Abstract
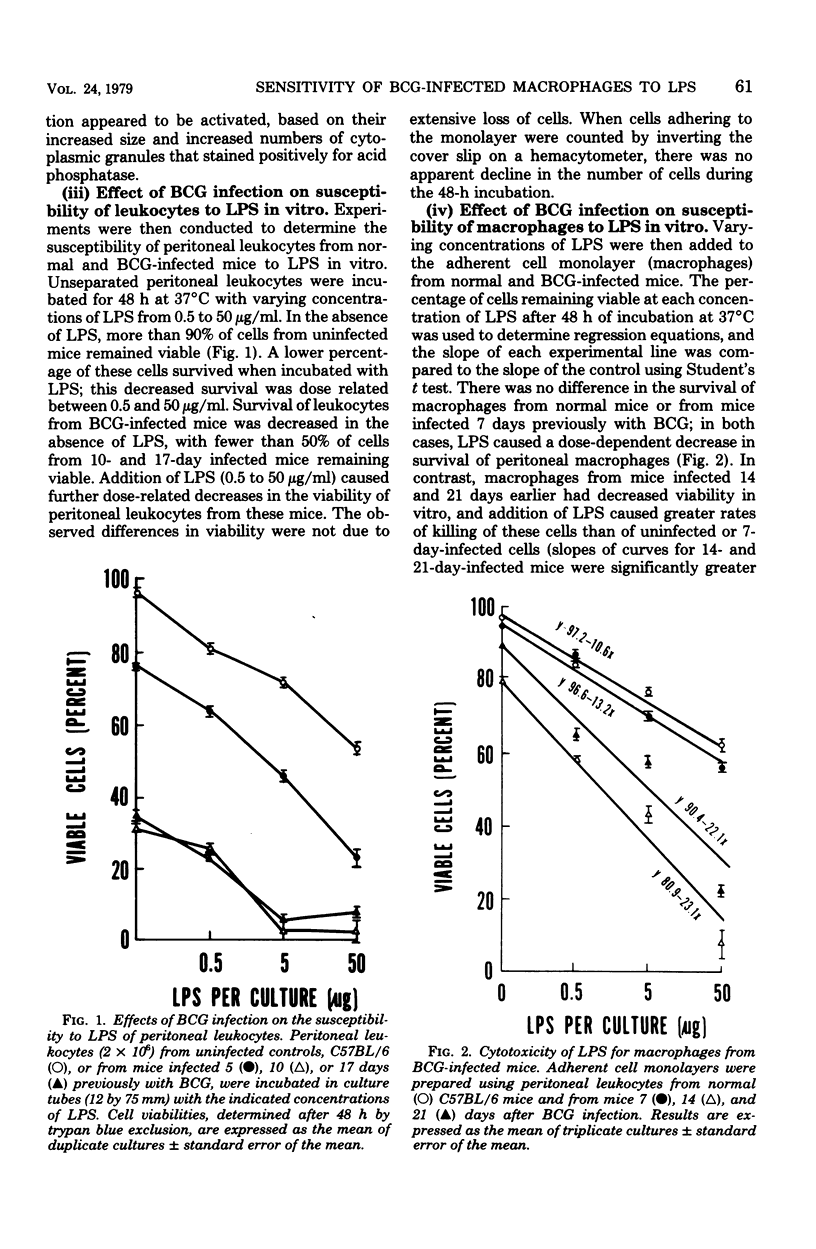
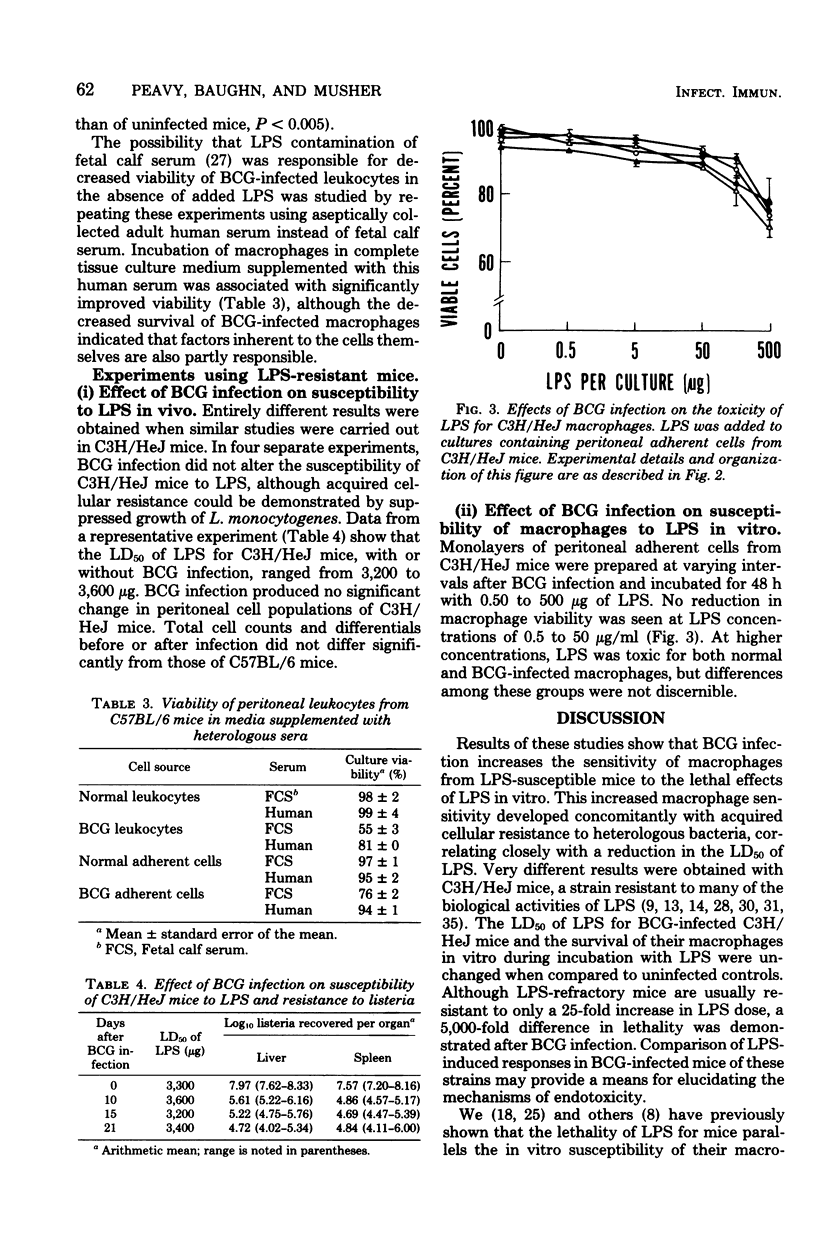
Mice infected intravenously with Mycobacterium bovis (BCG) are 100 to 1,000 times more sensitive to the lethal effects of bacterial lipopolysaccharides (LPS). Since BCG infection results in macrophage activation and LPS may cause pathophysiological effects through interaction with this cell type, it was of interest to determine whether macrophages from BCG-infected animals were more susceptible to the toxic effects of LPS in vitro. When LPS-susceptible, C57BL/6 mice were infected with BCG, a significant reduction in the 50% lethal dose of LPS was first observed after 7 days and persisted for several weeks. Macrophages from these animals had greatly increased susceptibility to LPS in vitro, which correlated with the development of acquired cellular resistance as determined by their ability to inhibit the growth of Listeria monocytogenes. In contrast, BCG infection of C3H/HeJ mice, a strain resistant to LPS, did not alter the 50% lethal dose of LPS for these animals or increase the sensitivity of their peritoneal macrophages to LPS in vitro. These results indicate that susceptibility of BCG-infected mice to the lethal effects of LPS parallels the susceptibility of their macrophages in vitro; release of vasoactive substances from LPS-susceptible activated macrophages in vivo may be, in part, responsible for lethality.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERNATHY R. S., BRADLEY G. M., SPINK W. W. Increased susceptibility of mice with brucellosis to bacterial endotoxins. J Immunol. 1958 Oct;81(4):271–275. [PubMed] [Google Scholar]
- Apte R. N., Hertogs C. F., Pluznik D. H. Regulation of lipopolysaccharide-induced granulopoiesis and macrophage formation by spleen cells. I. Relationship between colony-stimulating factor release and lymphocyte activation in vitro. J Immunol. 1977 Apr;118(4):1435–1440. [PubMed] [Google Scholar]
