Intestinal innate lymphoid cells
Keywords: inflammatory bowel disease, innate lymphoid cell, intestinal homeostasis, mucosal immunology
Abstract
Innate lymphoid cells (ILCs) are a recently appreciated immune cell population that is constitutively found in the healthy mammalian gastrointestinal (GI) tract and associated lymphoid tissues. Translational studies have revealed that alterations in ILC populations are associated with GI disease in patients, such as inflammatory bowel disease, HIV infection and colon cancer, suggesting a potential role for ILCs in either maintaining intestinal health or promoting intestinal disease. Mouse models identified that ILCs have context-dependent protective and pathologic functions either during the steady state, or following infection, inflammation or tissue damage. This review will discuss the associations of altered intestinal ILCs with human GI diseases, and the functional consequences of targeting ILCs in mouse models. Collectively, our current understanding of ILCs suggests that the development of novel therapeutic strategies to modulate ILC responses will be of significant clinical value to prevent or treat human GI diseases.
Introduction
Innate lymphoid cells (ILCs) are a family of innate immune cells that critically contribute to the state of health or disease in the mammalian gastrointestinal (GI) tract. The development, heterogeneity, plasticity and regulation of ILCs have been extensively reviewed elsewhere (1–7). In brief, ILCs develop from lymphoid precursors in a process regulated by the common γ-chain cytokine receptor and the transcription factors inhibitor of DNA binding 2 (Id2), T-cell factor 1 (TCF1) and GATA-binding protein 3 (GATA3). Mature ILCs express the IL-7 receptor (CD127), but lack surface markers associated with other immune cell lineages, including T cells, B cells, granulocytes and myeloid cells. Further, ILCs can be divided into three groups based on transcriptional regulation. Group 1 ILCs (ILC1) constitutively express T-bet but not retinoic acid-related orphan receptor γt (RORγt), group 2 ILCs (ILC2) constitutively express GATA3 but not RORγt and group 3 ILCs (ILC3) constitutively express RORγt.
There are numerous shared regulatory and effector pathways that are associated with each group of ILCs. For example, ILC1 respond to IL-12 and IL-18 to produce IFN-γ and TNF-α, ILC2 respond to IL-25, IL-33 and thymic stromal lymphopoietin (TSLP) to produce IL-5, IL-9, IL-13 and amphiregulin, and ILC3 respond to IL-1β and IL-23 to produce IL-17 and IL-22. However, significant heterogeneity has been found in ILC1 and ILC3 populations. Currently, ILC1 encompass classical NK cells (which will not be discussed in this review) and recently identified ILC1 that lack expression of eomesodermin and cytolytic potential, and can be heterogeneous in expression of natural cytotoxicity receptors (NCRs), such as NKp46 and NKp44. ILC3 encompass CCR6+ cells that most closely resemble fetal lymphoid tissue inducer (LTi) cells and CCR6− cells that can co-express T-bet and NCRs. The lineage relationships, plasticity and significance of heterogeneity in groups of ILCs remain unclear and are active areas of investigation.
Analyses of ILCs in humans and mice have revealed that the GI tract and associated lymphoid tissues constitutively contain populations of ILCs under homeostatic conditions. Further, studies in patient populations with diseases that affect the GI tract, including inflammatory bowel disease (IBD), HIV and cancer, have revealed significant alterations in either the composition or the functional potential of ILC populations. Mouse models have identified several critical pathways by which ILCs can directly modulate intestinal homeostasis, immunity, inflammation and tissue repair, suggesting that further interrogation of ILCs could provoke the development of novel preventative and therapeutic strategies for GI diseases. The focus of this review will be to discuss data supporting the idea that that alterations in ILC populations occur in the intestine of healthy versus diseased humans and to highlight functional studies in mice identifying context-dependent protective and pathologic roles for intestinal ILCs.
