Abstract
Importance
Many people meditate to reduce psychological stress and stress-related health problems. To counsel people appropriately, clinicians need to know what the evidence says about the health benefits of meditation.
Objective
To determine the efficacy of meditation programs in improving stress-related outcomes (anxiety, depression, stress/distress, positive mood, mental health quality of life, attention, substance use, eating, sleep, pain, and weight) in diverse adult clinical populations.
Evidence Review
We included randomized trials with active controls that controlled for placebo effects, identified through November 2012 from MEDLINE®, PsycINFO, EMBASE®, PsycArticles, SCOPUS, CINAHL, AMED, Cochrane Library, and hand searches. Independent reviewers screened citations and extracted data. We graded the strength of evidence using four domains (risk of bias, precision, directness, and consistency) and determined the magnitude and direction of effect by calculating the relative difference between groups in change from baseline. When possible, we conducted meta-analyses using standardized mean differences to obtain aggregate estimates of effect size (ES) with 95 percent confidence intervals (CI).
Findings
After reviewing 17,801 citations, we included 47 trials with 3,320 participants. Mindfulness meditation programs had moderate evidence to improve anxiety [ ES 0.38 (CI 0.12 to 0.64) at 8 weeks; ES 0.22 (0.02 to 0.43) at 3–6 months], depression [ES 0.30 (0.00 to 0.59) at 8 weeks; ES 0.23 (0.05 to 0.42) at 3–6 months] and pain [ES 0.33 (0.03 to 0.62)], and low evidence to improve stress/distress and mental health-related quality of life. We found either low evidence of no effect or insufficient evidence of any effect of meditation programs on positive mood, attention, substance use, eating, sleep, and weight. We found no evidence that meditation programs were better than any active treatment (drugs, exercise, other behavioral therapies).
Conclusions and Relevance
Clinicians should be aware that meditation programs can result in small to moderate reductions in multiple negative dimensions of psychological stress. Thus, clinicians should be prepared to talk with their patients about the role that a meditation program could have in addressing psychological stress. Stronger study designs are needed to determine the effects of meditation programs in improving positive dimensions of mental health and stress-related behavior.
Introduction
Many people use meditation to treat stress and stress-related conditions, as well as to promote general health.1, 2 To counsel people appropriately, clinicians need to know more about meditation programs and how they can affect health outcomes. Meditation training programs vary in several ways, including the type of mental activity promoted, amount of training, the use and qualifications of an instructor, and emphasis on religion or spirituality. Some meditative techniques are integrated into a broader alternative approach that includes dietary and/or movement therapies (e.g., ayurveda or yoga).
Meditative techniques are categorized as emphasizing “mindfulness,” “concentration,” and “automatic self-transcendence.” Popular techniques like transcendental meditation (TM) emphasize the use of a mantra in such a way that it transcends one to an effortless state where there is no focused attention.3–5 Other popular techniques, like mindfulness-based stress reduction (MBSR), emphasize training in present-focused awareness or “mindfulness”. Uncertainty remains about what these distinctions mean, and the extent to which these distinctions actually influence psychosocial stress outcomes.5, 6
Reviews to date report a small to moderate effect of both “mindfulness” and “mantra” meditation techniques in reducing emotional symptoms (e.g., anxiety, depression, and stress) and improving physical symptoms (e.g., pain).7–18, 19–26 These reviews have largely included both uncontrolled and controlled studies, and many of the controlled studies did not adequately control for placebo effects (e.g., wait-list or usual care controlled studies). Observational studies have a high risk of bias due to problems such as self-selection of interventions (people who believe in the benefits of meditation or who have prior experience with meditation are more likely to enroll in a meditation program and report that they benefited) and use of outcome measures that can be easily biased by participants’ beliefs in the benefits of meditation. Clinicians need to know whether there are beneficial effects of meditation training beyond self-selection biases and the non-specific effects of time, attention, and expectations for improvement. 27, 28
An informative analogy is the use of placebos in pharmaceutical trials. A placebo is typically designed to match non-specific aspects of the “active” intervention and thereby elicit the same expectations of benefit on the part of both provider and patient in the absence of the “active” ingredient. Office visits and patient-provider interactions, all of which influence expectations for outcome, are particularly important to control when the evaluation of outcome relies on patient reporting. In the situation where double blinding has not been feasible, the challenge to execute studies that are not biased by these nonspecific factors is more pressing.28 To develop evidence-based guidance on the use of meditation programs, we need to examine the specific effects of meditation in randomized controlled trials (RCTs) in which the non-specific aspects of the intervention are controlled.
The objectives of this systematic review are to evaluate the effects of meditation programs on negative affect (e.g. anxiety, stress) and positive affect (e.g. well-being), mental component of health-related quality of life, attention, health-related behaviors affected by stress (substance use, sleep, eating), pain, and weight, among those with a clinical condition. We include only RCTs that used one or more control groups in which the amount of time and attention provided by the control intervention was comparable to that of the meditation program.
Methods
Study Selection
We searched the following databases for primary studies: MEDLINE®, PsycINFO, EMBASE®, PsycArticles, SCOPUS, CINAHL, AMED, and The Cochrane Library through June, 2013. We developed a MEDLINE search strategy using PubMed® based on medical subject heading (MeSH®) terms and text words of key articles that we identified a priori. We used a similar strategy in the other electronic sources. We reviewed the reference lists of included articles, relevant review articles, and related systematic reviews to identify articles missed in the database searches. We did not impose any limits based on language or date of publication. The protocol for this systematic review is publicly available.29
Two trained investigators independently screened title and abstracts, excluding those that both investigators agreed met at least one of the exclusion criteria (Table 1). For those included after this first review, a second dual independent review of the full-text article occurred and differences regarding article inclusion were resolved through consensus.
Table 1.
Study inclusion and exclusion criteria
| Inclusion | Exclusion | |
|---|---|---|
| Population and Condition of Interest |
|
|
| Interventions | Structured meditation programs (any systematic or protocolized meditation programs that follow predetermined curricula) consisting of, at a minimum, at least 4 hours of training with instructions to practice outside the training session These include: Mindfulness-based:
Mantra-based:
Other meditation |
Meditation programs in which the meditation is not the foundation and majority of the intervention These include:
|
| Comparisons of Interest | Active control is defined as a program that is matched in time and attention to the intervention group for the purpose of matching expectations of benefit. Examples include “attention control,” “educational control,” or another therapy, such as progressive muscle relaxation, that the study compares to the intervention.
|
Studies that only evaluate a wait-list/usual-care control or do not include a comparison group |
| Study Design | RCTs with an active control | Non-randomized designs, such as observational studies. |
| Timing and Setting | Longitudinal studies that occur in general and clinical settings |
ACT = Acceptance and Commitment Therapy; DBT = Dialectical Behavioral Therapy; MBCT = Mindfulness-based Cognitive Therapy; MBSR = Mindfulness-based Stress Reduction; TM = Transcendental Meditation; RCT = Randomized Controlled Trials
We excluded articles with no original data (reviews, editorials, and comments), studies published in abstract form only, and dissertations.
We included RCTs in which the control group was matched in time and attention to the intervention group. We also required that studies include participants with a clinical condition. We defined a clinical condition broadly to include mental health/psychiatric conditions (e.g., anxiety or stress) and physical conditions (e.g., low back pain, heart disease, or advanced age). Additionally, since stress is of particular interest in meditation studies, we also included trials that studied stressed populations even though they may not have a defined medical or psychiatric diagnosis.
