Abstract
IL-1 receptor antagonist (IL-1rn) is a protein that binds to IL-1 receptors (IL-1r1) and inhibits the binding of IL-1α and IL-1β. In recent years, IL-1rn has been implicated to be associated with many human health problems. The effects of treatment of several inflammatory disorders with anakinra, which is an interleukin-1 (IL-1) receptor antagonist, have also been reported. Both positive and negative effects have been described. In this review, we systematically analyzed the expression, correlation, and regulation of IL-1rn and its 13 partner genes using available gene expression profiles from a variety of tissues in a well known transcriptome database, Genenetwork. The 13 partner genes include IL-1r1, IL-1β, IL-1α, Myd88, Irak1, Irak2, Irak4, Traf6, Tlr4, IL-1rap, Ikbkap, Nfkb1, and Nfkb2. Gene expression profiles are from 10 tissues including spleen, kidney, lung, whole brain, eye, prefrontal cortex, cerebellum, hippocampus, striatum, and nucleus accumbens. Our analysis indicated that the interactions among IL-1rn and its partner are complex and different from tissues to tissues, suggesting a broad spectrum of the effect of IL-1rn on biological and metabolic pathways. Transcripts and protein sequences resulted from different splicing, interaction with genomic background of individuals, and environmental factors affect function of IL-1rn. At present, our knowledge on the function of IL-1rn and its partner in various tissues or organs is very limited. The long term and extended effect of anakinra on human health needs further investigations. In the future, targeted sequences or oligos of Il-1rn might be ideal for therapeutic application with less toxic and more specific in the treatment of specific disease. Detailed study on the molecular function of IL-1rn and its interaction with other genes and environmental factors is essential for development therapeutic application using IL-1rn.
Keywords: Anakinra, Arthritis, Expression, Genomic background, IL-1 receptor antagonist, Inflammation
1. Introduction
IL-1 receptor antagonist (IL-1rn) is a protein that binds to IL-1 receptors (IL-1r1) and inhibits the binding of IL-1α and IL-1β. Its expression level is known to increase in the blood of patients with a variety of infectious, immune, and traumatic conditions. IL-1rn was initially cloned and described in 1990 [1]. In human genomics its gene is located on chromosome 2, between 113,864,791 and 113,891,593 bps (according to Ensembl, Human GRCH37). It has 9 transcripts, including five with known protein sequences. In mouse, it is located on chromosome 2: between 24,192,373 and 24,207,014 bps (according to Ensembl, Mouse NCBIM37). It has 6 transcripts including 4 with known protein sequences. The largest protein sequence of mouse (ENSMUSP00000110126, with 178aa) is 87% similar to that of human IL-1RN isoform #3.
2. IL-1 receptor antagonist, human diseases, and pharmaceutics application
Shortly following its discovery in 1999, a number of human diseases or disorders has been linked to the polymorphism or expression of IL-1rn or its partners. Several chronic inflammatory diseases have been linked to polymorphism of IL-1rn in humans. Those human disorders include arthritis [2], systemic lupus erythematosus [3], ulcerative colitis [4], alopecia areata [5], multiple sclerosis [6], diabetic nephropathy [7], and fat mass [8].
Recent reports on linkage of Il-1rn with human diseases seem to increase its spectrum to many pathological and physiological problems. Those problems include neonatal onset of sterile multifocal osteomyelitis, periostitis, and pustulosis [9], neonatal onset of pustular rash, osteopenia, lytic bone lesions, respiratory insufficiency, and thrombosis [10], generalized aggressive periodontitis [11,12], refractory adult-onset Still’s disease [13], coronary artery disease [14], schizophrenia [15], and risk of gastric cancer [16] and early stages of gastric carcinogenesis [17].