Human ILCs in GI health and disease
Subsets of ILCs were first discovered in the intestinal tract and associated lymphoid tissues of fetal and adult donors. In a seminal study by Colonna et al., a subset of innate immune cells was identified—in the tonsils, Peyer’s patches and lamina propria of adult donors—that constitutively expressed NKp44, RORC (which encodes RORγ isoforms) and IL-22 (8). Similarly, a report by Cupedo, Spits et al. identified a population of NCR− CD127+ innate immune cells present in the fetal intestine and mesenteric lymph nodes that expressed IL-17 and IL-22, and further characterized a post-natal development of NKp44+ CD127+ cells that express IL-22 and RORC in human tonsils (9). Subsequent studies suggested that IL-22-expressing NKp44+ cells might be a subset of immature NK cells (10) and highlighted many questions regarding the developmental and lineage relationships of innate IL-22- and IL-17-expressing cell types in the GI tract and associated lymphoid tissues of humans.
Since those initial reports, more recent studies have demonstrated that these cells are indeed a novel innate immune cell population, now termed ILC3, that are distinct from classical NK cells (11) and uniquely regulated by environmental and inflammatory stimuli (12–15). The ability of ILC3 to rapidly produce IL-22 and IL-17, two cytokines that induce expression of anti-microbial peptides and inflammatory mediators (16), provoked the hypothesis that they were critical regulators of intestinal immunity and inflammation.
Consistent with this, ILC3 were found to be the dominant source of IL-22 in the intestine and associated lymphoid tissue of healthy human donors (17) and, as discussed below, alterations in ILC3 populations were identified in multiple infectious, inflammatory and metabolic diseases affecting the GI tract. For example, it was reported by multiple groups that IL-22- and IL-17-expressing ILC3 are depleted from the GI tract of non-human primates following infection with a highly pathogenic strain of Simian immunodeficiency virus and induction of tissue damage in the intestine (18–20), a pathway critically linked to the progression of AIDS. Further, the presence of IL-22-producing ILC3 populations has been correlated with preserved integrity of the GI tract of HIV-infected individuals (21, 22), suggesting a beneficial role for ILC3 and IL-22 in maintaining intestinal health during chronic viral infection.
IBD patients have also been reported to exhibit significant alterations in intestinal ILCs. IL-22-producing NKp44+ ILC3 were found to be reduced in patients with Crohn’s disease (23, 24); however, these ILC3 were preserved in ankylosing spondylitis patients exhibiting subclinical intestinal inflammation (25), suggesting that this population of cells may play a role in limiting the development of clinical GI disease. This loss of a potentially ‘protective’ ILC3 population in IBD was also associated with an increase in potentially ‘pathologic’ ILCs (23, 24, 26). Powrie et al. identified an expansion of CD127+ IL-23-responsive, IL-17-producing ILC3 in the intestinal lamina propria of Crohn’s disease patients (26). In a seminal study by Spits et al., a subset of IFN-γ-producing ILC1 were found to be increased in Crohn’s disease patients relative to non-inflamed controls, and further expansion of human ILC1 could be driven by experimental intestinal damage in a humanized mouse model (24). Colonna et al. also identified a population of T-bet+ IFN-γ-producing ILC1 residing in the intra-epithelial cell compartment, which accumulated in Crohn’s disease patients (27). Collectively, these reports suggest that there are significant alterations in the composition or functional potential of intestine-resident ILCs during chronic infection or tissue inflammation in humans.