Data Abstraction and Data Management
We used Distiller SR (Evidence Partners, 2010) to manage the screening process. For each meditation program, we extracted information on measures of intervention fidelity including dose, training, and receipt of intervention. We recorded duration and maximal hours of structured training in meditation, amount of home practice recommended, description of instructor qualifications, and description of participant adherence, if any. Since there were numerous scales for the measures of negative or positive affect, we chose scales that were common to the other trials as well as most clinically relevant to make comparisons more meaningful.
To display outcome data, we calculated relative difference-in-change scores (i.e., the change from baseline in the treatment group minus the change from baseline in the control group, divided by the baseline score in the treatment group). We used the relative difference-in-change scores to estimate the direction and approximate magnitude of effect for all outcomes. We were unable calculate a relative difference-in-change score for six outcomes due to incompletely reported data for statistically insignificant findings. We considered a 5 percent relative difference-in-change score to be potentially clinically significant, since these studies were looking at short-term interventions and relatively low doses of meditation.
For the purpose of generating an aggregate quantitative estimate of the effect of an intervention and the associated 95 percent confidence interval, we performed random effects meta-analyses using standardized mean differences (effect sizes; Cohen’s d). We also used these to assess the precision of individual studies, which we factored into the overall strength of evidence. For each outcome, effect size estimates are displayed according to the type of control group and duration of followup. Trials did not give enough information to conduct a meta-analysis on 16 outcomes. We display the relative difference-in-change scores along with the effect size estimates from meta-analysis so that readers can see the full extent of the available data (Figure 1 and Appendix A).
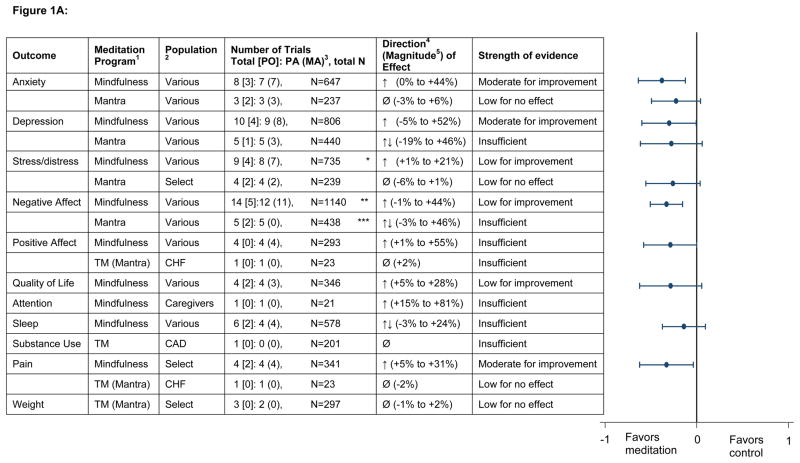
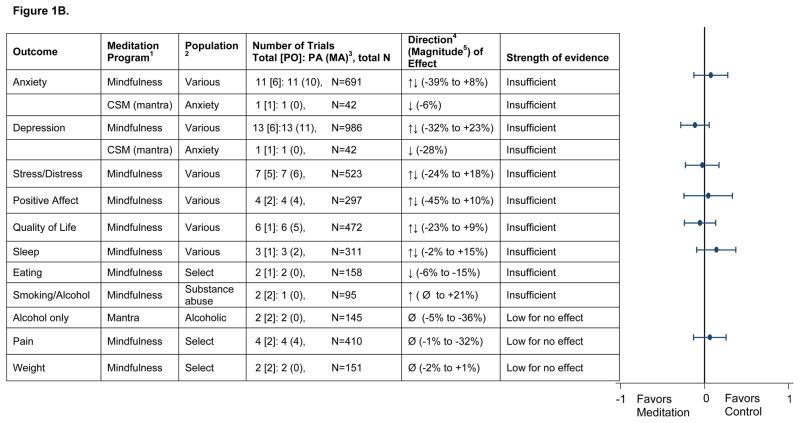
Figure 1.
Figure 1A: Summary across measurement domains of comparisons of meditation programs with non-specific active controls
Figure 1B. Summary across measurement domains of comparisons of meditation programs with specific active controls
- CSM = Clinically Standardized Meditation, a mantra meditation program; TM=Transcendental Meditation, a mantra meditation program
- CHF = Congestive Heart Failure; CAD = Coronary Artery Disease
- PO = Number of trials in which this was a primary outcome for the trial; PA = Primary Analysis; MA = Meta-analysis; N = sample size
-
Direction based on relative difference in change analysis:↑ indicates that the meditation group improved relative to the control group (with a relative difference generally greater than or equal to 5 percent across trials).↓ indicates the meditation group worsened relative to the control group (with a relative difference generally greater than or equal to 5 percent across trials).Ø indicates a null effect (with a relative difference generally less than 5 percent across trials).↑↓ indicates inconsistent findings (some trials reported improvement with meditation [relative to control] while others showed no improvement or improvement in the control group [relative to meditation]).
- Magnitude based on relative difference in the change score: This is a relative percent difference, using the baseline mean in the meditation group as the denominator. For example, if the meditation group improves from a 10 to 19 on a mental health scale and the control group improves from 11 to 16 on the same scale, the relative difference between groups in the change score is: (((19-10)-(16-11))/10)x100=40%. The interpretation is that there is a 40% relative improvement on the mental health scale in the meditation group compared with the control group. Improvement in all scales is indicated in the positive direction. A positive relative percent difference means that the score improved more in the intervention group than in the control group
Meta-analysis figure on far right shows Cohen’s d with the 95% CI
* Summary effect size not shown due to concern for publication bias for this outcome
**Negative affect combines the outcomes of anxiety, depression, stress/distress, and is thus duplicative of those outcomes
*** We did not perform meta-analysis on this outcome since it would duplicate the anxiety meta-analysis for mantra. Anxiety and depression are indirect measures of negative affect, and therefore resulted in a lower strength of evidence than for the outcome of mantra on anxiety.
We classified the type of control group as either a nonspecific active or specific active control (Table 1). Nonspecific active controls (e.g. education or attention control) control for the nonspecific effects of time, attention and expectation. Comparisons against these controls allow for assessments of the specific effectiveness of the meditation program above and beyond the nonspecific effects of time, attention, and expectation. This is similar to a comparison against a placebo pill in a drug trial. Specific active controls are therapies (e.g., exercise or progressive muscle relaxation) known or expected to change clinical outcomes. Comparisons against these controls allow for assessments of comparative effectiveness, similar to drug trials that compare one drug against another known drug. Since these study designs are expected to yield different conclusions (efficacy vs. comparative effectiveness), we separated them in our analyses.
Strength of the Body of Evidence
We assessed quality of the trials independently and in duplicate based on the recommendations in the Evidence-based Practice Center Methods Guide.30 We supplemented these tools with additional assessment questions based on the Cochrane Collaboration’s Risk of Bias Tool.31,32 Two reviewers graded the strength of evidence for each outcome using the grading scheme recommended by the Methods Guide for Conducting Comparative Effectiveness Reviews.33 This was followed by a discussion to review and achieve consensus on the assigned grades. In assigning evidence grades, we considered four domains: risk of bias, directness, consistency, and precision. We classified evidence into four basic categories: 1) “High” grade (indicating high confidence that the evidence reflects the true effect, and further research is very unlikely to change our confidence in the estimate of the effect); 2) “Moderate” grade (indicating moderate confidence that the evidence reflects the true effect, and further research may change our confidence in the estimate of the effect and may change the estimate); 3) “Low” grade (indicating low confidence that the evidence reflects the true effect, and further research is likely to change our confidence in the estimate of the effect and is likely to change the estimate); and 4) “Insufficient” grade (evidence is unavailable or inadequate to draw a conclusion).
Results
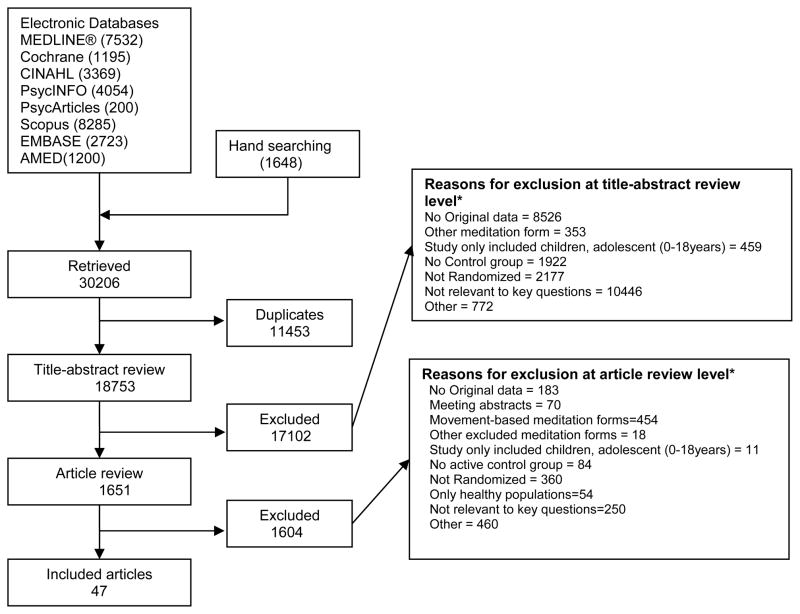
We screened 18,753 unique citations (Figure 2), and 1651 full-text articles. Forty seven trials met our inclusion criteria.34–80
Figure 2. Summary of the literature search.
* Total exceeds the number in the exclusion box because reviewers were allowed to mark more than 1 reason for exclusion
Most trials were short-term but ranged from 4 weeks to 9 years in duration (Table 2). Not all trials reported on amount of training or home practice recommended. MBSR programs typically provided 20–27.5 hours of training over 8 weeks. The other mindfulness meditation trials provided about half this amount. Transcendental meditation (TM) trials provided 16–39 hours over 3–12 months, while other mantra meditation programs provided about half this amount. Only five of the trials reported the trainers’ actual meditation experience (ranging between 4 months to 25 years) and six reported the trainers’ actual teaching experience (ranging between 0 and 15.7 years). Fifteen trials studied psychiatric populations including those with anxiety, depression, stress, chronic worry, and insomnia. Five trials studied smokers and alcoholics, 5 studied chronic pain populations, and 16 studied diverse medical populations including heart disease, lung disease, breast cancer, diabetes, hypertension, and HIV.
Table 2.
Study descriptions
| Author, year | Meditation Program |
Type of active control |
Study Quality |
Program Training (hrs) |
Home- work (hrs) |
Program duration (weeks), Study duration (months) |
Outcomes | Outcome at end of treatment |
Outcome at end of study |
Population | N |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Mindfulness Trials, Nonspecific Active Controls | |||||||||||
|
| |||||||||||
| Henderson, 201168 | MBSR | NSAC | Fair | 25 | ? | 8 weeks, 24 months | Anxiety | ns | ns | breast cancer | 100 |
| Depression |

