Recent studies have implicated sleep in the process of memory consolidation but the question remains open whether other altered states of consciousness can also permit memory processes to occur. One clinically important altered state of consciousness is the state of anesthesia. Of particular interest to the clinician is whether learning can occur during anesthesia. Can patients learn and remember what goes on in the operating room while anesthetized? These issues will be addressed in the present chapter. We will begin by defining learning and memory types and their cellular and network underpinnings in the brain. We will turn briefly to experiments that implicate sleep in memory consolidation and whether new learning can occur during sleep. Then we will complete the chapter with a review of experiments that illustrate that learning is drastically altered under anesthesia, although memory processing has not yet been investigated in this state.
Learning and Memory
Learning is a process of acquiring knowledge, skills, or thought patterns either by adding to or overriding existing learned items. Memory represents the retrievable storage of such learned items, and that storage represents a consolidation continuum from temporary working and intermediate-term memory, to permanent long-term memory. As we will see below, anesthetics seem to inhibit learning and impair more recent rather than remote memories.
Working Memory, Intermediate Memory, Sleep, and Anesthesia
The shortest-term memory is called working memory and is held within the prefrontal cortex for seconds to, at most, a few minutes of uninterrupted attention via enhanced neural activity, reverberation, or very short-term synaptic enhancement using a temporary rise in presynaptic Ca2+ (e.g. Ref. 1). Light doses of anesthetics leave intact the working memory system so that a patient under conscious sedation can hold a conversation and appear to be lucid.
However, even under conscious sedation, intermediate-term and long-term memory of the events that occurred during anesthesia is missing, probably because they are never transferred into the coding scheme of intermediate-term memory. Intermediate-term memory lasts from minutes to months, requires an extracellular and intracellular cascade of events for synaptic plasticity, and is governed by the hippocampus.
Similarly, when incompletely awakened from slow wave sleep (SWS), one can conduct a seemingly lucid working memory-based conversation that is not remembered the next day. The neurotransmitters and intracellular genetic cascade involved in intermediate-term and long-term memory are not available during deep sleep nor during some dissociated states such as incomplete arousal from deep sleep.
We will explore in the text associated with Figures 1, 2, the mechanisms of anesthetic action on synaptic plasticity that would explain why intermediate-term memory is not laid down under anesthesia or during sleep. Working memory, which does not use the same internal cellular mechanisms, is not affected by anesthetic agents to the same extent.
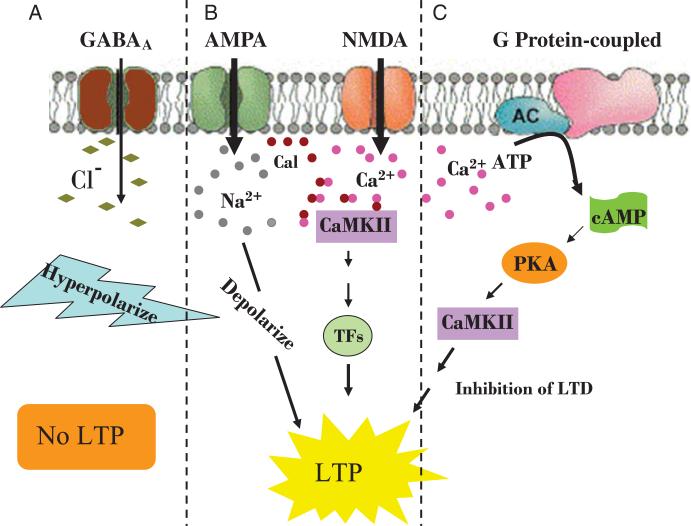
Figure 1.
Postsynaptic receptor-mediated neuronal activation of long-term potentiation. A, GABAA receptor hyperpolarizes the cell and prevents LTP. B, Activation of AMPA or NMDA receptors facilitates influx of positive ions, Na2 + and Ca2 +, respectively, which depolarizes the postsynaptic membrane. Calmodulin (Cal) inside the membrane combines with Ca2 + to form CaMKII, which promotes transcription factors to translocate to the nucleus and promotes protein synthesis and LTP. C, G protein-coupled activation by an increase in intracellular Ca2 + promotes second messenger, cAMP-dependent phosphorylation of CaMKII by PKA, which facilitates downstream induction of LTP.
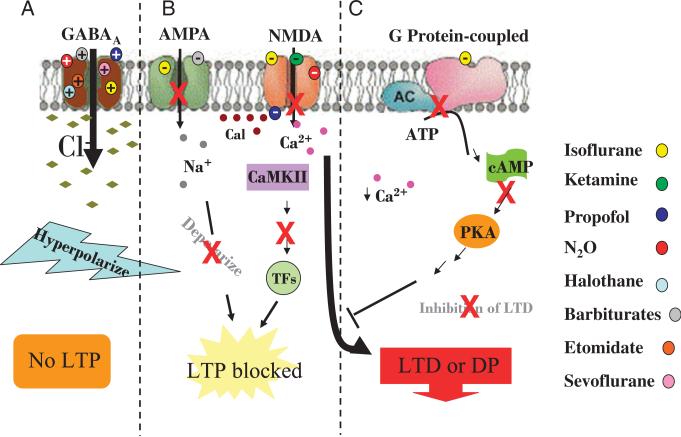
Figure 2.
Effects of anesthesia on postsynaptic targets. A, The inhibitory actions of GABA are enhanced by several anesthetics that bind the GABAA receptor and increase Cl- influx. Intracellular hyperpolarization prevents LTP-dependent memory formation. B, Anesthetic action on AMPA and NMDA receptors reduces membrane excitation, Ca2 + influx and blocks LTP and initiates LTD or depotentiation. C, Inhibition of G protein coupled receptor action allows depotentiation to ensue.
Deep anesthesia, like deep sleep itself, does not allow sensory information to be properly encoded even in the working memory system. Under deep anesthesia, inhibition processes are exaggerated, altering the basic firing properties of neurons and providing a level of sensory blockade.
Long-term Memory Stability and Anesthesia
Anesthetics and sleep do not generally impair long-term memories that are already consolidated into long-term storage in the cortex. Long-term memories remain intact because de novo synaptic plasticity events cannot be induced under anesthetics or during the state of nonrapid eye movement (NREM) sleep in the cell2 and the network3 are not necessary for long-term memory storage upkeep. Instead, we will see in the “Molecular Mechanisms for LTP/DP and Anesthetic Agents” section below that the long-term aspect of long-term memories is stabilized at synapses via a mutual phosphorylation cycle between calcium calmodulin protein kinase II (CaMKII) and mitogen-activated protein kinase (MAPK) that is unaffected by anesthetics and sleep.
Long-term Memory Reconsolidation Vulnerable to Anesthetics
Some of the intracellular events of synaptic plasticity that are vulnerable to anesthetics may remain important to the reconsolidation of memories learned within the time span of weeks to months in the hours after their recent retrieval. The vulnerability of retrieved memories to subsequent anesthesia depends on the degree to which those memories have been consolidated, which usually depends on the amount of time that has passed since the initial learning event. More remote memories are less vulnerable to interventions during the reconsolidation period after retrieval.4,5 This temporal principle of anesthetic effects works for all types of intermediate-term memory, and may explain developmental setbacks observed by parents and teachers of young children. Memory types and their differential susceptibility to synaptic plasticity interventions will be described next.
Types of Learning
Learning Classified by Cell Process Involved
Working Memory Mechanisms Learning types can be classified by the cellular processes underlying them. Short-term habituation and working memory use the short duration, rapidly degraded presynaptic and postsynaptic signals involving brief rises in intracellular Ca2+ levels.
Long-term Potentiation
Short-term and long-term potentiation (LTP) are continuums of the same process underlying Hebbian learning and can involve semi-simultaneous inputs to the postsynaptic cell (heterosynaptic) or simply strong driving along a single input (mono-synaptic). The timing of activity between the presynaptic and post-synaptic neurons determines whether LTP or long-term depression (LTD) or depotentiation (DP) of previously potentiated synapses will occur. The difference in synaptic strengthening versus weakening can be on the order of a few milliseconds by the rules of a well-investigated parameter set called spike timing dependent plasticity (STDP, Ref. 6). When a postsynaptic cell is strongly depolarized at the same time or shortly after a presynaptic cell depolarizes, then the synapses connecting the cells are strengthened. If however, the postsynaptic cell is depolarized without the presynaptic excitatory input, for example, when the presynaptic input arrives several milliseconds too late, then the presynaptic cell's connections to the postsynaptic cell are weakened. Thus, factors that cause relatively subtle changes in neuronal spike timing in a subset of cells could strongly effect the strengths of synaptic weights in a memory network made plastic in the hours (up to 24) after learning. Whether random inputs to the system destabilize it in a competitive fashion or whether strengthened synapses would be relatively immune to random synaptic events is under some debate.7,8
Implicit Learning and Priming?