The mechanisms that result in dysregulated human ILC populations in these chronic diseases currently remain unclear. Several studies suggest the loss of protective ILCs and accumulation of pathologic ILCs may occur through altered lineage plasticity or dysregulated exposure to regulatory cytokines or microbial stimuli (12–14, 24, 26, 28, 29). Further, a recent report identified that IL-22+ ILC3 are enriched in tumors of patients with colorectal cancer (30), suggesting that alterations in ILCs may be present in a broad spectrum of human GI diseases. Finally, although the presence of ILC2 in the intestine and associated lymphoid tissues has been identified in non-diseased fetal and adult donors (31), it remains poorly defined whether they are dysregulated in the context of intestinal diseases. However, one recent report identified an accumulation of IL-13-producing cells that resemble ILC2 in fibrotic Crohn’s disease patients (32), suggesting an involvement in pathologic collagen deposition. In future analyses of ILCs in the human intestine, it will be important to undertake extensive phenotypic and functional characterization to fully elucidate groups of ILCs in the healthy intestine and alterations that may occur in infectious, inflammatory or metabolic diseases.
The role of ILCs in maintaining intestinal health
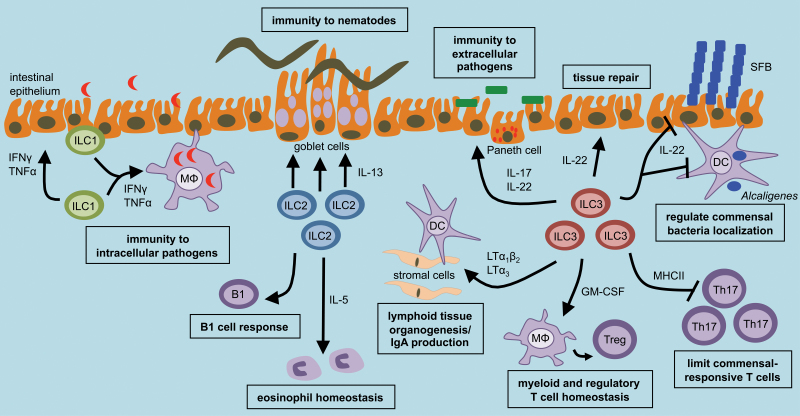
Functional studies in mice have revealed that ILCs can play a significant role in maintaining intestinal health by promoting immunity to pathogens, limiting inappropriate inflammatory responses to commensal bacteria or dietary antigens, or mediating repair following tissue damage. This is accomplished through expression of a number of factors that can influence stromal, epithelial, myeloid, granulocyte and adaptive immune cell responses (Fig. 1).
Fig. 1.
ILCs are critical regulators of intestinal health. Intestinal ILCs can promote optimal innate and adaptive immunity, maintain immune cell homeostasis and mediate tissue repair. This occurs through regulation of multiple epithelial, stromal, granulocyte, myeloid and adaptive immune cell lineages.
ILCs promote intestinal immunity to pathogens
ILCs can be a rapid source of protective cytokines following initial exposure to a variety of pathogens. For example, a recently described ILC1 population was found to be an important innate source of IFN-γ and TNF-α following oral infection with Toxoplasma gondii, which promoted the recruitment of inflammatory monocytes and control of parasite replication (33). ILC2 in the small intestine are critical for promoting tissue immunity via rapid production of IL-5 and IL-13, which induces intestinal goblet cell responses and expulsion of intestinal nematodes, such as Nippostrongylus brasiliensis (34, 35). Finally, ILC3 are a rapid source of IL-22 and IL-17, which induce intestinal epithelial cell expression of anti-microbial peptides and innate immunity to extracellular bacteria, such as Citrobacter rodentium (8, 16, 36, 37). Therefore, ILCs provide rapid cytokine production that is critical to control pathogen replication and clearance early following infection.
As previously reviewed (38), ILCs also play a significant role in modulating adaptive immunity in the intestine through either indirect interactions with stromal cells or direct interactions with adaptive immune cells. Studies by Mebius et al. (39, 40) identified a subset of ILC3, termed LTi cells, that interacts with stromal cells to induce secondary lymphoid organogenesis prior to birth. Following birth, a complex interplay between mammalian hosts and commensal bacteria facilitate the maturation of intestinal cryptopatches to isolate lymphoid follicles by RORγt+ ILC3 (41, 42).
