting a diagnsosis of ASD and requires administration by a research-reliable clinician (B.A.C.). A research algorithm total score of 7 or greater was required for inclusion in the ASD group. This measure was administered only to participants with ASD as it is not traditionally administered to typically developing individuals who were instead screened by the Social Communication Questionnaire (SCQ) described below.
Wechsler Abbreviated Scale of Intelligence (WASI)
The WASI (Wechsler, 1999) is a measure of cognitive ability used to obtain an estimate of intellectual functioning. The WASI includes two verbal subtests assessing word knowledge and verbal reasoning, and two non-verbal stubtests assessing visual spatial reasoning and construction ability. Inclusion in the study required an estimated composite IQ of 70 or higher for both groups of participants.
Social Communication Questionnaire (SCQ)
The SCQ (Rutter, 2003) was used as a screening tool for ASD (scores of ≥15 is suggestive of ASD) which can corroborate diagnosis, but was primarily used in the current study to rule out ASD in the TD group. The SCQ asks the parent to provide developmental information relevant to the ASD diagnosis pertaining to social and communication skills as well as repetitive, restrictive behaviors. The exclusion criteria for a typically developing child was a score ≥10; however, no participants were excluded based on this criteria.
ERP task and procedures
Stimuli
Stimuli included 51 color photographs of novel faces depicting young adults obtained from a standardized Radboud Faces Database set (Langner, Dotsch, Bijlstra, Wigboldus, Hawk & van Knippenberg, 2010), 51 color photographs of novel houses (façade view, obtained from realtor websites), and a drawing of a yellow smiley face. One of the novel faces and one of the houses were randomly selected to be repeated 50 times throughout the experiment, yielding a unique set of 50 repeated faces and houses for each participant. The remaining stimuli were presented once. A drawing of a yellow smiley face served as an attention probe and was presented 10 times throughout the session (brain responses to this stimulus were not included in the analysis). The onscreen size of the stimuli was 30 cm high and 25 cm wide. From the viewing distance of 90 cm, the stimuli subtended visual angles of 19° (h) x 16°(w). The yellow smiley face was 14.5 cm (9.21°) in diameter. The large stimulus size was selected to ensure that all stimulus features were clearly visible, and in the case of faces, were close to real life size. Large visual stimuli have been successfully used in previous ERP studies of face processing in persons with ASD (e.g., Elsabbagh et al., 2012; Webb et al., 2011).
Electrodes
A high-density array of 128 Ag/AgCl electrodes embedded in soft sponges (Geodesic Sensor Net, EGI, Inc., Eugene, OR) and high input-impedance amplifiers were used to record the ERPs. Electrode impedance levels were at or below 40 kOhm (Feree et al., 2001). During testing data were sampled at 250Hz with the filters set to .1–100 Hz. All electrodes were referenced to Cz (vertex) and then re-referenced offline to an average reference recommended for high-density arrays (Picton et al., 2000). The average reference has also been used successfully in prior ERP studies of memory (e.g., Curran & Cleary, 2003).
Procedure
All stimuli were presented in random order for 1500 ms with a random inter-stimulus interval of 1300–1600 ms to prevent habituation and development of trial onset expectations. To verify attention to the stimuli, participants were asked to press a response button when they saw the yellow smiley face. Stimulus presentation was controlled by E-prime (v.2.0, PST, Inc., Pittsburgh, PA). The entire task included 210 trials and lasted approximately 12 minutes. A researcher was present in the room to monitor participants’ behavior. If participants became inattentive or restless, stimulus presentation could be suspended until the participant was ready to continue with the task.
To examine test-retest stability of the ERPs reflecting differences in processing of repeated vs. single stimulus presentations, all participants repeated the ERP procedure approximately 3 weeks following the first session (M test-retest interval =20.6 days, SD = 3.34). New repeated stimuli were selected for each test session. The repeated face and house stimuli used in the first test session were excluded from the second test.
Neuropsychological assessment
NEPSY: Memory for Faces (Korkman, Kirk, & Kemp, 2007) was administered to assess social perception and memory for facial information. Participants viewed a series of 16 pictures of children’s faces presented for 5 seconds each and then were asked to identify those faces amidst an array of 3 non-presented choices, immediately and following a 15–25 minute delay. The broad average range is defined by scaled scores ranging from 7 to 13. The scaled scores for immediate and delayed memory for faces were used in the analyses.
Social Responsiveness Scale (SRS; Constantino & Gruber, 2005) is a 65-item questionnaire completed by care providers. It measures social functioning as related to awareness, communication, cognition, and motivation skills as well as restricted interests and behaviors. The Total T-score was used in the analyses. Higher scores indicate greater impairment. Specifically, T-scores below 60 are average, whereas, T-scores between 60-to-75 are clinically significant and represent the mild-to-moderate range of impairment, T-scores 76 or higher are strongly associated with more severe ASD symptoms and impairment.
Adaptive Behavior Assessment System (ABAS) (Harrison & Oakland, 2000) is a caregiver questionnaire that assesses 10 areas of adaptive functioning in three main domains of social functioning, conceptual reasoning and practical functioning. For this study, the ABAS was used to ascertain adaptive functioning related to social and communication skills. Scaled scores from 7-to-13 fall within the average range, whereas scores between 3-to-6 are clinically relevant and considered to be below average.
Data Analysis
Behavioral Data
To determine if participants maintained attention throughout the test session, number and reaction time of the responses to the smiley face probes were submitted to a repeated measures ANOVA with the diagnostic group as the between-subject factor and test session as the within-subject factor.
