Abstract
Confirmed clinical and veterinary cases of West Nile virus (WNV) infection in Mexico remain restricted to northern Mexico, supporting a unidirectional transmission model from the US into Mexico. Full-length genomic sequencing of nine WNV isolates obtained from Culex spp. mosquito pools in El Paso, Texas (n = 7) and Cuidad Juarez, Mexico (n = 2) from 2005 to 2010 demonstrates the co-circulation of three independent genetic groups, two of which belong to the southwestern (SW/WN03) genotype and the other to the North American (NA/WN02) genotype. These results indicate ongoing dynamic circulation of WNV between the United States and Mexico.
Keywords: West Nile virus, flavivirus, viral evolution, phylogenetics, viral epidemiology
Introduction
West Nile virus (WNV, Flaviviridae: Flavivirus) is a mosquito-borne neurotropic viral pathogen maintained in an enzootic cycle between mosquitoes and birds with equids, humans, and other mammals acting as dead-end hosts (Bernard and Kramer, 2001; Blitvich, 2008). Transmission of the original New York genotype (known as NY99) in resident Culex spp. mosquito and wild bird populations in 1999 initiated the expansion of WNV across the continental United States north into Canada and south into Mexico and Central/South America (Pepperell et al., 2003; Deardorff et al., 2006). Emergence of the North American (NA/WN02) genotype in 2002 displaced the original NY99 genotype as defined by a single amino acid substitution, V159A, in the envelope (E) protein, which is believed to facilitate more efficient viral dissemination in the mosquito reservoir compared to the original NY99 genotype (Ebel et al., 2004; Davis et al., 2005; Moudy et al., 2007); however, this hypothesis is still being debated (Anderson et al., 2012). Dual emergence of the Southwestern (SW/WN03) genotype in the US Southwest and northern Mexico in 2003 prompted the rapid regional displacement of the dominant NA/WN02 genotype between 2003 and 2008 (McMullen et al., 2011). McMullen et al. identified 13 conserved nucleotide changes characteristic of this novel genotype with positive selection for the encoded NS4A-A85T and NS5-K314R amino acid substitutions in the nonstructural (NS) proteins.
Despite continued circulation of WNV with numerous human and equine cases of infection in the United States and Canada, few veterinary or human clinical cases have been reported in Mexico. Nonetheless, serological screening of serum samples from Mexican equids in 2002 and 2006-2007 identified evidence of widespread WNV transmission with WNV-specific antibodies observed in up to 62.5% of horses sampled in over 14 Mexican States (Blitvich et al., 2003; Estrada-Franco et al., 2003; Lorono-Pino et al., 2003; Alonso-Padilla et al., 2009; Ibarra-Juarez et al., 2012). The prototype Mexican WNV strain (TM171-03) was isolated in southeastern Tabasco State from a dead raven in 2003 (Estrada-Franco et al., 2003). However, human WNV infection in Mexico remains limited to eight confirmed clinical cases of West Nile fever or neuroinvasive disease reported in the northern states of Chihuahua (n = 4), Nuevo Leon (n = 2), and Sonora (n = 2) with a few additional WNV isolates from mosquito pools, horses, and birds between 2003-2004 (Estrada-Franco et al., 2003; Blitvich et al., 2004; Elizondo-Quiroga et al., 2005; Deardorff et al., 2006).
The current paradigm for WNV introduction into Mexico supports movement via seasonal bird migrations across the Gulf-of-Mexico into the Yucatan Peninsula (Deardorff et al., 2006). The absence of the E-V159A substitution in the Tabasco 2003 strain (TM171-03) places this initial introduction prior to the emergence of the NA/WN02 genotype. However, identification of the characteristic E-V159A, NS4A-A85T, and NS5-K314R amino acid substitutions in all 2003-2004 northern Mexico WNV isolates suggests the separate introduction(s) of the SW/WN03 genotype into northern Mexico via a unidirectional WNV transmission model across the Southwest border (Blitvich et al., 2004; Elizondo-Quiroga et al., 2005; Deardorff et al., 2006). We report sequence and phylogenetic analysis of nine full-length WNV isolates collected from El Paso, Texas and Cuidad Juarez, Chihuahua State, Mexico between 2005 and 2010 indicating the co-circulation of both the NA/WN02 and SW/WN03 genotypes on the US-Mexican border. Furthermore, dual emergence of a divergent phylogenetic group encoding a distinct genetic profile supports a second introduction of WNV into Mexico with evidence of dynamic WNV transmission across the US-Mexican border that is not in agreement with the current unidirectional model.