An important development related to research on function of IL-1rn is the clinic trial of anakinra, which is an interleukin-1 (IL-1) receptor antagonist. In a very recent period of time, it has been reported that it is effective on the treatment of several human inflammatory disorders, such as Mevalonate kinase deficiency (MKD)[18], systemic juvenile idiopathic arthritis (SJIA) [19–21], TNF receptor-associated periodic syndrome (TRAPS) [22], refractory chronic tophaceous gout [23], and refractory adult-onset Still’s disease [13], Schnitzler syndrome [24], cryopyrin-associated periodic syndromes [25], refractory Sweet’s syndrome [26], autoinflammatory Muckle–Wells syndrome [27], persistent knee effusion [28], familial Mediterranean fever [29], Castleman’s disease [30], and arthrofibrosis [31].
At the same time, at least several negative data or data suggesting precaution have been reported. Those data include the onset of focal bone erosion in neonatal-onset multisystem inflammatory disease [32], failure of anakinra treatment of pyoderma gangrenosum in an IBD patient [33], and hepatotoxicity due to tocilizumab and anakinra in rheumatoid arthritis [34]. Treatment with Anakinra improves disposition index but not insulin sensitivity in nondiabetic subjects with the metabolic syndrome [35].
These observations point out the complex role of IL-1rn in diseases and the function in human body. Those reports also suggest that IL-1rn may express or function differently in different tissues and IL-1rn and its partners may play different roles in the pathogenesis and pathophysiology in different tissues, as previously described by Luheshi and colleagues [36].
3. Expression levels of probes of IL-1rn and its partners in mouse tissues
To identify a group of genes that are relevant to IL-1rn, we input interleukin-1 receptor, type I; receptor for interleukin-1 alpha (IL-1A), beta (IL-1B), and interleukin-1 receptor antagonist protein (IL-1RA) on the Genenetwork (http://www.genenetwork.org/webqtl/main.py) [37], which consists of a set of linked resources for systems genetics. We then obtained the interaction partner genes of IL-1rn based on STRING analysis. As expected, as shown in Fig. 1, IL-1rn participate in different gene networks. Based on the Gene net work and our experience, we examined the expression level of 14 genes, Il-1r1, Il-1b, Il-1a, Myd88, Irak1, Irak2, Irak4, Traf6, Il-1rn, Tlr4, Il-1rap, Ikbkap, Nfkb1, and Nfkb2 and their interactions with each other. The expression levels in 10 tissues of those genes are from the same set of RI strain, the BXD strains. The combined BXD strain set at present is the largest RI mapping panel of mouse models (http://www.genenetwork.org/mouseCross.html#BXD). The BXD RI strains including 46 recently generated strains at Dr. Williams’ laboratory [38] and 36 previously generated strains from the Jackson laboratory [39]. All of them are derived from C57BL/6J (B6) X DBA/2J (D2).
Fig. 1.
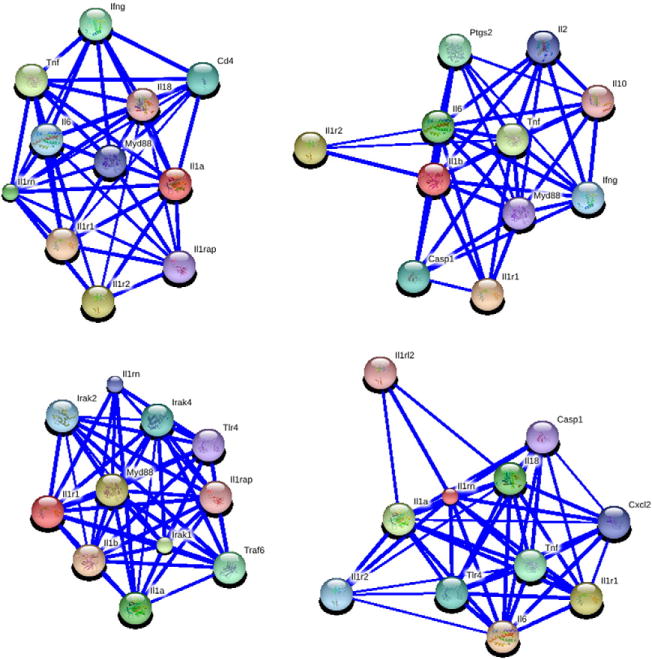
Potential gene network of IL-1rn. Data are generated using STRING 9.0. Nodes are colored for predicted functional links. Stronger associations are represented by thicker lines.
