Abstract
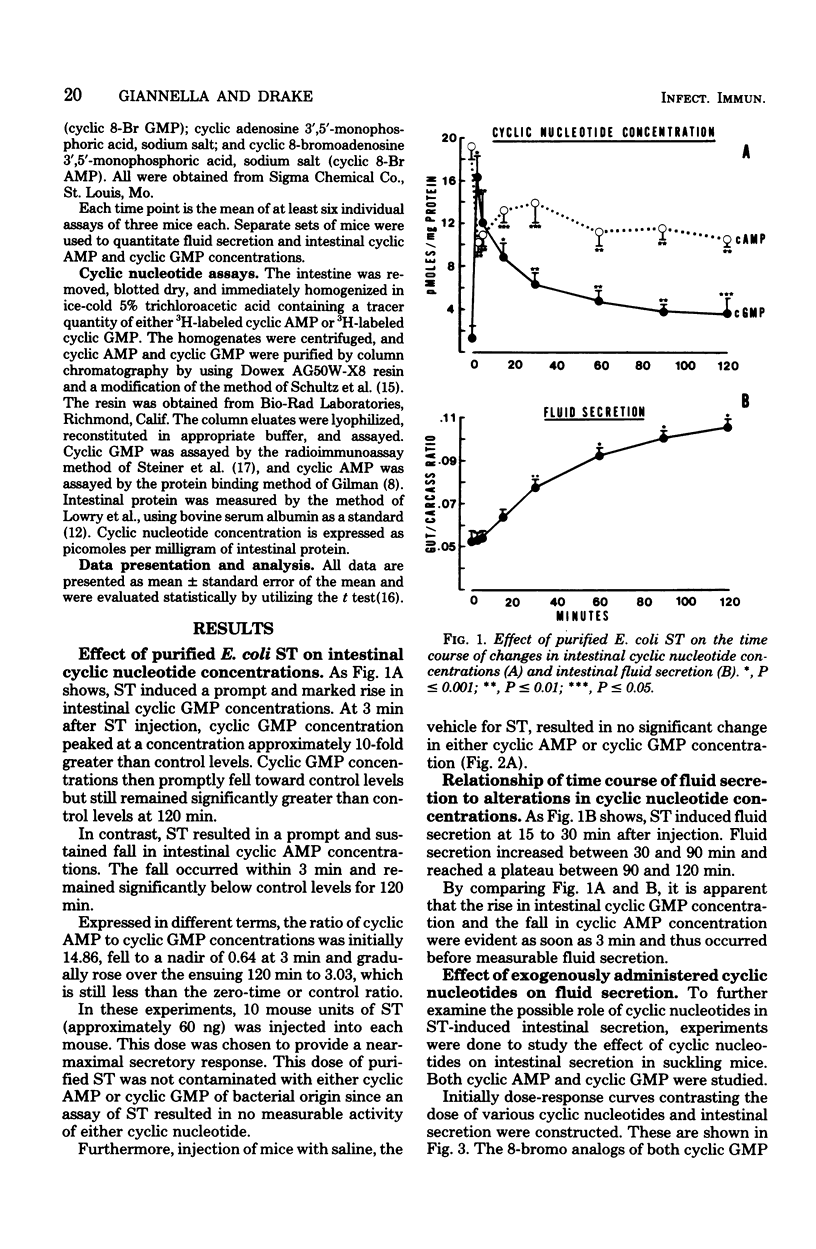
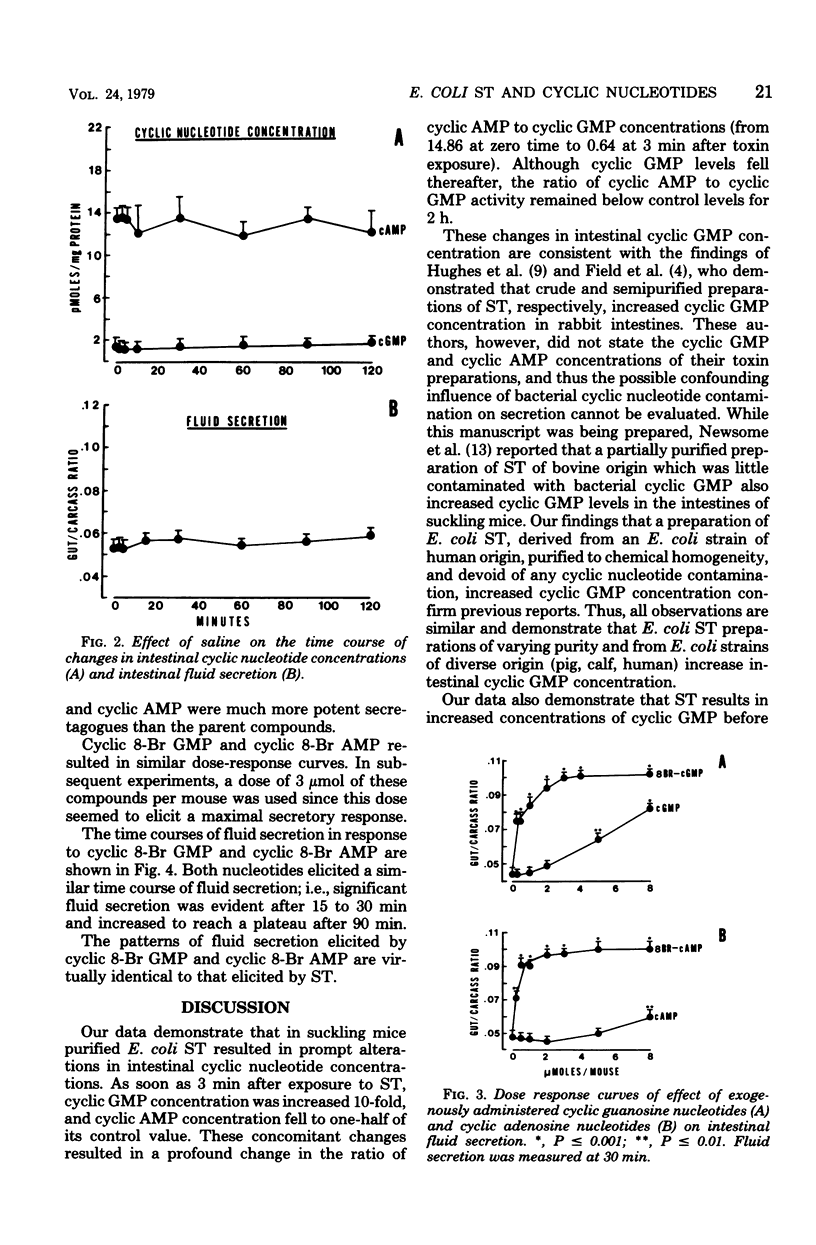
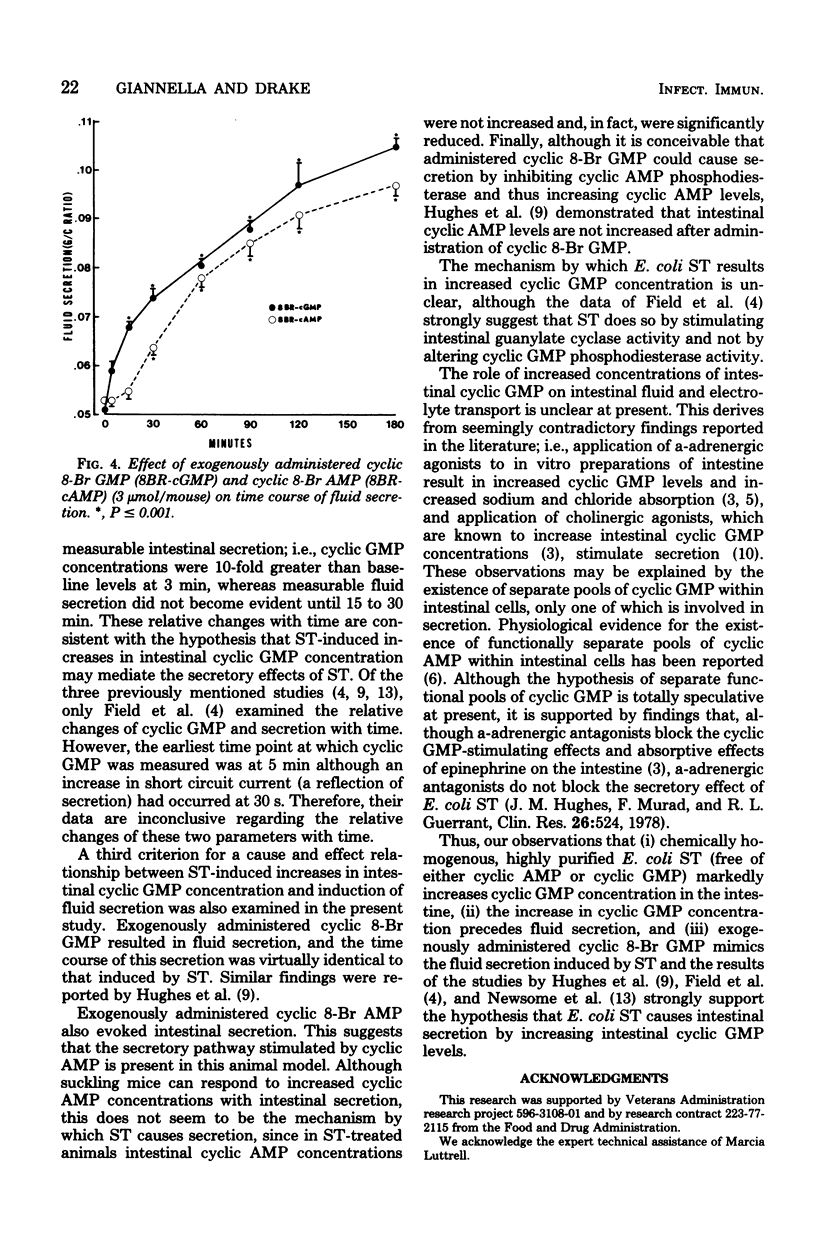
Enterotoxigenic Escherichia coli cause diarrhea by elaborating two enterotoxins. The large-molecular-weight, heat-labile toxin causes intestinal secretion by stimulating cyclic adenosine 5′-monophosphate production. The mechanism by which the small-molecular-weight, heat-stable enterotoxin induces secretion is unclear. The present study tested the hypothesis that heat-stable enterotoxin induces secretion by altering intestinal cyclic nucleotide concentrations. This was studied in suckling mice by using highly purified E. coli heat-stable enterotoxin obtained from a strain pathogenic for humans. At 3 min after administration of this toxin, intestinal cyclic guanosine 5′-monophosphate (GMP) levels were increased 10-fold. Cyclic GMP levels decreased thereafter, but still were greater than control levels at 120 min. Cyclic adenosine 5′-monophosphate levels fell to one-half of control levels at 3 min and remained below control levels for 120 min. When the time course of enterotoxin-induced secretion was compared with changes in cyclic GMP levels, fluid secretion was not evident until 15 to 30 min after enterotoxin administration. Thus, the increase in intestinal cyclic GMP concentration preceded measurable fluid secretion. And finally, administration of the 8-bromo analog of cyclic GMP evoked fluid secretion, the time course of which was similar