Results
Genomic characterization of the WNV isolates
Nine WNV isolates collected in El Paso, Texas, US and Cuidad Juarez, Chihuahua State, Mexico were obtained from the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA) at the University of Texas Medical Branch and from the Texas State Department of Health Services in Austin (Table 1). Seven isolates were from pools of mosquitoes collected in El Paso, Texas between 2005 and 2010 with one isolate each from 2005, 2007, and 2008, two from 2009, and two from 2010. Two additional isolates, one each in 2008 and 2009, were from mosquitoes collected in Cuidad Juarez, Mexico. Six of the seven El Paso isolates (all but TX5282) were isolated from Culex tarsalis mosquitoes. The two Cuidad Juarez isolates and TX5282 were isolated from Cx. quinquefasciatus mosquito pools.
Table 1.
West Nile virus isolates described in the conducted genetic and phylogenetic analyses, 1998-2010a
| Strain | Location | Collection year | Sourceb | GenBank accession no. |
|---|---|---|---|---|
| IS-98 ISD | Eilat, Israel | 1998 | White stork | AF481864 |
| NY99-flamingo382-99 | New York, NY, USA | 1999 | Chilean flamingo | AF196835 |
| TM171-03 | Tabasco, Mexico | 2003 | Raven | AY660002 |
| Colorado 3258 | Colorado, USA | 2003 | Magpie | DQ164203 |
| WNV-1/US/BID-V4585/2003 | Connecticut, USA | 2003 | Cx. salinarius | HM488220 |
| TX AR 5-2686 | El Paso, Texas, USA | 2005 | Cx. tarsalis | JX015515 |
| 011WG-TX06EP | Texas, USA | 2006 | Human | GQ507470 |
| 013WG-TX07EP | Texas, USA | 2007 | Human | GQ507471 |
| WNV-1/US/BID-V4093/2007 | New York, USA | 2007 | American crow | HM488201 |
| TX AR 7-6745 | El Paso, Texas, USA | 2007 | Cx. tarsalis | JX015516 |
| WNV-1/US/BID-V4622/2008 | New York, USA | 2008 | American Crow | HM488237 |
| TX AR 8-6866 | Cuidad Juarez, Mexico | 2008 | Cx. quinquefasciatus | JX015518 |
| TX AR 8-5947 | El Paso, Texas, USA | 2008 | Cx. tarsalis | JX015517 |
| TX7827 | Texas, USA | 2009 | Blue jay | JF415924 |
| TX AR 9-6115 | Cuidad Juarez, Mexico | 2009 | Cx. quinquefasciatus | JX015520 |
| TX AR 9-5282 | El Paso, Texas, USA | 2009 | Cx. quinquefasciatus | JX015519 |
| TX AR 9-7465 | El Paso, Texas, USA | 2009 | Cx. tarsalis | JX015521 |
| TX AR 10-5718 | El Paso, Texas, USA | 2010 | Cx. tarsalis | JX015522 |
| TX AR 10-6572 | El Paso, Texas, USA | 2010 | Cx. tarsalis | JX015523 |
Strains in bold were sequenced in this study.
El Paso, Texas and Cuidad Juarez, Mexico isolates were collected from Culex (Cx.) spp. mosquito pools in 2005-2010.
Comparison of the nine isolates with the prototype NY99 (NY99-flamingo382-99) strain identified 43-72 nucleotide (nt) differences (0.39-0.65%) per 11,029 nt genome with increased nt divergence (0.63-0.92%) relative to the original TM171-03 Mexican isolate (Table 2) (Lanciotti et al., 1999). Each El Paso and Cuidad Juarez isolate encodes 12 of the 13 nucleotide changes characteristic of the NA/WN02 genotype (Davis et al., 2005); however, none of these isolates encoded the SW/WN03 genotype C to U mutation at position 3774 in the NS2A gene (data not shown). Furthermore, the nine isolates differed at 39 residues in the encoded polyprotein relative to the prototype NY99 strain with 5-13 (0.12-0.38%) substitutions per isolate.
Table 2.