We examined the expression levels among IL-1ra and its partners in ten tissues, spleen, kidney, lung, whole brain, eye, prefrontal cortex, cerebellum, hippocampus, striatum, and nucleus accumbens. Microarray data are from Affymetrix M430.v2. The 430v2 array consists of 25-nucleotide probes that estimate the expression of genes and/or transcripts.
Table 1 lists the probes of each gene and their average expression levels in mice tested. Although numbers of mouse strains used in different tissues are different, all 14 genes express at a relatively high level across all 10 tissues. Among 14 genes, 10 have multiple probes: Il-1r1, Irak1, Irak4, Traf6, Il-1rn, Tlr4, Il-1rap, Ikbkap, Nfkb1, and Nfkb2. While the expression levels of different genes showed differences, majority of probes from the same gene also showed different levels too. 1) In general, probes from exons are different from probes from 3′ UTR. Particularly, the expression levels of two probes of Ikbkap, one at mid 3′ UTR while the other from exons, showed a significant difference (P<0.0002). One is at 10.18 while the other is at 7.61 (Table 1). Il-1rap has five probes from different location of the gene. The expression level of probe 1449585 in exon region is much lower than that of other four probes, which are at the different distances of 3′ UTR. 2) The difference seems not only between probes from exons and 3′ UTR but also among probes from different positions from 3′ UTR. For example, there are three probes from Il-1rn, one is from the exons while the other two are from 3′ UTR. The two probes from 3′ UTR are from different positions, one is mid 3′ UTR while the other is from distal 3′ UTR. The expression levels of all three probes of Il-1rn are different one from the other. Irak1 has four probes from exons and 3′ UTR, all of them showed significant difference from each other. Four out of 6 probes of Traf6 showed significant difference from each other.
Table 1.
Mean expression level of IL-1rn and its partner genes.
| Probe ID | Gene symbol |
Description | Location | Tissues
|
Everage | P value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hippocampus | Cerebellum | Whole brain |
Eye | Nucleus Accumbens |
Prefrontal Cortex |
Striatum | Kidney M |
Kidney F |
Spleen | Lung | ||||||
| 1451254_at | Ikbkap | inhibitor of kappa light polypeptide enhancer in B-cells, kinase complex-associated protein; mid 3′ UTR | Chr4: 56.763911 | 10.703 | 10.242 | 10.302 | 10.98 | 11.306 | 9.646 | 12.048 | 7.847 | 7.848 | 10.607 | 10.41 | ||
| 1424142_at | Ikbkap | inhibitor of kappa light polypeptide enhancer in B-cells, kinase complex-associated protein; four exons (exons 26, 27, 28, 29) | Chr4: 56.800653 | 8.407 | 8.135 | 7.823 | 7.408 | 8.186 | 6.211 | 6.871 | 7.718 | 7.673 | 8.369 | 6.933 | 7.612182 | 0.000999 |
| 1421473_at | Il1a | interleukin 1 alpha; mid distal 3′ UTR | Chr2: 129.125444 | 6.21 | 5.912 | 6.732 | 6.565 | 5.664 | 3.761 | 6.651 | 6.426 | 6.422 | 7.676 | 7.286 | 6.300455 | 3.84E-05 |