ERP Data
Collected EEGs were filtered using a 30Hz low-pass filter. Individual ERPs were derived by segmenting the ongoing EEG on stimulus onset to include a 100-ms prestimulus baseline and a 900 ms post-stimulus interval. The resulting trials for faces and houses were grouped into presented once (“single”) and repeated categories. All trials contaminated by ocular and movement artifacts were excluded from further analysis using an automated screening algorithm in NetStation followed by a manual review. The automated screening criteria were set as follows: for the eye channels, voltage in excess of 140 μV was interpreted as an eye blink and voltage above 55 μV was considered to reflect eye movements. Any channel with voltage exceeding 200 μV was marked as bad. Data for electrodes with poor signal quality within a trial were reconstructed using spherical spline interpolation procedures. If more than 20% of the electrodes within a trial were deemed bad, the entire trial was discarded. The retention rates per condition were comparable across groups and test sessions (ASD: T1=20.81+/−7.92; T2=22.00+/−8.08; TD: T1=23.91+/−8.88; T2 =25.25+/−7.47; p’s >.05), exceeded the minimum number of trials considered acceptable in prior memory studies (e.g., Curran & Cleary, 2003), and were comparable to those in studies of face perception in ASD (e.g., Elsabbagh et al., 2012; Webb et al., 2011).
Following artifact screening, individual ERPs were averaged, re-referenced to an average reference, and baseline-corrected by subtracting the average microvolt value across the 100-ms prestimulus interval from the post-stimulus segment. To reduce the number of electrodes in the analysis, only data from selected electrodes corresponding to frontal, parietal, and occipito-temporal locations within each hemisphere (see electrode diagrams in Figures 1 and 2) were used in the remaining statistical analyses. These clusters were selected a priori and were identical to the ones used in a previously published ERP study of face familiarity (Curran & Hancock, 2007). These locations have been identified as optimal for the frontal N400 and parietal P600 memory-related effects, as well as for occipito-temporal P1 and N170 responses.
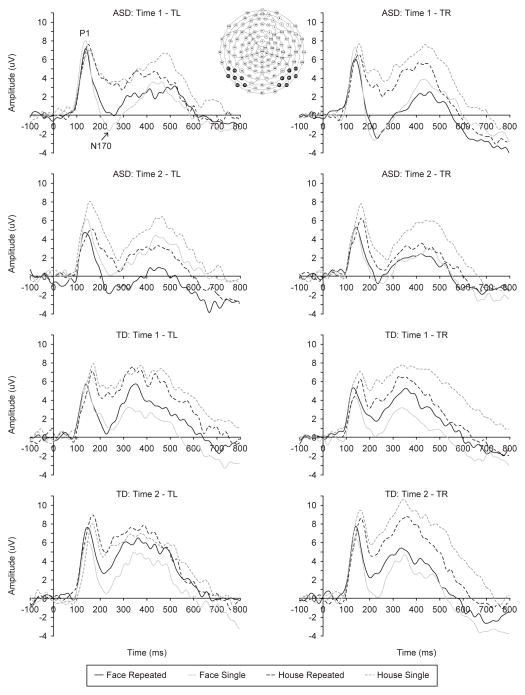
Figure 1.
ERP waveforms in response to repeated and single stimuli at left and right occipito-temporal clusters for children with ASD and TD.
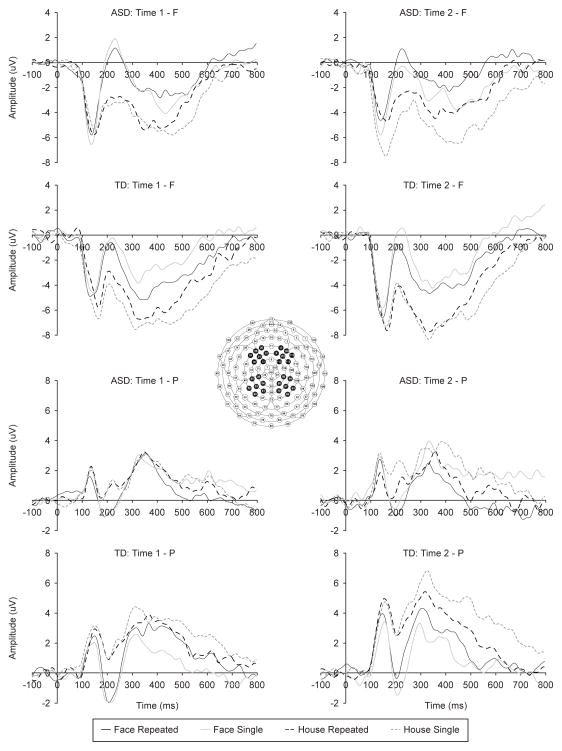
Figure 2.
ERP waveforms in response to repeated and single stimuli at frontal and parietal clusters (averaged across hemispheres) for children with ASD and TD.
Next, mean ERP amplitudes were derived for the FN400 in the 300–500 ms window, and for the posterior P600 in the 300–500 and 500–800ms post-stimulus intervals. Because prior studies identified repetition-related effects mainly for the amplitude of the ERP responses, latency measures were not included in the analyses. The specific time intervals were selected a priori based on temporal windows utilized in previously published ERP studies of recognition and recall in visual paradigms (e.g., Curran & Hancock, 2007), and confirmed by visual inspection of the grand-averaged waveforms. Additionally, mean amplitudes for occipito-temporal P1 were calculated over the 100–180 ms window, and for the N170 in the 180–300 ms window. These windows were determined based on the visual inspection of the grand-averaged waveforms and consistent with the intervals used in prior ERP studies of face perception in children with autism (e.g., Grice et al., 2005).
The resulting mean amplitude values for each peak (P1, N170, FN400, early P600, late P600) were averaged across the electrodes within the pre-selected electrode clusters and entered into separate repeated-measures ANOVAs with Group as the between-subject factor and Time (2: test, retest) x Stimulus (2: faces, houses) x Memory condition (2: single, repeated) x Hemisphere (2: left/right frontal, parietal, or occipito-temporal) within-subject factors with Huynh-Feldt correction. Significant interactions were further explored using one-way ANOVAs and pair-wise comparisons with Bonferroni correction. These analyses focused only on contrasts relevant to the hypotheses, such as stimulus-related (faces vs. houses) and memory differences (repeated vs. single presentations) as well as test-retest differences.