Nucleotide and amino acid divergence between El Paso and Cuidad Juarez WNV isolatesa
| Group 1 | Group 2 | Group 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | NY99 | TM171-03 | TX5282 | TX7465 | TX5718 | TX6866 | TX5947 | TX6115 | TX2686 | TX6745 | TX6572 |
| NY99 | - | 0.41 | 0.61 | 0.64 | 0.65 | 0.43 | 0.46 | 0.54 | 0.41 | 0.39 | 0.53 |
| TM171-03 | 0.09 | - | 0.87 | 0.89 | 0.92 | 0.69 | 0.73 | 0.81 | 0.67 | 0.63 | 0.77 |
| TX5282 | 0.32 | 0.44 | - | 0.15 | 0.06 | 0.74 | 0.78 | 0.86 | 0.72 | 0.68 | 0.82 |
| TX7465 | 0.26 | 0.38 | 0.12 | - | 0.19 | 0.79 | 0.83 | 0.92 | 0.76 | 0.76 | 0.90 |
| TX5718 | 0.41 | 0.52 | 0.09 | 0.20 | - | 0.80 | 0.83 | 0.92 | 0.79 | 0.75 | 0.89 |
| TX6866 | 0.26 | 0.38 | 0.52 | 0.47 | 0.61 | - | 0.07 | 0.17 | 0.53 | 0.52 | 0.65 |
| TX5947 | 0.26 | 0.38 | 0.52 | 0.47 | 0.61 | 0.06 | - | 0.19 | 0.56 | 0.56 | 0.70 |
| TX6115 | 0.35 | 0.47 | 0.61 | 0.55 | 0.70 | 0.15 | 0.09 | - | 0.64 | 0.63 | 0.76 |
| TX2686 | 0.20 | 0.35 | 0.38 | 0.35 | 0.50 | 0.35 | 0.35 | 0.44 | - | 0.33 | 0.46 |
| TX6745 | 0.15 | 0.26 | 0.29 | 0.23 | 0.38 | 0.29 | 0.29 | 0.38 | 0.17 | - | 0.26 |
| TX6572 | 0.26 | 0.38 | 0.47 | 0.41 | 0.55 | 0.41 | 0.41 | 0.47 | 0.29 | 0.17 | - |
Percent nucleotide divergence from NY99 and TM171-03 is indicated above the diagonal with amino acid divergence shown below the diagonal. Sequences with high nucleotide and/or amino acid similarity are annotated in bold.
Evidence of regional evolution of WNV on the US-Mexican border
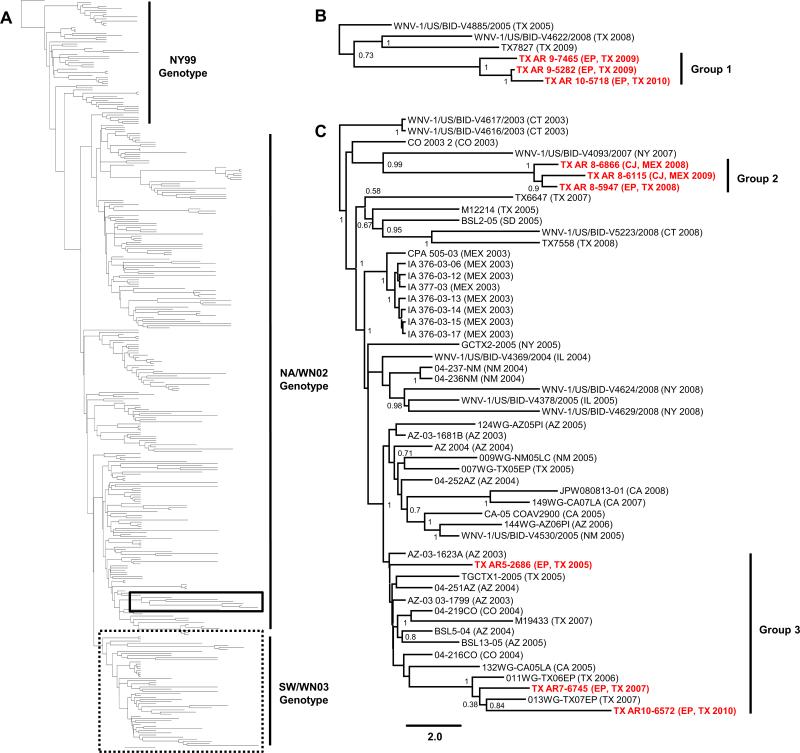
Neighbor-joining (NJ), maximum likelihood (ML), and relaxed clock Bayesian inference methods using the GTR+I+Γ4 substitution model with 1000 bootstrap replicates were used to analyze the polyprotein sequences of the nine El Paso and Cuidad Juarez isolates with all 347 published full-length North American isolates. Phylogenetic analyses produced consistent tree topologies rooted to the Israeli IS-98 STD isolate. Inclusion of the newly sequenced 2005-2010 El Paso and Cuidad Juarez isolates retained the topologic distribution of the NY99, NA/WN02, and SW/WN03 genotypes proposed by McMullen et al. (2011) (Fig. 1A). Furthermore, conserved nt divergence between the nine isolates sequenced in this study indicates the co-circulation of three distinct genetic groups (Fig. 1B and C). Group 1 (0.06-0.19% divergence) consists of three 2009-2010 El Paso isolates: TX7465, TX5282, and TX5718; Group 2 (0.07-0.19%) includes the TX6686, TX5947, and TX6115 El Paso and Cuidad Juarez isolates collected from 2008-2009; and Group 3 (0.26-0.46%) consists of three 2005-2010 El Paso isolates: TX2686, TX6745, and TX6572 (Figure 1). Each amino acid substitution conserved in more than one isolate was limited to a single genetic group with the exception of the E-V159A, NS4AA85T, and NS5-K314R substitutions (Table 3). In particular, the Group 1 isolates lack the NS4A-A85T substitution whereas the Group 2 isolates do not encode the NS5-K314R substitution. All three amino acid substitutions are absent from the TM171-03 Mexican isolate.
