Abstract
Objective
The OPRM1 gene was studied for DNA methylation in opioid dependence and possible paternal contribution to epigenetic inheritance of altered methylation profiles.
Participants and methods
DNA was extracted from blood and sperm from 13 male opioid addicts and 21 male control subjects. DNA methylation was determined by pyrosequencing in 24 CpG sites at the OPRM1 promoter region.
Results
The authors found significantly increased overall methylation in blood DNA from addicted subjects (Kruskal-Wallis [K-W] p = 0.013) Seven CpG sites showed significantly hypermethylated blood DNA from cases when compared with blood DNA controls (p < 0.05 at CpGs 5, 9, 10, 11, 18, 23, and 24). In sperm-derived DNA from addicts, the methylation was significantly increased at CpG 2 (p = 0.012), and overall methylation did not reach significant difference ( K-W p = 0.523).
Conclusions
Increased DNA methylation in the OPRM1 gene is associated with opioid dependence. Hypermethylated CpG sites located in OPRM1 promoter may potentially block the binding of Sp1 and other transcription activators, thus leading to OPRM1 silencing. The increased DNA methylation in sperm may suggest a way of epigenetic heritability of opioid abuse or dependence phenotypes.
Keywords: OPRM1, DNA methylation, CpG island, opioid dependence
INTRODUCTION
Genetic components play an important role in the drug abuse vulnerability, as evident from previous adoption, familial, and twin studies.1,2 However, the incomplete heritability of addiction indicates that the prevalence of opioid dependence cannot be explained by genetics alone. Environmental factors can interact with genomes and modify DNA structure, and the resulting phenotypic effects may be inherited by the offspring through epigenetic transmission of parental behavior.3,4 There are records for nonheritable influences in the biology of addiction; however, still there is no undeniable confirmation for any major factors of overall heritable epigenetic control.5
There are three opioid receptor genes, OPRM1, OPRD1, and OPRK1, encoding the μ-, δ-, and κ-receptors. The μ-opioicl receptor (μ-1) is the primary target for many opioids, including morphine, heroin, fentanyl, and methadone. As such, the OPRM1 gene (HGNC: 8156) has been the focus of much research in the etiology of opioid abuse and dependence. Connection between transcription regulation of genes and the level of DNA methylation of their promoters6 has been established, and a number of epigenetic mechanisms for the methylation-induced gene repression have been proposed. Methylation of DNA is involved in transcriptional silencing of genes, regulation of expression of imprinted genes, histone modification and silencing of tumor suppressor genes, and silencing of genes located on the inactive X chromosome.7 CpG islands are regions rich in cytosineguanine dinucleotides, in which the cytosine is more susceptible to methylation. Throughout the genome, the majority of CpG dinucleotides that are outside of CpG islands are mostly methylated. In normal cells, this methylation may be a way to maintain the transcriptional inactivity of noncoding DNA.8 About 60 percent of human genes' promoters include CpG islands,9 and the μ-1 opioid receptor gene (OPRMl) falls in this class.
Recent study found significantly increased lymphocyte DNA methylation in the 5′ regulatory region of the μ-1 gene in methadone-maintained former heroin addicts when compared with controls.10 These findings are especially salient given that the implicated CpG sites are positioned within potential binding sites for Sp1 activator, which is a transcription factor that can activate or repress transcription in response to physiological and pathological stimuli, thus affecting gene expression. Follow-up analyses of this region have also demonstrated ethnic differences in DNA methylation,11 and previous reports have associated hypermethylation of OPRM1 promoter region to reduced mRNA gene expression in mouse P19 cells.12
At the same time, the adverse effects of opioids, especially the aforementioned μ-1 receptor agonises, on reproductive physiology have been revealed. Significant reduction of spermatozoid motility is observed following incubation with morphine (but not in the presence of naloxone, a μ-1 antagonist), suggesting a possible role of μ-1 agonists in regulation of sperm functioning.13 These findings are consistent with earlier animal studies, which showed that chronic opioid administration adversely affected male rat fertility and produced a number of deficits in their offspring,14 and it also dramatically reduced the size and secretory activity of the rat's secondary sex organs.15 As methylation of DNA during genesis and development of parental gametes has a key role on this process, analyses of gametic DNA methylation or sex-specific patterns of transmission can supply evidence for heritable epigenetic features. Given that elevated levels of DNA methylation are observed at numerous regions in poor quality sperm,16 environmentally induced imperfect reprogramming of the male gametic line might theoretically alter spermatogenesis and affect sperm DNA methylation and, hence, lead to possible epigenetic inheritance.