- BERRY L. J., SMYTHE D. S., YOUNG L. G. Effects of bacterial endotoxin on metabolism. I. Carbohydrate depletion and the protective role of cortisone. J Exp Med. 1959 Sep 1;110:389–405. doi: 10.1084/jem.110.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. P., Meltzer M. S., Zbar B. Tumor cytotoxicity in vitro by macrophages from mice infected with mycobacterium bovis strain BCG. J Natl Cancer Inst. 1974 Jun;52(6):1887–1895. doi: 10.1093/jnci/52.6.1887. [DOI] [PubMed] [Google Scholar]
- DANNENBERG A. M., Jr, BENNETT W. E. HYDROLASES OF MONONUCLEAR EXUDATE CELLS AND TUBERCULOSIS. I. EXUDATE CHARACTERISTICS, ESTERASES, PROTEINASES, AND LIPASE. Arch Pathol. 1963 Nov;76:581–591. [PubMed] [Google Scholar]
- Germain R. N., Williams R. M., Benacerraf B. Specific and nonspecific antitumor immunity. II. Macrophage-mediated nonspecific effector activity induced by BCG and similar agents. J Natl Cancer Inst. 1975 Mar;54(3):709–720. [PubMed] [Google Scholar]
- Glode L. M., Jacques A., Mergenhagen S. E., Rosenstreich D. L. Resistance of macrophages from C3H/HeJ mice to the in vitro cytotoxic effects of endotoxin. J Immunol. 1977 Jul;119(1):162–166. [PubMed] [Google Scholar]
- Glode L. M., Mergenhagen S. E., Rosenstreich D. L. Significant contribution of spleen cells in mediating the lethal effects of endotoxin in vivo. Infect Immun. 1976 Sep;14(3):626–630. doi: 10.1128/iai.14.3.626-630.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD J. G., BIOZZI G., HALPERN B. N., STIFFEL C., MOUTON D. The effect of Mycobacterium tuberculosis (BCG) infection on the resistance of mice to bacterial endotoxin and Salmonella enteritidis infection. Br J Exp Pathol. 1959 Jun;40(3):281–290. [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr Heterocytolysis by macrophages activated by bacillus Calmette-Guérin: lysosome exocytosis into tumor cells. Science. 1974 Apr 26;184(4135):468–471. doi: 10.1126/science.184.4135.468. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Chapman H. A., Jr, Weinberg J. B. Macrophage tumor killing: influence of the local environment. Science. 1977 Jul 15;197(4300):279–282. doi: 10.1126/science.327547. [DOI] [PubMed] [Google Scholar]