to that induced by enterotoxin. These, and other data, strongly suggest that E. coli heat-stable enterotoxin induces intestinal secretion by increasing intestinal cyclic GMP levels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Robertson D. C. Purification and chemical characterization of the heat-stable enterotoxin produced by porcine strains of enterotoxigenic Escherichia coli. Infect Immun. 1978 Mar;19(3):1021–1030. doi: 10.1128/iai.19.3.1021-1030.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banwell J. G., Sherr H. Effect of bacterial enterotoxins on the gastrointestinal tract. Gastroenterology. 1973 Sep;65(3):467–497. [PubMed] [Google Scholar]
- Brasitus T. A., Field M., Kimberg D. V. Intestinal mucosal cyclic GMP: regulation and relation to ion transport. Am J Physiol. 1976 Jul;231(1):275–282. doi: 10.1152/ajplegacy.1976.231.1.275. [DOI] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., McColl I. Ion transport in rabbit ileal mucosa. 3. Effects of catecholamines. Am J Physiol. 1973 Oct;225(4):852–857. doi: 10.1152/ajplegacy.1973.225.4.852. [DOI] [PubMed] [Google Scholar]
- Field M., Sheerin H. E., Henderson A., Smith P. L. Catecholamine effects on cyclic AMP levels and ion secretion in rabbit ileal mucosa. Am J Physiol. 1975 Jul;229(1):86–92. doi: 10.1152/ajplegacy.1975.229.1.86. [DOI] [PubMed] [Google Scholar]
