Abstract
An efficient, step-economical, and scalable synthesis of a diene-bearing AB spiroketal fragment of spongistatin 1, and a demonstration of its efficient coupling to an aldehyde derived from silylformylation of a homopropargyl alcohol to produce the entire complex C(13)–C(17) linker region are described. The scalability of the synthesis of the AB spiroketal fragment was demonstrated by the preparation of 34.5 grams by one chemist in ~60 workdays, and more than 40 grams overall. With this material in hand and having established a method for its efficient coupling to the CD fragment, we have set the stage for the rapid synthesis and evaluation of a series of analogs of the CD spiroketal.
Introduction
Natural products – by virtue of their structural complexity and variety – provide a rich forum for reaction design and chemical invention and innovation. When they are possessed of truly extraordinary biological activity and at the same time are available in significant quantity only through total chemical synthesis, they provide much more than that, and it would be difficult to identify a natural product that more clearly exemplifies this than spongistatin 1. This extraordinarily complex and exceedingly precious anti-mitotic agent was first reported nearly simultaneously by three research groups in 1993,1–3 and has been reported to have an average IC50 value against the NCI panel of 60 human cancer cell lines of 0.12 nM.4 Seven research groups have reported syntheses of spongistatin 1 and/or 2,5–11 and, notably, the Smith team ultimately produced 1 gram of fully synthetic spongistatin 1.7c–e Despite all of this excellent and pioneering synthetic chemistry, the possibility of developing a synthesis that could deliver the kinds of amounts of spongistatin 1 or an analog thereof that will be needed for clinical development and beyond still seems quite remote. Thus, while the world does not need an eighth synthesis of one of the spongistatins, it certainly does need better chemotherapeutics, and one important part of addressing the extremely daunting challenge of turning spongistatin 1 or more likely a designed analog thereof into an effective cancer drug will be the development of significantly more efficient, step-economical, and scalable synthetic chemistry.
Study design
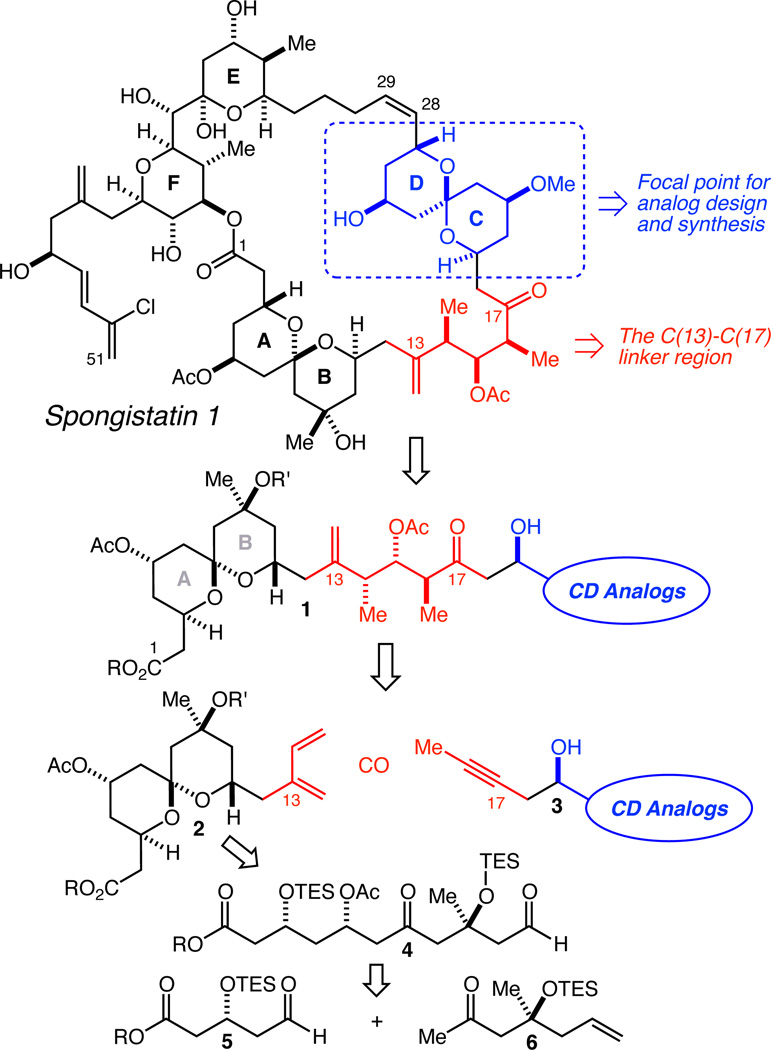