To determine if stimulus- and memory-related ERP findings were related to cognitive and behavioral functioning, exploratory correlation analyses were performed. Due to the small sample size of the groups, group membership was collapsed to allow for the examination of possible relationships between variables. The combined sample was thought to represent a continuum of social functioning.
Results
Behavioral data
Responses to attention probes (smiley face) revealed that all participants maintained attention to the stimuli during the test session. There were no group or test session related differences in the number of responses to the attention probe (p’s >.24). Similarly, there were no test or group differences in the reaction time (p’s>.11; see Table 2 for the summary data). Reaction times to attention probes did correlate with the composite IQ only in children with ASD (T1: r=−.765, p=.004; T2: r=−.679, p=.015). In typical children, there were no correlations between the reaction time and IQ (p’s =.50–.70).
Table 2.
Summary of behavioral performance (means and standard deviations) for children with ASD, typical development, and the combined sample.
| ASD | TD | Group Difference | |||
|---|---|---|---|---|---|
| Mean (range) | SD | Mean (range) | SD | p-value | |
| NEPSY Memory for Faces | |||||
| Immediate (scaled) | 8.08 (3–14) | 3.01 | 11.64 (6–17) | 2.91 | .008 |
| Delayed (scaled) | 8.31 (4–13) | 2.43 | 11.30 (7–15) | 2.54 | .009 |
| SRS total (scaled) | 74.38 (57–88) | 10.19 | 41.45 (34–54) | 5.15 | <.001 |
| ABAS | |||||
| Communication (Scaled) | 7.15 (2–13) | 3.08 | 13.64 (11–15) | 1.03 | <.001 |
| Social (scaled) | 4.46 (1–9) | 2.82 | 12.18 (7–15) | 2.23 | <.001 |
| Responses to attention probe | |||||
| number @ T1 | 9.31 | 1.44 | 9.91 | 0.54 | .204 |
| number @ T2 | 9.46 | 1.33 | 9.82 | 0.40 | .402 |
| RT (ms) @T1 | 691.85 | 219.70 | 567.09 | 116.32 | .105 |
| RT (ms) @T2 | 629.57 | 168.90 | 533.85 | 52.54 | .085 |
SRS – Social Responsiveness Scale; ABAS – Adaptive Behavior Assessment System; RT – reaction time
ERP data
In the sections below, significant findings related to perceptual and memory processes are described first, followed by the description of the test-retest effects on observed ERPs.
Early Perceptual Responses
Occipito-temporal P1 (100–180ms)
There was a main effect of Stimulus, F(1,22)=17.393, p<.001, partial eta sq.=.442, due to more positive mean amplitudes in response to houses than faces (Figure 1). Group differences were evident in the interactions of Memory x Group, F(1,22)=6.421, p=.019, partial eta sq.=.226. Although there were no significant between- group differences in responses to either repeated or single stimuli, the within-group analyses revealed that only children with ASD demonstrated a smaller P1 amplitude to repeated compared to single presentations, t(12)=2.375, p=.035, d=.659. There were no significant memory-related differences in P1 amplitude for typical children (p=.252).
Occipito-temporal N170 (180–300ms)
The N170 response was characterized by the main effect of Stimulus, F(1,22)=54.473, p<.001, partial eta sq.=.712, as well as by Stimulus x Memory, F(1,22)=12.345, p=.002, partial eta sq.=.359, and Stimulus x Memory x Hemisphere interactions, F(1,22)=5.181, p=.033, partial eta sq.=.191. Faces elicited larger left and right N170 responses than houses both in the repeated and single condition, t(23)=3.960–8.300, p ≤ .001, d=.808–1.694 (Figure 1). Follow-up analyses also revealed that repeated compared to single houses were associated with larger right N170 response, t(23)=4.096, p<.001, d=.836.
Repetition Detection Responses
FN400 (300–500ms)
The FN400 response was characterized by the main effect of Stimulus, F(1,22)=98.455, p<.001, partial eta sq.=.817, due to larger (more negative) amplitudes in response to houses compared to faces, and a Memory x Group interaction, F(1,22)=4.434, p=.047, partial eta sq.=.168. Group differences were evident in response to the repeated stimuli only, F(1,22)=5.924, p=.024, with larger (more negative) responses in typical children than children with ASD (−5.26 μV vs. −3.10μV, respectively). Within-group analyses indicated that only children with ASD showed memory-related differences in the FN400 amplitude with more negative responses to single vs. repeated stimuli, t(12)=2.240, p=.045, d=.621 (Figure 2). In children with typical development, this contrast was not significant (p=.572).
Early Parietal P600 (300–500ms)
This response was associated with the main effects of Stimulus, F(1,22)=12.226, p=.002, partial eta sq.=.357, and Hemisphere, F(1,22)=7.134, p=.014, partial eta sq.=.245, as well as Stimulus x Hemisphere, F(1,22)=8.070, p=.010, partial eta sq.=.268 and Stimulus x Memory interaction, F(1,22)=4.713, p=.041, partial eta sq.=.176. Group differences were present in the form of Stimulus x Group, F(1,22)=4.613, p=.043, partial eta sq.=.173, and Stimulus x Memory x Group interactions, F(1,22)=6.171, p=.021, partial eta sq.=.219. For all participants, houses were associated with more positive amplitudes than faces over the right hemisphere, t(23)=3.473, p=.002, d=.709. Although there were no between-group differences in response to any particular image type, within-group analyses revealed that only children with typical development demonstrated a larger positive amplitude response to repeated vs. single faces, t(10)=2.629, p=.025, d=.793 (Figure 2), but not houses (p=.096). Children with ASD did not show the same response to faces (p=.152) and instead elicited a reduced amplitude to repeated than single houses, t(12)=2.633, p=.022, d=.730.