In this study, we replicate in an independent sample the findings that hypermethylation of blood DNA in the OPRM1 promoter region is associated with opioid addiction10 and examine whether these effects are also observed in sperm DNA from case subjects.
SUBJECTS AND METHODS
This study was conducted with the approval of Human Research Protection Office at the Washington University Medical School, and informed consent was obtained from all participating subjects. Cases were recruited through advertisements at drug treatment centers in the Saint Louis metropolitan area. Volunteers seeking treatment for addiction to heroin, methadone, and/or other prescription opioids called an informational number and came into the institution's offices to be screened, and those meeting the Diagnostic and Statistical Manual of Mental Disorders (fourth edition, DSM-IV) criteria for opioid addiction, were included in the study. Addiction is seldom treated for a single drug of abuse, and therefore, blood or urine testing was not performed to identify the specific opioid drug with preciseness. Following informed consent, addicts were administered the Addiction Severity Index (ASI),17 and they donated semen, blood, and/or saliva samples.
Control subjects were recruited from two sources: local key informants at the Washington University, and sperm donors at the Reproductive Endocrinology and Infertility Clinic (REIC). Local recruits were seen in the same manner as the experimental group, with volunteers coming to the institution to be administered the ASI and donate samples. At the REIC, following ASI analysis by clinic Staff, volunteers were asked to donate the remainder of their semen samples after analyzed by the clinic, and an extra tube of blood for DNA extraction. All identifying information was stripped from the samples, except for age and ethnicity. The mean age was 29.3 years for controls and 42.5 years for the cases. DNA samples from semen and venous blood from 13 male heroin addicts and 21 male control subjects were analyzed as part of this study.
DNA was extracted using the QIAamp DNA Maxi Kit of QIAGEN (Geimantown, MD). DNA aliquots were sent out to EpigenDx Inc. (Worcester, MA) for bisulfite conversion and pyrosequencing methylation analysis. Two polymerase chain reaction (PCR) amplicons of 253 and 273 bp and six pyrosequencing assays were designed to cover 24 of the 33 CpG sites in the OPRM1 promoter CpG island, spanning over 422 bp (Chr.6: 154360587-154361008; primer sequences available on request from EpigenDx Inc.). Bisulfite modification was done using EZ Methylation kit of Zymo Research Corp. (Irvine, CA), and 500 ng of sample DNA was used for bisulfite modification followed by the PCR amplification . PCR reactions were performed with HotStar Taq Polymerase of QIAGEN, using 3.0 μL (1 ×) 10× PCR buffer (contains 15 μM MgCl2), 1.8 μL (1.5 mM) MgCl2, 0.6 μL (200 μM of each) deoxyribonucleotide triphosphates, 0.6 μL (6 pmol) and 0.6 μL (6 pmol) forward and reverse primers, 0.15 μL (0.75 U) HotStar Taq Polymerase, 3 μL of bisulfite-treated DNA and were adjusted to 30 μL total reaction volume. PCR cycling conditions were as follows: 95°C 5 minutes; 45 × 95°C 30 seconds; 56°C 30 seconds; 72°C 30 seconds); 72°C 5 minutes. Bisulfite modification, PCR, and pyrosequencing assays were validated by mixing in vitro highly methylated and demethylated DNA control at different ratios. Fourteen randomly picked samples were run in duplicates, blind to the performing laboratory, to test for reproducibility and reliability of measurements. Assays were run on a PSQ™ 96HS system (Biotage, Uppsala, Sweden). Initial data for the CpG island sequence were extracted from the Human Feb. 2009 assembly (GRCh37/hg19).