More recently, a University of Pennsylvania and Eisai team led by Smith demonstrated that the CD spiroketal is most likely not directly involved in the binding of spongistatin 1 to β-tubulin by the synthesis of a “diminutive congener” wherein the CD spiroketal and C(13)–C(17) linker region were replaced with a simple tether and the subsequent demonstration that significant anti-mitotic activity was retained, albeit with a reduction in potency.12 Inspired by this sophisticated and elegant work, and, in part, because the synthesis of the CD spiroketal has been one of the most difficult challenges in this arena, we have initiated a program whose ultimate long-term goal is the preparation and biological evaluation of a series of CD spiroketal-modified analogs of spongistatin 1 (Figure 1). In addition to an efficient synthesis and large supply of the “EF Half” of spongistatin 1 (C(29)–C(51), not shown), this would entail the synthesis of a series of ABCD fragments (1) with the targeted CD spiroketal analogs incorporated. Since we were and remain quite sober about the likely necessity of preparing many such analogs in order to have a reasonable chance at identifying one that retained the sub-nanomolar potency of the natural product while being significantly easier to synthesize, we were convinced that the only way this would be feasible would be to first develop new synthetic chemistry with two main objectives: 1) a highly efficient and step-economical method for the rapid union of the AB spiroketal with a variety of CD spiroketal analogs in a way that would directly establish the entire complex C(13)–C(17) linker region between the spiroketals from simple precursors, and 2) a significantly more step-economical and scalable synthesis of the AB spiroketal than any yet advanced that could then be used to synthesize a large enough supply to support all of the CD spiroketal analog work and beyond. For the former objective, we envisioned application of our recently reported complex fragment coupling by crotylation methodology13 in combination with the silylformylation/crotylation/Tamao oxidation/diastereo-selective tautomerization methodology.13,14 For the latter objective, we envisioned the use of another recently developed method, asymmetric aldehyde isoprenylation,13 with aldehyde 4 followed by spiroketalization to produce 2, and an aldol coupling of fragments 5 and 6 to produce 4. Herein we describe the development of a synthesis of an AB spiroketal of type 2 that has allowed us to produce a large supply, and a demonstration of its efficient coupling with a model aldehyde derived from a homopropargyl alcohol of type 3 to directly produce the entire C(13)–C(17) linker region.