- Giannella R. A. Suckling mouse model for detection of heat-stable Escherichia coli enterotoxin: characteristics of the model. Infect Immun. 1976 Jul;14(1):95–99. doi: 10.1128/iai.14.1.95-99.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. M., Murad F., Chang B., Guerrant R. L. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature. 1978 Feb 23;271(5647):755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- Isaacs P. E., Corbett C. L., Riley A. K., Hawker P. C., Turnberg L. A. In vitro behavior of human intestinal mucosa. The influence of acetyl choline on ion transport. J Clin Invest. 1976 Sep;58(3):535–542. doi: 10.1172/JCI108498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T. M., Wu B. J. Biochemical properties of Escherichia coli low-molecular-weight, heat-stable enterotoxin. Infect Immun. 1974 Feb;9(2):342–347. doi: 10.1128/iai.9.2.342-347.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Newsome P. M., Burgess M. N., Mullan N. A. Effect of Escherichia coli heat-stable enterotoxin on cyclic GMP levels in mouse intestine. Infect Immun. 1978 Oct;22(1):290–291. doi: 10.1128/iai.22.1.290-291.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B. Human diarrheal disease caused by enterotoxigenic Escherichia coli. Annu Rev Microbiol. 1975;29:333–353. doi: 10.1146/annurev.mi.29.100175.002001. [DOI] [PubMed] [Google Scholar]
- Schultz G., Böhme E., Hardman J. G. Separation and purification of cyclic nucleotides by ion-exchange resin column chromatography. Methods Enzymol. 1974;38:9–20. doi: 10.1016/0076-6879(74)38005-6. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]