- Kincade P. W. Defective colony formation by B lymphocytes from CBA/N and C3H/HeJ mice. J Exp Med. 1977 Feb 1;145(2):249–263. doi: 10.1084/jem.145.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S., Hoffmann M. K., Thomas L. Induction of phenotypic lymphocyte differentiation in LPS unresponsive mice by an LPS-induced serum factor and by lipid A-associated protein. J Immunol. 1977 May;118(5):1910–1911. [PubMed] [Google Scholar]
- Kramer J. J., Granger G. A. The in vitro induction and release of a cell toxin by immune C57B1-6 mouse peritoneal macrophages. Cell Immunol. 1972 Jan;3(1):88–100. doi: 10.1016/0008-8749(72)90229-8. [DOI] [PubMed] [Google Scholar]
- Levin J., Tomasulo P. A., Oser R. S. Detection of endotoxin in human blood and demonstration of an inhibitor. J Lab Clin Med. 1970 Jun;75(6):903–911. [PubMed] [Google Scholar]
- Li C. Y., Yam L. T., Lam K. W. Acid phosphatase isoenzyme in human leukocytes in normal and pathologic conditions. J Histochem Cytochem. 1970 Jul;18(7):473–481. doi: 10.1177/18.7.473. [DOI] [PubMed] [Google Scholar]
- Peavy D. L., Baughn R. E., Musher D. M. Mitogenic activity of bacterial lipopolysaccharides in vivo: morphological and functinal characterization of responding cells. Infect Immun. 1978 Jan;19(1):71–78. doi: 10.1128/iai.19.1.71-78.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy D. L., Baughn R. E., Musher D. M. Strain-dependent cytotoxic effects of endotoxin for mouse peritoneal macrophages. Infect Immun. 1978 Jul;21(1):310–319. doi: 10.1128/iai.21.1.310-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy D. L., Shands J. W., Jr, Adler W. H., Smith R. T. Mitogenicity of bacterial endotoxins: characterization of the mitogenic principle. J Immunol. 1973 Aug;111(2):352–357. [PubMed] [Google Scholar]
- Piessens W. F., Churchill W. H., Jr, David Macrophages activated in vitro with lymphocyte mediators kill neoplastic but not normal cells. J Immunol. 1975 Jan;114(1 Pt 2):293–299. [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Defective tumoricidal capacity of macrophages from C3H/HeJ mice. J Immunol. 1978 Jan;120(1):329–334. [PubMed] [Google Scholar]