Implicit learning is learning that does not require explicit memory of the learned items or even the learning session but is nevertheless apparent. For example, when normally associated pairs of words, like SWEET:SOUR, are presented during training, and then at testing presentation of the word “SWEET” elicits the response “SOUR” from the subject rather than another commonly associated word like “SUGAR.” This phenomenon is called priming, a type of implicit learning. Implicit learning tasks are generally spared in persons suffering from amnesia due to temporal lobe damage; that is, implicit learning does not require the hippocampus. The cellular events that underlie priming are not well understood, but this learning class is relevant to the study of anesthesia and learning because some studies show that priming, in particular, is intact in persons under general anesthesia. Priming appears not to involve LTP processes, but perhaps instead a shorter term habituation process that lies somewhere between intermediate term and working memory, degenerating after a period of a few hours.9
We will discuss the findings of implicit learning and anesthesia in detail toward the end of this chapter because there is some controversy as to its preservation under anesthesia. To best answer whether learning can occur during anesthesia, we first turn our attention to the well-studied cellular processes of associative learning such as learning a list of not normally paired words like MARCH:SHAVE, and such as spatial learning, which involves connecting a set of different types of cues. The LTP processes underlying associative learning are often targets of anesthetic agents that inhibit their normal function.
Learning by Brain Area
Learning is also classified by the types of brain structures involved in the learning process. Almost every stimulus modality has its own encoding structures in the brain, and comprehensive review of them lies outside the scope of this chapter. Instead, we will spend most of our time on the most well studied learning area in the brain, the hippocampus, because its discussion includes most of the issues relevant to learning and anesthesia.
The Hippocampus for Associative Learning
The hippocampus is the initial assembly site of complex associative learning where the cellular building blocks for intermediate-term and long-term memory, LTP, LTD, and depotentiation are rapidly employed. The hippocampus can be considered the highest association cortex. This structure receives input from sensory and motor associative areas of the neocortex through the entorhinal cortex and rapidly modifies the gain of synapses between well interconnected neurons within the hippocampus. Then memory consolidation slowly turns over the memory encoding responsibility back to the neocortex over weeks to months.
The Neocortex for Memory Storage
The long-term storage of associative memories is thought to be distributed back to all the relevant encoding sites of the neocortex. For example, the scent of perfume may set in motion a whole series of complex memories involving the one who you know usually wears that scent. Such memories could include what they look like, a pleasant experience involving that person, the sound of their voice, their name, and various learned semantic facts about them like their birthday and favorite flower. All of these memory fragments are stored in their modality-specific brain areas and are connected by a multisynaptic network of neurons formed during the consolidation process.10,11 Thus the sites of initial associative memory acquisition and long-term storage are not necessarily one and the same, which may be why consolidation can require so much time. Multimodal associative memories comprised through a network of neurons in remote areas, many synapses removed from one another, require simultaneous activity to occur again and again—perhaps many more times than the original exposure provided, to potentiate the whole network.
Hippocampal Place Learning
Place learning is one such type of hippocampus-dependent complex associative process, involving associations between such inputs as direction sense, the configuration of any number of available sensory cues, and other factors such as the time of day, etc. that would indicate how an animal should behave in that context. The many sensory areas projecting to the hippocampus feeding the information to this central location for association may be where the memories are ultimately stored, though they are far from each other. Offline processing, such as can occur in sleep, may be necessary to solidify the network encoding these disparate components of complex memories.
The hippocampus can be bilaterally lesioned after the learned association has been consolidated into long-term memory elsewhere. Even with complete ablation of the hippocampal learning structure, the memory of the associated items will persist.12,13
Amygdala and Fear Conditioning
Unlike the hippocampus, the amygdala is necessary for both the acquisition of fearful memories and their long-term storage and retrieval.14,15 Olfactory and other sensory memories probably function in much the same way. That is, if the primary encoding site is damaged or tonically inhibited during SWS or anesthesia, recall of the memories stored there would necessarily be impaired. Anesthetics that target the brain areas storing the memories would, of course, interfere with the recall of those memories, though as soon as the anesthesia wore off, the long-term memory would again be accessible. Recently consolidated fear memories are made vulnerable again through the process of recall. Agents which interfere in the process of reconsolidation could disrupt the memory storage and subsequent recall. Recently, clinician researchers have had some success anesthetizing patients after recall of trauma and alleviating symptoms of posttraumatic stress disorder.4
Sleep State Influences
The relationship between sleep and anesthesia can be delineated by describing the physiologic, molecular, and behavioral characteristics of each.16 Anesthesia, as with sleep, is characterized by a depression of consciousness. Evidence suggests that this state is achieved by mechanisms that are common to sleep and anesthesia (Chapter 6). Typically, sleep is composed of 2 main stages, NREM sleep and rapid eye movement (REM) sleep. NREM can be divided further into 4 stages that transition from light to deep sleep, also called SWS. During SWS, when cortical cells are tonically and rhythmically hyperpolarized, normal memory recall would be inhibited just as consciousness itself is compromised. The state of REM is quite different from SWS and more like the waking state, in neuronal polarity, synchrony/desynchrony, neurochemical milieu of acetylcholine and γ-aminobutyric acid (GABA) and patterns of neural activity. The preponderance of Φ oscillations in the hippocampal neuronal network is most similar to the active waking, learning state. Given the memory content of REM dream reports and the physiologic characteristics of the state, the ability to reactivate memories in the REM state is likely intact.
Learning “Not,” Development, and the Potential for Anesthesia Interference
Learning what is “not” is just as important to the overall learning process. For example, when learning the features that a chick will ever after identify as “mother,” the chick also erases other synapses that were initially present and ready to encode nearly any combination of features that would present themselves during the critical imprinting period. Sounds, movements, shapes, textures, etc that appear during the critical period are associated together in a specialized area of the cortex whereas cells that would have responded to other sounds not presented during this time are not active and the synapses that would have encoded them are trimmed away from the originally plural encoding network of synapses.
Strengthening of synapses occurs between simultaneously active cells while synaptic pruning occurs among cells not simultaneously active. It is a concept most important in the consideration of anesthesia, learning, and memory, because neuronal activity level changes are some of the first observable effects of anesthetics. STDP is important in any plastic brain network in both the adult and the developing brain. STDP happens in the visual cortex, for example, when an animal learns to see. Initially, input from both eyes activates- neurons in the primary visual cortex. With visual experience, some cells in the primary visual cortex become selectively activated by inputs from the eye to which it is most highly connected, and that disparity is amplified by heterosynaptic LTP. Inputs from the eye less likely to depolarize the postsynaptic cell become weakened, and are then removed in an active pruning process. The strengthening of simultaneously active and weakening of nonsimultaneously active cells creates the classic optical dominance columns of the primary visual cortex.17 Interestingly, such plasticity is enhanced by the state of sleep.18
Such general-to-specific innervation pattern changes also develop in the muscle system and many other places in the nervous system. Certain anesthesia combinations excite some cells and inhibit others during rapid development of the nervous system in its metaplastic phases. Heterosynaptic activity is caused by anesthetics inactivating presynaptic or postsynaptic targets while leaving their output or inputs, respectively, active. Heterosynaptic activity leads to the first step in unwiring a network. A series of studies by Jevtovic-Todorovic et al and others19–21 demonstrated startling results in the form of widespread cell death from the developing rat under a common cocktail of anesthetic agents administered for only 6 hours. It would be interesting to know whether the brain areas most compromised were those undergoing a critical period of plasticity, making them more vulnerable to the effects of heterosynaptic depotentiation, LTD, pruning, and presynaptic cell loss.