Late Parietal P600 (500–800ms)
There were main effects of Stimulus, F(1,22)=6.758, p=.016, partial eta sq.=.235, and Memory, F(1,22)=9.260, p=.006, partial eta sq.=.296, as well as the interactions of Stimulus x Group, F(1,22)=5.557, p=.028, partial eta sq.=.202, and Stimulus x Memory x Group, F(1,22)=9.230, p=.006, partial eta sq.=.296. Follow-up analyses did not identify any significant group differences in the amplitude of the P600 response to any stimulus type or memory condition. Within-group analysis revealed that children with ASD elicited a smaller P600 to repeated vs. single faces, t(12)=2.633, p=.022, d=.730 (Figure 2), but not to houses (p=.221). Children with typical development exhibited a similar pattern of reduction in the P600 amplitude for repeated vs.single houses, t(12)=2.667, p=.024, d=.804, but not to faces (p=.227).
Test-Retest effects
Occipito-temporal P1 (100–180ms)
Test session differences were evident in the interactions of Time x Group, F(1,22)=6.643, p=.017, partial eta sq.=.232, and Time x Memory x Hemisphere x Group, F(1,22)=11.204, p=.003, partial eta sq.=.337. Follow-up analyses indicated that there were no group or test-retest differences in the amplitude of the occipito-temporal P1 response to any particular stimulus. However, the reduction in P1 amplitude for repeated stimuli in children with ASD described above was present only during the retest session (second visit) and only over the left hemisphere, t(12)=2.939, p=.012, d=.815. Children with typical development elicited more positive left P1 amplitudes to repeated than single stimuli during the retest, t(10)=3.461, p=.006, d=1.044.
Occipito-temporal N170 (180–300ms)
Test-retest effects were reflected in the interactions of Time x Memory x Group, F(1,22)=4.552, p=.044, partial eta sq.=.171, and Time x Memory x Hemisphere x Group, F(1,22)=6.350, p=.019, partial eta sq.=.224. There were no group or test-retest differences in the amplitude of the occipito-temporal N170 response to any particular stimulus. However, during the retest session, children with ASD evidenced a larger (more negative) N170 response to repeated than single stimuli over the left hemisphere, t(12)=3.258, p=.007, d=.904, while no significant memory-related differences were detected in the N170 response of children with typical development.
FN400 (300–500ms)
Test-retest differences were present in the form of Time x Hemisphere x Group interaction, F(1,22)=5.546, p=.028, partial eta sq.=.201. Follow-up analyses revealed no significant group differences in FN400 amplitudes at any electrode location or test session. Within-group analyses also did not identify any test-retest or left-right frontal differences for either participant group (p’s=.146– .986).
Early Parietal P600 (300–500ms)
Test-retest effects were noted as a Time x Stimulus, F(1,22)=4.605, p=.043, partial eta sq.=.173, and Time x Memory interactions, F(1,22)=4.435, p=.047, partial eta sq.=.168. There were no significant test-retest differences in brain responses for any stimulus type or memory conditions. However, during the retest visit, images of houses compared to faces elicited larger positive amplitudes, t(23)=3.281, p=.003, d=.670.
Late Parietal P600 (500–800ms)
There were no significant main effects of interactions involving the Time factor.
ERP connections with cognition and behavior
To further examine whether ERP responses to repeated vs. single stimuli are related to behavioral characteristics such as age and handedness as well as the measures of IQ, attention during the task (number of attention probes detected, RT) and social skills (SRS, ABAS), correlational analyses were performed for the combined sample thought to represent a continuum of social functioning. To reduce the number of ERP variables in these analyses, the ERP index of memory was computed separately for faces and houses as a difference score by subtracting the amplitudes in response to single stimulus presentation from those elicited by the repeated images. This was done only for the early and late parietal P600 responses and the occipito-temporal N170, as these were the only ERP variables showing Stimulus x Memory interactions. These ERP data as well as the measures of attention during the task (responses to the attention probe) were averaged across the test-retest sessions, because Time was not a factor in the interactions.
False discovery rate (FDR; Benjamini & Hochberg, 1995) was used to control for multiple significance tests. Unlike the Bonferroni correction that adjusts alpha levels based on the total number of tests conducted, this statistical method controls for the proportion of incorrect rejections of the null hypothesis among the tests for which the null hypothesis was rejected (i.e., tests with p-values less than .05).
There were no significant associations between any of the ERP variables and age, handedness, IQ, or attention during the task. Correlations with standardized behavioral measures of social skills were significant only for the early parietal P600, where more positive response to repeated than single faces was associated with better delayed memory for faces (scaled score; r=.506, p=.014), lower scores on SRS (scaled; r=−.522, p=.009) and higher ABAS communication scores (scaled; r=.580, p=.003).
To examine the possible association between memory for the repeated vs. single stimuli indexed by the difference in the amplitude of the early and late parietal P600 responses and the early perceptual responses (reflected by the occipito-temporal N170), a separate set of correlations was performed. In addition to the contrast between repeated vs. single presentations, face-specificity of the early perceptual responses was quantified separately for repeated and single stimuli and computed as the difference in the amplitude of the left and right N170 responses to faces vs. houses. Following the FDR procedure, only the correlations between the early parietal P600 and right occipito-temporal N170 remained significant. Better memory for repeated vs. single faces (larger early P600 amplitude difference) was associated with a larger right N170 in response to faces than houses in the single presentation condition, r=−.476, p=.019, and a reduction of the right N170 amplitude for repeated vs. single faces, r=.573, p=.003.
Discussion
The purpose of this study was to examine whether ERP measures of incidental memory due to stimulus repetition in a passive visual paradigm involving social and nonsocial stimuli reflect differences in the salience of faces, and potentially in social motivation, between children with ASD and typical development. Our results revealed no significant diagnostic group differences in the early perceptual processing of the stimuli (P1, N170) but supported the hypothesized difference in memory for social stimuli (early parietal P600): only the typical children demonstrated ERP evidence of memory traces for the repeated vs. single faces, while the two participant groups did not differ in memory for houses. Furthermore, the observed ERP markers of memory were stable over the 3-week test-retest period, suggesting that they may reflect trait-like characteristics.