| 1449399_a_at | Il1b | interleukin 1 beta; last exon and mid and proximal 3′ UTR | Chr2: 129.190645 | 7.634 | 7.745 | 7.323 | 6.563 | 7.437 | 5.6 | 7.33 | 8.068 | 8.002 | 9.625 | 9.238 | 7.687727 | 1.26E-06 |
| 1459777_at | Il1r1 | interleukin 1 receptor, type 1; distal 3′ UTR | Chr1: 40.260465 | 6.001 | 4.807 | 4.285 | 5.134 | 4.681 | 3.073 | 5.311 | 5.69 | 5.677 | 5.456 | 5.29 | 5.036818 | 4.12E-07 |
| 1448950_at | Il1r1 | interleukin 1 receptor, type 1; distal 3′ UTR | Chr1: 40.372447 | 8.367 | 8.441 | 8.676 | 9.17 | 8.071 | 6.785 | 6.553 | 9.51 | 9.469 | 9.969 | 10.28 | 8.663 | 8.34E-06 |
| 1449585_at | Il1rap | interleukin 1 receptor accessory protein | Chr16: 26.701452 | 6.054 | 5.424 | 4.848 | 5.59 | 5.745 | 3.672 | 6.791 | 6.154 | 6.275 | 6.245 | 7.149 | 5.813364 | 0.000133 |
| 1421843_at | Il1rap | interleukin 1 receptor accessory protein | Chr16: 26.716065 | 7.671 | 7.96 | 8.56 | 6.862 | 7.947 | 5.562 | 7.732 | 7.636 | 7.647 | 6.863 | 7.719 | 7.469 | 0.012041 |
| 1421844_at | Il1rap | interleukin 1 receptor accessory protein (hippocampal DG highly specific expression signature); far 3′ UTR | Chr16: 26.717242 | 8.03 | 8.181 | 9.037 | 7.731 | 8.589 | 5.689 | 8.957 | 7.301 | 7.366 | 7.912 | 8.811 | 7.964 | 0.02834 |
| 1442614_at | Il1rap | interleukin 1 receptor accessory protein | Chr16: 26.723185 | 8.628 | 7.365 | 8.205 | 6.725 | 7.63 | 7.202 | 7.49 | 6.224 | 6.194 | 6.291 | 5.83 | 7.071273 | 0.278097 |
| 1439697_at | Il1rap | interleukin 1 receptor accessory protein; far 3′ UTR | Chr16: 26.729648 | 8.884 | 7.8 | 8.772 | 7.398 | 7.501 | 7.274 | 8.474 | 5.959 | 5.976 | 5.731 | 5.786 | 7.232273 | 0.606911 |
| 1423017_a_at | Il1rn | interleukin 1 receptor antagonist; last three exons | Chr2: 24.203031 | 6.314 | 7.636 | 7.979 | 8.053 | 7.09 | 5.362 | 6.864 | 8.925 | 8.893 | 7.587 | 8.399 | 7.554727 | 0.003972 |
| 1451798_at | Il1rn | interleukin 1 receptor antagonist; mid 3′ UTR | Chr2: 24.205596 | 6.005 | 6.118 | 6.26 | 7.572 | 5.716 | 3.61 | 6.885 | 6.197 | 6.227 | 7.49 | 8.36 | 6.403636 | 0.017659 |
| 1425663_at | Il1rn | interleukin 1 receptor antagonist; distal 3′ UTR | Chr2: 24.206409 | 7.029 | 8.786 | 7.995 | 7.491 | 6.869 | 5.184 | 7.409 | 8.421 | 8.44 | 6.655 | 7.552 | 7.439182 | 3.06E-07 |
| 1460649_at | Irak1 | interleukin-1 receptor-associated kinase 1; mid 3′ UTR | ChrX: 71.259671 | 8.866 | 10.236 | 10.056 | 9.216 | 8.891 | 7.212 | 10.025 | 10.678 | 10.46 | 10.431 | 9.912 | 9.634818 | 0.181412 |
| 1438120_x_at | Irak1 | interleukin-1 receptor-associated kinase 1 | ChrX: 71.260147 | 8.547 | 8.806 | 8.004 | 9.152 | 9.745 | 7.898 | 10.621 | 8.962 | 8.729 | 11.045 | 9.21 | 9.156273 | 0.001677 |
| 1438857_x_at | Irak1 | interleukin-1 receptor-associated kinase 1 | ChrX: 71.260147 | 7.861 | 8.668 | 7.722 | 8.296 | 8.414 | 6.262 | 9.388 | 8.829 | 8.707 | 8.987 | 7.882 | 8.274182 | 9.65E-05 |
| 1448668_a_at | Irak1 | interleukin-1 receptor-associated kinase 1 | ChrX: 71.260552 | 8.641 | 9.878 | 9.854 | 8.705 | 9.521 | 7.615 | 9.821 | 9.854 | 9.594 | 11.326 | 9.917 | 9.520545 | 0.010975 |