Figure 1.
Bayesian inferred 70% majority-rule phylogenetic tree of all published, full-length North American West Nile virus isolates, 1999-2010, for a total of 356 polyprotein sequences. Isolates are clustered into the NY99, North American (NA/WN02), and Southwest (SW/WN03) genotypes (A). Enlargement of the sequenced Group 1 isolates in the solid-lined box (B) and SW/WN03 genotype in the dash-lined box (C) containing the Group 2 and Group 3 isolates. Posterior probabilities >50% are indicated in the two sub-trees except in the indicated phylogenetic groups. Bolded red, El Paso, Texas and Cuidad Juarez, Mexico isolates sequenced in this study. Scale bar in Panel C indicates divergence time in years.
Table 3.
Amino acid substitutions in WNV isolates from Mexico and El Paso, Texas, 2005-2010a
| Group 1 |
Group 2 |
Group 3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene/Position | NY99b | TX7465 | TX5282 | TX5718 | TX6866 | TX5947 | TX6115 | TX2686 | TX6745 | TX6572 | |
| C | 119 | A | V | V | V | . | . | . | . | . | . |
| 121 | V | A | A | A | . | . | . | . | . | . | |
| prM | 140 | V | I | I | . | . | . | . | . | A | |
| E | 51 | A | T | T | T | . | . | . | . | . | . |
| 159 | V | A | A | A | A | A | A | A | A | A | |
| NS1 | 314 | R | . | . | . | K | K | K | . | . | . |
| NS2A | 26 | K | R | R | R | . | . | . | . | . | . |
| 89 | F | L | L | L | . | . | . | . | . | . | |
| 119 | H | . | . | . | Y | Y | Y | . | . | . | |
| 224 | A | . | . | . | V | V | V | . | . | . | |
| NS2B | 116 | L | . | . | . | . | M | M | . | . | . |
| 119 | V | L | L | L | . | . | . | . | . | . | |
| NS3 | 249 | P | . | L | L | . | . | . | . | . | . |
| 258 | V | . | . | . | I | I | I | . | . | . | |
| 355 | Y | . | . | . | F | F | F | . | . | . | |
| NS4A | 85 | A | . | . | . | T | T | T | T | T | T |
| 135 | V | . | M | M | . | . | . | . | . | . | |
| NS5 | 202 | Y | . | . | . | F | F | F | . | . | . |
| 314 | K | R | R | R | . | . | . | R | R | R | |
| 860 | A | . | . | . | . | . | . | . | T | T | |
C, capsid; prM, pre-membrane; E, envelope; NS, nonstructural. Nucleotide and amino acid changes are relative to the NY99 genotype strain [AF196835]. Dots indicate no change from the NY99 isolate.
Substitutions indicated for conserved changes in >1 isolate.
Distribution of the Group 1 isolates within the NA/WN02 genotype was not focused to a single monophyletic node whereas Groups 2 and 3 exhibited restricted clustering within the SW/WN03 genotype in all applied phylogenetic methods (Fig. 1B and C). In addition, the Group 1 cluster demonstrates consistent 100% internal bootstrap and posterior values despite a diverse temporal and geographic range of isolates including a 2003 Connecticut (WNV-1/US/BIDV4586/2003), 2008 New York (WNV-1/US/BID-V4622/2008), and 2009 Texas (TX7827) isolate sharing a common monophyletic lineage. Mean bootstrap values <15 and posterior probabilities ranging from 26-73%, dependent on the applied phylogenetic method, coupled with 0.53-0.73% sequence divergence fail to support robust monophyletic phylogenetic relationships of the Group 1 cluster among published NA/WN02 genotype isolates.