Examination of the early ERP responses revealed no diagnostic group differences in perceptual processes. Occipito-temporal P1, thought to reflect processing of low-level visual features of the stimuli (Rossion & Caharel, 2011), was larger in response to houses than faces regardless of the memory (repeated vs. single) condition, likely due to greater variability in perceptual characteristics of the houses relative to faces (e.g., greater color variability, etc.) and no explicit instructions directing attention to categorical differences between the stimulus types (face vs. house). Larger N170 responses were recorded to faces than houses both in the repeated and single condition, as would be expected based on prior ERP studies of face vs. object processing (e.g., Bentin & Deouell, 2000). These results are consistent with recent findings by Webb et al. (2010a) showing no group differences in similar occipito-temporal responses to repeated and novel faces in adults with ASD and typical development. Our findings extend those observations to 7–13-year-old children and support prior results suggesting that deficits in face perception in persons with ASD are not uniform and may be limited to more cognitively demanding tasks (see Jemel et al., 2006, Weigelt et al., 2012 for reviews). Furthermore, previous studies comparing early perceptual face processing in individuals with ASD and typical peers using face vs. object stimulus contrasts typically observed group differences in the latency but not the amplitude of the P1 and N170 responses (McPartland et al., 2004; Hileman et al., 2011), suggesting a comparable extent of the perceptual processing but possible differences in the timing.
Although P1 and N170 responses are typically thought of as reflecting mainly perceptual processes, in the present study they also were sensitive to memory effects as evident in their amplitude modulation by stimulus repetition. Across both groups, repeated compared to single houses were associated with larger right N170 responses, possibly reflecting increased perceptual experience due to repeated exposures to the same image (Tanaka & Curran, 2001). Also, during the retest session, children with ASD evidenced larger left P1 and smaller (less negative) bilateral N170 amplitudes for single than repeated stimuli, while children with typical development showed more positive left P1 amplitudes to repeated than single stimuli. Because these differences were not observed in the first test session, they have to be interpreted with caution until further replication. Nevertheless, the opposite direction of stimulus repetition impact on P1 amplitude could reflect differential effects of increased familiarity with the experimental procedure: perceptual habituation to visual features of the repeated stimuli in children with ASD versus increased attention to unexpected repetition within a highly diverse visual stream in children with TD.
We hypothesized that memory for repeated images would be evidenced by a less negative FN400 and/or more positive parietal P600 responses compared to those elicited by the single-presentation stimuli. Furthermore, we expected that these effects would be more pronounced for faces due to their inherently higher interest or motivational value as a social stimulus in typically developing populations, which could contribute to a stronger memory trace. We also anticipated that children with typical development would demonstrate better memory for the repeated faces than children with ASD. Our results partially supported these hypotheses.
For children with ASD, detection of repeated stimuli was reflected in the amplitude of the FN400, where all repeated stimuli elicited smaller (less negative) response than the single images. The FN400 response is thought to reflect stimulus recognition based on perceptual characteristics, and therefore suggests that children with ASD were sensitive to the perceptual differences between the repeated and single images. The absence of the FN400 effect in the typical group should not be interpreted as the absence of stimulus recognition, however. Instead, it is likely reflecting group differences in the information processing strategies. Previous studies of the old/new effect in typical adults using novel faces reported the absence of the FN400 (but present parietal effect) when a face was judged as “old” but no specific information about it (e.g., name, occupation) could be recalled (MacKenzie & Paller, 2007). In line with prior findings in ASD suggesting increased reliance on individual features for face recognition (e.g., Lahaie et al., 2006), it is possible that children with ASD recognized particular details of the repeated face or house, resulting in a larger FN400 response.
Conversely, typical children demonstrated the anticipated increased parietal positivity (early P600) in response to repeated vs. single faces but not to houses. Based on the functional interpretation of the posterior P600 response (e.g., Rugg et al., 1998), we consider these amplitude differences between the conditions to reflect memory trace development for the repeated faces. Because our experimental paradigm was designed to be passive and nonverbal, we did not collect any behavioral evidence of explicit stimulus recollection to confirm this interpretation. However, prior studies involving active memorization and recognition/recall noted that misremembered stimuli (e.g., old items incorrectly classified as new or new items incorrectly judged as old) did not elicit the more positive P600 response observed for the correctly recalled stimuli (Wilding & Rugg, 1997).
The early parietal P600 response findings suggest that typical children processed repeated social stimuli to a greater extent than would be sufficient for the establishment of perceptual familiarity. On the other hand, children with ASD detected perceptual familiarity of the repeated stimuli (FN400 effect) but did not engage in more extensive processing that would result in the development of a stronger memory trace (no early parietal P600 effect), likely due to the low salience of faces and no specific task or explicit instructions directing attention to such stimuli. Similar reduction in the extent of novel vs. familiar stimulus processing has been reported in 9-month-old infants at familial risk for ASD (Key & Stone, 2012). Furthermore, the observed group differences in memory for repeated faces cannot be explained by differences in perceptual processing as there were no group differences in the amplitude of N170, which was larger to faces than houses in all participants. This group similarity in early perceptual responses and difference in later memory for the repeated faces between children with and without ASD is also consistent with the observations made in a multi-study review of face memory in ASD by Weigelt et al. (2012).
Correlations between behavioral measures of social functioning and the early parietal P600 index of memory for repeated faces but not houses further suggest that our passive repetition detection paradigm was likely tapping into processes relevant to social interest, salience, or motivation. As predicted, better memory for repeated faces reflected in the ERPs was related to better performance on NEPSY’s delayed memory for faces, ABAS communication scores, and social responsiveness.