- SAITO K., SUTER E. LYSOSOMAL ACID HYDROLASES IN MICE INFECTED WITH BCG. J Exp Med. 1965 May 1;121:727–738. doi: 10.1084/jem.121.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E., KIRSANOW E. M. Hyperreactivity to endotoxin in mice infected with mycobacteria. Induction and elicitation of the reactions. Immunology. 1961 Oct;4:354–365. [PMC free article] [PubMed] [Google Scholar]
- SUTER E., ULLMAN G. E., HOFFMAN R. G. Sensitivity of mice to endotoxin after vaccination with BCG (Bacillus Calmette-Guérin). Proc Soc Exp Biol Med. 1958 Oct;99(1):167–169. doi: 10.3181/00379727-99-24282. [DOI] [PubMed] [Google Scholar]
- Shands J. W., Jr, Peavy D. L., Gormus B. J., McGraw J. In vitro and in vivo effects of endotoxin on mouse peritoneal cells. Infect Immun. 1974 Jan;9(1):106–112. doi: 10.1128/iai.9.1.106-112.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shands J. W., Jr, Senterfitt V. C. Endotoxin-induced hepatic damage in BCG-infected mice. Am J Pathol. 1972 Apr;67(1):23–40. [PMC free article] [PubMed] [Google Scholar]
- Shiigi S. M., Mishell R. I. Sera and the in vitro induction of immune responses. I. Bacterial contamination and the generation of good fetal bovine sera. J Immunol. 1975 Sep;115(3):741–744. [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Morrison D. C., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J Immunol. 1975 Feb;114(2 Pt 2):770–775. [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Weigle W. O., Riblet R., Watson J. Immunologic properties of bacterial lipopolysaccharide (LPS). III. Genetic linkage between the in vitro mitogenic and in vivo adjuvant properties of LPS. J Exp Med. 1976 Jan 1;143(1):143–150. doi: 10.1084/jem.143.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of host responses to endotoxin. Infect Immun. 1972 Jan;5(1):107–113. doi: 10.1128/iai.5.1.107-113.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M., Goodman G. W. Endotoxin protein: a B-cell mitogen and polyclonal activator of C3H/HeJ lymphocytes. J Exp Med. 1976 Sep 1;144(3):821–827. doi: 10.1084/jem.144.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. II. A gene that influences a membrane component involved in the activation of bone marrow-derived lymphocytes by lipipolysaccharides. J Immunol. 1975 May;114(5):1462–1468. [PubMed] [Google Scholar]