| 1436507_at | Irak2 | interleukin-1 receptor-associated kinase 2; distal half of 3′ UTR | Chr6: 113.644249 | 8.01 | 9.369 | 9.186 | 9.642 | 8.948 | 7.061 | 8.929 | 9.385 | 9.386 | 10.154 | 9.054 | 9.011273 | 1.24E-08 |
| 1421670_a_at | Irak4 | interleukin-1 receptor-associated kinase 4 | Chr15: 94.384350 | 6.051 | 5.531 | 4.624 | 5.631 | 5.144 | 3.499 | 5.444 | 6.329 | 6.3 | 6.933 | 6.062 | 5.595273 | 0.000338 |
| 1451749_at | Irak4 | interleukin-1 receptor-associated kinase 4 | Chr15: 94.388763 | 5.868 | 6.341 | 6.261 | 6.627 | 6.566 | 4.201 | 6.47 | 6.796 | 6.74 | 8.724 | 7.658 | 6.568364 | 4.18E-05 |
| 1451750_at | Irak4 | interleukin-1 receptor-associated kinase 4 | Chr15: 94.398178 | 7.403 | 7.135 | 8.086 | 7.696 | 7.396 | 5.052 | 7.44 | 7.11 | 7.156 | 9.354 | 8.551 | 7.489 | 0.016957 |
| 1419272_at | Myd88 | myeloid differentiation primary response gene 88 | Chr9: 119.245183 | 7.776 | 7.116 | 7.386 | 8.462 | 7.222 | 5.537 | 8.413 | 9.119 | 9.151 | 10.395 | 10.34 | 8.265364 | 0.000276 |
| 1427705_a_at | Nfkb1 | nuclear factor of kappa light chain gene enhancer in B-cells 1, p105; mid proximal 3′ UTR | Chr3: 135.247911 | 8.89 | 9.628 | 9.836 | 9.931 | 9.325 | 7.631 | 8.867 | 9.357 | 9.597 | 12.089 | 11.05 | 9.654909 | 4.1E-06 |
| 1442949_at | Nfkb1 | nuclear factor of kappa light chain gene enhancer in B-cells 1, p105; intron 6 | Chr3: 135.294808 | 6.171 | 7.281 | 7.099 | 6.846 | 7.392 | 5.218 | 5.806 | 7.234 | 7.192 | 6.746 | 6.167 | 6.650182 | 0.002332 |
| 1425902_a_at | Nfkb2 | nuclear factor of kappa light polypeptide gene enhancer in B-cells 2, p49/p100; last three exons | Chr19: 46.385793 | 6.873 | 8.055 | 7.742 | 8.374 | 7.865 | 6.218 | 6.02 | 9.341 | 9.176 | 10.894 | 9.524 | 8.189273 | 0.060248 |
| 1429128_x_at | Nfkb2 | nuclear factor of kappa light polypeptide gene enhancer in B-cells 2, p49/p100 | Chr19: 46.386535 | 8.7 | 8.965 | 9.118 | 8.751 | 9.13 | 7.974 | 7.494 | 8.935 | 8.877 | 10.085 | 9.014 | 8.822091 | 4.8E-05 |
| 1418163_at | Tlr4 | toll-like receptor 4; last exon and proximal 3′ UTR | Chr4: 66.502349 | 7.164 | 7.076 | 6.398 | 6.995 | 6.485 | 4.922 | 6.502 | 7.774 | 7.731 | 8.505 | 8.729 | 7.116455 | 0.000151 |
| 1418162_at | Tlr4 | toll-like receptor 4; distal 3′ UTR | Chr4: 66.503254 | 6.082 | 4.889 | 4.754 | 6.785 | 5.158 | 3.347 | 6.096 | 6.138 | 6.22 | 7.883 | 8.314 | 5.969636 | 0.203133 |
| 1442827_at | Tlr4 | toll-like receptor 4; far 3′ UTR | Chr4: 66.504146 | 6.157 | 5.273 | 5.11 | 5.8 | 5.297 | 3.465 | 6.024 | 5.882 | 5.893 | 6.62 | 7.725 | 5.749636 | 0.343018 |
| 1430695_at | Tlr4 | toll-like receptor 4; deep 3′ UTR (alternative 3′ UTR?) | Chr4: 66.590773 | 6.47 | 5.633 | 5.252 | 5.391 | 5.021 | 3.366 | 5.727 | 6.33 | 6.311 | 5.638 | 5.468 | 5.509727 | 3.82E-05 |
| 1446940_at | Traf6 | Tnf receptor-associated factor 6 | Chr2: 101.525934 | 7.227 | 7.624 | 8.2 | 7.241 | 7.59 | 5.513 | 6.6 | 7.227 | 7.18 | 6.967 | 6.738 | 7.100636 | 0.755648 |