ILC3 promote the development of GALT through expression of lymphotoxin (LT) and interactions with stromal cells, allowing the recruitment of immune cells to discrete organized locations (40). The formation of secondary lymphoid tissues in the intestine by ILC3 is critical for orchestrating optimal adaptive immunity. For example, regulation of these functions of ILC3 by dietary retinoids significantly impacts the magnitude of protective adaptive immune cell responses to mucosal viruses (43). Expression of surface-bound LTα1β2 or soluble LTα3 by ILC3 is also essential for generating host-protective IgA in the GI tract in a T-cell-independent and T-cell-dependent manner, respectively (44, 45). ILC2 have recently been described to regulate eosinophil homeostasis in the intestine through constitutive production of IL-5 (46). While the homeostatic functions of intestinal eosinophils are poorly understood, emerging evidence suggests they can contribute to optimal induction of IgA and Treg cells (47).
ILCs can also directly interact with adaptive immune cells to promote tissue immunity. ILC3 can promote B-cell responses through production of GM-CSF, B-cell activating factor (BAFF) and co-stimulatory receptors, and ILC2 have been shown to be critical regulators of B1 cell proliferation (14, 34, 48). ILC3 can also directly promote maintenance of memory CD4+ T-cell responses to pathogens through expression of Ox40 ligand (OX40L) and CD30L (49). Collectively, ILCs can orchestrate intestinal immunity to pathogens through rapid cytokine-dependent responses, or by facilitating optimal adaptive immune cell responses
ILCs limit inflammatory responses to commensal bacteria and dietary antigens
A growing body of evidence suggests that ILCs play a critical role in limiting inappropriate immune responses to non-harmful environmental stimuli, such as commensal bacteria and dietary antigens. In the absence of pathogen infection, loss of ILC3 or IL-22 results in peripheral dissemination of commensal bacteria and systemic inflammation (17). Interestingly, disseminating bacteria were found to be of the genus Alcaligenes or Achromobacter (17), which were characterized to constitutively colonize the GALT of healthy humans, non-human primates and mice (50), suggesting that the ILC3–IL-22 pathway selectively regulates the anatomical containment of commensal bacteria in order to limit chronic inflammation.
Consistent with this, it has been demonstrated that regulation of ILC3 and IL-22 by the aryl hydrocarbon receptor (Ahr) is critical to limit colonization of intestinal epithelial cells with segmented filamentous bacteria (SFB). Ahr-deficient mice exhibit an overgrowth of SFB and a subsequent increase in pro-inflammatory Th17 cells (51). Collectively, these studies suggest that the ILC3–IL-22 pathway is critical to maintain intestinal homeostasis by limiting the dissemination and replication of commensal bacteria that are intimately associated with mammalian hosts, such as Alcaligenes and Achromobacter that are resident in lymphoid tissue, or SFB that are associated with intestinal epithelial cells.
Emerging evidence suggests that ILC3 may also limit inappropriate immune responses to commensal bacteria through other effector pathways. For example, it was recently shown that ILC3 can directly limit pro-inflammatory CD4+ T-cell responses to commensal bacteria through expression of MHC class II (MHCII) (52). Loss of ILC-intrinsic MHCII resulted in dysregulated effector CD4+ Th17 cell responses to commensal bacteria and spontaneous intestinal inflammation (52). A recent report by Merad et al. also identified that ILC3 are a dominant source of GM-CSF in the intestine and critically regulate myeloid cell homeostasis (53). Intriguingly, ILC3-mediated regulation of myeloid cells was required to maintain optimal Treg cell responses and tolerance to dietary antigens (53). Collectively, these results suggest that ILC3 play a central role in regulating intestinal homeostasis by limiting inappropriate immune responses to normally beneficial or harmless environmental stimuli.