|
↑ | |||||||||
| Positive affect |

|
⊘ | |||||||||
|
| |||||||||||
| Gaylord, 201143 | MBSR | NSAC | Fair | 23* | Y | 8 weeks, 3 months | Anxiety | ⊘ |

|
IBS | 75 |
| Depression | ⊘ | ⊘ | |||||||||
| Stress/Distress | ⊘ |

|
|||||||||
| Pain |

|

|
|||||||||
|
| |||||||||||
| Schmidt, 201064 | MBSR | NSAC | Fair | 27 | 42 | 8 weeks, 4 months | Anxiety | ⊘ |

|
fibromyalgia | 109 |
| Depression | ⊘ | ↑ | |||||||||
| Sleep | ⊘ | ⊘ | |||||||||
| Pain | ↑ | ⊘ | |||||||||
|
| |||||||||||
| Gross, 201044 | MBSR | NSAC | Fair | 27 | Y | 8 weeks, 6 months | Anxiety | ↑ | ↑ | organ transplant | 137 |
| Depression | ↑ | ↑ | |||||||||
| Positive affect | ⊘ | ↑ | |||||||||
| Mental QoL | ⊘ | ⊘ | |||||||||
| Sleep | ↑ |

|
|||||||||
| Pain | ⊘ | ⊘ | |||||||||
|
| |||||||||||
| Morone, 200955 | MBSR | NSAC | Good | 12 | 42 | 8 weeks, 6 months | Pain | ↑ | ⊘ | Low back pain | 35 |
|
| |||||||||||
| Whitebird, 201272 | MBSR | NSAC | Fair | 25 | 26.7 | 8 weeks, 6 months | Anxiety | ⊘ | ⊘ | dementia caregivers | 78 |
| Depression |

|
↑ | |||||||||
| Stress/Distress |

|

|
|||||||||
| Mental QoL |

|

|
|||||||||
|
| |||||||||||
| SeyedAlinaghi, 201267 | MBSR | NSAC | Poor | 25* | y | 8 weeks, 14 months | Stress/Distress | ↑ | ↓ | HIV | 171 |
|
| |||||||||||
| Pbert L, 201260 | MBSR | NSAC | Good | 26 | 24 | 8 weeks, 10 months | Stress/Distress | ↑ |

|
Asthmatics | 82 |
| Mental QoL | ↑ |

|
|||||||||
|
| |||||||||||
| Oken, 201058 | MM | NSAC | Fair | 9 | Y | 7 weeks | Depression | ↑ | dementia caregivers | 19 | |
| Stress/Distress | ↑ | ||||||||||
| Sleep | ⊘ | ||||||||||
|
| |||||||||||
| Garland, 201042 | MM | NSAC | Fair | ? | 17.5 | 10 weeks | Stress/Distress |

|
alcohol | 37 | |
|
| |||||||||||
| Mularski, 200956 | MM | NSAC | Poor | 8 | Y | 8 weeks | Stress/Distress | ⊘ | COPD | 49 | |
| Mental QoL | ↑ | ||||||||||
|
| |||||||||||
| Lee, 200650 | MM | NSAC | Fair | 8 | Y | 8 weeks | Anxiety |

|
anxiety | 41 | |
| Depression | ↑ | ||||||||||
|
| |||||||||||
| Malarkey, 201252 | MM | NSAC | Good | 9 | 18.5 | 8 weeks | Depression | ns | CRP>3.0 | 186 | |
| Stress/Distress | ns | ||||||||||
| Sleep | ns | ||||||||||
|
| |||||||||||
| Chiesa, 201239 | MBCT | NSAC | Fair | 16 | ? | 8 weeks | Anxiety | ↑ | depression | 18 | |
| Depression |

|
||||||||||
| Positive affect |

|
||||||||||
|
| |||||||||||
| Hoge, 201378 | MBSR | NSAC | Fair | 20 | 18.7 | 8 weeks | Anxiety |

|
anxiety | 89 | |
| Sleep |

|
||||||||||
|
| |||||||||||
| Nakamura, 201379 | MM | NSAC | Fair | 6 | ? | 3 weeks, 3 months | Depression | ⊘ | ↑ | Cancer & insomnia | 38 |
| Stress/Distress | ↑ | ↑ | |||||||||
| Positive affect | ⊘ | ⊘ | |||||||||
| Sleep | ↑ | ↑ | |||||||||
| Author, year | Meditation Program |
Type of active control |
Study Quality |
Program Training (hrs) |
Home- work (hrs) |
Program duration (weeks), Study duration (months) |
Outcomes | Outcome at end of treatment |
Outcome at end of study |
Population | N |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Mindfulness Trials, Specific Active Controls | |||||||||||
|
| |||||||||||
| Wong, 201174 | MBSR | Pain AC | Good | 27 | Y | 8 weeks, 6 months | Anxiety | ⊘ | ⊘ | chronic pain | 99 |
| Depression | ⊘ | ⊘ | |||||||||
| Mental QoL | ⊘ | ⊘ | |||||||||
| Pain | ⊘ | ⊘ | |||||||||
|
| |||||||||||
| Gross, 201145 | MBSR | drug | Fair | 26 | 36 | 8 weeks, 5 months | Anxiety | ⊘ | ↑ | insomnia | 27 |
| Depression | ↓ | ↓ | |||||||||
| Mental QoL | ⊘ | ||||||||||
| Sleep | ↑ | ⊘ | |||||||||
|
| |||||||||||
| Koszycki, 200771 | MBSR | CBGT | Poor | 27.5 | 28 | 8 weeks | Anxiety | ↓ | anxiety | 53 | |
| Depression | ⊘ | ||||||||||
|
| |||||||||||
| Barrett, 201234 | MBSR | Exercise | Fair | 20 | 42 | 8 weeks, 5 months | Anxiety | ⊘ | ⊘ | cold/URI in past year | 98 |
| Stress/Distress | ⊘ | ⊘ | |||||||||
| Positive affect | ⊘ | ⊘ | |||||||||
| Mental QoL | ⊘ | ⊘ | |||||||||
| Sleep | ⊘ | ⊘ | |||||||||
|
| |||||||||||
| Jazaieri, 201248 | MBSR | Exercise | Poor | 25 | 28.3 | 8 weeks, 5 months | Anxiety | ↑ | ⊘ | Social anxiety disorder | 56 |
| Depression | ↑ | ↑ | |||||||||
| Stress/Distress | ↑ | ||||||||||
| Positive affect | ↑ | ||||||||||
|
| |||||||||||
| Moritz, 200654 | MBSR | Spirituality | Good | 12* | Y | 8 weeks, 3 months | Anxiety |