Extinction
Extinction of previously learned associations is also a learning process. When learning that the cheese has moved to another part of the environment, the animal must also extinguish the behavior of checking the old cheese place to find food efficiently. A place once associated with the presence of a predator may now be the best source of fresh water after the predator has moved away. Extinction learning is being intensely investigated by neuroscientists in the learning community. Extinction may be key to avoiding posttraumatic stress disorder such as experienced by patients who are aware during anesthesia. Extinction of fear seems to involve the medial prefrontal cortex and is thought to be represented as a “not” or “not now” learned association on top of the original fear association in the amygdala.22 If the medial prefrontal cortex is anesthetized during extinction training, for example, when the subject is presented repeatedly with the stimulus that was previously associated with pain, but the painful stimulus is absent time after time, the fear persists. Learning the “not any more” association of the original learning is why you may remember that you used to believe that monsters were under the bed, even though you have learned that they do not exist. It is not that you have erased the original idea of monsters from your memory networks once you learn that they are not, but you may no longer respond with faster heart rate and perspiration because you have learned the “not” clause better than the original fearful association.
Some synapses appear to be actually actively erased from the memory network, however. Such can occur even in a mature network through a process called depotentiation. CB1 receptors (the endogenous cannabiniod receptor) appear to be important in this depotentiation processes as its blockage during extinction training causes fear reactions to persist despite many repeated extinction trials,23 and augmentation enhances extinction.24 Low levels of norepinephrine at the b-adrenergic receptors during heterosynaptic activation also can undo LTP of synapses, essentially unwiring a previously strengthened network.25 At the source of cortical norepinephrine, the locus coeruleus of the brainstem, noradrenergic neurons are tonically active during all behavioral states except REM sleep.26 The effect of various anesthetic agents on locus coeruleus activity may allow us to better predict which agents would preferentially lead to poor memory recall and/or active erasure of learned associations made active by recall just before anesthetic administration (see Reconsolidation, below).
Consolidation and Reconsolidation
In humans, memories are consolidated over a period of hours to years from the assembly plants of the hippocampus to the neocortical network. Once consolidated, memories are subject to reconsolidation errors after each subsequent recall of the memory or reactivation of the memory-structured network. The length of time that a memory network is vulnerable to reconsolidation errors is controversial. But recent evidence indicates that there is a time after which long-term memories are fairly permanent.27 Certainly much remains to be understood about the exact coding structure of memories on the intracellular, synaptic, and network levels (for review, see Ref. 28). However much evidence collected over many years, since the discovery of activity-induced changes in network connectivity by Lomo and Bliss in the early 1970s,29,30 points to a few essential building blocks of memory.
Cellular/Network Building Blocks for Learning and Memory
The neocortical network of memory must be modified to encode new things, and such is accomplished via the process of LTP. LTP is the cellular basis for learning and strengthening synaptic connections between neurons. The opposite process, LTD is also necessary and normally involved in memory storage (see Learning “Not,” above). Both strengthening and weakening synapses between neurons are involved in reshaping the neural network to encode a novel memory and to incorporate that new material to learn into the greater knowledge schema.
LTP, LTD, and its relative, depotentiation, all use some common intracellular pathways which are the molecular building blocks underlying the persistent modifications of long-term memory. Some of these pathways have been identified as targets for various anesthetic agents, as will be discussed in the next section. A recent thorough review of the molecular changes underlying synaptic plasticity can be found in Ref. 31. Here we will concentrate on those cellular underpinnings of synaptic plasticity underlying learning that share something in common with anesthesia mechanisms, namely, the N-methyl-d-aspartate type receptors (NMDA), GABA receptors, specifically GABAA receptors, and AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors.
Activation of NMDA Receptors
The NMDA receptor begins to function to initiate LTP after a prolonged (10+ ms) depolarization of the postsynaptic membrane. When glutamate is released from the presynaptic excitatory neuron, it activates the sensitive ligand-gated AMPA glutamate receptors, which open and allow sodium ions to enter and depolarize the postsynaptic cell. If enough glutamate is present at the synapse or a prolonged release of glutamate occurs to continuously depolarize the postsynaptic cell, or if the postsynaptic cell membrane is simultaneously depolarized by another agent such as by acetylcholine (which in the hippocampus drives a y rhythm depolarization lasting tens of milliseconds), then the postsynaptic membrane potential is made positive enough inside to release the large magnesium ions blocking the nonspecific cation channel in the NMDA receptors. The NMDA channel opens allowing calcium to enter the postsynaptic cell. A large rise in the carefully regulated intracellular calcium triggers a cascade of molecular events in the cell that leads to the phosphorylation of AMPA receptors to increase their insertion in the postsynaptic membrane and make the postsynaptic cell more sensitive to presynaptic glutamate release. Phosphorylation also alters the subunit composition of AMPA receptors to increase the probability of channel opening and the length of open time. Increase in numbers and longer opened AMPA receptors makes the postsynaptic cell more responsive to each presynaptic excitatory drive. Because all the described Ca2+ entry and phosphorylation changes are local to the synapse, LTP is a local, synapse-specific phenomenon. Thus, the neural network that encodes memories tends to reside in a very specific synaptic connectivity pattern. A single neuron with its hundreds of thousands of inputs and tens of thousands of output synaptic connections can be involved in encoding at least tens of different memories. Any agent altering the function of the NMDA receptor, the AMPA receptor, or the probability of Ca2+ entry to a cell would have profound impact on the plasticity of the system.
Molecular Mechanisms for LTP/DP and Receptor Targets of Anesthetic Agents
Anesthetics exert their actions on various membrane-bound targets. Many of these targets are key to establishing and maintaining the synaptic changes that underlie intermediate and long term memory. The long-term component of LTP is thought to reside in the iterative phosphorylation of CaMKII and its reciprocal activation of MAPK. The intervention that would stop and reverse LTP, then is to break that CaMKII-MAPK reciprocal strengthening loop, for example, through dephosphorylating CaMKII. A rise in intracellular calcium triggers a cascade of events inside the cell that activates MAPK, beginning the reciprocal phosphorylation cycle with CaMKII.
A rise in intracellular Ca2+ through NMDA can directly activate the adenyl cyclase unit of the b-adrenergic receptor, which leads to a rise in adenosine 3,5‘-cyclic monophosphate (cAMP), the activation of cAMP-dependent protein kinase A (PKA) and thus the activation of MAPK/ ERK kinase and MAPK. The changes are restricted to the dendrites of postsynaptic cells, and some presynaptic axon terminals, but MAPK and PKA, set in motion at the synapse, can also translocate to the nucleus, where they regulate gene transcription.
The activation of PKA can also occur through the ligand (noradrenaline) activation of the β-adrenergic receptor or by another G protein-coupled receptor, which has the additional LTP preserving benefit of activating adenylyl cyclase, which itself inhibits the dephosphorylator of CaMKII called protein phosphatase 2b, also known as calcineurin. The opposite of LTP happens when only small amounts of calcium enter or are released inside the cell. A small rise in intracellular calcium can trigger the activation of calcineurin, which, as was described above, will lead to the dephosphorylation of CaMKII and the reversal of LTP. Thus, activation of Gs proteins will prevent the dephosphorylation of CaMKII and thus prevent the reversal of LTP. Noradrenaline (or norepinephrine) or other PKA-activating G-protein receptor ligands inhibits the depotentiation of synapses, an essential step in reshaping neural networks to incorporate novel information with the extant memory network structure. Other ligands that bind G-protein receptors like noradrenaline are isoflurane, and ketamine. Thus these anesthetic agents have a biochemical pathway to influence depotentiation. The activation of G-protein-coupled receptors through their ligands (eg, noradrenaline at the beta adrenergic receptor) also preserves LTP by activating an inhibitor of calcineurin, which would otherwise dephosphorylate CaMKII. Thus noradrenaline (aka norepinephrine) and other G-protein receptor ligands inhibit the depotentiation of synapses. A mechanism whereby anesthetics can interfere with LTP is through blockade of these G-protein coupled receptors and their intracellular pathways. Anesthetic ligands that bind and inhibit the activity of G-protein receptors are, for example, isoflurane, sevoflurane,32 halothane and ketamine, which inhibit the high-affinity states of the G-protein-linked receptors33 (Fig. 2C). Isoflurane at the beta adrenergic receptor, for example, acts through the intracellular calcineurin cascade to allow reversal of LTP (depotentiation) when only low levels of Ca2+ enter the cell. Isoflurane allows only a small rise in intracellular calcium because it interferes with the postsynaptic rise of both Na+ and Ca2+ by depressing the presynaptic release of glutamate.34
Activation of GABA Receptors
Thus far we have discussed the effects of anesthetic agents on excitatory systems involved with learning and LTP/depotentiation. Here, we turn to the most common effect of anesthetics: activation of GABA receptors to hyperpolarize cell membranes.