Fig. 1.
Study design and retrosynthesis.
Results and discussion
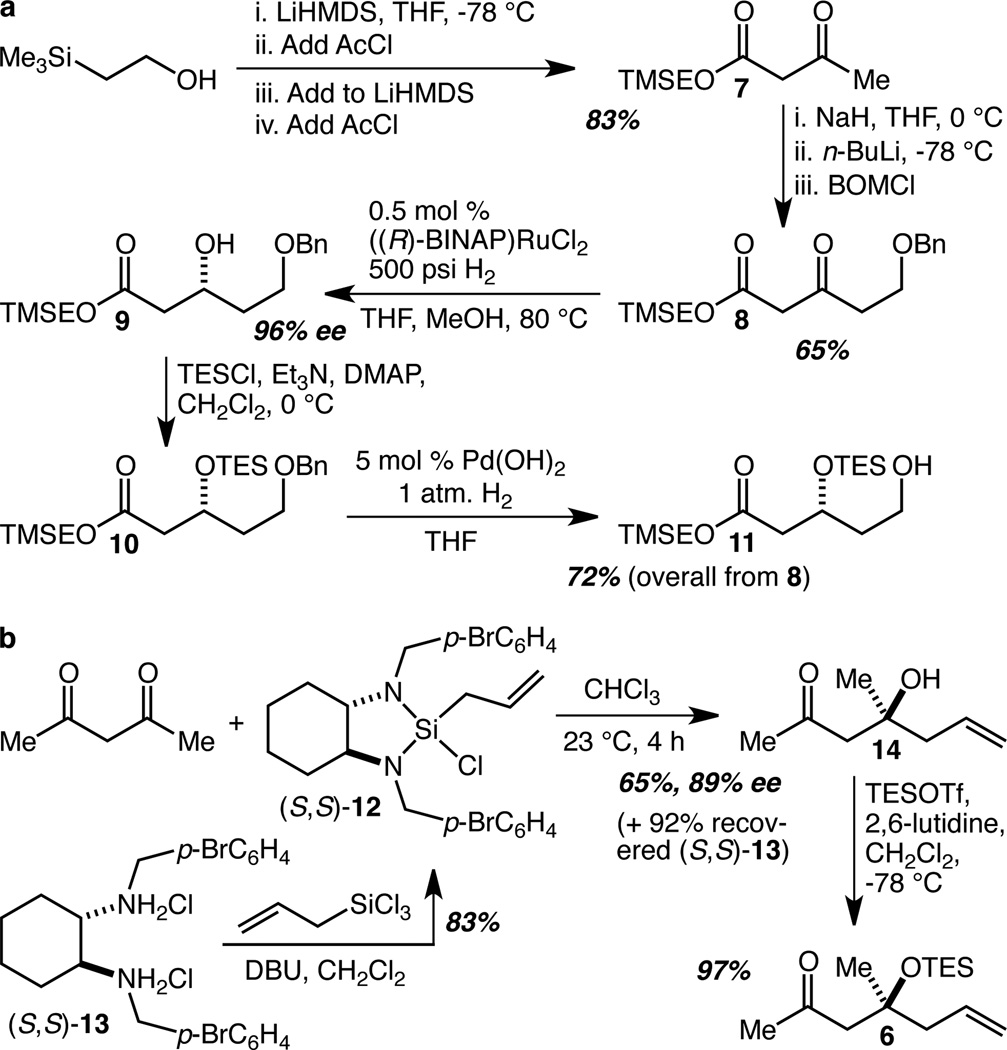
Our synthesis of the precursor to an aldehyde of type 5 commenced with conversion of 2-(trimethylsilyl)ethanol (TMSEOH) to β-ketoester 7 in 83% yield (Scheme 1a). The mixed Na/Li dienolate of 7 was alkylated with PhCH2OCH2Cl to give 8 in 65% yield (86% based on recovered 7).15 This set the stage for the Noyori hydrogenation16 which proceeded smoothly with 0.5 mol % of ((R)-BINAP)RuCl2 to give 9 in 96% enantiomeric excess (ee). After protection of the alcohol with triethylsilyl chloride (TESCl) to give 10, the benzyl (Bn) group was removed by hydrogenation, allowing the isolation of alcohol 11 in 72% overall yield from 8.
Scheme 1.
Synthesis of Fragments 5 and 6.
The synthesis of ketone 6 began with an application of our recently reported direct and enantioselective allylation of β-diketones,17 in this case acetylacetone (Scheme 1b). Allyl-silane (S,S)-12 (commercially available, and easily prepared on tens of grams scale from (S,S)-13 and allyltrichlorosilane) reacts smoothly with acetylacetone to provide 14. The only technical challenge we faced as we scaled this reaction up was the volatility of 14, which required the use of low boiling point solvents for the workup and purification. Once optimized, the reaction proved capable of delivering ~10 g (~70 mmol) of 14 in a single run in 65% yield and 89% ee. In addition, the diamine (S,S)-13 was recovered in 92% yield, and recycled. This reaction greatly simplifies the synthesis of the AB spiroketal. The ketone that is to be used in the aldol reaction and that becomes the spiroketal (see Scheme 2) is purchased in the form of a commodity chemical, acetylacetone, and is never protected or masked. At the same time the tertiary carbinol – one of the more challenging aspects of the target – is directly installed in a single step from acetylacetone. Protection of the tertiary alcohol as its TES ether proceeded smoothly in 97% yield, and provided the methyl ketone 6 in just two steps.