|
mood disturbance (POMS) | 110 | |
| Depression | ↓ | ||||||||||
| Stress/Distress |

|
↓ | |||||||||
| Positive affect |

|
||||||||||
| Mental QoL |

|
↓ | |||||||||
| Pain | ↓ | ||||||||||
|
| |||||||||||
| Plews-Ogan, 200563 | MBSR | Massage | Poor | 20 | Y | 8 weeks, 3 months | Mental QoL | ↓ | ↑ | chronic pain | 23 |
| Pain | ↓ | ↓ | |||||||||
|
| |||||||||||
| Hebert, 200146 | MBSR | Nutrition Education | Fair | 45* | ? | 15 weeks, 12 months | Eating | ⊘ | ⊘ | breast cancer | 106 |
| Weight | ⊘ | ⊘ | |||||||||
|
| |||||||||||
| Philippot, 201161 | MBCT | Relaxation | Fair | 13.5 | Y | 6 weeks, 3 months | Anxiety | ↑ | ↑ | Tinnitus | 25 |
| Depression | ↑ | ⊘ | |||||||||
|
| |||||||||||
| Segal, 201066 | MBCT | drug | Good | 23* | Y | 8 weeks, 20 months | Depression | ↑ | depression | 84 | |
|
| |||||||||||
| Kuyken, 200849 | MBCT | drug | Good | 24* | 37.5 | 8 weeks, 15 months | Depression | ↑ | ↑ | depression | 123 |
| Mental QoL |

|

|
|||||||||
|
| |||||||||||
| Piet, 201062 | MBCT | CBGT | Fair | 16 | 28 | 8 weeks | Anxiety | ↓ | social phobia | 26 | |
| Depression | ↓ | ||||||||||
| Stress/Distress | ↓ | ||||||||||
|
| |||||||||||
| Delgado, 201040 | MM | PMR | Fair | 10 | Y | 5 weeks | Anxiety | ⊘ | worriers | 32 | |
| Depression | ↑ | ||||||||||
| Stress/Distress | ⊘ | ||||||||||
| Positive affect | ⊘ | ||||||||||
|
| |||||||||||
| Wolever, 201273 | MM | Viniyoga | Fair | 14 | ? | 12 weeks | Depression | ↑ | stressed employees | 186 | |
| Stress/Distress | ⊘ | ||||||||||
| Sleep | ⊘ | ||||||||||
| Pain | ↓ | ||||||||||
|
| |||||||||||
| Miller, 201253 | MM | Smart Choices | Poor | 25 | Y | 12 weeks, 6 months | Eating | ↓ | ↓ | diabetes | 52 |
| Weight | ⊘ | ⊘ | |||||||||
|
| |||||||||||
| Brewer, 201137 | MM | Lung Assoc FFS | Poor | 12 | Y | 4 weeks, 4 months | Smoking | ↑ |

|
smokers | 71 |
|
| |||||||||||
| Brewer, 200936 | MM | CBT | Poor | 9 | ? | 9 weeks | Alcohol | ⊘ | alcohol | 118 | |
|
| |||||||||||
| Arch, 201375 | MM | CBT | Fair | 18 | 29.2 | 10 weeks, 6 months | Anxiety | ⊘ | ↑ | anxiety | 105 |
| Depression | ↑ | ⊘ | |||||||||
|
| |||||||||||
| Omidi, 201380 | MBCT | CBT | Poor | 16 | 56 | 8 weeks | Anxiety | ↓ | depression | 60 | |
| Depression | ↓ | ||||||||||
|
| |||||||||||
| Ferraioli, 201377 | MM | SBPT | Poor | 16 | ? | 8 weeks, 5 months | Stress/Distress |

|

|
Stressed parents | 15 |
| Author, year | Meditation Program |
Type of active control |
Study Quality |
Program Training (hrs) |
Home- work (hrs) |
Program duration (weeks), Study duration (months) |
Outcomes | Outcome at end of treatment |
Outcome at end of study |
Population | N |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Mantra Trials, Non-Specific Active Controls | |||||||||||
|
| |||||||||||
| Paul-Labrador, 200659 | TM | NSAC | Good | 39 | Y | 16 weeks | Anxiety | ⊘ | CAD | 103 | |
| Depression | ↓ | ||||||||||
| Stress/Distress | ↓ | ||||||||||
|
| |||||||||||
| Jayadevappa, 200747 | TM | NSAC | Good | 22.5* | 90 | 12 weeks, 6 months | Depression | ↑ | ↑ | CHF | 23 |
| Stress/Distress | ⊘ | ⊘ | |||||||||
| Positive affect | ⊘ | ⊘ | |||||||||
| Pain | ⊘ | ↑ | |||||||||
|
| |||||||||||
| Schneider, 201265 | TM | NSAC | Good | ~78* | 1310 | 12 weeks, 5.4 yrs | Depression | ↑ | CAD | 201 | |
| Weight | ns | ||||||||||
|
| |||||||||||
| Smith, 197669 | TM | NSAC | Poor | ? | 87.5 | 4 weeks, 6 months | Anxiety | ⊘ | anxious people | 41 | |
|
| |||||||||||
| Elder, 200641 | TM | NSAC | Fair | ? | 90 | ? | Weight | ⊘ | diabetes | 54 | |
|
| |||||||||||
| Castillo-Richmond, 200038,38 | TM | NSAC | Poor | ? | 120.6 | 12 weeks | Weight | ⊘ | hypertensive AA | 60 | |
|
| |||||||||||
| Chattre, 201376 | TM | NSAC | Fair | 24 | 112 | 12 weeks, 6 months | Depression | ↑ | HIV | 20 | |
| Stress/Distress | ↑ | ||||||||||
|
| |||||||||||
| Bormann, 200635 | Mantra | NSAC | Fair | 7.5 | Y | 10 weeks, 6 months | Anxiety | ↑ | ⊘ | HIV | 93 |
| Depression | ⊘ | ↓ | |||||||||
| Stress/Distress | ⊘ | ⊘ | |||||||||
| Author, year | Meditation Program |
Type of active control |
Study Quality |
Program Training (hrs) |
Home- work (hrs) |
Program duration (weeks), Study duration (months) |
Outcomes | Outcome at end of treatment |
Outcome at end of study |
Population | N |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Mantra Trials, Specific Active Controls | |||||||||||
|
| |||||||||||
| Taub, 199470 | TM | Biofeedbck | Fair | 19 | ? | 4 weeks | Alcohol | ⊘ | alcohol | 118 | |
|
| |||||||||||
| Lehrer, 198351 | CSM | PMR | Fair | 7.5 | y | 5 weeks, 6 months | Anxiety | ⊘ | ↓ | anxiety | 42 |
| Depression | ↓ | ||||||||||
|
| |||||||||||
| Murphy, 198657 | CSM | running | Poor | 8 | 37.5 | 8 weeks | Alcohol |