ILCs mediate intestinal tissue repair
Production of IL-22 is critical in mediating repair of the intestinal epithelium in the context of tissue damage (16). Studies by Eberl et al. have highlighted that ILC3 rapidly respond to experimental tissue damage in the intestine and mediate IL-22-dependent tissue repair (54). Interestingly, in a mouse model of graft versus host disease, ILC3-derived IL-22 maintained intestinal epithelial barrier integrity following hematopoietic stem cell transplantation by directly protecting intestinal stem cells from immune-mediated damage (55). Acting directly on intestinal stem cells may be one mechanism by which a relatively rare population, such as ILC3, can substantially influence homeostasis in the intestine. Collectively, ILCs promote multiple beneficial processes in order to maintain a state of health in the mammalian intestine.
The role of ILCs in promoting intestinal disease
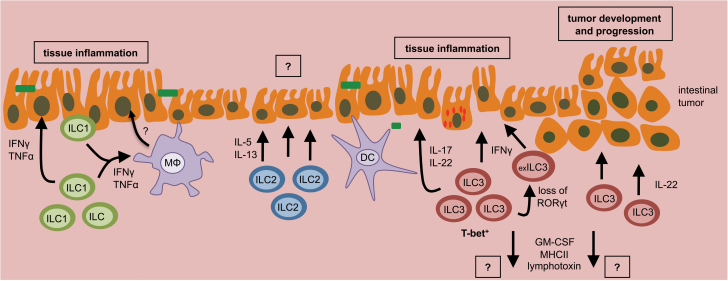
In contrast to the tissue protective roles of intestinal ILCs, dysregulated ILC responses or changes in the composition of intestinal ILC populations can promote intestinal inflammation and the development or progression of intestinal cancers (Fig. 2).
Fig. 2.
Dysregulated ILC responses can promote intestinal disease. Dysregulated ILC responses, or changes in the composition of ILC populations, can promote chronic intestinal inflammation or initiate the development and progression of intestinal tumors. This can occur through loss of regulation or sustained activation.
Dysregulated ILC responses promote intestinal inflammation
Pioneering research by Powrie et al. (56) identified that IL-23 production in response to Helicobacter hepaticus infection, or following dysregulated activation of dendritic cells via anti-CD40 antibody administration, results in the expansion of a T-bet+ ILC3 population that promotes colitis in an IL-17- and IFN-γ-dependent manner. Later research identified that T-bet co-expression in ILC3 is associated with IFN-γ production and enterocolitis following Salmonella infection (57). IL-22 production by ILC3 can also promote colitis in some contexts (58), which may be dependent on co-expression of other pro-inflammatory cytokines (16).
In addition to ILC3, recent research suggests that dysregulated intra-epithelial ILC1 responses can promote IFN-γ-dependent intestinal inflammation following anti-CD40 antibody administration (27). Emerging evidence suggest that a shift from protective to pathologic ILC responses in the intestine may involve lineage plasticity, altered expression of transcriptional regulators or engagement of alternative activation pathways (24, 28, 29). For example, recent work suggests that loss of RORγt expression in ILC3 is accompanied with an up-regulation of T-bet and IFN-γ expression (24, 28). As RORγt is a defining transcription factor of ILC3, these cells have been termed ‘ex-ILC3’, but are distinct from ILC1, which have no history RORγt expression (33). Collectively, these data suggest that dysregulation or expansion of pro-inflammatory ILC populations may directly promote colitis through the production of cytokines IL-17, IL-22, TNF-α and IFN-γ. Additional studies are required to carefully define the mechanistic pathways that promote dysregulation of the composition or functional potential of intestinal ILCs, and further, a role for ILC2 in intestinal inflammation.
Dysregulated ILC responses support intestinal cancers
In addition to chronic inflammation, dysregulated ILC responses have been linked to the development of intestinal cancers. Administration of a carcinogen following H. hepaticus infection results in the formation of aberrant crypt lesions, which were enriched in expression of IL-23, IL-22 and IL-17 (30). ILC3 and IL-22 were required for cancer progression in this model (30), suggesting that sustained activation of ILC3 can promote the formation of intestinal tumors.