| 1421376_at | Traf6 | Tnf receptor-associated factor 6; last exon and proximal 3′ UTR | Chr2: 101.537566 | 6.435 | 7.312 | 7.884 | 7.356 | 7.542 | 5.418 | 7.013 | 7.141 | 7.068 | 7.651 | 7.837 | 7.150636 | 9.22E-06 |
| 1421377_at | Traf6 | Tnf receptor-associated factor 6 | Chr2: 101.538253 | 5.835 | 6.554 | 6.374 | 6.389 | 6.352 | 4.148 | 5.777 | 6.722 | 6.678 | 6.592 | 7.132 | 6.232091 | 1.69E-05 |
| 1435350_at | Traf6 | Tnf receptor-associated factor 6 | Chr2: 101.541347 | 7.156 | 7.943 | 8.095 | 8.565 | 8.181 | 5.749 | 8.293 | 7.172 | 7.178 | 9.212 | 9.144 | 7.880727 | 0.000218 |
| 1443288_at | Traf6 | Tnf receptor-associated factor 6 | Chr2: 101.542176 | 6.868 | 6.513 | 6.706 | 6.699 | 6.716 | 4.324 | 7.896 | 6.506 | 6.483 | 6.277 | 7.456 | 6.585818 | 0.000378 |
| 1446977_at | Traf6 | Tnf receptor-associated factor 6 | Chr2: 101.542894 | 6.334 | 5.31 | 5.333 | 5.702 | 5.421 | 3.332 | 6.793 | 6.071 | 6.045 | 6.277 | 7.364 | 5.816545 | 2.65E-06 |
The universal expression and diversity pathways of IL-1rn and its partners among tissues and/or organs raise a critical issue on how a drug and/or molecule might work in human body. Current data accumulated in genenetwork suggest that IL-1rn and its partners are expressed in multiple tissues or organs, or perhaps they all are expressed universally in mouse or human body. Thus any drug or a targeted molecule that mimics the protein sequences or the function of IL-1rn will interact with genes in whatever tissues or organs that the drug or the molecule reaches to.
4. Relative expression levels of probes of genes among different tissues
The average expression levels of 37 probes of 14 genes in 10 tissues are similar except in the prefrontal cortex in which the expression levels of those probes are significantly lower than all other tissues. However, the absolute values of expression levels of genes in different tissues appear to have no effect on the relative expression levels among genes in the same tissues. To examine whether a relative expression level of a probe among all probes in different tissues are the same or not, we analyzed the correlation of gene expression among different tissues. Our analysis (Table 2) indicated that the expression levels among different tissue are from moderately positively correlated to highly positively correlated to each other. Among them, similar tissue types showed more positive correlated than non-similar tissues. For example, gene relative expression levels in whole brain, cerebellum, prefrontal cortex, and nucleus accumbens are highly correlated with the R of more than 0.9. While relative expression levels of probes in kidneys from male and female mice are near identical, with R=0.9979. There is a modest positive correlation between brain tissues (cerebellum, and prefrontal cortex, and nucleus accumbens) and internal organ tissues (kidney, spleen). The relative expression levels of genes in eye have a modest positive correlation with all other tissues, while the relative expression in lung has the lowest positive correlation with all other tissues.