Statistical analyses were performed using the Stata software (StataCorp LP, College Station, TX). The analysis of DNA methylation measurements was done using the Kruskal-Wallis (K-W) rank test, which is a nonparametric analog of a one-way ANOVA. Advantage of the K-W test is that it does not assume the data are normally distributed. First, a screen test was performed to assess group differences over the whole CpG island, and then on a single CpG site basis with correction for multiple testing for a false discovery rate of 5 percent.18 However, this approach is known to be overly conservative when the outcomes of interest are highly correlated (as is commonly the case for methylation levels assessed at closely spaced CpG sites). Thus, we complemented this analysis with the linear discriminant analysis (LDA) and the leave-one-out strategy for ascertaining classification errors. Because of the expected issue of multicollinearity, these analyses are provided with both the lasso and the ridge penalty adjustments.
RESULTS
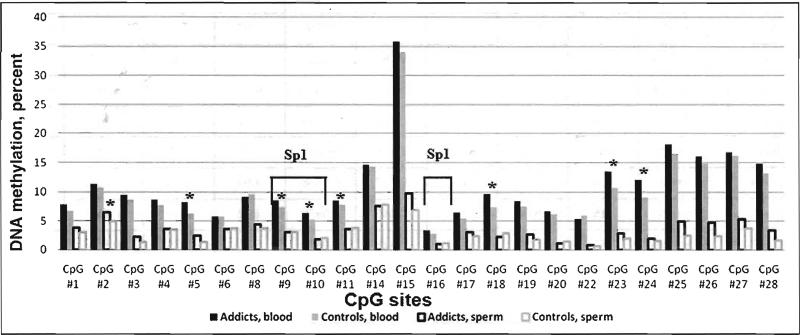
Comparing the average levels of DNA methylation between cases and controls over the whole CpG island, we found significant difference in blood DNA (K-W p = 0.013), while the difference in sperm DNA did not reach statistical significance (K-W p = 0.523). At the single CpG site level (Figure 1), the discriminant analysis confirmed the significant hypermethylation at seven CpG sites (CpGs 5, 9, 10, 11, 18, 23, and 24) in blood DNA from addicts, when compared with blood DNA from controls (p < 0.05; Table 1). In sperm-derived DNA from addicts, the level of methylation was significantly increased at CpG 2 when compared with sperm DNA from controls (p = 0.010). Finally, using the top hits and LDA, we arrive at a correct classification rate of 62 percent for cases and 68 percent for controls (leave-one-out strategy).
Figure 1.
Average methylation levels of 24 CpG sites at the OPRM1 promoter region in blood and sperm DNA from opioid addicts and control subjects. Sp1, potential binding site for the Sp1 transcription activator;(*) p<0.05, Kruskal-Wallis rank test.
Table 1.