In support of this, it was recently highlighted that uncontrolled IL-22-dependent signals, occurring through a loss of the regulatory IL-22 binding protein (IL-22BP), results in increased tumorigenesis in the intestine following tissue damage (59). Further, it has also been shown that loss of intestinal barrier function can contribute to sustained activation of the IL-23–IL-17 pathway and promote growth of intestinal tumors (60). It is possible to speculate that the ILC3–IL-22 axis may be particularly poised to promote tumorigenesis by its ability to act directly on intestinal stem cells, as previously observed following hematopoietic stem cell transplantation (55).
Therefore, sustained activation of ILCs, likely the result of chronic infection or impaired intestinal barrier function, can promote the development and progression of inflammation and cancer within the GI tract. Despite these advances, little is known about whether other factors expressed by ILC3, such as LT, MHCII or GM-CSF, can in some contexts promote the development of intestinal inflammation or tumors, and this will be an important area of future investigation.
Summary and outstanding questions
As discussed above, translational studies in healthy human donors and patient populations have revealed a significant association of altered intestinal ILC responses with human disease, and basic mouse models have demonstrated that ILCs have the functional potential to promote both tissue protective and pathologic responses. Therefore, it is likely that developing novel targets to manipulate ILCs will be of significant value to prevent or limit human intestinal diseases. Additional analyses are required to identify the regulatory pathways that control the composition and functional potential of ILC responses in the intestine. Recent reports have highlighted that microbial and dietary signals can significantly influence intestinal ILCs (43, 54, 61), suggesting that not only will this be important to consider in our interpretation of basic and translational studies, but also that this may be a useful strategy to identify pathways to boost protective ILC responses while potentially limiting pathologic ILC responses.
Funding
Research in the Sonnenberg laboratory is supported by the National Institutes of Health (DP5OD012116); the NIAID Mucosal Immunology Studies Team (MIST) Scholar Award in Mucosal Immunity; a pilot grant from the NIDDK P30 center for Molecular Studies in Digestive and Liver Diseases (P30DK50306) and the Institute for Translational Medicine and Therapeutics Transdisciplinary Program in Translational Medicine and Therapeutics (UL1-RR024134 from the US National Center for Research Resources).
Acknowledgements
Members of the Sonnenberg laboratory are thanked for discussions and critical reading of the manuscript.
References
- 1. Spits H., Cupedo T. 2012. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 30:647. [DOI] [PubMed] [Google Scholar]
- 2. Cherrier M., Ohnmacht C., Cording S., Eberl G. 2012. Development and function of intestinal innate lymphoid cells. Curr. Opin. Immunol. 24:277. [DOI] [PubMed] [Google Scholar]
- 3. Walker J. A., Barlow J. L., McKenzie A. N. 2013. Innate lymphoid cells–how did we miss them? Nat. Rev. Immunol. 13:75. [DOI] [PubMed] [Google Scholar]
- 4. Tanriver Y., Diefenbach A. 2014. Transcription factors controlling development and function of innate lymphoid cells. Int. Immunol. 26:119. [DOI] [PubMed] [Google Scholar]
- 5. Sonnenberg G. F., Mjösberg J., Spits H., Artis D. 2013. SnapShot: innate lymphoid cells. Immunity 39:622. [DOI] [PubMed] [Google Scholar]
- 6. Sonnenberg G. F., Artis D. 2012. Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity 37:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spits H., Artis D., Colonna M., et al. 2013. Innate lymphoid cells–a proposal for uniform nomenclature. Nat. Rev. Immunol. 13:145. [DOI] [PubMed] [Google Scholar]
- 8. Cella M., Fuchs A., Vermi W., et al. 2009. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cupedo T., Crellin N. K., Papazian N., et al. 2009. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat. Immunol. 10:66. [DOI] [PubMed] [Google Scholar]