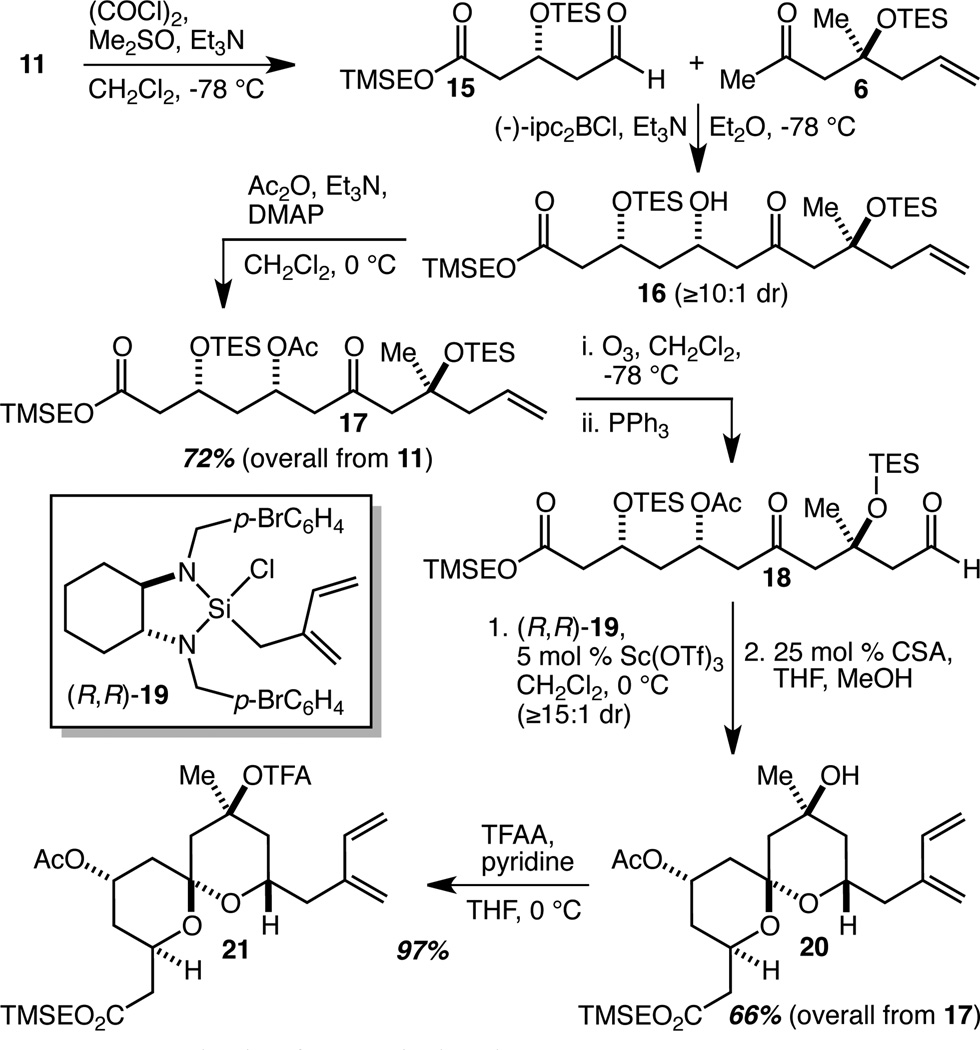
Scheme 2.
Synthesis of AB Spiroketal Fragment 21.
Swern oxidation18 of 11 provided access to aldehyde 15 and set the stage for the aldol coupling reaction (Scheme 2). For stereochemical induction, we turned to the use of a chiral boron enolate derived from (−)-B-chlorodiisopinocampheyl-borane ((−)-ipc2BCl), according to the procedure reported by Paterson.19 (−)-Ipc2BCl is inexpensive and its use on larger scales typically presents only one (indirect) technical problem: the oxidative workup creates two equivalents of ipcOH, which can be, and almost always is in practice, difficult to separate from the desired aldol product. In the present case, the aldol coupling of 6 with 15 proceeded smoothly to give 16 (with ≥10:1 diastereoselectivity as judged by 1H NMR spectroscopic analysis of the unpurified reaction mixture). Instead of struggling with a difficult separation at this stage, however, the mixture of 16 and ipcOH was simply acetylated. Separation of the desired product 17 from the ipcOAc was far more straightforward, and delivered 17 in 72% overall yield from alcohol 11. This 3-step sequence proved readily scalable, and indeed, could be and was used to prepare ~15 g of 17 per run. Ozonolysis of alkene 17 provided aldehyde 18 which was, without purification, subjected to an asymmetric isoprenylation reaction according to our recently reported procedure using (R,R)-19.13 The unpurified product (analysis of which by 1H NMR spectroscopy revealed a diastereoselectivity for the isoprenylation reaction of ≥15:1) was subjected to spiroketalization with camphorsulfonic acid (CSA), leading to the smooth production of 20 in 66% overall yield from 17. Finally, the liberated tertiary alcohol was trifluoroacetylated to give completed AB spiroketal fragment 21 in 97% yield.