Table 2.
Similarity in gene expression of IL-1rn and its partner genes among tissues.
| Hippocampus | Cerebellum | Whole brain | Eye | Nucleus accumbens | Prefrontal cortex | Striatum | Kidney M | Kidney F | Spleen | Lung | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hippocampus | 1 | 0.829 | 0.830 | 0.763 | 0.864 | 0.924 | 0.811 | 0.560 | 0.565 | 0.589 | 0.508 |
| Cerebellum | 1.000 | 0.946 | 0.874 | 0.940 | 0.916 | 0.769 | 0.825 | 0.826 | 0.709 | 0.627 | |
| Whole brain | 1.000 | 0.848 | 0.918 | 0.901 | 0.743 | 0.713 | 0.718 | 0.631 | 0.590 | ||
| Eye | 1.000 | 0.884 | 0.856 | 0.771 | 0.776 | 0.786 | 0.829 | 0.800 | |||
| Nucleus accumbens | 1.000 | 0.952 | 0.827 | 0.710 | 0.710 | 0.730 | 0.634 | ||||
| Prefrontal cortex | 1.000 | 0.779 | 0.680 | 0.679 | 0.676 | 0.553 | |||||
| Striatum | 1.000 | 0.530 | 0.530 | 0.595 | 0.585 | ||||||
| Kidney M | 1.000 | 0.998 | 0.813 | 0.747 | |||||||
| Kidney F | 1.000 | 0.816 | 0.762 | ||||||||
| Spleen | 1.000 | 0.893 | |||||||||
| Lung | 1.000 |
In our examination of gene expression, correlation, and regulation, we used the same type of microarray chips of the same platform and the same set of RI strains derived from the same parental strains. Data from each of those studies are carefully inspected by investigators in each case (http://www.genenetwork.org/webqtl/main.py). Nevertheless, we realize that the number of strains used for different tissues or organs are different. Considering the fact that our data show the expression patterns of kidney tissues between female and male are nearly identical, and expression patterns from similar tissues are similar (such as tissues from brain), we feel confident of the conclusion from the analysis from those data.
5. Correlation of expression of probes among genes in different tissues
In order to examine whether the similarity of relative expression levels of probes in different tissues resulted a similar pattern in the gene–gene co-expression or correlation among probes of genes, we examined the correlation among probes of genes within tissues and compared their similarities. Because we have a total of 37 probes for the 14 genes, for each tissue, a total of 37×37 matrix Spearman Rank Correlations were produced from Genenetwork (Supplementary Table 1). Among those tissues, gene correlationships among kidney in male and female mice are measured separately, while others are predominantly female. The correlation among gene correlationship in those seven tissues shown in Table 3. As expected, gene correlationship of probes of genes from male and female kidney tissues are very correlated to each other, with R=0.72. However, in general, gene–gene co expressions are poorly correlated among of most of those tissues (Table 3). In particular, correlationship of genes derived from lung is at the weakest in relation to the correlationship of genes from other tissues, mostly with R equal or less than 0.1. The tissues from brain, including cerebellum, hippocampus, and whole brain have relatively higher R scores, between 0.2 and 0.3. Surprisingly, the gene correlation among striatum is independent of other tissues, including brain tissues, with R equal or less than 0.07.
Table 3.