|
alcohol | 27 | |
=estimated; Ø=no effect (within + or − 5%);
 = improved and statistically significant; ↑= favors medita on > 5% but non significant; ↓=favors control > 5% but non significant;
= improved and statistically significant; ↑= favors medita on > 5% but non significant; ↓=favors control > 5% but non significant;
 = worsened & statistically significant; ?= unclear; Y= yes, homework was prescribed but amount not specified; ns= not significant, not reported
= worsened & statistically significant; ?= unclear; Y= yes, homework was prescribed but amount not specified; ns= not significant, not reported
MBSR = mindfulness-based stress reduction; MM = mindfulness meditation; MBCT = mindfulness-based cognitive therapy; TM = transcendental meditation; CSM = clinically standardized meditation
Mental QoL = Mental component of health-related quality of life; COPD = chronic obstructive pulmonary disease; FFS = Freedom From Smoking program; HIV = Human Immunodeficiency Virus; AA = African Americans; NSAC = Nonspecific active control; IBS = irritable bowel syndrome; PMR = progressive muscle relaxation; CBGT = cognitive behavioral group therapy; Pain AC = pain active control; Risk of Bias: L=low, M= Medium, H=high; SBPT = Skills-Based Parent Training program
The strength of evidence on the outcomes is shown in Figures 1A and 1B. We found it difficult to draw comparative effectiveness conclusions due to the large heterogeneity of type and strength of the many comparators. Therefore, we present our results first for all the comparisons with non-specific active controls (efficacy), and then for the specific active controls (comparative effectiveness).
The direction and magnitude of effect is derived from the relative difference between groups in the change score. In our efficacy analysis (Fig 1A), we found low evidence of no effect or insufficient evidence that mantra meditation programs had an effect on any of the psychological stress and well-being outcomes we examined. Mindfulness meditation programs had moderate evidence to improve anxiety [effect size (ES) = 0.38 (0.12 to 0.64) at 8 weeks; ES = 0.22 (.02 to .43) at 3–6 months], depression [ES = 0.30 (0.00 to +0.59) at 8 weeks; ES = 0.23 (.05 to .42) at 3–6 months] and pain [ES = 0.33 (.03 to .62)], and low evidence to improve stress/distress and mental health-related quality of life. We found either low evidence of no effect or insufficient evidence of an effect of meditation programs on positive mood, attention, sleep and weight. We also found insufficient evidence that meditation programs had an effect on health-related behaviors affected by stress including substance use and sleep.
In our comparative effectiveness analyses (Figure 1B), we found low evidence of no effect or insufficient evidence that any of the meditation programs were more effective than exercise, progressive muscle relaxation, cognitive-behavioral group therapy, or other specific comparators in changing any outcomes of interest.
Few trials reported on potential harms of meditation programs. Of the nine trials reporting this information, none reported any harms of the intervention.
Assessment of Potential Publication Bias
We could not conduct any quantitative tests (e.g., funnel plots) for publication bias since few studies were available for most outcomes and many were excluded from the meta-analysis due to missing data. We reviewed the clinicaltrials.gov registration database to identify trials completed three or more years ago that prespecified our outcomes of interest and did not publish at all or did not publish all prespecified outcomes. We found five trials that appeared to have been completed before Jan 1, 2010 that did not publish all the outcomes they had prespecified, and found nine trials for which we could not find an associated publication. Since only six outcomes were excluded from the analyses of the relative difference-in-change scores between groups, whereas 16 outcomes were excluded from the meta-analyses, our findings from the primary analyses are less likely to be affected by publication bias than the meta-analyses.
Discussion
Our review indicates that meditation programs can reduce negative dimensions of psychological stress. Mindfulness meditation programs, in particular, show small improvements in anxiety, depression, and pain with moderate evidence, and small improvements in stress/distress and the mental health component of health-related quality of life with low evidence when compared to nonspecific active controls. Mantra meditation programs did not improve any of the outcomes examined, but the strength of this evidence varied from low to insufficient. While meditation programs generally seek to improve the positive dimensions of health, the evidence from a small number of studies did not show any effects on positive affect or well-being for any meditation program. We found no evidence for any harms of meditation programs, although few trials reported on harms. A strength of our review is the focus on RCTs with active controls, which should give clinicians greater confidence that the reported benefits are not due to nonspecific effects (e.g., attention and expectations) as seen in trials using a wait-list or usual care control.
Anxiety, depression and stress/distress are different components of negative affect. When we combined each component of negative affect, we saw a small and consistent signal that any domain of negative affect is improved in mindfulness programs when compared with a non-specific active control. The effect sizes were small, yet significant for some of these individual outcomes, and seen over a broad range of clinical conditions as shown in Table 2. Over the course of 2–6 months, mindfulness meditation program effect size estimates ranged from 0.22–0.38 for anxiety symptoms and 0.23–0.30 for depressive symptoms. These small effects are comparable with what would be expected from the use of an antidepressant in a primary care population, without the associated toxicities. In a study using patient-level meta-analysis, Fournier et al. found that for patients with mild to moderate depressive symptoms, antidepressants had an effect size of 0.11 (−0.18, +0.41), while those with severe depression had an effect size of 0.17 (−0.08, +0.43) compared with placebo.81
Among the nine RCTs evaluating the effect on pain, we found moderate evidence that MBSR reduces pain severity to a small degree when compared with a nonspecific active control, yielding an effect size of 0.33 from the meta-analysis. This effect is variable across painful conditions and is based on four trials, of which two were conducted in musculoskeletal pain patients, one in patients with irritable bowel syndrome, and one in a non-pain population. Visceral pain had a large and statistically significant relative 30 percent improvement in pain severity, while musculoskeletal pain showed 5–8 percent improvements that were considered nonsignificant.
Overall, the evidence was insufficient to indicate that meditation programs alter health-related behaviors affected by stress, and the evidence was low to suggest that meditation programs do not influence weight. While uncontrolled studies have usually found a benefit of meditation, very few controlled studies have found a similar benefit for the effects of meditation programs on health-related behaviors affected by stress.17–19
In the 20 RCTs examining comparative effectiveness, mindfulness and mantra programs did not show significant effects when the comparator was a known treatment or therapy. A lack of statistically significant superiority compared to a specific active control (e.g., exercise) only addresses the question of equivalency or non-inferiority if the trial was suitably powered to detect any difference. Sample sizes in the comparative effectiveness trials were small (average size of 37 per group), and none appeared adequately powered to assess noninferiority or equivalence.
A number of observations provide context to our conclusions. First, very few mantra meditation programs met our inclusion criteria. This significantly limited our ability to draw inferences about the effects of mantra meditation programs on psychological stress-related outcomes, which did not change when we evaluated TM separately from other mantra training.
Second, there may be differences between trials for which the outcomes are a primary versus secondary focus, although we did not find any evidence for this. The samples included in these trials resembled a general primary care population, and there may not be room to measure an effect if symptom levels of the outcomes were low to start with (i.e., a “floor” effect). This may explain the null results for mantra meditation programs, since three TM trials enrolled cardiac patients, while only one enrolled anxiety patients.