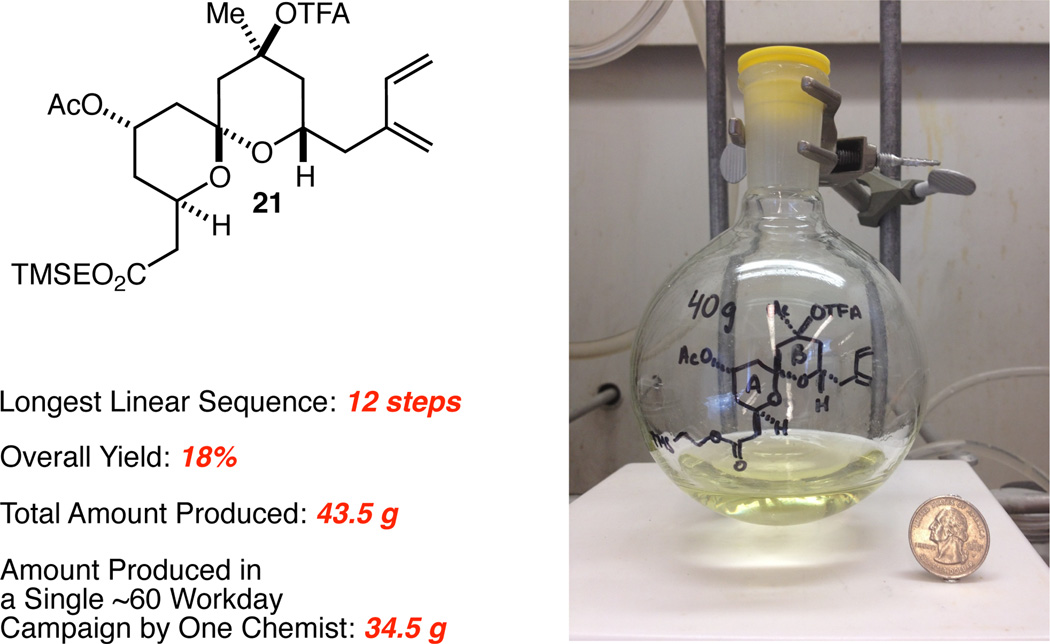
The synthesis of AB spiroketal 21 thus proceeds with a longest linear sequence of 12 steps from TMSEOH in 18% overall yield (Figure 2). Because 6 is accessed in only 2 steps, the total step count is only 14 steps (this count does not include the preparation of 12 and 19). This comprises by far the most step-economical synthesis of a fully configured AB spiroketal ready for coupling to the CD spiroketal fragment yet reported. While step-economy can be valuable in and of itself (for example for the more rapid production of analogs), it is not the same thing as scalability. The most useful and efficient syntheses will combine step-economy with true scalability, and while scalability can be easily claimed, it is, in the end, best demonstrated. The synthesis described has thus far been used to prepare 43.5 g of 21. Of course, even this is not particularly informative without an accompanying description of how much time and effort was required of how many chemists. In that regard, we can report that 34.5 g of 21 was prepared in a single campaign carried out entirely by one of us (S.K.R.) in ~60 workdays. It is perhaps not unreasonable to suggest based on this that the route could serve as the starting point for the development of a process that could deliver hundreds of grams or more of 21 by an industrial process chemistry team, should a clinical candidate ultimately emerge.
Fig. 2.
Analysis of synthetic efficiency for AB spiroketal 21.