Interestingly, both participant groups demonstrated reduced parietal responses in 300–500 ms and 500–800ms windows following onset of the repeated vs. single houses, potentially suggesting habituation-related decrease in brain responses. This pattern of results appears consistent with the findings in repetition detection studies using fMRI. Jessen et al. (2002) reported reduced activation of memory-related structures (e.g., hippocampus) in response to repeated pictures of nonsocial scenes, which was interpreted to suggest habituation. Conversely, repetition-related enhancement of brain activity was observed in fMRI studies in cases of increased attention to the stimuli or incidental recollection of the repeated items (see Segaert et al., 2013 for review). Thus, it is possible that neither children with ASD nor typically developing children found the pictures of houses particularly interesting or deserving of extensive processing beyond basic perceptual analysis and suggesting that at least some aspects of memory functioning are similar across the two groups. In the future, it would be important to examine whether more salient nonsocial stimuli that would be of high personal interest to the participants (e.g., objects of restricted interest for persons with ASD) might result in similar enhancement of ERP amplitudes as observed for repeated faces in typical children in the present study.
It is important to note that the observed stimulus-specific memory results were not image-specific because a different set of pictures was used for the repeated condition for each participant and test session. Also of note is the absence of correlations between ERP indices of social memory and participants’ IQ scores. Thus, the passive task and the corresponding neural measures of stimulus processing successfully evaluated basic memory processes independent of participants’ level of intellectual functioning. Additionally, early parietal P600 evidence of memory was also unrelated to the speed of response to the attention probe, suggesting that a passive viewing paradigm with minimal explicit attention and memory demands is nevertheless effective in creating a memory trace for salient and/or intrinsically interesting stimuli even if the degree of participants’ attention varies throughout the test session. Memory indices were computed within each stimulus category (e.g., repeated vs. single faces and repeated vs. single houses), and thus any potential differences in low-level perceptual features between the photographs of faces and houses are not likely to explain the observed results. Finally, the reported ERP markers of memory appear to be stable over the 3-week test-retest interval, as evidenced by the absence of Time x Stimulus x Memory interactions. The observed interactions with Time did not identify any test-retest differences in response to any particular stimulus or memory condition, were limited primarily to early perceptual responses, and were attributed to amplitude differences in the retest session, likely reflecting general familiarity with the perceptual features of the task (picture types, their appearance on the computer screen, etc.).
Although novel, this study has several limitations. Our sample size was not atypical for an ERP study, yet it was relatively small and diverse in age. Power analyses indicated that the study was adequately powered to detect only large effect sizes for between-group differences and correlations. While it is possible that smaller differences were not detected in the present study, examination of the waveforms, the magnitude of the observed effect sizes, and the evidence of the test-retest stability of the observed memory effects suggest that the reported group differences in memory for the repeated stimuli are indeed face specific. Also, while a larger sample size could allow to detect smaller differences, our present results suggest that the proposed passive ERP paradigm generates large effects and therefore is likely to have clinical and research value as a nonverbal measure of stimulus salience, social interest, and motivation.
While the two participant groups did not differ on their performance IQ, they did not match on verbal IQ. Even though verbal IQ differences between children with ASD and typical development would be expected, our sample of typical peers had higher than average scores. This is not uncommon for university-based community samples but could bring into question the representativeness of this group. While a closer age and verbal IQ match would be desirable, there were no significant correlations between these variables and ERP indices of memory. Furthermore, our experimental paradigm was deliberately designed to be nonverbal and did not require any explicit evaluation of the stimuli in order to keep the cognitive load low and suitable for use in participants of all levels of functioning. Thus, we strongly believe that the potential differences in IQ did not confound the results. Nevertheless, future studies using the proposed passive repetition detection paradigm should try to control for IQ differences.
The passive design of the paradigm precluded us from collecting overt behavioral evidence of memory for repeated faces and houses. However, correlations between ERP responses and NEPSY memory for faces scores support the proposed interpretation that the observed brain responses reflected memory-related processes. Anecdotal data from participants’ spontaneous comments also suggest that many of them became aware of the stimulus repetition at some point during the test session. It is possible that children with autism did not attend to face stimuli as closely as the typical children. However, our main research question was about the individual differences in the spontaneous response to faces in the absence of an explicit “face task”. Thus, any variability in attention to the screen would be informative about the stimulus salience, and the possibility of lower than typical attention would not argue against our interpretation. Similarly, slower reaction times to the attention probe in children with autism compared to their typically developing peers do not necessarily suggest reduced engagement with the task. The probe was peripheral to the purpose of the study (5% of the trials) and used only to provide additional confirmation (beyond the actual observation by a researcher during the test) that participants looked at the screen. The near-perfect accuracy indicates that indeed all participants were processing the visual stimuli enough to detect the probe. Differences in the speed of the response did not correlate with the ERP measures of memory and therefore are unlikely to explain the observed pattern of results. Future studies examining trial-to-trial variability in ERPs and formally documenting frequencies of off-task behaviors could help address the question of attention differences in more detail.
Finally, our study represented just a single snapshot of possible memory processes in preadolescent children. Given existing studies suggesting developmental differences in face processing skills in persons with ASD, future longitudinal studies are needed to document the developmental course of social memory and to test the power of the ERP markers identified in the present study for quantifying plasticity of memory for social information due to maturation and/or treatment. These future studies are particularly important because brain evidence of altered information processing is often present in advance of behavioral symptoms, and thus could contribute to the development of biomarkers of treatment effects or developmental outcomes.
Despite these weaknesses, our findings suggest that ERPs in a passive nonverbal face memory paradigm based on repetition detection may be a promising marker of social stimulus salience, and potentially social motivation, in children. Within the framework of social motivation as involving detecting and orienting to social information, seeking and liking social interactions, and maintaining social bonds (Chevallier et al., 2012b), our current findings suggest that individuals with ASD may be comparable to typical peers in orienting to social information (P1/N170 responses) but exhibit a possible impairment in spontaneous memory for faces, the ability likely important for establishing and maintaining social bonds. The use of simple stimuli with clear figure-ground organization and the lack of explicit instructions directing attention to social stimuli preclude us from making conclusions about the seeking component of social motivation. The non-invasive nature and relatively low cost of the ERPs, the low cognitive demand of the passive task as well as the stability of the identified ERP responses over time make our paradigm particularly well suited for future use as a marker of risk for adverse developmental outcomes and for repeated applications over the course of development or treatment.