Third, the lack of effect on stress-related outcomes may relate to the way the research community conceptualizes meditation programs, the challenges in acquiring such skills or meditative states, and the limited duration of RCTs. Historically meditation was not conceptualized as an expedient therapy for health problems.3,6,82 It was a skill or state one learns and practices over time to increase one’s awareness and through this awareness gain insight and understanding into the various subtleties of their existence. Training the mind in awareness, in nonjudgmental states, or in the ability to become completely free of thoughts or other activity are daunting accomplishments. The interest in meditation that has grown over the past 30 years in Western cultures comes out of Eastern traditions that emphasize life-long growth. The translation of these traditions into research studies remains challenging. Long-term trials may be optimal to examine the impact of meditation on many health outcomes, such as those that have evaluated mortality.65 However, many of the studies included in this review were short-term (e.g., 2.5 hours a week for 8 weeks) and the participants likely did not achieve a level of expertise needed to improve outcomes that depend on mastery of mental and emotional processes.
Finally, none of our conclusions yielded a high SOE grade for a positive or null effect. Thus, further studies in primary care and disease-specific populations are indicated to address uncertainties caused by inconsistencies in the body of evidence, deficiencies in power, and risk of bias.
Limitations
Some of the trials we reviewed were implemented before modern standards for clinical trials were established. Thus, many did not report key design characteristics to enable an accurate assessment of the risk of bias. Most trials were not registered, did not standardize training using trainers who met specified criteria, did not specify primary and secondary outcomes a priori, did not power the trial based on the primary outcomes, did not use CONSORT recommendations for reporting results, or did not operationalize and measure the practice of meditation by study participants.83
We could not draw definitive conclusions about effect modifiers such as dose and duration of training because of the limited details provided in the publications of the trials. Despite our focus on RCTs using active controls, we were unable to detect a specific effect of meditation on most outcomes, with the majority of our evidence grades being insufficient or low. These evidence grades were mostly driven by two important evaluation criteria: the quality of the trial and inconsistencies in the body of evidence. Trials suffered primarily from four biases: lack of blinding of outcome assessment, high attrition, lack of allocation concealment, and lack of intention to treat analysis. The reasons for inconsistencies in the body of evidence may have included the differences in the particular clinical conditions, as well as the type of control groups studies used. Another possibility is that the programs had no real effect on many of the outcomes that had inconsistent findings.
Clinical Implications and Future Directions
Despite the limitations of the literature, the evidence suggests that mindfulness meditation programs could help reduce anxiety, depression and pain in some clinical populations. Thus, clinicians should be prepared to talk with their patients about the role that a meditation program could have in addressing psychological stress.
Future research in meditation would benefit by addressing methodological and conceptual issues that remain. All forms of meditation, including both mindfulness and mantra, imply that more time spent meditating will yield larger effects. Most forms, but not all, also present meditation as a skill that requires expert instruction and time dedicated to practice. Thus, more training with an expert and practice in daily life should lead to greater competency in the skill or practice, and greater competency or practice would presumably lead to better outcomes. However, when compared with other skills that require training, such as writing, the amount of training or “dose” afforded in the trials was quite small and generally the training was offered over a fairly short period of time. These three components – trainer expertise, amount of practice, and skill – require further investigation. We were unable to examine the extent to which trainer expertise influences clinical outcome, since teacher qualifications were not reported in detail in most trials. Trials need to document the amount of training instructors provide and patients receive and the amount of home practice patients complete. This will allow future investigators to examine questions about “dosing” related to outcome.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the support of our AHRQ Task Order Officer, Shilpa H. Amin, M.D. We extend our appreciation to our Key Informants and members of our Technical Expert Panel, all of whom provided thoughtful advice and input during our research process.
The EPC thanks Swaroop Vedula for conducting meta-analysis and assisting with their interpretation. The EPC also thanks Shonali Saha, Manisha Reuben, Deepa Pawar, Oluwaseun Shogbesan and Yohalakshmi Chelladurai for their contributions to this project.
Funding Source: Agency for Healthcare Research and Quality (AHRQ) HHSA 290 2007 10061I
Footnotes
Financial Disclosure Statement
None of the investigators has any affiliations or financial involvement that conflicts with the material presented in this report.
Disclosure: The authors of this article are responsible for its contents, including any clinical or treatment recommendations. No statement in this article should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services.
Prepared by:
Johns Hopkins University Evidence-based Practice Center, Baltimore, MD.
All authors had full access to all the data. Dr. Goyal takes full responsibility for the completeness and integrity of the data.
Reference List
- 1.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;(12):1–23. [PubMed] [Google Scholar]
- 2.Goyal M, Haythornthwaite J, Levine D, et al. Intensive meditation for refractory pain and symptoms. J Altern Complement Med. 2010;16(6):627–31. doi: 10.1089/acm.2009.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rapgay L, Bystrisky A. Classical mindfulness: an introduction to its theory and practice for clinical application. Ann N Y Acad Sci. 2009;1172:148–62. doi: 10.1111/j.1749-6632.2009.04405.x. [DOI] [PubMed] [Google Scholar]
- 4.Travis F, Shear J. Focused attention, open monitoring and automatic self-transcending: Categories to organize meditations from Vedic, Buddhist and Chinese traditions. Conscious Cogn. 2010 Dec;19(4):1110–8. doi: 10.1016/j.concog.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Chiesa A, Malinowski P. Mindfulness-based approaches: are they all the same? J Clin Psychol. 2011;67 (4):404–24. doi: 10.1002/jclp.20776. [DOI] [PubMed] [Google Scholar]
- 6.Sedlmeier P, Eberth J, Schwarz M, Zimmermann D, Haarig F, Jaeger S, Kunze S. The psychological effects of meditation: a meta-analysis. Psychol Bull. 2012;138(6):1139–71. doi: 10.1037/a0028168. [DOI] [PubMed] [Google Scholar]
- 7.Bohlmeijer E, Prenger R, Taal E, Cuijpers P. The effects of mindfulness-based stress reduction therapy on mental health of adults with a chronic medical disease: a meta-analysis. J Psychosom Res. 2010;68(6):539–44. doi: 10.1016/j.jpsychores.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Chambers R, Gullone E, Allen NB. Mindful emotion regulation: An integrative review. Clin Psychol Rev. 2009;29(6):560–72. doi: 10.1016/j.cpr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Chiesa A, Serretti A. Mindfulness-based stress reduction for stress management in healthy people: a review and meta-analysis. J Altern Complement Med. 2009;15(5):593–600. doi: 10.1089/acm.2008.0495. [DOI] [PubMed] [Google Scholar]