- 10. Hughes T., Becknell B., McClory S., et al. 2009. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin-22. Blood 113:4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crellin N. K., Trifari S., Kaplan C. D., Cupedo T., Spits H. 2010. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J. Exp. Med. 207:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hughes T., Becknell B., Freud A. G., et al. 2010. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity 32:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crellin N. K., Trifari S., Kaplan C. D., Satoh-Takayama N., Di Santo J. P., Spits H. 2010. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity 33:752. [DOI] [PubMed] [Google Scholar]
- 14. Cella M., Otero K., Colonna M. 2010. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc. Natl Acad. Sci. USA 107:10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoorweg K., Peters C. P., Cornelissen F., et al. 2012. Functional differences between human NKp44(-) and NKp44(+) RORC(+) innate lymphoid cells. Front. Immunol. 3:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sonnenberg G. F., Fouser L. A., Artis D. 2011. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 12:383. [DOI] [PubMed] [Google Scholar]
- 17. Sonnenberg G. F., Monticelli L. A., Alenghat T., et al. 2012. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 336:1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klatt N. R., Estes J. D., Sun X., et al. 2012. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol. 5:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu H., Wang X., Liu D. X., Moroney-Rasmussen T., Lackner A. A., Veazey R. S. 2012. IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques. Mucosal Immunol. 5:658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reeves R. K., Rajakumar P. A., Evans T. I., et al. 2011. Gut inflammation and indoleamine deoxygenase inhibit IL-17 production and promote cytotoxic potential in NKp44+ mucosal NK cells during SIV infection. Blood 118:3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim C. J., Nazli A., Rojas O. L., et al. 2012. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol. 5:670. [DOI] [PubMed] [Google Scholar]
- 22. Fernandes S. M., Pires A. R., Ferreira C., et al. 2014. Enteric mucosa integrity in the presence of a preserved innate IL-22 compartment in HIV-1 treated individuals. J. Infect Dis. [DOI] [PubMed] [Google Scholar]
- 23. Takayama T., Kamada N., Chinen H., et al. 2010. Imbalance of NKp44(+)NKp46(-) and NKp44(-)NKp46(+) natural killer cells in the intestinal mucosa of patients with Crohn’s disease. Gastroenterology 139:882. [DOI] [PubMed] [Google Scholar]
- 24. Bernink J. H., Peters C. P., Munneke M., et al. 2013. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 14:221. [DOI] [PubMed] [Google Scholar]
- 25. Ciccia F., Accardo-Palumbo A., Alessandro R., et al. 2012. Interleukin-22 and interleukin-22-producing NKp44+ natural killer cells in subclinical gut inflammation in ankylosing spondylitis. Arthritis Rheum. 64:1869. [DOI] [PubMed] [Google Scholar]
- 26. Geremia A., Arancibia-Cárcamo C. V., Fleming M. P., et al. 2011. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 208:1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fuchs A., Vermi W., Lee J. S., et al. 2013. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity 38:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vonarbourg C., Mortha A., Bui V. L., et al. 2010. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes. Immunity 33:736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Glatzer T., Killig M., Meisig J., et al. 2013. RORγt⁺ innate lymphoid cells acquire a proinflammatory program upon engagement of the activating receptor NKp44. Immunity 38:1223. [DOI] [PubMed] [Google Scholar]
- 30. Kirchberger S., Royston D. J., Boulard O., et al. 2013. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med. 210:917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mjösberg J. M., Trifari S., Crellin N. K., et al. 2011. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 12:1055. [DOI] [PubMed] [Google Scholar]
- 32. Bailey J. R., Bland P. W., Tarlton J. F., et al. 2012. IL-13 promotes collagen accumulation in Crohn’s disease fibrosis by down-regulation of fibroblast MMP synthesis: a role for innate lymphoid cells? PLoS One 7:e52332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klose C. S., Flach M., Möhle L., et al. 2014. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 157:340. [DOI] [PubMed] [Google Scholar]
- 34. Moro K., Yamada T., Tanabe M., et al. 2010. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463:540. [DOI] [PubMed] [Google Scholar]