Compared to the Group 1 isolates, the Group 2 isolates formed a stable outgroup (43-100% posterior probabilities) of the SW/WN03 genotype with a 2007 New York (WNV-1/US/BID-V4093/2007) and 2003 Colorado (Colorado 3258) isolate. In particular, genetic comparison of the 2007 New York and Group 2 isolates indicates 0.52-0.63% nt and 0.26-0.35% deduced amino acid divergence, with the conserved absence of the NS5-K314R substitution. The Group 3 El Paso isolates cluster within the SW/WN03 genotype with the conserved distribution of the 2005 TX2686 isolate as an outgroup to several 2003-2007 Arizona, California, Colorado, and Texas isolates. Both the TX6745 and TX6572 isolates form stable monophyletic lineages with the 2006 and 2007 Texan isolates (011WG-TX06EP and 013WG-TX07EP) exhibiting 13-84% bootstrap frequencies and <0.29% nucleotide divergence among these four isolates. Significant sequence divergence (0.52-0.76%) between the Group 2 and 3 isolates in comparison to the prototype NY99 (0.39-0.54%) and Mexican TM171-03 (0.64-0.81%) strains supports the disparate emergence of these two phylogenetic clusters from a common ancestral SW/WN03 genotype strain.
Discussion
Inclusion of nine newly sequenced 2005-2010 WNV isolates collected from both El Paso, Texas and Cuidad Juarez, Mexico in a complete phylogenetic analysis of all published North American strains in this study allowed investigation of the dynamics of WNV evolution on the US-Mexican border. Complete genomic sequencing of the two WNV isolates from Cuidad Juarez permitted the expansion of these analyses upon the limited number of characterized WNV isolates collected from wild birds (n = 9) and a single horse in northern and southeastern Mexico (Beasley et al., 2004; Deardorff et al., 2006). Our results demonstrate the co-circulation of three distinct genetic pools of WNV isolates in the El Paso, Texas and Cuidad Juarez principality exhibiting conserved phylogenetic clustering within the NA/WN02 (Fig. 1B) and SW/WN03 (Fig. 1C) US genotypes. Significant nucleotide divergence of the two Cuidad Juarez and 2008 El Paso isolates from the prototype NY99 (>0.43%) and TM171-03 (>0.69%) strains in addition to other El Paso isolates (>0.52%) demonstrates evidence of active WNV transmission on the US-Mexican border between 2005 and 2010.
Emergence of the SW/WN03 genotype in the southwestern US between 2003 and 2008 resulted in the rapid regional displacement of the NA/WN02 genotype (McMullen et al., 2011). Undetected circulation of either genotype in northern Mexico since the initial epizootic in 2003 contrasts the continued detection and evolution of WNV in the United States. However, surveillance of WNV transmission in Mexico remains limited to the few state-wide and national serum sampling campaigns of equid populations plus rare reports of West Nile fever in human clinical cases. Possible explanations for the comparative absence of WNV activity in Mexico include 1) serologic cross-protection and/or competition with other endemic flaviviruses such as dengue or St. Louis encephalitis viruses (Tesh et al., 2002, Rodriguez et al., 2010); 2) under-reporting or clinical misdiagnosis of West Nile fever under the dengue fever clinical umbrella; 3) passage of an attenuated WNV phenotype in the Culex spp. mosquito population or wild bird amplifying hosts; or 4) a range of other potential environmental and socioeconomic factors. Inoculation of the prototype TM171-03 and 2004 northern Mexico Tecate strains into susceptible indigenous bird hosts resulted in comparable peak viremia, tissue tropism, and lethality (Guerrero-Sanchez et al., 2011); however, the TM171-03 strain demonstrated an attenuated phenotype compared to the virulent 382-99 NY99 strain in the American crow (Brault et al., 2011). Critical differences in Culex spp. and wild bird speciation, distribution, and susceptibility to WNV infection offer additional alternative explanations for the lack of clinical disease while supporting circulation of a distinct attenuated Mexican WNV phenotype.