- 10.Chiesa A, Calati R, Serretti A. Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clin Psychol Rev. 2011;31(3):449–64. doi: 10.1016/j.cpr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Chiesa A, Serretti A. Mindfulness based cognitive therapy for psychiatric disorders: a systematic review and meta-analysis. Psychiatry Res. 2011;187(3):441–53. doi: 10.1016/j.psychres.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann SGSAWAOD. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J Consult Clin Psychol. 2010;78(2):169–83. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krisanaprakornkit T, Ngamjarus C, Witoonchart C, Piyavhatkul N. Meditation therapies for attention-deficit/hyperactivity disorder (ADHD) Cochrane Database Syst Rev. 2010;(6):CD006507. doi: 10.1002/14651858.CD006507.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ledesma D, Kumano H. Mindfulness-based stress reduction and cancer: a meta-analysis. Psychooncology. 2009;18(6):571–9. doi: 10.1002/pon.1400. [DOI] [PubMed] [Google Scholar]
- 15.Matchim Y, Armer JM, Stewart BR. Mindfulness-based stress reduction among breast cancer survivors: a literature review and discussion. Oncol Nurs Forum. 2011;38(2):E61–71. doi: 10.1188/11.ONF.E61-E71. [DOI] [PubMed] [Google Scholar]
- 16.Piet J, Hougaard E. The effect of mindfulness-based cognitive therapy for prevention of relapse in recurrent major depressive disorder: a systematic review and meta-analysis. Clin Psychol Rev. 2011;31(6):1032–40. doi: 10.1016/j.cpr.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Wanden-Berghe RG, Sanz-Valero J, Wanden-Berghe C. The application of mindfulness to eating disorders treatment: a systematic review. Eat Disord. 2011;19(1):34–48. doi: 10.1080/10640266.2011.533604. [DOI] [PubMed] [Google Scholar]
- 18.Winbush NY, Gross CR, Kreitzer MJ. The effects of mindfulness-based stress reduction on sleep disturbance: a systematic review. Explore (NY) 2007;3(6):585–91. doi: 10.1016/j.explore.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Zgierska A, Rabago D, Chawla N, Kushner K, Koehler R, Marlatt A. Mindfulness meditation for substance use disorders: a systematic review. Subst Abus. 2009;30(4):266–94. doi: 10.1080/08897070903250019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernardy K, Fuber N, Kollner V, Hauser W. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome - a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37(10):1991–2005. doi: 10.3899/jrheum.100104. [DOI] [PubMed] [Google Scholar]
- 21.Rainforth MV, Schneider RH, Nidich SI, Gaylord-King C, Salerno JW, Anderson JW. Stress reduction programs in patients with elevated blood pressure: a systematic review and meta-analysis. Curr Hypertens Rep. 2007;9(6):520–8. doi: 10.1007/s11906-007-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson JW, Liu C, Kryscio RJ. Blood pressure response to transcendental meditation: a meta-analysis. Am J Hypertens. 2008;21(3):310–6. doi: 10.1038/ajh.2007.65. [DOI] [PubMed] [Google Scholar]
- 23.Canter PH, Ernst E. The cumulative effects of Transcendental Meditation on cognitive function--a systematic review of randomised controlled trials. Wien Klin Wochenschr. 2003;115(21–22):758–66. doi: 10.1007/BF03040500. [DOI] [PubMed] [Google Scholar]
- 24.SOKT, Orme-Johnson DW. Three randomized experiments on the longitudinal effects of the Transcendental Meditation technique on cognition. Intelligence. 2001:419–40. [Google Scholar]
- 25.Travis FGS, Stixrud W. ADHD, Brain Functioning, and Transcendental Meditation Practice. Mind & Brain, The Journal of Psychiatry. 2011:73–81. [Google Scholar]
- 26.Chen KW, Berger CC, Manheimer E, et al. Meditative therapies for reducing anxiety: a systematic review and meta-analysis of randomized controlled trials. Depress Anxiety. 2012;29(7):545–62. doi: 10.1002/da.21964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chambless DL, Hollon SD. Defining empirically supported therapies. J Consult Clin Psychol. 1998;66(1):7–18. doi: 10.1037//0022-006x.66.1.7. [DOI] [PubMed] [Google Scholar]
- 28.Hollon SD, Ponniah K. A review of empirically supported psychological therapies for mood disorders in adults. Depress Anxiety. 2010;27(10):891–932. doi: 10.1002/da.20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Research protocol. http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=981&pageaction=displayproduct.
- 30.Methods Guide for Conducting Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; Aug, 2007. AHRQ Publication No. 10(11)-EHC063-EF. [PubMed] [Google Scholar]
- 31.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of Interventions Version 5.1.0. London: The Cochrane Collaboration; [Accessed February 17, 2012]. Updated March 2011. Available at: http://www.cochrane.org/training/cochrane-handbook. [Google Scholar]
- 33.Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions--agency for healthcare research and quality and the effective health-care program. J Clin Epidemiol. 2010;63(5):513–23. doi: 10.1016/j.jclinepi.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Barrett B, Hayney MS, Muller D, et al. Meditation or exercise for preventing acute respiratory infection: a randomized controlled trial. Ann Fam Med. 2012;10(4):337–46. doi: 10.1370/afm.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bormann JE, Gifford AL, Shively M, et al. Effects of spiritual mantram repetition on HIV outcomes: a randomized controlled trial. J Behav Med. 2006;29(4):359–76. doi: 10.1007/s10865-006-9063-6. [DOI] [PubMed] [Google Scholar]
- 36.Brewer JA, Sinha R, Chen JA, et al. Mindfulness training and stress reactivity in substance abuse: results from a randomized, controlled stage I pilot study. Subst Abus. 2009;30(4):306–17. doi: 10.1080/08897070903250241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brewer JA, Mallik S, Babuscio TA, et al. Mindfulness training for smoking cessation: Results from a randomized controlled trial. Drug Alcohol Depend. 2011 doi: 10.1016/j.drugalcdep.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castillo-Richmond A, Schneider RH, Alexander CN, et al. Effects of stress reduction on carotid atherosclerosis in hypertensive African Americans. Stroke. 2000;31(3):568–73. doi: 10.1161/01.str.31.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiesa A, Mandelli L, Serretti A. Mindfulness-based cognitive therapy versus psycho-education for patients with major depression who did not achieve remission following antidepressant treatment: a preliminary analysis. J Altern Complement Med. 2012;18(8):756–60. doi: 10.1089/acm.2011.0407. [DOI] [PubMed] [Google Scholar]
- 40.Delgado LC, Guerra P, Perakakis P, Vera MN, Reyes del Paso G, Vila J. Treating chronic worry: Psychological and physiological effects of a training programme based on mindfulness. Behav Res Ther. 2010;48(9):873–82. doi: 10.1016/j.brat.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Elder C, Aickin M, Bauer V, Cairns J, Vuckovic N. Randomized trial of a whole-system ayurvedic protocol for type 2 diabetes. Altern Ther Health Med. 2006;12(5):24–30. [PubMed] [Google Scholar]
- 42.Garland EL, Gaylord SA, Boettiger CA, Howard MO. Mindfulness training modifies cognitive, affective, and physiological mechanisms implicated in alcohol dependence: results of a randomized controlled pilot trial. J Psychoactive Drugs. 2010;42(2):177–92. doi: 10.1080/02791072.2010.10400690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaylord SA, Palsson OS, Garland EL, et al. Mindfulness training reduces the severity of irritable bowel syndrome in women: results of a randomized controlled trial. Am J Gastroenterol. 2011;106(9):1678–88. doi: 10.1038/ajg.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gross CR, Kreitzer MJ, Thomas W, et al. Mindfulness-based stress reduction for solid organ transplant recipients: a randomized controlled trial. Altern Ther Health Med. 2010;16(5):30–8. [PMC free article] [PubMed] [Google Scholar]
- 45.Gross CR, Kreitzer MJ, Reilly-Spong M, et al. Mindfulness-based stress reduction versus pharmacotherapy for chronic primary insomnia: a randomized controlled clinical trial. Explore (NY) 2011;7(2):76–87. doi: 10.1016/j.explore.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hebert JR, Ebbeling CB, Olendzki BC, et al. Change in women’s diet and body mass following intensive intervention for early-stage breast cancer. J Am Diet Assoc. 2001;101(4):421–31. doi: 10.1016/S0002-8223(01)00109-2. [DOI] [PubMed] [Google Scholar]
- 47.Jayadevappa R, Johnson JC, Bloom BS, et al. Effectiveness of transcendental meditation on functional capacity and quality of life of African Americans with congestive heart failure: a randomized control study. Ethn Dis. 2007 Summer;17(3):595. [PMC free article] [PubMed] [Google Scholar]; Ethnicity & Disease. 2007;17(1):72–7. [PMC free article] [PubMed] [Google Scholar]
- 48.Jazaieri H, Goldin PR, Werner K, Ziv M, Gross JJ. A Randomized Trial of MBSR Versus Aerobic Exercise for Social Anxiety Disorder. J Clin Psychol. 2012 doi: 10.1002/jclp.21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuyken W, Byford S, Taylor RS, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psychol. 2008;76(6):966–78. doi: 10.1037/a0013786. [DOI] [PubMed] [Google Scholar]