Chromosomal location of human OPRM1 CpG sites and percent methylation in blood and sperm DNA in addicts and controls
| CpG site | Coordinates, bp | Blood DNA | p-value | Sperm DNA | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| GRCh37/hg 19 Chr. 6 | Relative toATG | Mean methylation, percent | Mean methylation, percent | |||||||
| Addicts (n = 13) | Controls (n = 20) | K-W | FDR | Addicts (n = 13) | Controls (n = 21) | K-W | FDR | |||
| CpGl | 154360587 | −93 | 7.80 | 6.71 | 0.084 | 0.0250 | 3.78 | 3.03 | 0.232 | 0.0146 |
| CpG2 | 154360590 | −90 | 11.34 | 10.67 | 0.179 | 0.0375 | 6.54 | 5.00 | 0.010 | 0.0021 |
| CpG3 | 154360600 | −80 | 9.49 | 8.67 | 0.103 | 0.0333 | 2.31 | 1.39 | 0.191 | 0.0125 |
| CpG4 | 154360609 | −71 | 8.69 | 7.74 | 0.167 | 0.0354 | 3.62 | 3.53 | 0.445 | 0.0229 |
| CpG5 | 154360620 | −60 | 8.22 | 6.31 | 0.002* | 0.0021 | 2.46 | 1.35 | 0.103 | 0.0083 |
| CpG6 | 154360630 | −50 | 5.78 | 5.74 | 0.086 | 0.0271 | 3.66 | 3.67 | 0.607 | 0.0333 |
| CpG8 | 154360655 | −25 | 9.16 | 9.56 | 0.076 | 0.0208 | 4.39 | 3.71 | 0.257 | 0.0167 |
| CpG9 | 154360662 | −18 | 8.53 | 7.45 | 0.021 | 0.0125 | 3.07 | 3.04 | 0.958 | 0.0479 |
| CpGlO | 154360666 | −14 | 6.42 | 5.26 | 0.002* | 0.0042 | 1.89 | 2.08 | 0.669 | 0.0396 |
| CpG 11 | 154360670 | −10 | 8.52 | 7.88 | 0.033 | 0.0146 | 3.65 | 3.77 | 0.736 | 0.0417 |
| CpGl4 | 154360706 | 27 | 14.67 | 14.32 | 0.094 | 0.0292 | 7.65 | 7.82 | 0.929 | 0.0458 |
| CpG15 | 154360732 | 53 | 35.92 | 33.98 | 0.568 | 0.0500 | 9.76 | 6.96 | 0.559 | 0.0292 |
| CpGl6 | 154360763 | 84 | 3.41 | 2.74 | 0.050 | 0.0167 | 1.05 | 1.17 | 0.898 | 0.0438 |
| CpG17 | 154360805 | 126 | 6.46 | 5.44 | 0.097 | 0.0313 | 3.11 | 2.38 | 0.092 | 0.0063 |
| CpG18 | 154360814 | 135 | 9.70 | 7.45 | 0.014 | 0.0104 | 2.31 | 2.85 | 0.642 | 0.0375 |
| CpG19 | 154360819 | 140 | 8.44 | 7.49 | 0.065 | 0.0188 | 2.65 | 1.75 | 0.330 | 0.0188 |
| CpG20 | 154360824 | 145 | 6.73 | 6.18 | 0.080 | 0.0229 | 1.09 | 1.40 | 0.507 | 0.0250 |
| CpG22 | 154360838 | 159 | 5.39 | 5.97 | 0.245 | 0.0396 | 0.85 | 0.60 | 0.568 | 0.0313 |
| CpG23 | 154360861 | 182 | 13.54 | 10.73 | 0.011 | 0.0083 | 2.91 | 2.00 | 0.613 | 0.0354 |
| CpG24 | 154360865 | 186 | 12.05 | 9.12 | 0.005* | 0.0063 | 1.97 | 1.57 | 0.984 | 0.0500 |
| CpG25 | 154360885 | 206 | 18.13 | 16.46 | 0.347 | 0.0438 | 4.90 | 2.46 | 0.125 | 0.0104 |
| CpG26 | 154360894 | 215 | 16.03 | 14.85 | 0.347 | 0.0458 | 4.72 | 2.40 | 0.083 | 0.0042 |
| CpG27 | 154360916 | 237 | 16.80 | 16.23 | 0.428 | 0.0479 | 5.36 | 3.75 | 0.555 | 0.0271 |
| CpG28 | 154360922 | 243 | 14.84 | 13.17 | 0.311 | 0.0417 | 3.45 | 1.62 | 0.395 | 0.0208 |
| Over whole CpG island | 11.07 | 10.02 | 0.013 | - | 3.63 | 2.89 | 0.524 | - | ||
Abbreviations: K-W, Kruskal-Wallis rank test; FDR. false discovery rate.