Acknowledgments
This work was supported in part by NICHD Grant P30 HD15052 to Vanderbilt Kennedy Center (VKC), NIMH R01 MH085717 to Dr. Corbett, and by a VKC Hobbs Discovery Award to Drs. Corbett & Key. We would like to thank Dorita Jones and Amber Vinson for assistance with ERP data acquisition and processing, and David Simon and Deanna Swain for coordianting recruitment and behavioral assessments of the participants.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 2000. Text Revision (DSM-IV-TR) [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57(1):289–300. [Google Scholar]
- Bentin S, Deouell LY. Structural encoding and identification in face processing: ERP evidence for separate mechanisms. Cognitive Neuropsychology. 2000;17:35–55. doi: 10.1080/026432900380472. [DOI] [PubMed] [Google Scholar]
- Boucher J, Lewis V, Collis G. Familiar face and voice matching and recognition in children with autism. Journal of Child Psychology and Psychiatry. 1998;39(2):171–181. [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68(5):815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Grèzes J, Molesworth C, Berthoz S, Happé F. Brief report: Selective social anhedonia in high functioning autism. Journal of Autism and Developmental Disorders. 2012a;42(7):1504–1509. doi: 10.1007/s10803-011-1364-0. [DOI] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012b;16(4):231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Molesworth C, Happé F. Diminished social motivation negatively impacts reputation management: autism spectrum disorders as a case in point. PLoS ONE. 2012c;7(1):e31107. doi: 10.1371/journal.pone.0031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale. Los Angeles: Western Psychological Services; 2005. [Google Scholar]
- Corbett BA, Carmean V, Ravizza S, Wendelken C, Henry ML, Carter C, Rivera S. A functional and structural study of emotion and face processing in children with autism. Psychiatry Research. 2009;173(3):196–205. doi: 10.1016/j.pscychresns.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T, Cleary AM. Using ERPs to dissociate recollection from familiarity in picture recognition. Brain research Cognitive brain research. 2003;15(2):191–205. doi: 10.1016/s0926-6410(02)00192-1. [DOI] [PubMed] [Google Scholar]
- Curran T, Hancock J. The FN400 indexes familiarity-based recognition of faces. NeuroImage. 2007;36(2):464–471. doi: 10.1016/j.neuroimage.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Carver L, Meltzoff AN, Panagiotides H, McPartland J, Webb SJ. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Development. 2002;73(3):700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Developmental Neuropsychology. 2005;27(3):403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Deruelle C, Rondan C, Gepner B, Tardif C. Spatial frequency and face processing in children with autism and Asperger syndrome. Journal of Autism and Developmental Disorders. 2004;34(2):199–210. doi: 10.1023/b:jadd.0000022610.09668.4c. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Winward L, Hayward D, Knight RT. Dissociable neural correlates for familiarity and recollection during the encoding and retrieval of pictures. Brain research Cognitive brain research. 2004;18(3):255–272. doi: 10.1016/j.cogbrainres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Ellis HD, Young AW. Faces in their social and biological context. In: Young AW, editor. Face and mind. New York: Oxford University Press; 1998. pp. 67–95. [Google Scholar]
- Elsabbagh M, Volein A, Csibra G, Holmboe K, Garwood H, Tucker L, Krljes S, Baron-Cohen S, Bolton P, Charman T, Baird G, Johnson M. Neural correlates of eye gaze processing in the infant broader autism phenotype. Biological Psychiatry. 2009;65(1):31–38. doi: 10.1016/j.biopsych.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clinical Neurophysiology. 2001;112(3):536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Friedman D, Johnson R. Event-related potential (ERP) studies of memory encoding and retrieval: a selective review. Microscopy research and technique. 2000;51(1):6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Grice SJ, Halit H, Farroni T, Baron-Cohen S, Bolton P, Johnson MH. Neural correlates of eye-gaze detection in young children with autism. Cortex. 2005;41:342–353. doi: 10.1016/s0010-9452(08)70271-5. [DOI] [PubMed] [Google Scholar]
- Harrison PL, Oakland T. Adaptive Behavior Assessment System. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Hileman CM, Henderson H, Mundy P, Newell L, Jaime M. Developmental and individual differences on the P1 and N170 ERP components in children with and without autism. Developmental Neuropsychology. 2011;36(2):214–236. doi: 10.1080/87565641.2010.549870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemel B, Mottron L, Dawson M. Impaired face processing in autism: Fact or artifact? Journal of Autism and Developmental Disorders. 2006;36(1):91–106. doi: 10.1007/s10803-005-0050-5. [DOI] [PubMed] [Google Scholar]
- Jessen F, Manka C, Scheef L, Granath DO, Schild HH, Heun R. Novelty detection and repetition suppression in a passive picture viewing task: A possible approach for the evaluation of neuropsychiatric disorders. Human Brain Mapping. 2002;17(4):230–236. doi: 10.1002/hbm.10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key AP, Dykens EM. Event-related potential index of age-related differences in memory processes in adults with Down syndrome. Neurobiology of Aging. 2014;35(1):247–253. doi: 10.1016/j.neurobiolaging.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key APF, Stone WL. Same but different: 9-month-old infants at average and high risk for autism look at the same facial features but process them using different brain mechanisms. Autism Research. 2012;5(4):253–266. doi: 10.1002/aur.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key A, Stone W, Williams SM. What do infants see in faces? ERP evidence of different roles of eyes and mouth for face perception in 9-month-old infants. Infant and Child Development. 2009;18(2):149–162. doi: 10.1002/icd.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Sparrow SS, de Bildt A, Cicchetti DV, Cohen DJ, Volkmar FR. A normed study of face recognition in autism and related disorders. Journal of Autism and Developmental Disorders. 1999;29(6):499–508. doi: 10.1023/a:1022299920240. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S. NEPSY. 2. San Antonio, TX: Harcourt Assessment; 2007. [Google Scholar]