- 50.Lee SH, Ahn SC, Lee YJ, Choi TK, Yook KH, Suh SY. Effectiveness of a meditation-based stress management program as an adjunct to pharmacotherapy in patients with anxiety disorder. J Psychosom Res. 2007;62(2):189–95. doi: 10.1016/j.jpsychores.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 51.Lehrer PM. Progressive relaxation and meditation: A study of psychophysiological and therapeutic differences between two techniques. Behav Res Ther. 1983;21(6):651–62. doi: 10.1016/0005-7967(83)90083-9. [DOI] [PubMed] [Google Scholar]
- 52.Malarkey WB, Jarjoura D, Klatt M. Workplace based mindfulness practice and inflammation: A randomized trial. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller CK, Kristeller JL, Headings A, Nagaraja H, Miser WF. Comparative Effectiveness of a Mindful Eating Intervention to a Diabetes Self-Management Intervention among Adults with Type 2 Diabetes: A Pilot Study. J Acad Nutr Diet. 2012;112(11):1835–42. doi: 10.1016/j.jand.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moritz S, Quan H, Rickhi B, et al. A home study-based spirituality education program decreases emotional distress and increases quality of life--a randomized, controlled trial. Altern Ther Health Med. 2006;12(6):26–35. [PubMed] [Google Scholar]
- 55.Morone NE, Rollman BL, Moore CG, Li Q, Weiner DK. A mind-body program for older adults with chronic low back pain: results of a pilot study. Pain Med. 2009;10(8):1395–407. doi: 10.1111/j.1526-4637.2009.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mularski RA, Munjas BA, Lorenz KA, et al. Randomized controlled trial of mindfulness-based therapy for dyspnea in chronic obstructive lung disease. J Altern Complement Med. 2009;15(10):1083–90. doi: 10.1089/acm.2009.0037. [DOI] [PubMed] [Google Scholar]
- 57.Murphy TJ, Pagano RR, Marlatt GA. Lifestyle modification with heavy alcohol drinkers: effects of aerobic exercise and meditation. Addict Behav. 1986;11(2):175–86. doi: 10.1016/0306-4603(86)90043-2. [DOI] [PubMed] [Google Scholar]
- 58.Oken BS, Fonareva I, Haas M, et al. Pilot controlled trial of mindfulness meditation and education for dementia caregivers. J Altern Complement Med. 2010;16(10):1031–8. doi: 10.1089/acm.2009.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paul-Labrador M, Polk D, Dwyer JH, et al. Effects of a randomized controlled trial of transcendental meditation on components of the metabolic syndrome in subjects with coronary heart disease. Arch Intern Med. 2006;166(11):1218–24. doi: 10.1001/archinte.166.11.1218. [DOI] [PubMed] [Google Scholar]
- 60.Pbert L, Madison JM, Druker S, et al. Effect of mindfulness training on asthma quality of life and lung function: A randomised controlled trial. 2012;67(9):769–76. doi: 10.1136/thoraxjnl-2011-200253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Philippot P, Nef F, Clauw L, Romree M, Segal Z. A Randomized Controlled Trial of Mindfulness-Based Cognitive Therapy for Treating Tinnitus. Clin Psychol Psychother. 2011 doi: 10.1002/cpp.756. [DOI] [PubMed] [Google Scholar]
- 62.Piet J, Hougaard E, Hecksher MS, Rosenberg NK. A randomized pilot study of mindfulness-based cognitive therapy and group cognitive-behavioral therapy for young adults with social phobia. Scand J Psychol. 2010;51(5):403–10. doi: 10.1111/j.1467-9450.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 63.Plews-Ogan M, Owens JE, Goodman M, Wolfe P, Schorling J. A pilot study evaluating mindfulness-based stress reduction and massage for the management of chronic pain. J Gen Intern Med. 2005;20(12):1136–8. doi: 10.1111/j.1525-1497.2005.0247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmidt S, Grossman P, Schwarzer B, Jena S, Naumann J, Walach H. Treating fibromyalgia with mindfulness-based stress reduction: results from a 3-armed randomized controlled trial. Pain. 2011;152(2):361–9. doi: 10.1016/j.pain.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 65.Schneider RH, Grim CE, Rainforth MV, et al. Stress Reduction in the Secondary Prevention of Cardiovascular Disease: Randomized, Controlled Trial of Transcendental Meditation and Health Education in Blacks. (1941–7705 (Electronic). 1941–7713 (Linking)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Segal ZV, Bieling P, Young T, et al. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psychiatry. 2010;67(12):1256–64. doi: 10.1001/archgenpsychiatry.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.SeyedAlinaghi S, Jam S, Foroughi M, et al. Randomized controlled trial of mindfulness-based stress reduction delivered to human immunodeficiency virus-positive patients in Iran: effects on CD4[sup]+[/sup] T lymphocyte count and medical and psychological symptoms. Psychosom Med. 2012;74(6):620–7. doi: 10.1097/PSY.0b013e31825abfaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Henderson VP, Clemow L, Massion AO, Hurley TG, Druker S, Hebert JR. The effects of mindfulness-based stress reduction on psychosocial outcomes and quality of life in early-stage breast cancer patients: a randomized trial. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith JC. Psychotherapeutic effects of transcendental meditation with controls for expectation of relief and daily sitting. J Consult Clin Psychol. 1976;44(4):630–7. doi: 10.1037//0022-006x.44.4.630. [DOI] [PubMed] [Google Scholar]
- 70.Taub E, Steiner SS, Weingarten E, Walton KG. Effectiveness of broad spectrum approaches to relapse prevention in severe alcoholism: A long-term, randomized, controlled trial of Transcendental Meditation, EMG biofeedback and electronic neurotherapy. Alcoholism Treatment Quarterly. 1994;11(1–2):187–220. [Google Scholar]
- 71.Koszycki D, Benger M, Shlik J, Bradwejn J. Randomized trial of a meditation-based stress reduction program and cognitive behavior therapy in generalized social anxiety disorder. Behav Res Ther. 2007;45(10):2518–26. doi: 10.1016/j.brat.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 72.Whitebird RR, Kreitzer M, Crain AL, Lewis BA, Hanson LR, Enstad CJ. Mindfulness-Based Stress Reduction for Family Caregivers: A Randomized Controlled Trial. Gerontologist. 2012 doi: 10.1093/geront/gns126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolever RQ, Bobinet KJ, McCabe K, et al. Effective and viable mind-body stress reduction in the workplace: a randomized controlled trial. J Occup Health Psychol. 2012;17(2):246–58. doi: 10.1037/a0027278. [DOI] [PubMed] [Google Scholar]
- 74.Wong SY, Chan FW, Wong RL, et al. Comparing the Effectiveness of Mindfulness-based Stress Reduction and Multidisciplinary Intervention Programs for Chronic Pain: A Randomized Comparative Trial. Clin J Pain. 2011;27(8):724–34. doi: 10.1097/AJP.0b013e3182183c6e. [DOI] [PubMed] [Google Scholar]
- 75.Arch JJ, Ayers CR, Baker A, Almklov E, Dean DJ, Craske MG. Randomized clinical trial of adapted mindfulness-based stress reduction versus group cognitive behavioral therapy for heterogeneous anxiety disorders. 2013;51(4–5):185–96. doi: 10.1016/j.brat.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 76.Chhatre S, Metzger DS, Frank I, et al. Effects of behavioral stress reduction Transcendental Meditation intervention in persons with HIV. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferraioli SJ, Harris SL. Comparative Effects of Mindfulness and Skills-Based Parent Training Programs for Parents of Children with Autism: Feasibility and Preliminary Outcome Data. 2013;4(2):89–101. [Google Scholar]
- 78.Hoge EA, Bui E, Marques L, Metcalf CA, et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. Clin Psychiatry. 2013 doi: 10.4088/JCP.12m08083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakamura Y, Lipschitz DL, Kuhn R, Kinney AY, Donaldson GW. Investigating efficacy of two brief mind-body intervention programs for managing sleep disturbance in cancer survivors: A pilot randomized controlled trial. 2013;7(2):165–82. doi: 10.1007/s11764-012-0252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Omidi A, Mohammadkhani P, Mohammadi A, Zargar F. Comparing mindfulness based cognitive therapy and traditional cognitive behavior therapy with treatments as usual on reduction of major depressive disorder symptoms. 2013;15(2):142–6. doi: 10.5812/ircmj.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010 Jan 6;303(1):47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hart W. The Art of Living: Vipassana Meditation as taught by SN Goenka. Igatpuri, India: Vipassana Research Institute; 2005. [Google Scholar]
- 83.Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010 Mar 23;340:C332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.