Significant for 5 percent FDR multiple correction procedure.18
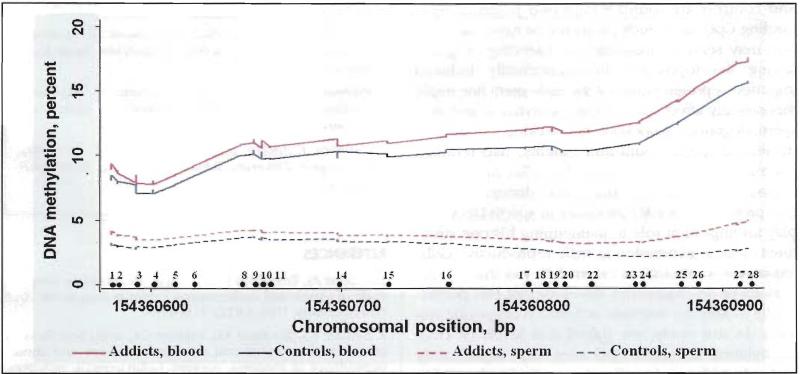
Overall methylation levels in sperm DNA were much lower than those in DNA from blood, and the distribution across the whole CpG island had similar methylation patterns between the addicts and controls (Figure 2). The percent methylation obtained from the mixing study of highly methylated and demethylated DNA for the two PCR assays was highly correlated with the expected methylation percentages, producing an R2 > 0.88. The pyrosequencing assays proved highly reproducible and reliable, with test-retest ratio of 0.97 and correlation coefficient of 0.96 (p < 0.001) in the duplicated blood and sperm DNA samples.
Figure 2.
Methylation patterns of OPRM1 CpG island and distribution between blood and sperm DNA from opioid addicts and control subjects. (•) denotes single CpG sites and their respective location on chromosome 6.
DISCUSSION
Our results, in a sample of active opioid-dependent subjects, are consistent with previous works reporting increased methylation levels at the OPRM1 promoter region in former heroin addicts undergoing methadone maintenance.10 The current findings show that blood DNA methylation at the OPRM1 promoter region is significantly higher in the opioid addicted group when compared with controls. The replication is further enhanced by the use of two different platforms for methylation assays: ABI Prism 3700 sequencer in the cited report, and PSQ™ 96HS pyrosequencing system in our study. Of note is that the other study used peripheral blood lymphocytes or whole blood as a source of DNA, whereas we used only whole blood. There might be some variability in methylation across blood cell types, and further research is warranted to investigate the impact of opioids on DNA methylation in specific cell types. We observed nonsignificant elevated DNA methylation in sperm from addicts, and we also found that the overall methylation level of the OPRM1 5′ region in DNA extracted from sperm is considerably lower than the methylation level of blood DNA. This is not unexpected, as genome-wide restriction landmark scanning on a large number of loci in mice indicates that methylation levels in testicular DNA are eight times lower than in other somatic tissues.19
There is reported connection between transcription regulation of genes and the level of DNA methylation in their promoter regions,6 and a number of mechanisms for the methylation-induced gene repression have been proposed. Sequence analyses of the OPRM1 promoter region suggest binding sites for several transcription factors (including Sp1, YY1, and E2F1), which may be actively involved in modulating OPRM1 transcription in lymphocytes.20 Recent report revealed that the promoter activity of OPRM1 is initiated by Sp1 that functions as an activator of transcription solely in the course of binding to nonmethylated DNA.21 It is thus significant that in the study of Nielsen et al.,10 as well as in our independent sample, significant differences in methylation levels between addicts and controls are found within two potential Sp1-binding CpG sites. Such promoter de novo methylation may serve to maintain the silencing of genes during development.22 Environmentally induced imperfect reprogramming of the male germ line might theoretically affect sperm DNA methylation and alter spermatogenesis. Knockout mice lacking μ-l showed decreased sperm count and motility, had reduced mating activity, and produced smaller litter size.23 Although marginal, the consistently distinct methylation profiles of OPRM1 promoter in sperm DNA may play an important role in maintaining histone structures or gene expression in male reproductive