Current phylogenetic models of WNV evolution in North America support the unidirectional introduction of WNV into the Yucatan Peninsula prior to 2003 with subsequent expansion into northern Mexico from the southwestern US between 2003 and 2004 (Deardorff et al., 2006). Here we demonstrate the recent emergence of three independent genetic groups of WNV isolates with ≥0.52% nucleotide divergence in the El Paso and the Cuidad Juarez area, which indicate selective pressures distinct from the surrounding southwestern US region. Furthermore, limited nucleotide and amino acid conservation (i.e. E-V159A substitution) between the Cuidad Juarez isolates and the 2003-2004 northern Mexico and the Tabasco TM171-03 strains precludes the emergence of the characterized Group 2 isolates from either northern (Cuidad Juarez) or southern (Tabasco) Mexican origin.
McMullen et al. (2011) demonstrated the phylogenetic clustering of the published northern Mexico WNV isolates within the SW/WN03 genotype distinct from the confirmed grouping of the prototype TM171-03 isolate in the NY99 genotype (Deardorff et al., 2006). The inferred monophyletic lineage of the Group 2 isolates with a 2003 Colorado and 2007 New York strain further supports the incongruent origin and divergent evolution of this outgroup from an ancestral SW/WN03 genotype strain consistent with a second introduction of WNV into northern Mexico between 2003 and 2008. Robust phylogenetic clustering and limited sequence divergence (<0.19%) between the Group 2 isolates compared to US isolates of close temporal and geographic distribution support the subsequent reintroduction of the 2008 El Paso isolate from Cuidad Juarez following circulation of an adapted SW/WN03 genotype strain in northern Mexico.
Selective pressure from circulating Mexican WNV strains or possibly other related flaviviruses is a potential ecological stimulus for the diverse genetic profile observed in the 2005-2010 El Paso and Cuidad Juarez isolates. Inferred co-circulation of both the NA/WN02 and SW/WN03 genotypes further emphasizes the divergent genomic sequences of the identified Group 2 isolates. In effect, the proposed model of dynamic WNV transmission between Mexico and the United States offers a novel route influencing the sustained evolution of WNV in the southwestern US. Continued isolation and phenotypic characterization of WNV strains from the southwestern US and northern Mexico will be required to confirm the underlying dynamics of WNV transmission and evolution in this region of North America.
Methods
Sequencing of isolates
Nine WNV isolates collected from Culex spp. mosquito pools in El Paso, Texas, US and Cuidad Juarez, Chihuahua State, Mexico during 2005-2010 were obtained from the World Reference Center for Emerging Viruses and Arboviruses at the University of Texas Medical Branch and from the Texas Department of State Health Services in Austin (Table 1). Extraction of viral RNA, subsequent reverse-transcriptase (RT) PCR, and consensus Sanger sequence analysis were performed as previously described in our lab (Davis et al., 2005; McMullen et al., 2011).
Phylogenetic analysis and nucleotide/amino acid comparisons
Sequences were edited using ContigExpress in the VectorNTI Advance 11 program suite (Invitrogen, Carlsbad, CA, USA) and assembled in BioEdit v7.0.9.0 (Hall 1999) with 347 published North American WNV isolate sequences in GenBank (as of December 2011) and the Israeli isolate WNV IS-98 STD. Full-length coding sequences for all 356 WNV isolates were aligned using the MUSCLE algorithm on the EMBL-EBI server (Edgar 2004).
Neighbor-Joining (NJ), maximum likelihood (ML), and relaxed clock Bayesian analyses were processed with Seaview v4.3.0 (Gouy et al., 2010), RAxML v7.2.8 Blackbox (Stamatakis 2006; Stamatakis et al., 2008), and BEAST v1.6.2 (Drummond and Rambaut 2007) on the CIPRES Science Gateway teragrid server (Miller et al., 2010) using the GTR+I+Γ4 substitution model and 1000 bootstrap replicates. Inferred phylogenetic trees were edited and formatted with FigTree v1.3.1. The IS-98 STD isolate was utilized as a common outgroup in phylogenetic alignments of all North American WNV isolates.
Research Highlights.
➢ West Nile virus is endemic in the United States.