- Kuusikko-Gauffin S, Jansson-Verkasalo E, Carter A, Pollock-Wurman R, Jussila K, Mattila ML, Rahko J, Ebeling H, Pauls D, Moilanen I. Face memory and object recognition in children with high-functioning autism or Asperger syndrome and in their parents. Research in Autism Spectrum Disorders. 2011;5(1):622–628. [Google Scholar]
- Lahaie A, Mottron L, Arguin M, Berthiaume C, Jemel B, Saumier D. Face perception in high-functioning autistic adults: Evidence for superior processing of face parts, not for a configural face-processing deficit. Neuropsychology. 2006;20(1):30–41. doi: 10.1037/0894-4105.20.1.30. [DOI] [PubMed] [Google Scholar]
- Langner O, Dotsch R, Bijlstra G, Wigboldus D, Hawk S, van Knippenberg A. Presentation and validation of the Radboud Faces Database. Cognition & Emotion. 2010;24(8):1377–1388. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Luyster RJ, Wagner JB, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Neural Correlates of Familiar and Unfamiliar Face Processing in Infants at Risk for Autism Spectrum Disorders. Brain Topography. 2011;24(3–4):220–228. doi: 10.1007/s10548-011-0176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie G, Donaldson DI. Dissociating recollection from familiarity: Electrophysiological evidence that familiarity for faces is associated with a posterior old/new effect. NeuroImage. 2007;36(2):454–463. doi: 10.1016/j.neuroimage.2006.12.005. [DOI] [PubMed] [Google Scholar]
- McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ. Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2004;45(7):1235–1245. doi: 10.1111/j.1469-7610.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- Menon V, White CD, Eliez S, Glover GH, Reiss AL. Analysis of a distributed neural system involved in spatial information, novelty, and memory processing. Human Brain Mapping. 2000;11(2):117–129. doi: 10.1002/1097-0193(200010)11:2<117::AID-HBM50>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C, Thomas K, de Haan M, Wewerka S. Delayed recognition memory in infants and adults as revealed by event-related potentials. International Journal of Psychophysiology. 1998;29:145–165. doi: 10.1016/s0167-8760(98)00014-2. [DOI] [PubMed] [Google Scholar]
- O'Connor K, Hamm JP, Kirk IJ. Neurophysiological responses to face, facial regions and objects in adults with Asperger's syndrome: An ERP investigation. International Journal of Psychophysiology. 2007;63(3):283–293. doi: 10.1016/j.ijpsycho.2006.12.001. [DOI] [PubMed] [Google Scholar]
- O'Hearn K, Schroer E, Minshew N, Luna B. Lack of developmental improvement on a face memory task during adolescence in autism. Neuropsychologia. 2010;48(13):3955–3960. doi: 10.1016/j.neuropsychologia.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pascalis O, De Schonen S, Morton J, Deruelle C, Fabre-Grenet M. Mother’s face recognition by neonates: A replication and an extension. Infant Behavior and Development. 1995;18:79–85. [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Miller G, Ritter W, Ruchkin D, Rugg M, Taylor M. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37(2):127–152. [PubMed] [Google Scholar]
- Riby D, Hancock PJB. Looking at movies and cartoons: eye-tracking evidence from Williams syndrome and autism. Journal of Intellectual Disability Research. 2009;53(2):169–181. doi: 10.1111/j.1365-2788.2008.01142.x. [DOI] [PubMed] [Google Scholar]
- Rossion B, Caharel S. ERP evidence for the speed of face categorization in the human brain: Disentangling the contribution of low-level visual cues from face perception. Vision Research. 2011;51(12):1297–1311. doi: 10.1016/j.visres.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Mark RE, Walla P, Schloerscheidt AM, Birch CS, Allan K. Dissociation of the neural correlates of implicit and explicit memory. Nature. 1998;392(6676):595–598. doi: 10.1038/33396. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Tanaka JW, Curran T. A neural basis for expert object recognition. Psychological Science. 2001;12(1):43–47. doi: 10.1111/1467-9280.00308. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. Faces call for attention: Evidence from patients with visual extinction. Neuropsychologia. 2000;38:693–700. doi: 10.1016/s0028-3932(99)00107-4. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Jones EJH, Merkle K, Murias M, Greenson J, Richards T, Aylward E, Dawson G. Response to familiar faces, newly familiar faces, and novel faces as assessed by ERPs is intact in adults with autism spectrum disorders. International Journal of Psychophysiology. 2010a;77(2):106–117. doi: 10.1016/j.ijpsycho.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Jones EJH, Merkle K, Namkung J, Toth K, Greenson J, Murias M, Dawson G. Toddlers with elevated autism symptoms show slowed habituation to faces. Child Neuropsychology. 2010b;16(3):255–278. doi: 10.1080/09297041003601454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Jones EJH, Merkle K, Venema K, Greenson J, Murias M, Dawson G. Developmental change in the ERP responses to familiar faces in toddlers with autism spectrum disorders versus typical development. Child Development. 2011;82(6):1868–1886. doi: 10.1111/j.1467-8624.2011.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Weigelt S, Koldewyn K, Kanwisher N. Face identity recognition in autism spectrum disorders: A review of behavioral studies. Neuroscience & Biobehavioral Reviews. 2012;36(3):1060–1084. doi: 10.1016/j.neubiorev.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Wilding EL. In what way does the parietal ERP old/new effect index recollection? International Journal of Psychophysiology. 2000;35(1):81–87. doi: 10.1016/s0167-8760(99)00095-1. [DOI] [PubMed] [Google Scholar]
- Wilding EL, Rugg MD. An event-related potential study of memory for words spoken aloud or heard. Neuropsychologia. 1997;35(9):1183–1195. doi: 10.1016/s0028-3932(97)00048-1. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46(3):441–517. [Google Scholar]