GABA receptor-dependent currents generally have a hyperpolarizing effect on neuronal cell membranes. Anesthetic agents that modulate these currents act on the presynaptic or postsynaptic neuron or at the synaptic cleft. The most commonly identified receptor type that is targeted by most anesthetics is the GABAA receptor. Representative agents that have been identified to impact this receptor are isoflurane, sevoflurane, propofol, benzodiazepines and the barbiturates.
Typically, a depolarization within the presynaptic GABA-ergic neuron descends to an axon terminal and facilitates an increase in intracellular calcium. The increase of Ca2+ induces the migration of GABA-containing vesicles to the cell membrane. Fusion of these vesicles with the cell membrane is followed by release of GABA into the synaptic cleft. While the concentration of GABA increases it is met with an insertion of GABA-ergic receptors in the postsynaptic terminal. Subsequent to binding the receptor and channel opening, an increase in Cl– ions flow into the postsynaptic cell, which causes hyperpolarization. Deactivation occurs by 2 processes; (1) the concentration of the inhibitory neurotransmitter rapidly decays (2) GABAA-bound ligand is released, but at a much slower rate than the clearance of GABA from the cleft. The latter response signals the decrease of ionic currents into the cell.
Anesthetics act in a myriad of ways to affect the normal activating function of GABA at its receptor. Here, the GABAA receptor will be the focus of discussion. Anesthetic effects on GABA receptors include increasing hyperpolarization by facilitating Cl– influx, prolonging receptor-ligand binding kinetics, interfering with gating properties, or inducing conformational changes within the receptor channel that increases Cl- influx. Some of these modulatory mechanisms are described below.
Common Receptor Targets for LTP and Anesthetic Agents
The following examples are taken from scientific reviews by several researchers, which illustrate the effects of selected volatile and intravenous anesthetics on their cellular and molecular targets (Fig. 2). In general, these compounds reduce or prevent LTP-inducing memory formation by affecting the kinetics, conformation, gating, or catalytic properties of neuronal membrane-bound proteins. In some cases, the activities of these anesthetics are well described, however additional investigations are needed to fully understand the complexity of these pharmacologic compounds in inducing unconsciousness.
Isoflurane
Isoflurane is an inhalant anesthetic that is commonly employed during surgical procedures for the purpose of amnesia and hypnosis. Its potency varies with the different receptor types it engages. In general, the GABA receptor is the primary target of isoflurane, though it does display effects at other receptors. Isoflurane weakly effects AMPA receptors.35 This weak interaction contributes to the challenge to measure its effects, which seem to reach a measurable level when combined with the impact of isoflurane on NMDA receptors. A functionally significant decrement in the total charge transfer between the extracellular and intracellular environments36 is the direct effect of isoflurane on the AMPA and NMDA receptor system. Ming and coworkers37 have found a robust inhibition of NMDA receptor-dependent currents that is observable in cortical neurons, which becomes less evident over development. This decreased impact of isoflurane could reflect a lower sensitivity of the NMDA R2 subunit, which increases expression with development of the neuronal network. Kainate receptors (GluR6) on GABA interneurons38 can facilitate GABA release due to isoflurane's excitarory action on a subunit of the kainite receptor that is impacted by volatile anesthetics.39 Topf and colleagues40 report that isoflurane attenuates the channel gating closure, keeping the Cl– channel in an open state. Hence, LTP-inducing depolarization is prevented by the transfer of these negative ions across the membrane.
Sevoflurane
Sevoflurane inhibits neuronal excitation in the hippocampus that would otherwise induce LTP. Sevoflurane does display activity in the spinal cord and other organs, but the focus here is on amnestic effects in the brain. Several studies have shown that sevoflurane increases the affinity of GABA for its receptor. In the hippocampus, GABAA receptor-mediated chloride currents are enhanced by the presence of sevoflurane at clinically relevant concentrations41,42 and effectively restrict excitatory activity among CA1 neurons.41 Ishizeki and colleagues41 showed that tetanic stimulation (100 Hz, 1 s) to presynaptic hippocampal neurons failed to induce LTP in postsynaptic pyramidal cells in rat brain in the presence of sevoflurane. They also demonstrated that sevoflurane decreased glutamate release from presynaptic terminals as detected by an increased latency to paired stimulation (paired pulse facilitation), a stimulus response that is indicative of reduced presynaptic, calcium-mediated neurotransmitter release that is possibly due to decreased stimulus-induced calcium mobilization of synaptic vesicles in the presynaptic nerve terminal. The investigators concluded that these effects of sevoflurane may confer its amnestic properties.
N2O
N2O is another inhalant anesthetic that has a long history of usage for anesthetic purposes (over 150 y), as well as becoming a drug of abuse. Pharmacologically, it exerts significant inhibitory effects on NMDA receptors at clinically relevant concentrations.43,44 Studies have shown that N2O is mildly inhibitory to native44,45 and recombinant Glur2-containing AMPA receptors. It is further described as a noncompetitive NMDA receptor antagonist that only weakly enhances GABA receptor currents,43,46 but little else is known about its mechanism of action.
Propofol
Propofol is administered intravenously, and has been shown to have a regulatory effect on NMDA receptor-dependent activation of intracellular events. Propofol disrupts serine facilitated phosphorylation of the NMDA subunit 1 which decreases the Ca2+ influx into the cell and attenuates LTP production.47 Propofol also attenuates ERK phosphorylation in a concentration-dependent manner.47 ERK attenuation significantly decreases subsequent transcriptional events that occur in the nucleus of neuronal cells after LTP induction. In addition, there are reports that demonstrate the impact of propofol on GABAA receptors at clinically relevant doses.48,49 Studies suggest that propofol can stimulate GABA-mediated events through direct activation of GABAA receptors, which opens Cl– channels. A site (β2 subunit of TM3 at extracellular interface) that is distinctly different than the receptor ligand has been identified as the target for propofol. Specifically, a result of propofol binding is the prolongation of inhibitory postsynaptic potentials50,51 and the increase of inhibitory postsynaptic currents.52–55 A delay in the desensitization of GABAA receptors contributes to the long-standing inhibitory currents.56,57 All of these actions of propofol facilitate hyperpolarization of the postsynaptic neuron and the dampening of neural activation for which unconsciousness is the physiologic end point.
Etomidate
Etomidate has been shown to act as a selective modulator of GABAA receptors at clinically relevant concentrations. A result of its binding is enhancement of GABA receptor-mediated inhibition of neurotransmission. Its binding affinity is similar to GABA58 although the binding site of etomidate is distinctly different from that of GABA. This binding site has been identified as the b3 subunit of GABAA receptor, particularly on the TM2 spanning unit.59,60 Enhancement of Cl– currents occurs as a result of etomidate binding. Hyperpolarization of the postsynaptic membrane facilitates a reduction in membrane excitability61; which can also occur in the absence of GABA at higher concentrations.62 Etomidate has demonstrated some affinity for the α2 adrenoreceptor, but it exerts sedating effects rather than amnesia.63 Presently, there is no evidence of etomidate action on other ligand-gated channels. See Ref. 64 for a more detailed interpretation of etomidate involvement with GABAA receptors.
Ketamine
Ketamine is classified as a dissociative anesthetic and is a strong, noncompetitive blocker of NMDA receptors with little effect on AMPA receptors.44,65–69 It is also an injectable anesthetic agent that has been described mechanistically to have 2 modes of action. It appears to act as an open-channel blocker of NMDA receptors70 capable of concentration-dependent decreases in mean open channel time.71 Second, ketamine decreases NMDA channel opening frequency. It has been shown to exert differential effects because it possesses affinity for multiple subunit combinations.44 There are no apparent effects of ketamine on GABA receptors.