➢ Evidence to date supports a unidirectional transmission model from US into Mexico
➢ Co-circulation of three genetic groups in El Paso, Texas and Cuidad Juarez, Mexico
➢ Ongoing dynamic circulation of WNV between the United States and Mexico
Acknowledgements
This work was supported in part by NIH grant AI 067847 and contract HHSN272201000040I/HHSN27200004/D04. In addition, we thank Mary D'Anton and the Texas Department of State Health Services for the generous contribution of the WNV samples characterized in this study in addition to review of the final manuscript. We also thank Andrew Beck for helpful discussions and phylogenetic insight.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso-Padilla J, Loza-Rubio E, Escribano-Romero E, Cordoba L, Cuevas S, Mejia F, Calderon R, Milian F, Travassos da Rosa A, Weaver SC, Estrada-Franco JG, Saiz JC. The continuous spread of West Nile virus (WNV): seroprevalence in asymptomatic horses. Epidemiol. Infect. 2009;137:1163–1168. doi: 10.1017/S0950268809002325. [DOI] [PubMed] [Google Scholar]
- Anderson JF, Main AJ, Cheng G, Ferrandino FJ, Fikrig E. Horizontal and vertical transmission of West Nile virus genotype NY99 by Culex salinarius and genotypes NY99 and WN02 by Culex tarsalis. Am. J. Trop. Med. Hyg. 2012;86(1):134–139. doi: 10.4269/ajtmh.2012.11-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley DWC, Davis CT, Estrada-Franco J, Navarro-Lopez R, Campomanes-Cortes A, Tesh RB, Weaver SC, Barrett ADT. Genome sequencing and attenuating mutations in West Nile virus isolate from Mexico. Emerg. Infect. Dis. 2004;10(12):2221–2224. doi: 10.3201/eid1012.040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard KA, Kramer LD. West Nile virus activity in the United States, 2001. Viral Immunol. 2001;14(4):319–338. doi: 10.1089/08828240152716574. [DOI] [PubMed] [Google Scholar]
- Blitvich BJ. Transmission dynamics and changing epidemiology of West Nile virus. Anim. Health Res. Rev. 2008;9(1):71–86. doi: 10.1017/S1466252307001430. [DOI] [PubMed] [Google Scholar]
- Blitvich BJ, Fernandez-Salas I, Contreras-Cordero JF, Marlenee NL, Gonzalez-Rojas JI, Komar N, Gubler DJ, Calisher CH, Beaty BJ. Serologic evidence of West Nile virus infection in horses, Coahuila State, Mexico. Emerg. Infect. Dis. 2003;9(7):853–856. doi: 10.3201/eid0907.030166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitvich BJ, Fernandez-Salas I, Contreras-Cordero JF, Lorono-Pino MA, Marlenee NL, Diaz FJ, Gonzalez-Rojas JI, Obregon-Martinez N, Chiu-Garcia JA, Black WC, IV, Beaty BJ. Phylogenetic analysis of West Nile virus, Nuevo Leon State, Mexico. Emerg. Infect. Dis. 2004;10(7):1314–1317. doi: 10.3201/eid1007.030959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault AC, Langevin SA, Ramey WN, Fang Y, Beasley DWC, Barker CM, Sanders TA, Reisen WK, Barrett ADT, Bowen RA. Reduced avian virulence and viremia of West Nile virus isolates from Mexico and Texas. Am. J. Trop. Med. Hyg. 2011;85(4):758–767. doi: 10.4269/ajtmh.2011.10-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CT, Ebel GD, Lanciotti RS, Braut AC, Guzman H, Siirin M, Lambert A, Parsons RE, Beasley DWC, Novak RJ, Elizondo-Quiroga D, Green EN, Young DS, Stark LM, Drebot MA, Artsob H, Tesh RB, Kramer LD, Barrett ADT. Phylogenetic analysis of North American West Nile virus isolates, 2001-2004: evidence for the emergence of a dominant genotype. Virology. 2005;342:252–265. doi: 10.1016/j.virol.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Davis CT, Li L, May FJ, Bueno R, Jr., Dennett JA, Bala AA, Guzman H, Elizondo-Quiroga D, Tesh RB, Barrett ADT. Genetic stasis of dominant West Nile virus genotype, Houston, Texas. Emerg. Infect. Dis. 2007;13(4):601–604. doi: 10.3201/eid1304.061473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff E, Estrada-Franco JG, Brault AC, Navarro-Lopez R, Campomanes-Cortes A, Paz-Ramirez P, Solis-Hernandez M, Ramey WN, Davis CT, Beasley DWC, Tesh RB, Barrett ADT, Weaver SC. Introductions of West Nile virus strains into Mexico. Emerg. Infect. Dis. 2006;12(2):314–318. doi: 10.3201/eid1202.050871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel GD, Carricaburu J, Young D, Bernard KA, Kramer LD. Genetic and