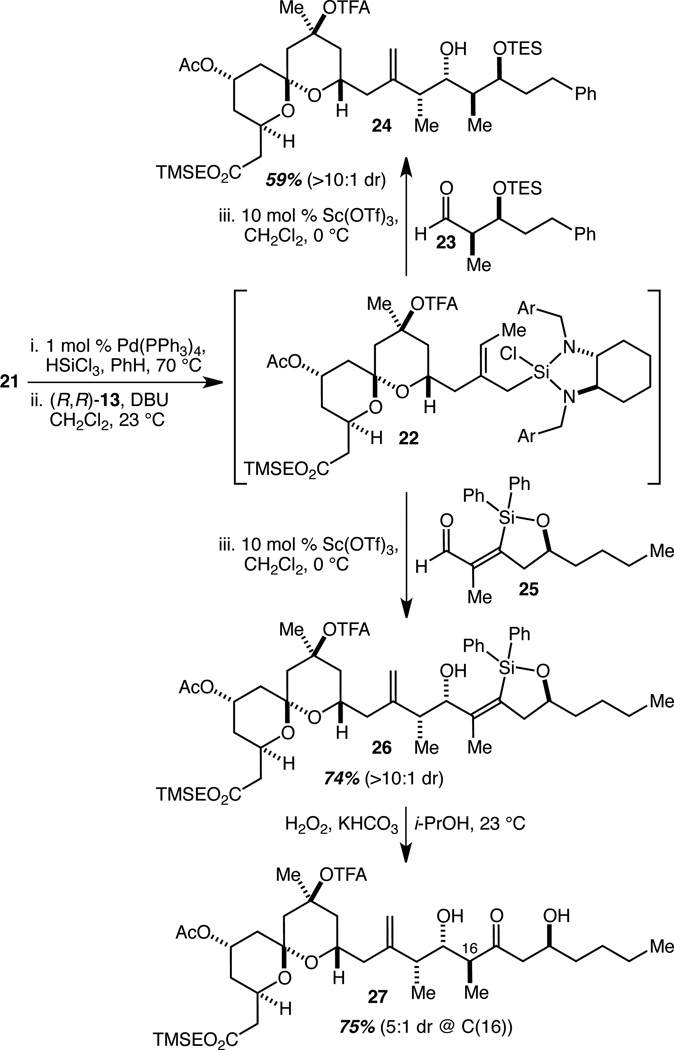
Of course, none of this will mean very much without an efficient method to both couple AB spiroketal fragment 21 to the CD spiroketal fragment or analogs thereof, and at the same time establish the entire complex C(13)–C(17) array of a 1,1-disubstituted alkene, three stereocenters, and a ketone that comprises the linking region between the spiroketals. We have reported the development of a fragment coupling by crotylation method13,20 that was designed to accomplish exactly that as demonstrated with simplified model dienes, and given such a large supply of 21, we thought it prudent to evaluate its performance in the fragment coupling process. In that sequence, the diene is subjected to a Pd(PPh3)4-catalyzed hydrosilylation reaction with HSiCl3 according to Tsuji’s protocol,21 and then to chiral diamine complexation. The resulting complex crotylsilane species is then reacted with an aldehyde partner in a Sc(OTf)3-catalyzed crotylsilylation reaction.22 We applied the first two operations to 21 to generate complex crotylsilane 22 (Scheme 3). This was split in half and used in the Sc(OTf)3-catalyzed crotylation reactions of two different aldehydes which contain the necessary functionality for the installation of the C(16) methyl-bearing stereocenter and the C(17) ketone. Aldehyde 23 represented the more conservative choice, and its crotylation reaction with 22 produced 24 in 59% yield and with >10:1 diastereoselectivity. We were at first concerned about the moderate efficiency of this reaction relative to the 73–79% yields that we had consistently achieved with model dienes,13 but quickly discovered that a significant amount of the product had undergone TES ether cleavage during the work-up (with n-Bu4NF•(H2O)3), a presumably fixable problem. Confirmation that diene 21 performs well was then provided by the fragment coupling crotylation reaction with aldehyde 25, which, as we have previously reported,13 arises very efficiently from the silylformylation of the corresponding homopropargylic alcohol (e.g. 3, Fig. 1). This reaction produced 26 in 74% yield and with >10:1 diastereoselectivity. Finally, subjection of 26 to our optimized conditions for a Tamao oxidation/diastereoselective tautomerization reaction13 resulted in the isolation of 27 as the major product of a 5:1 mixture of diastereomers (at C(16)) in 75% yield. These model fragment coupling transformations thus serve as convincing proofs of concept for the planned coupling of the AB spiroketal 21 with a variety of CD spiroketal analogs and concurrent establishment of the C(13)–C(17) linker region in a rapid and highly efficient fashion.
Scheme 3.
Fragment Coupling by Crotylation and Tamao Oxidation/Diastereoselective Tautomerization.
Conclusions
We have described the development of a highly step-economical and scalable synthesis of the AB spiroketal fragment of spongistatin 1. The scalability and the step-economy were demonstrated by the synthesis of 34.5 g of 21 by one chemist in a ~60 workday campaign. This unprecedented level of synthetic efficiency was achieved primarily through methodological innovations such as the β-diketone allylation, aldehyde isoprenylation, and fragment coupling by crotylation reactions that both facilitated efficient and scalable access to the AB spiroketal target and rendered the target simpler than the corresponding AB fragments used in other spongistatin syntheses. In addition, the suitability of this material for use in the fragment coupling by crotylation methodology was demonstrated in coupling reactions with two different model aldehydes. By taking the time to innovate and develop this chemistry, we think we have taken significant steps towards the ultimate goal of a synthesis of spongistatin 1 or an analog thereof that can deliver the kinds of amounts that would be needed for its clinical development. By leveraging the synthetic efficiency that derived from that innovation to build a “war chest” of more than 40 g of 21, and by developing the fragment coupling by crotylation chemistry we have further put ourselves in a strong position with regard to our ability rapidly to synthesize and evaluate a series of CD spiroketal analogs of the natural product, and subsequently and rapidly to produce significant quantities of any such analogs that showed promise for full pre-clinical evaluation. A correspondingly efficient and scalable synthesis of the EF fragment and some good ideas about which analogs to target are the other major pieces of the puzzle, and our efforts along those lines continue.