cells. Exposures to internal or external factors during vital periods of an organism's development can permanently modify the structure or function of specific systems. In our study, we found that levels of DNA methylation at certain CpG sites were significantly higher in addicts in blood and may also be elevated in semen. This suggests that opioid dependence may alter DNA structure maintenance or gene expression not only in somatic cells but might influence methylation at some loci in gametic cells as well. This is consistent with previous reports on the effects of morphine incubation on human spermatozoa.13
Although suspected in humans, epigenetic inheritance of opioid addiction remains a controversial hypothesis.5 At the same time, epigenetic factors such as DNA methylation, chromatin regulation, and gene imprinting play important role in psychiatric and substance dependence disorders as well.24,25 Our study adds evidence to the finding that chronic opioid misuse alters OPRM1 promoter methylation, an effect that may also be reflected in impaired germ cell development and possible epigenetic transmission of heroin addiction phenotypes. Study limitations include the moderate age difference and sample size, and although we obtained relatively high LDA classification rates for cases and controls (>60 percent), the results should be interpreted with caution.
ACKNOWLEDGMENTS
This study was conducted with the support of NIH grants: DA021330 (TJC), AA017681 (VMC), DAO18823 (AAT), and MH083823 (AAT).
Footnotes
The authors thank all the participants of thiS study, and they report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Vesselin M. Chorbov, Department of Psychiatry, Washington University School of Medicine, Saint Louis, Missouri..
Alexandre A. Todorov, Department of Psychiatry, Washington University School of Medicine, Saint Louis, Missouri..
Michael T. Lynskey, Department of Psychiatry, Washington University School of Medicine, Saint Louis, Missouri..
Theodore J. Cicero, Department of Psychiatry, Washington University School of Medicine, Saint Louis, Missouri..
REFERENCES
- 1.Cadoret RJ, Troughton E, O'Gorman TW, et al. An adoption study of genetic and environmental factors in drug abuse. Arch Gen Psychiatry. 1986;43(12):1131–1136. doi: 10.1001/archpsyc.1986.01800120017004. [DOI] [PubMed] [Google Scholar]
- 2.Kendler KS, Jacobson KC, Prescott CA, et al. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160(4):687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson PS, Ronnback L, Hansson E. Do persistent morphine effects involve interactions with the genome? Drug Alcohol Depend. 1989;24(1):39–43. doi: 10.1016/0376-8716(89)90006-9. [DOI] [PubMed] [Google Scholar]
- 4.Champagne F, Meaney MJ. Like mother, like daughter: Evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- 5.Uhl GR, Drgon T, Johnson C, et al. Molecular genetics of addiction and related heritable phenotypes: Genome-wide association approaches identify “connectivity constellation” and drug target genes with pleiotropic effects. Ann NY Acad Sci. 2008;1141:318–381. doi: 10.1196/annals.1441.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bird AP, Wolffe AP. Methylation-induced repression—Belts, braces, and chromatin. Cell. 1999;99(5):451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- 7.Fahrner JA, Eguchi S, Herman JG, et al. Dependence of histone modifications and gene expression on DNA hypermethylation in cancer. Cancer Res. 2002;62(24):7213–7218. [PubMed] [Google Scholar]