Barbiturates
Barbiturates, like isoflurane, have the GABA receptor as its primary target. However it has demonstrated an inhibitory affect on AMPA receptor currents. The effects are dependent upon the specific subunit composition of the receptor. For example, GluR1 is not affected by barbiturates, although Glur1/Glur2 heteromers are strongly inhibited.71–73 Barbiturates also block AMPA receptors. Yamakura and coworkers reported that there may be significance to the editing of the Q/R site in Glur2 that facilitates the blocking action of barbiturates. This site is a determinant of Ca2+ permeability and channel rectification72 when AMPA is bound and desensitized.74 Barbiturates effectively bind GABAA receptors at clinical levels. The barbiturate-binding site appears to be localized to the junction between the a and g subunits of GABAA. In this position, barbiturates lock the receptor in a conformation that increases GABA affinity for the receptor. This orientation increases the probability of Cl– channel opening, which facilitates an increase in Cl– currents and subsequently hyperpolarizes the cell membrane. Effects of barbiturates on the NMDA receptor type remain unclear. Additional studies are necessary to reveal the functional significance of barbiturates as an anesthetic agent.
Role of Sleep in Learning and Memory
Sleep is an entirely different state than anesthesia (Chapter 6). However some features are shared, such as the activation of the GABAA receptors. Pharmacologic activation of this GABA receptor produces SWS. It is therefore not surprising that SWS is also associated with amnesia.
There is growing body of literature, which argues strongly in favor of a role for sleep in the processes of learning and memory. The studies have been targeted at probing the relationship between sleep and learning and also sleep and the consolidation of the learned content, that is, memory consolidation. However, this memory reprocessing state is different from memory acquisition. It has been shown that new memories are not gained during the state of sleep. The subject must be awakened by exposure to the stimulus to gain any knowledge of it and must also remain awake until sleep inertia (defined here as a transition state between sleep and full wakefulness) wears off.75,76 This explains why people awakened by a phone call from the highly inertial state of deep SWS (stage 4) can hold a brief conversation and fall right back into sleep and have no memory of that conversation the next morning. There is a period of about 2 minutes before sleep onset for which people are amnestic to exposed stimuli.
The fact that people do not acquire new knowledge while asleep is not surprising if one considers the high GABA activity and overall thalamic bock of sensory information to the neocortex. Hyperpolarized networks cannot overcome the Mg2+ block of the NMDA channel that does not allow voltage gated Ca2+ channels to open. It is also not possible to induce LTP through external electrical stimuli during the state of SWS3,77 though the experiment has not been attempted when stimuli are confined to the depolarized phase of the slow waves. The acetylcholine system, so important for memory formation, is also actively inhibited during NREM sleep. When acetylcholine is disrupted in awake animals, learning is strongly impaired (for example Ref. 78). Although few anesthetic agents have direct cholinergic action, amplification of the neocortical GABAergic system would effect an inhibition of cholinergic cells in the basal forebrain thus contributing to the amnesia associated with anesthesia and subanesthetic doses of volatile anesthetics such as isoflurane and sevoflurane reduce activation of the nicotinic acetylcholine receptor,79 which would have negative learning consequences.
REM Sleep
It is interesting to note that much of the amnestic properties of sleep are not true during REM sleep, when the cholinergic system of the forebrain is highly active, the neural networks are not synchronously inhibited by high GABA tone, and LTP can be electrically induced.
There is much that remains unknown. For example, the molecular basis of priming-related learning is not even speculated. Priming generally occurs in well-established neural networks. For example in priming of related word pairs, the associations between words of a pair have been established for many years before the experimenter tests that connection. If reconsolidation is a temporary vulnerability, then those primed memories would not be vulnerable to random perturbations in the network. Thus it may be that priming learning can occur even under deep anesthesia because no LTP mechanisms are activated, no true learning occurs and Ca2+ does not enter the cell to either potentiate or depotentiate the synapses. Retrieval of well-consolidated associations may not involve any of the plasticity inducing events of recall of less fully consolidated memories. It could also be that these strong, well-learned associations are encoded in so many different network pathways that even if one were reactivated and depotentiated or reconsolidated incorrectly after anesthesia, the degradation of the memory would be too small to notice.
Priming and Anesthesia
Further consideration of the effects of anesthetics on different types of memories illuminates the debate over memory loss or preservation under anesthesia. It is likely to intuit that these agents would interrupt or prevent formation of memory based on data that identifies molecular targets where they exert hypnotic and analgesic effects. Indeed, this is the case with some of these compounds. However, many studies suggest that anesthesia does not eliminate all memory encoding or learning.
Several experimental methods have been devised to attempt to clarify this issue. However, speculation remains as to the effectiveness of the tests to parse out whether a subject is retaining memories while in the unconscious state or if it is simply a matter of an insufficient depth of anesthesia. Indicators such as heart rate, blood pressure, respiratory rate, and movement, among other clinically employed signs, are not reliable measures of depth of consciousness. Mechanical methods, such as the Bi-spectral Index (BIS) monitor are also inadequate to fully assess consciousness. Studies have been conducted to examine the effectiveness of the BIS monitor at assessing consciousness.80–82 Some researchers have chosen to use a combination of the BIS monitor and carefully designed memory tests to eliminate some of the inherent challenges of either method alone.83
Ghoneim published a review in which he describes various methods of testing implicit and explicit memory.84 He also cites studies of others who have used one or more of these investigative tools, individually or in combination, to assess the character of memory that may be obtained in the unconscious state.
Implicit memory formation can be facilitated by priming.85 Test subjects are presented with information during a learning phase and then prompted to recall the information after some time interval during which an intervention (pharmacologic or otherwise) is presented. Then, the second part of the test consists of 2 challenges. The first is word stem completion, during which a subject is presented with word lists and instructed to judge the words by some attribute. After a retention period, subjects are presented with a list of 3 letter words and asked to supply the first words that come to mind beginning with those letters. The second part of the tests consists of word identification.
There are primarily 3 powerful tests of explicit memory. The yes/no recognition test is one such method where items are presented singly as a random order sequence of old and new objects. Individuals are required to judge objects as old (yes) or new (no). Forced choice recognition is another method where subjects are presented old items that are grouped with new items. The respondent is then asked to choose which items are old. A third test involves modified process association, which is a derivative of the previous ones.83,84,86
Evidence suggests that implicit memory priming can be facilitated by surgical stimulation under anesthesia.87,88 In part, this seems plausible because cortisol is released into circulating blood as a response to surgical stress, which then activates the amygdala.89 Involvement by this part of the limbic system is responsible for emotional memory. It may follow that any memory formation coincident to this stress-induced event would have some emotional context. Further, items that are more emotionally salient would tend to be reinforced rather than neutral ones.
Deeprose and Andrade83 hypothesized that under anesthesia, learning of words is facilitated by surgical stimulation. In other words, they compared the effect of priming during the interval between induction of unconsciousness and commencement of surgery to that of priming after the first surgical incision. They chose to employ yes/no recognition and word stem completion to assess explicit and implicit memory, respectively.
Briefly, after test subjects were anesthetized with propofol anesthesia they were presented words (randomly) from 2 of 4 lists before surgical stimulation or after the initial incision. One list was designated as rehearsed and the other as distractor. Postsurgically, the subjects were played random word stems from each list and were challenged to respond with “yes” or “no” if they remembered hearing them during anesthesia. Additionally, the participants were prompted with stems of the words from the remainder of the previous lists and were given the task of responding with the first completion, impulsively. Finally, a postsurgical interview was conducted during which subjects were asked to describe events before anesthesia or intraoperatively. The yes/no recognition test was employed to assess explicit memory. Implicit memory was examined by word stem completion. Deeprose and Andrade concluded that there was no difference in explicit memory with or without surgical stimulation. Although slight improvement in implicit memory performance was detected with stimulation, the difference was insignificant.
Electrical Indirect stimulation of the brainstem is another methodology that is applied to assess neural responses. In this regard, the elicited responses are taken to represent neural activation that may indicate postoperative auditory memory traces. These patterns of stimulation are called auditory-evoked potentials (AEPs). Components of the resultant brain wave responses are indicative of brainstem activation. The particular brain regions that are activated by the AEPs are the median geniculate nucleus90 and the primary and association cortical areas.91 The duration of the stimulus response (latency) and the amplitude of the signal are the measurable parameters.83 Thornton and Sharpe92 discovered that anesthesia reduces the amplitude and increases the latency of the evoked auditory response.