Supplementary Material
Acknowledgements
This work was supported by a grant from the National Institute of General Medical Sciences (GM58133).
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures and spectroscopic data. See DOI: 10.1039/b000000x/
Notes and references
- 1.Pettit GR, Cichacz ZA, Gao F, Herald CL, Boyd MR, Schmidt JM, Hooper JNA. J. Org. Chem. 1993;58:1302–1304. [Google Scholar]
- 2.Fusetani N, Shinoda K, Matsunaga S. J. Am. Chem. Soc. 1993;115:3977–3981. [Google Scholar]
- 3.(a) Kobayashi M, Aoki S, Sakai H, Kawazoe K, Kihara N, Sasaki T, Kitagawa I. Tetrahedron Lett. 1993;34:2795–2798. [Google Scholar]; (b) Kobayashi M, Aoki S, Sakai H, Kihara N, Sasaki T, Kitagawa I. Chem. Pharm. Bull. 1993;41:989–991. doi: 10.1248/cpb.41.989. [DOI] [PubMed] [Google Scholar]; (c) Kobayashi M, Aoki S, Kitagawa I. Tetrahedron Lett. 1994;35:1243–1246. [Google Scholar]
- 4.Boyd MR. In: Principles and Practices of Oncology Updates. 3. DeVita VT Jr, Hellman S, Rosenberg SA, editors. Vol. 10. 1994. pp. 1–12. [Google Scholar]
- 5.(a) Evans DA, Coleman PJ, Dias LC. Angew. Chem. Int. Ed. 1997;36:2738–2741. [Google Scholar]; (b) Evans DA, Trotter BW, Côté B, Coleman PJ. Angew. Chem. Int. Ed. 1997;36:2741–2744. [Google Scholar]; (c) Evans DA, Trotter BW, Côté B, Coleman PJ, Dias LC, Tyler AN. Angew. Chem. Int. Ed. 1997;36:2744–2747. [Google Scholar]; (d) Evans DA, Trotter BW, Coleman PJ, Côté B, Dias LC, Rajapakse HA, Tyler AN. Tetrahedron. 1999;55:8671–8726. [Google Scholar]
- 6.(a) Guo J, Duffy KJ, Stevens KL, Dalko PI, Roth RM, Hayward MM, Kishi Y. Angew. Chem. Int. Ed. 1998;37:187–192. [Google Scholar]; (b) Hayward MM, Roth RM, Duffy KJ, Dalko PI, Stevens KL, Guo J, Kishi Y Y. Angew. Chem. Int. Ed. 1998;37:192–196. [Google Scholar]
- 7.(a) Smith AB, III, Doughty VA, Lin Q, Zhuang L, McBriar MD, Boldi AM, Moser WH, Murase N, Nakayama K, Sobukawa M. Angew. Chem. Int. Ed. 2001;40:191–195. doi: 10.1002/1521-3773(20010105)40:1<191::AID-ANIE191>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]; (b) Smith AB, III, Lin Q, Doughty VA, Zhuang L, McBriar MD, Kerns JK, Brook CS, Murase N, Nakayama K. Angew. Chem. Int. Ed. 2001;40:196–199. [PubMed] [Google Scholar]; (c) Smith AB, III, Tomioka T, Risatti CA, Sperry JB, Sfouggatakis C. Org. Lett. 2008;10:4359–4362. doi: 10.1021/ol801792k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Smith AB, III, Lin Q, Doughty VA, Zhuang L, McBriar MD, Kerns JK, Boldi AM, Murase N, Moser WH, Brook CS, Bennett CS, Nakayama K, Sobukawa M, Trout REL. Tetrahedron. 2009;65:6470–6488. doi: 10.1016/j.tet.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Smith AB, III, Sfouggatakis C, Risatti CA, Sperry JB, Zhu W, Doughty VA, Tomioka T, Gotchev DB, Bennett CS, Sakamoto S, Atasoylu O, Shirakami S, Bauer D, Takeuchi M, Koyanagi J, Sakamoto Y. Tetrahedron. 2009;65:6489–6509. doi: 10.1016/j.tet.