phenotypic variation of West Nile virus in New York, 2000-2003. Am. J. Trop. Med. Hyg. 2004;71(4):493–500. [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acid Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizondo-Quiroga D, Davis CT, Fernandez-Salas I, Escobar-Lopez R, Olmos DV, Gastalum LCS, Acosta MA, Elizondo-Quiroga A, Gonzalez-Rojas JI, Cordero JFC, Guzman H, Travassos da Rosa A, Blitvich BJ, Barrett ADT, Beaty BJ, Tesh RB. West Nile virus isolation in human and mosquitoes, Mexico. Emerg. Infect. Dis. 2005;11(9):1449–1452. doi: 10.3201/eid1109.050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Franco JG, Navarro-Lopez R, Beasley DWC, Coffey L, Carrara AS, Travassos da Rosa A, Clements T, Wang E, Ludwig GV, Cortes AC, Ramirez PP, Tesh RB, Barrett ADT, Weaver SC. West Nile virus in Mexico: evidence of widespread circulation since July 2002. Emerg. Infect. Dis. 2003;9(12):1604–1607. doi: 10.3201/eid0912.030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M, Guidon S, Gascuel O. Seaview version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Bio. Evol. 2010;27(2):221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- Guerrero-Sanchez S, Cuevas-Romero S, Nemeth NM, Trujillo-Olivera MTJ, Worwa G, Dupuis A, Brault AC, Kramer LD, Komar N, Estrada-Franco JG. West Nile virus infection of birds, Mexico. Emerg. Infect. Dis. 2011;17(12):2245–2252. doi: 10.3201/eid1712.110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- Ibarra-Juarez L, Eisen L, Bolling BG, Beaty BJ, Blitvich BJ, Sanchez-Casas RM, Ayala-Sulca YO, Fernandez-Salas I. Detection of West Nile virus-specific antibodies and nucleic acid in horses and mosquitoes, respectively, in Nuevo Leon State, northern Mexico, 2006-2007. Med. Vet. Entomol. 2012 doi: 10.1111/j.1365-2915.2012.01014.x. [Epub 2012 Apr 10] [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, Hall RA, MacKenzie JS, Cropp CB, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286(5448):2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- Lorono-Pino MA, Blitvich BJ, Farfan-Ale JA, Puerto FI, Blanco JM, Marlenee NL, Rosado-Paredes EP, Garcia-Rejon JE, Gubler DJ, Calisher CH, Beaty BJ. Serologic evidence of West Nile virus infection in horses, Yucatan State, Mexico. Emerg. Infect. Dis. 2003;9(7):857–859. doi: 10.3201/eid0907.030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen AR, May FJ, Li L, Guzman H, Bueno R, Jr., Dennett JA, Tesh RB, Barrett ADT. Evolution of new genotype of West Nile virus in North America. Emerg. Infect. Dis. 2011;17(5):785–793. doi: 10.3201/eid1705.101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees.. Proceedings of the Gateway Computing Environments Workshop (GCE); New Orleans, LA. 14 Nov 2010.2010. pp. 1–8. [Google Scholar]
- Moudy RM, Meola MA, Morin LLL, Ebel GD, Kramer LD. A newly emergent genotype of West Nile virus is transmitted earlier and more efficiently by Culex mosquitoes. Am. J. Trop. Med. Hyg. 2007;77(2):365–370. [PubMed] [Google Scholar]
- Pepperell C, Rau N, Krajden S, Kern R, Humar A, Mederski B, Simor A, Low DE, McGeer A, Mazzulli T, Burton J, Jaigobin C, Fearon M, Artsob H, Drebot MA, Halliday W, Brunton J. West Nile virus infection in 2002: morbidity and mortality among patients admitted to hospital in southcentral Ontario. CMAJ. 2003;168(11):1399–1405. [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Rodriguez D, Blitvich BJ, Lopez M, Fernandez-Salas I, Jimenez JR, Farfan-Ale JA, Tamez RC, Longoria CM, Aguilar M, Rivas-Estilla AM. Serologic surveillance for West Nile virus and other flaviviruses in febrile patients, encephalitic patients, and asymptomatic blood donors in northern Mexico. Vector Borne Zoonotic Dis. 2010;10(2):151–157. doi: 10.1089/vbz.2008.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. A fast bootstrapping algorithm for the RAxML web-servers. Syst. Biol. 2008;57(5):758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Tesh RB, Travassos da Rosa APA, Guzman H, Araujo TP, Xiao SY. Immunization with heterologous flaviviruses protects against fatal West Nile encephalitis. Emerg. Infect. Dis. 2002;8:245–251. doi: 10.3201/eid0803.010238. [DOI] [PMC free article] [PubMed] [Google Scholar]