Ghoneim and colleagues93 compared AEP patients with assessed implicit and explicit memory formation. The patients were assigned to 1 of 4 groups that were treated with different anesthetics. The first of 2 AEP recordings was taken 5 minutes after the initial incision. One of 2 childhood stories (Three Little Pigs or Wizard of Oz) was played along with target words repeated for 30 min. Then the second AEPs were recorded.
These investigators were interested in exploring the temporal relationships of postoperative interview on memory. In some cases, the interviews were held within 24 hours postsurgery or 3 to 4 days later, during which time the patients were asked to recall events after anesthesia or during surgery. This procedure was known as the “structured interview.”
Implicit memory was assessed by instructing the patients to draw associations to target words from the stories; one was target and the other was control. After a series of distractor questions, the subjects were asked to provide associations to target words once again. Explicit memory formation was tested by telling the patients to choose which of the 2 stories they heard.
In summation, the patients who exhibited recall of intraoperative events had received more opioids with high levels of analgesia and lower levels of anesthesia. However, story recognition was insignificant. Anesthetics had a markedly enhanced effect in attenuating the latency component of AEPs. Opioids had less significant effects than anesthetic groups. Priming was observed, of particular prominence, upon conduction of the second association test by the opioid group. This result suggests that intraoperative priming may be associated with light anesthetic plane. Therefore memory may not have been assembled during the anesthetized state as similarly discovered by Schwender and others.94
Conclusions
Given the strong effects of anesthetics on the molecular underpinnings of learning such as LTP, it is not surprising that learning is profoundly inhibited under anesthesia. Priming, which should be least susceptible to the agents that typically depress learning, is also least vulnerable to anesthetic agents, but even priming only works under light anesthesia conditions. The reasons why a small percentage (~0.2%) of anesthetized patients remember events during their supposed unconsciousness are unknown, but may relate to a differential expression of some NMDA receptor subtype, for example, not targeted by anesthetic agents, or a GABA receptor subtype also not available to anesthetic agents. Another possibility besides genetic considerations is that the level of anesthetic dose was too light for the individual. Yet another fascinating possibility, as yet unexplored, is that of a common mechanism between awareness during sleep and awareness during anesthesia. Many people with insomnia report having been aware of time passing and of their environment all night long, while their electroencephalogram record showed normal amounts of sleep stages throughout the same period.95 There may be an awareness center whose activity is not measurable through standard electroencephalography metrics and which, in certain individuals, can be uncommonly vigilant throughout otherwise normal states of sleep and anesthesia.
Obviously, more research is needed in this fascinating and important crossroads between sleep, anesthesia, awareness, and memory if we are to reduce or eliminate instances of memory during anesthesia while maximizing learning before and after the anesthetic procedure. Very few researchers are functioning at this junction. Given what we are beginning to understand about learning and memory, anesthetic action, and the role of sleep and states of consciousness in learning and memory processing, it is a fruitful area of research.
References
- 1.Mongillo G, Barak O, Tsodyks M. Synaptic theory of working memory. Science. 2008;319:1543–1546. doi: 10.1126/science.1150769. [DOI] [PubMed] [Google Scholar]
- 2.Cirelli C, Huber R, Gopalakrishnan A, et al. Locus coeruleus control of slow-wave homeostasis. J Neurosci. 2005;25:4503–4511. doi: 10.1523/JNEUROSCI.4845-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bramham CR, Srebro B. Synaptic plasticity in the hippocampus is modulated by behavioral state. Brain Res. 1989;4931:74–86. doi: 10.1016/0006-8993(89)91001-9. [DOI] [PubMed] [Google Scholar]
- 4.Bustos SG, Maldonado H, Molina VA. Disruptive effect of Midazolam on fear memory reconsolidation: decisive influence of reactivation time span and memory age. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.75. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Zhai H, Wu P, Chen S, et al. Effects of scopolamine and ketamine on reconsolidation of morphine conditioned place preference in rats. Behav Pharmacol. 2008;3:211–216. doi: 10.1097/FBP.0b013e3282fe88a0. [DOI] [PubMed] [Google Scholar]
- 6.Toyoizumi T, Pfister JP, Aihara K, et al. Optimality model of unsupervised spike-timing-dependent plasticity: synaptic memory and weight distribution. Neural Comput. 2007;19:639–671. doi: 10.1162/neco.2007.19.3.639. [DOI] [PubMed] [Google Scholar]
- 7.Song S, Miller KD, Abbott LF. Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat Neurosci. 2000;39:919–926. doi: 10.1038/78829. [DOI] [PubMed] [Google Scholar]
- 8.van Rossum MC, Bi GQ, Turrigiano GG. Stable Hebbian learning from spike timing-dependent plasticity. J Neurosci. 2000;2023:8812–8821. doi: 10.1523/JNEUROSCI.20-23-08812.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimamura AP, Squire LR. Independence of recognition memory and priming effects: a neuropsychological analysis. J Exp Psychol Learn Mem Cogn. 1985;11:37–44. doi: 10.1037//0278-7393.11.1.37. [DOI] [PubMed] [Google Scholar]
- 10.Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobio-logical perspective. Curr Opin Neurobiol. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 11.McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 12.Kim JJ, Faneslow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 13.Squire LR, Bayley PJ. The neuroscience of remote memory. Curr Opin Neurobiol. 2007;17:185–196. doi: 10.1016/j.conb.2007.02.006. Epub March 2, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilensky AE, Schafe GE, Kristensen MP, et al. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmerman JM, Rabinak CA, McLachlan IG, et al. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn Mem. 2007;14:634–644. doi: 10.1101/lm.607207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lydic RL, Baghdoyan HA. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiol. 2005;103:1268–1295. doi: 10.1097/00000542-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Cabelli RJ, Hohn A, Shatz CJ. Activity-dependent regulation of NMDAR1 immunoreactivity in the developing visual cortex. J Neurosci. 1997;17:8376–8390. doi: 10.1523/JNEUROSCI.17-21-08376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 19.Joksovic PM, Brimelow BC, Murbartián J, et al. Contrasting anesthetic sensitivities of T-type Ca2+ channels of reticular thalamic neurons and recombinant Ca(v)3.3 channels. Br J Pharmacol. 2005;144:59–70. doi: 10.1038/sj.bjp.0706020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikonomidou C, Bittigau P, Koch C, et al. Neurotransmitters and apoptosis in the developing brain. Biochem Pharmacol. 2001;62:401–405. doi: 10.1016/s0006-2952(01)00696-7. [DOI] [PubMed] [Google Scholar]
- 21.Olney JW. New insights and new issues in developmental neurotoxicology. Neurotoxicology. 2002;23:659–668. doi: 10.1016/S0161-813X(01)00092-4. [DOI] [PubMed] [Google Scholar]
- 22.Quirk GJ, Garcia R, González-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Varvel SA, Anum EA, Lichtman AH. Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology (Berl) 2005;179:863–872. doi: 10.1007/s00213-004-2121-2. Epub December 24, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Chhatwal JP, Davis M, Maguschak KA, et al. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;3:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- 25.Thomas KL, Davis S, Hunt SP, et al. Alterations in the expression of specific glutamate receptor subunits following hippocampal LTP in vivo. Learn Mem. 1996;2-3:197–208. doi: 10.1101/lm.3.2-3.197. [DOI] [PubMed] [Google Scholar]