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Paterson I I, Chen DY-K, Coster MJ, Aceña JL, Bach J, Gibson KR, Keown LE, Oballa RM, Trieselmann T, Wallace DJ, Hodgson AP, Norcross RD. Angew. Chem. Int. Ed. 2001;40:4055–4060. [PubMed] [Google Scholar]; (b) Paterson I, Coster MJ, Chen DY-K, Oballa RM, Wallace DJ, Norcross RD. Org. Biomol. Chem. 2005;3:2399–2409. doi: 10.1039/b504146e. [DOI] [PubMed] [Google Scholar]; (c) Paterson I, Coster MJ, Chen DY-K, Gibson KR, Wallace DJ. Org. Biomol. Chem. 2005;3:2410–2419. doi: 10.1039/b504148a. [DOI] [PubMed] [Google Scholar]; (d) Paterson I, Coster MJ, Chen DY-K, Aceña JL, Bach J, Keown LE, Trieselmann T. Org. Biomol. Chem. 2005;3:2420–2430. doi: 10.1039/b504149j. [DOI] [PubMed] [Google Scholar]; (e) Paterson I, Chen DY-K, Coster MJ, Aceña JL, Bach J, Wallace DJ. Org. Biomol. Chem. 2005;3:2431–2440. doi: 10.1039/b504151a. [DOI] [PubMed] [Google Scholar]
- 9.Crimmins MT, Katz JD, Washburn DG, Allwein SP, McAtee LF. J. Am. Chem. Soc. 2002;124:5661–5663. doi: 10.1021/ja0262683. [DOI] [PubMed] [Google Scholar]
- 10.Heathcock CH, McLaughlin M, Medina J, Hubbs JL, Wallace GA, Scott R, Claffey MM, Hayes CJ, Ott GR. J. Am. Chem. Soc. 2003;125:12844–12849. doi: 10.1021/ja030317+. [DOI] [PubMed] [Google Scholar]
- 11.Ball M, Gaunt MJ, Hook DF, Jessiman AS, Kawahara S, Orsini P, Scolaro A, Talbot AC, Tanner HR, Yamanoi S, Ley SV. Angew. Chem. Int. Ed. 2005;44:5433–5438. doi: 10.1002/anie.200502008. [DOI] [PubMed] [Google Scholar]
- 12.Smith AB, III, Risatti CA, Atasoylu O, Bennet CS, Liu J, Cheng H, TenDyke K, Xu Q. J. Am. Chem. Soc. 2011;133:14042–14053. doi: 10.1021/ja2046167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reznik SK, Marcus BS, Leighton JL. Chem. Sci. 2012;3:3326–3330. doi: 10.1039/C2SC21325G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spletstoser JT, Zacuto MJ, Leighton JL. Org. Lett. 2008;10:5593–5596. doi: 10.1021/ol802489w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks DW, Kellogg RP, Cooper CS. J. Org. Chem. 1987;52:192–196. [Google Scholar]
- 16.Kitamura M, Ohkuma T, Inoue S, Sayo N, Kumobayashi H, Akutagawa S, Ohta T, Takaya H, Noyori R. J. Am. Chem. Soc. 1988;110:629–631. [Google Scholar]
- 17.Chalifoux WA, Reznik SK, Leighton JL. Nature. 2012;487:86–89. doi: 10.1038/nature11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mancuso AJ, Swern D. Synthesis. 1981:165–185. [Google Scholar]
- 19.Paterson I, Oballa RM, Norcross RD. Tetrahedron Lett. 1996;37:8581–8584. [Google Scholar]
- 20.Nakata and coworkers have also reported a fragment coupling by crotylation approach to the ABCD fragment of spongistatin 1. See: Terauchi T, Tanaka T, Terauchi T, Morita M, Kimijima K, Sato I, Shoji W, Nakamura Y, Tsukada T, Tsunoda T, Hayashi G, Kanoh N, Nakata M. Tetrahedron Lett. 2003;44:7747–7751.
- 21.Tsuji J, Hara M, Ohno K. Tetrahedron. 1974;30:2143–2146. [Google Scholar]
- 22.Kim H, Ho S, Leighton JL. J. Am. Chem. Soc. 2011;133:6517–6520. doi: 10.1021/ja200712f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.