- 35. Neill D. R., Wong S. H., Bellosi A., et al. 2010. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464:1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sonnenberg G. F., Monticelli L. A., Elloso M. M., Fouser L. A., Artis D. 2011. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 34:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Satoh-Takayama N., Vosshenrich C. A., Lesjean-Pottier S., et al. 2008. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29:958. [DOI] [PubMed] [Google Scholar]
- 38. Hepworth M. R., Sonnenberg G. F. 2014. Regulation of the adaptive immune system by innate lymphoid cells. Curr. Opin. Immunol. 27:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mebius R. E., Rennert P., Weissman I. L. 1997. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7:493. [DOI] [PubMed] [Google Scholar]
- 40. Mebius R. E. 2003. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 3:292. [DOI] [PubMed] [Google Scholar]
- 41. Eberl G., Marmon S., Sunshine M. J., Rennert P. D., Choi Y., Littman D. R. 2004. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5:64. [DOI] [PubMed] [Google Scholar]
- 42. Bouskra D., Brézillon C., Bérard M., et al. 2008. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456:507. [DOI] [PubMed] [Google Scholar]
- 43. van de Pavert S. A., Ferreira M., Domingues R. G., et al. 2014. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 508:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kruglov A. A., Grivennikov S. I., Kuprash D. V., et al. 2013. Nonredundant function of soluble LTα3 produced by innate lymphoid cells in intestinal homeostasis. Science 342:1243. [DOI] [PubMed] [Google Scholar]
- 45. Tsuji M., Suzuki K., Kitamura H., et al. 2008. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity 29:261. [DOI] [PubMed] [Google Scholar]
- 46. Nussbaum J. C., Van Dyken S. J., von Moltke J., et al. 2013. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chu V. T., Beller A., Rausch S., et al. 2014. Eosinophils promote generation and maintenance of immunoglobulin-a-expressing plasma cells and contribute to gut immune homeostasis. Immunity 40:582. [DOI] [PubMed] [Google Scholar]
- 48. Magri G., Miyajima M., Bascones S., et al. 2014. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat. Immunol. 15:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Withers D. R., Gaspal F. M., Mackley E. C., et al. 2012. Cutting edge: lymphoid tissue inducer cells maintain memory CD4 T cells within secondary lymphoid tissue. J. Immunol. 189:2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Obata T., Goto Y., Kunisawa J., et al. 2010. Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc. Natl Acad. Sci. USA 107:7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Qiu J., Guo X., Chen Z. M., et al. 2013. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity 39:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hepworth M. R., Monticelli L. A., Fung T. C., et al. 2013. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 498:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mortha A., Chudnovskiy A., Hashimoto D., et al. 2014. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343:1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sawa S., Lochner M., Satoh-Takayama N., et al. 2011. RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat. Immunol. 12:320. [DOI] [PubMed] [Google Scholar]
- 55. Hanash A. M., Dudakov J. A., Hua G., et al. 2012. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 37:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Buonocore S., Ahern P. P., Uhlig H. H., et al. 2010. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464:1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Klose C. S., Kiss E. A., Schwierzeck V., et al. 2013. A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature 494:261. [DOI] [PubMed] [Google Scholar]
- 58. Eken A., Singh A. K., Treuting P. M., Oukka M. 2014. IL-23R+ innate lymphoid cells induce colitis via interleukin-22-dependent mechanism. Mucosal Immunol. 7:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huber S., Gagliani N., Zenewicz L. A., et al. 2012. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Grivennikov S. I., Wang K., Mucida D., et al. 2012. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Spencer S. P., Wilhelm C., Yang Q., et al. 2014. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 343:432. [DOI] [PMC free article] [PubMed] [Google Scholar]