- 26.Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981;8:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris RG, Inglis J, Ainge JA, et al. Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron. 2006;50:479–489. doi: 10.1016/j.neuron.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Eichenbaum H. The secret life of memories. Neuron. 2006;50:350–352. doi: 10.1016/j.neuron.2006.04.017. Review. [DOI] [PubMed] [Google Scholar]
- 29.Lomo T. The discovery of long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:617–620. doi: 10.1098/rstb.2002.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blitzer RD, Iyengara R, Landau EM. Postsynaptic signaling networks: cellular cogwheels underlying long-term plasticity. Biol Psychiatry. 2005;57:113–119. doi: 10.1016/j.biopsych.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka S, Tsuchida H. Effects of halothane and isoflurane on betaadrenoceptor-mediated responses in the vascular smooth muscle of rat aorta. Anesthesiol. 1998;89:1209–1217. doi: 10.1097/00000542-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 33.Seeman P, Kapur S. Anesthetics inhibit high-affinity states of dopamine D2 and other G-linked receptors. Synapse. 2003;50:35–40. doi: 10.1002/syn.10221. [DOI] [PubMed] [Google Scholar]
- 34.Ranft A, Kurz J, Deuringer M, et al. Isoflurane modulates glutamatergic and GABAergic neurotransmission in the amygdala. Eur J Neurosci. 2004;20:1276–1280. doi: 10.1111/j.1460-9568.2004.03603.x. [DOI] [PubMed] [Google Scholar]
- 35.Dildy-Mayfield JE, Eger El II, Harris RA. Anesthetics produce subunit-selective actions on glutamate receptors. J Pharamocol Exp Ther. 1996;276:157–160. [PubMed] [Google Scholar]
- 36.de Sousa SL, Dickinson R, Lieb WR, et al. Contrasting synaptic actions of the inhalational general anesthetics isoflurane and xenon. Anesthesiology. 2000;92:1055–1066. doi: 10.1097/00000542-200004000-00024. [DOI] [PubMed] [Google Scholar]
- 37.Ming Z, Knapp DJ, Mueller RA, et al. Differential modulation of GABA and NMDA-gated currents by ethanol and isoflurane in cultured rat cerebral cortical neurons. Brain Res. 2001;920:117–124. doi: 10.1016/s0006-8993(01)03044-x. [DOI] [PubMed] [Google Scholar]
- 38.Huettner JE. Kainite receptors and synaptic transmission. Prog Neurobiol. 2003;70:387–407. doi: 10.1016/s0301-0082(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 39.Akk G, Mennerick S, Steinbach JH. Schüttler J, Schwilden H, editors. Actions of Anesthetics on Excitatory Transmitter-Gated Channels. [May 18, 2008];Biological Psychiatry Handb Exp Pharamacol [serial online] 2008 Jan 8;182:53–84. doi: 10.1007/978-3-540-74806-9_3. Available from: Springerlink. [DOI] [PubMed] [Google Scholar]
- 40.Topf N, Jenkins A, Buron N, et al. Effects of isoflurane on gamma-aminobutyric acid type A receptors activated by full and partial agonists. Anesthesiology. 2003;98:306–311. doi: 10.1097/00000542-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Ishizeki J, Nishikawa K, Kubo K, et al. Amnesic concentrations of sevoflurane inhibit synaptic plasticity of hippocampal CA1 neurons through gamma-aminobutyric acid-mediated mechanisms. Anesthesiology. 2008;108:447–456. doi: 10.1097/ALN.0b013e318164cfba. [DOI] [PubMed] [Google Scholar]
- 42.Nishikawa K, Harrison NL. The actions of sevoflurane and desflurane on the gamma-amino-butyric acid type A: effects of TM2 mutations in alpha and beta subunits. Anesthesiology. 2003;993:678–684. doi: 10.1097/00000542-200309000-00024. [DOI] [PubMed] [Google Scholar]
- 43.Jevtovic-Todorovic V, Todorovic SM, Mennerick S, et al. Nitrous oxide (laughing gas) is an NMDA antagonist, neuroprotectant and neurotoxin. Nature Med. 1998;4:460–463. doi: 10.1038/nm0498-460. [DOI] [PubMed] [Google Scholar]
- 44.Yamakura T, Harris RA. Effects of gaseous anesthetics nitrous oxide and xenon on ligand-gated ion channels: Comparison with isoflurane and ethanol. Anesthesiology. 2000;93:1095–1101. doi: 10.1097/00000542-200010000-00034. [DOI] [PubMed] [Google Scholar]
- 45.Mennerick S, Jevtovic-Todorovic V, Todorovic SM, et al. Effect of nitrous oxide on excitatory and inhibitory synaptic transmission in hippocampal cultures. J Neurosci. 1998;23:9716–9726. doi: 10.1523/JNEUROSCI.18-23-09716.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dzoljic M, Van Duijn B. Nitrous oxide-induced enhancement of gamma-aminobutyric acidA-mediated chloride currents in acutely dissociated hippocampal neurons. Anesthesiology. 1998;88:473–480. doi: 10.1097/00000542-199802000-00026. [DOI] [PubMed] [Google Scholar]
- 47.Kozin J, Mao L, Arora A, et al. Inhibition of Glutamatergic action of extracellular signal-reglated protein kinases in hippocampal neurons by intravenous anesthetic propofol. Anesthesiology. 2006;105:1182–1191. doi: 10.1097/00000542-200612000-00018. [DOI] [PubMed] [Google Scholar]
- 48.Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cell Mol Life Sci. 1999;5510:1278–1303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krasowski MD, Koltchine VV, Rick CE, et al. Propofol and other intravenous anesthetics have sites of action on the gamma-aminobutyric acid type A receptor distinct from that for isoflurane. Mol Pharmacol. 1998;533:530–538. doi: 10.1124/mol.53.3.530. [DOI] [PubMed] [Google Scholar]
- 50.Nicoll RA, Eccles JC, Oshima T, et al. Prolongation of hippocampal inhibitory postsynaptic potentials by barbiturates. Nature. 1975;258:625–627. doi: 10.1038/258625a0. [DOI] [PubMed] [Google Scholar]
- 51.Scholfield CN. Potentiation of inhibition by general anaesthetics in neurons of the olfactory cortex in vitro. Pflugers Archiv. 1980;383:249–255. doi: 10.1007/BF00587527. [DOI] [PubMed] [Google Scholar]
- 52.Jones MV, Harrison NL. Effects of volatile anesthetics on the kinetics of inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurophysiol. 1993;70:1339–1349. doi: 10.1152/jn.1993.70.4.1339. [DOI] [PubMed] [Google Scholar]
- 53.Banks MI, Pearce RA. Dual actions of volatile anesthetics on GABAA IPSCs: dissociation of blocking and prolonging effects. Anesthesiology. 1999;90:120–134. doi: 10.1097/00000542-199901000-00018. [DOI] [PubMed] [Google Scholar]
- 54.Harrison NL, Vivini S, Narker JL. A steroid anaesthetic prolongs inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurosci. 1987;7:604–609. doi: 10.1523/JNEUROSCI.07-02-00604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacIver MB, Tanelian DL, Mody I. Two mechanisms for anesthetic-induced enhancement of GABA-mediated neuronal inhibition. Ann N Y Acad Sci. 1991;625:91–96. doi: 10.1111/j.1749-6632.1991.tb33832.x. [DOI] [PubMed] [Google Scholar]
- 56.Orser BA, Wang LY, Pennefather PS, et al. Propofol modulates activation and desensitization of GABAA receptors in cultured murine hippocampal neurons. J Neurosci. 1994;14:7747–7760. doi: 10.1523/JNEUROSCI.14-12-07747.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orser BA, McAdam LC, Roder S, et al. General anaesthetics and their effects on GABAA receptor desensitization. Toxicol Lett. 1998;100:217–224. doi: 10.1016/s0378-4274(98)00188-x. [DOI] [PubMed] [Google Scholar]
- 58.Robertson B. Actions of anesthetics and avermectin on GABAA chloride channels in mammalian dorsal root ganglion neurons. Br J Pharmacol. 1989;98:167–176. doi: 10.1111/j.1476-5381.1989.tb16878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tomlin SL, Jenkins A, Lieb WR, et al. Stereoselective effects of etomidate optical isomers on gamma-aminobutyric acid type A receptors and animals. Anesthesiology. 1998;88:708–717. doi: 10.1097/00000542-199803000-00022. [DOI] [PubMed] [Google Scholar]