- 8.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylatlon. N Engl J Med. 2003;349(21):2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 9.Antequera F. Structure, function and evolution of CpG island promoters. Cell Mol Life Sci. 2003;60(8):1647–1658. doi: 10.1007/s00018-003-3088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen DA, Yuferov V, Hamon S, et al. Increased OPRM1 DNA methylation in lymphocytes of methadone-maintained former heroin addicts. Neuropsychopharmacology. 2009;34(4):867–873. doi: 10.1038/npp.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen DA, Hamon S, Yuferov V, et al. Ethnic diversity of DNA methylation in the OPRM1 promoter region in lymphocytes of heroin addicts. Hum Genet. 2010;127(6):639–649. doi: 10.1007/s00439-010-0807-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang CK, Song KY, Kim CS, et al. Evidence of endogenous μ opioid receptor regulation by epigenetic control of the promoters. Mol Cell Biol. 2007;27(13):4720–4736. doi: 10.1128/MCB.00073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agirregoitia E, Valdivia A, Carracedo A, et al. Expression and localization of δ-, κ-, and μ-opioid receptors in human spermatozoa and implications for sperm motility. J Clin Endocrinol Metab. 2006;91(12):4969–4975. doi: 10.1210/jc.2006-0599. [DOI] [PubMed] [Google Scholar]
- 14.Cicero TJ, Dovis LA, LaRegina MC, et al. Chronic opiate exposure in the male rat adversely affects fertility. Pharmacol Biochem Behav. 2002;72(1-2):157–163. doi: 10.1016/s0091-3057(01)00751-1. [DOI] [PubMed] [Google Scholar]
- 15.Cicero TJ, Meyer ER, Wiest WG, et al. Effects of chronic morphine administration on the reproductive system of the male rat. J Pharmacol Exp Ther. 1975;192(3):542–548. [PubMed] [Google Scholar]
- 16.Houshdaran S, Cortessis VK, Siegmund K, et al. Widespread epigenetic abnormalities suggest a broad DNA methylation erasure defect in abnormal human sperm. PLoS One. 2007;2(12):e1289. doi: 10.1371/journal.pone.0001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLellan AT, Lubarsky L, Woody GE, et al. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289–300. [Google Scholar]
- 19.Oakes CC, La Salle S, Smiraglia DJ, et al. Developmental acquisition of genome-wide DNA methylation occurs prior to meiosis in male genn cells. Dev Biol. 2007;307(2):368–379. doi: 10.1016/j.ydbio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Liu H, Wang Z, et al. The role of transcription factors Sp1 and YY1 in proximal promoter region in initiation of transcription of the μ opioid receptor gene in human lymphocytes. J Cell Biochem. 2008;104(1):237–250. doi: 10.1002/jcb.21616. [DOI] [PubMed] [Google Scholar]
- 21.Hwang CK, Kim CS, Kim do K, et al. Up-regulation of the μ-opioicl receptor gene is mediated through chromatin remodeling and transcriptional factors in differentiated neuronal cells. Mol Pharmacol. 2010;78(1):58–68. doi: 10.1124/mol.110.064311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber M, Schubeler D. Genomic patterns of DNA methylation: Targets and function of an epigenetic mark. Curr Opin Cell Biol. 2007;19(3):273–280. doi: 10.1016/j.ceb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Tian M, Broxmeyer HE, Fan Y, et al. Altered hematopoiesis, behavior, and sexual function in μ opioid receptor-deficient mice. J Exp Med. 1997;185(8):1517–1522. doi: 10.1084/jem.185.8.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novikova SI, He F, Bai J, et al. Maternal cocaine administration in mice alters DNA methylation and gene expression in hippocampal neurons of neonatal and prepubertal offspring. PLoS One. 2008;3(4):e1919. doi: 10.1371/journal.pone.0001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renthal W, Nestler EJ. Chromatin regulation in drug addiction and depression. Dialogues Clin Neurosci. 2009;11(3):257–268. doi: 10.31887/DCNS.2009.11.3/wrenthal. [DOI] [PMC free article] [PubMed] [Google Scholar]