- 60.Belelli D, Lambert JJ, Peters JA, et al. The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid. Proc Natl Acad Sci U S A. 1997;94:11031–11036. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Proctor WR, Mynlieff M, Dunwiddie TV. Facilitatory action of etomidate and pentobarbital on recurrent inhibition in rat hippocampal pyramidal neurons. J Neurosci. 1986;6:3161–3168. doi: 10.1523/JNEUROSCI.06-11-03161.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evans RH, Hill RG. GABA-mimetic action of etomidate. Experientia. 1978;34:1325–1327. doi: 10.1007/BF01981448. [DOI] [PubMed] [Google Scholar]
- 63.Rüsch D, Zhong H, Forman A. Gating allosterism at a single class of etomidate sites on a1b2g2L. J Biol Chem. 2004;30:20982–20999. doi: 10.1074/jbc.M400472200. [DOI] [PubMed] [Google Scholar]
- 64.Herd MB, Haythomthwaite AR, Rosahl TW, et al. The expression of GABAA beta subunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells. J Physiol. 2008;586:989–1004. doi: 10.1113/jphysiol.2007.146746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anis NA, Berry SC, Burton NR, et al. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharamocol. 1983;79:565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris NL, Simmonds MA. Quantitative studies on some antagonists of N-methyl D-aspartate in slices of rat cerebral cortex. Br J Pharamcol. 1985;84:381–391. doi: 10.1111/j.1476-5381.1985.tb12922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thomson AM, West DC, Lodge D. An N-methyl-aspartate receptor-mediated synapse in rat cerebral cortex: a site of action of ketamine? Nature. 1995;313:479–481. doi: 10.1038/313479a0. [DOI] [PubMed] [Google Scholar]
- 68.Orser BA, Pennefather PS, MacDonald JF. Multiple mechanisms of ketamine blockade of N-methyl-D-aspartate receptors. Anesthesiology. 1997;86:903–917. doi: 10.1097/00000542-199704000-00021. [DOI] [PubMed] [Google Scholar]
- 69.MacDonald JF, Milijkovic Z, Pennefather P. Use-dependent block of excitatory amino acid currents in cultured CNS neurons and clonal pituitary cells. J Neurophysiol. 1987;58:251–256. doi: 10.1152/jn.1987.58.2.251. [DOI] [PubMed] [Google Scholar]
- 70.MacDonald JF, Bartlett MC, Mody I, et al. Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurons. J Physiol. 1991;432:483–508. doi: 10.1113/jphysiol.1991.sp018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taverna FA, Cameron BR, Hampson DL, et al. Sensitivity of AMPA receptors to pentobarbital. Eur J Pharmacol. 1994;267:R3–R5. doi: 10.1016/0922-4106(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 72.Yamakura T, Sakimura K, Mishima M, et al. The sensitivity of AMPA-selective glutamate receptor channels to pentobarbital is determined by a single amino acid residue of the alpha 2 subunit. FEBS Lett. 1995;374:412–414. doi: 10.1016/0014-5793(95)01163-9. [DOI] [PubMed] [Google Scholar]
- 73.Joo DT, Xiong Z, MacDonald JF, et al. Blockade of glutamate receptors and barbiturate anesthesia: increased sensitivity to pentobarbital-induced anesthesia despite reduced inhibition of AMPA receptors in GluR2 null mutant mice. Anesthesiology. 1999;91:1329–1341. doi: 10.1097/00000542-199911000-00025. [DOI] [PubMed] [Google Scholar]
- 74.Jackson MF, Joo DT, Al-Mahrouki AA, et al. Desensitization of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors facilitates use-dependent inhibition by pentobarbital. Mol Pharamocol. 2003;64:395–406. doi: 10.1124/mol.64.2.395. [DOI] [PubMed] [Google Scholar]
- 75.Wyatt JK, Bootzin RR, Allen JJ, et al. Mesograde amnesia during the sleep onset transition: replication and electrophysiological correlates. Sleep. 1997;20:512–522. [PubMed] [Google Scholar]
- 76.Wyatt JK, Bootzin RR, Anthony J, et al. Sleep onset is associated with retrograde and anterograde amnesia. Sleep. 1994;17:502–511. doi: 10.1093/sleep/17.6.502. [DOI] [PubMed] [Google Scholar]
- 77.Leonard AS, Lin IA, Hamsworth DE, et al. Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 1999;96:3239–3244. doi: 10.1073/pnas.96.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mizumori SJ, Perez GM, Alvarado MC, et al. Comparison of spatial and temporal characteristics of neuronal activity in sequential stages of hippocampal processing. Prog Brain Res. 1990;83:287–300. doi: 10.1016/s0079-6123(08)61257-1. [DOI] [PubMed] [Google Scholar]
- 79.Rada EM, Tharakan EC, Flood P. Volatile anesthetics reduce affinity at nicotinic acetylcholine receptors in the brain. Anesth Analg. 2003;1:108–111. doi: 10.1097/00000539-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 80.Levinson BW. States of awareness during general anaesthesia. Preliminary communication. Br J Anaesth. 1965;37:544–546. doi: 10.1093/bja/37.7.544. [DOI] [PubMed] [Google Scholar]
- 81.Schneider G, Wagner K, Reeker W, et al. Bi-spectral Index (BIS) may not predict awareness reaction to intubation in surgical patients. J Neurosurg Anesthesiol. 2002;1:7–11. doi: 10.1097/00008506-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 82.Bruhn J, Bouillon TW, Radulescu L, et al. Correlation of approximate entropy, bispectral index, and spectral edge frequency 95 (SEF95) with clinical signs of “anesthetic depth” during co-administration of propofol and remifentanil. Anesthesiology. 2003;98:621–627. doi: 10.1097/00000542-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 83.Deeprose C, Andrade J. Is priming during anesthesia unconscious? Conscious Cogn. 2006;15:1–23. doi: 10.1016/j.concog.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 84.Ghoneim MM. Review article: drugs and human behavior memory (Part 1). Clinical, theoretical, and methodological issues. Anesthesiology. 2004;100:987–1002. doi: 10.1097/00000542-200404000-00033. [DOI] [PubMed] [Google Scholar]
- 85.Merikle PM, Daneman M. Memory for unconsciously perceived events: evidence from anesthetized patients. Conscious Cogn. 1996;4:525–541. doi: 10.1006/ccog.1996.0031. [DOI] [PubMed] [Google Scholar]
- 86.Jacoby LL. A process dissociation framework: separating automatic and intentional uses of memory. J Mem Lang. 1991;30:513–541. [Google Scholar]
- 87.Deeprose C, Andrade J, Harrison N, et al. Unconscious auditory priming during surgery with propofol and nitros oxide anaesthesia: a replication. Br J Anaesth. 2004;94:57–62. doi: 10.1093/bja/aeh289. [DOI] [PubMed] [Google Scholar]
- 88.Gidron Y, Barak M, Kearse L, et al. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane and alfentanil in healthy volunteers. Anesthesiology. 2000;86:836–847. doi: 10.1097/00000542-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 89.Cahill I, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trend Neurosci. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- 90.Kaga K, Hink R, Shinoda Y. Evidence for a primary cortical origin of a middle latency auditory evoked potential in cats. Electroencephalogr Clin Neurol. 1990;50:254–266. doi: 10.1016/0013-4694(80)90153-4. [DOI] [PubMed] [Google Scholar]
- 91.Lovrich D, Novick B, Vaughn H. Topographic analysis of auditory event-related potentials associated with acoustic and semantic processing. Electroencephalogr Clin Neurophysiol. 1988;71:40–54. doi: 10.1016/0168-5597(88)90018-4. [DOI] [PubMed] [Google Scholar]
- 92.Thornton C, Sharpe RM. The Auditory Evoked Responses and Memory During Anesthesia. In: Ghoneim MM, editor. Awareness During Anesthesia. Butterworth-Heinmann; Oxford: 2001. pp. 112–117. [Google Scholar]
- 93.Ghoneim MM, Block RI, Dhanaraj VJ, et al. Auditory evoked responses and learning and awareness during general anesthesia. Acta Anaesthesiol Scand. 2000;442:33–43. doi: 10.1034/j.1399-6576.2000.440202.x. [DOI] [PubMed] [Google Scholar]
- 94.Schwender D, Kaiser A, Klasing S, et al. Mid-latency auditory evoked potentials and explicit and implicit memory in patients undergoing cardiac surgery. Anesthesiology. 1994;80:493–501. doi: 10.1097/00000542-199403000-00004. [DOI] [PubMed] [Google Scholar]
- 95.Mercer JD, Bootzin RR, Lack LC. Insomniacs’ perception of wake instead of sleep. Sleep. 2002;25:564–571. [PubMed] [Google Scholar]