Similarity of gene–gene interactions among tissues.
| Eye | Cerebellum | Lung | Kidney M | Whole brain | Hippocampus | Kidney F | nucleus accumbens | Prefrontal cortex | Striatum | |
|---|---|---|---|---|---|---|---|---|---|---|
| Eye | 1.000 | 0.266 | 0.244 | 0.159 | 0.191 | 0.216 | 0.157 | 0.125 | 0.175 | −0.015 |
| Cerebellum | 1.000 | 0.020 | 0.271 | 0.279 | 0.396 | 0.230 | 0.152 | 0.123 | −0.014 | |
| Lung | 1.000 | 0.044 | 0.078 | 0.091 | 0.055 | 0.110 | 0.100 | 0.072 | ||
| Kidney M | 1.000 | 0.205 | 0.266 | 0.723 | 0.135 | 0.089 | 0.078 | |||
| Whole Brain | 1.000 | 0.299 | 0.149 | 0.197 | 0.316 | 0.045 | ||||
| Hippocampus | 1.000 | 0.226 | 0.121 | 0.236 | 0.049 | |||||
| Kidney F | 1.000 | 0.108 | 0.057 | 0.014 | ||||||
| Nucleus accumbens | 1.000 | 0.200 | 0.058 | |||||||
| Prefrontal cortex | 1.000 | 0.052 | ||||||||
| Striatum | 1.000 |
One important note is that, while both positive and negative correlations among genes and/or probes are detected from 9 tissues, only positive correlations were shown from eye gene profiles of 71 RI strains.
Furthermore, because the molecular pathways and regulations of IL-1rn and its partners in different tissues are different from each other, the reaction with a drug or molecule in different tissues may trigger different molecular pathways, or result in the different phenotypes. Therefore, targeted specific tissue and accurate dosage in the drug application may be a key in the therapeutic application of a drug such as anakinra. Researches on delivery method and dosages seem to be essential for the drug development in the therapeutic application of drugs derived from of IL-1rn and its partners.
6. The potential influence of different splicing
The complications of interactions and regulations among Il-1rn and its partner genes at least partially due to the different splicing. For example, in Ensembl database (http://useast.ensembl.org/Mus_musculus/Info/Index), it listed 6 transcripts of mouse IL-1rn due to different splicing (Supplementary Table 2). While the Il1rn-001 and Il1rn-002 have a long (>1000 bp) 3′ end untranslated sequences, the other four have very short sequences at 3′ end. Although the tissue specificity of each transcript has not been clearly defined, the different expression levels detected by the three probes from IL-1rn may be a result of combination of expression levels of different transcripts in the different tissues. As tissue specific splicing has become known as the important regulatory mechanism of gene function [40–42], detailed consideration in target design of a gene or a pathway seems a critical issue. Regarding to anakinra, its molecule is a recombinant, non-glycosylated version of human IL-1RA with 153 amino acids. In humans, according to Ensembl database, IL-1rn gene encodes for nine transcripts, five of them have protein sequences. The anakinra molecule is larger than two and small than the other three predicted proteins sequences of IL-1RN in humans, according to Ensembl database. Thus, the anakinra likely represents the function of at least three isoforms of IL-1RN proteins in human body. Unfortunately there is a limited report of the detailed study on the expression and tissue specificity of isoforms in either humans or mice [43].
7. Potential transcriptome mapping of Il-1r1 and IL-1rn in variety tissues
In order to investigate whether different probes are regulated differently, we analyzed the transcriptome map of those two genes. Il-1r1 has two probes on the M430.v2 chip from the distal 3′ UTR, but those two probes showed a significant difference in expression levels in different tissues. IL-1rn has three probes on the chip, one from exons, one from mid 3′ UTR and another is from distal 3′ UTR. Expression levels of those three probes showed a significant difference to each other. Surprisingly, our analysis indicated that there is no similarity among tissues for the transcriptome map of those probes (data not shown). Furthermore, there is no similarity even in kidney between female and male mice. Fig. 2 shows the transcriptome maps of two probes of Il-1r1 and three probes of IL-1rn between female and male kidneys. For probes of Il-1r1, in male there are suggestive regulatory loci on chromosome 2, 8, 12, and 16 for the second probe, the probe ID 1448950, located on distal 3′ UTR at 40,372,447 on chromosome 1. In female, however, there are two loci on chromosomes 3 and 19 that regulate the first probe, ID 1459777_at which is located at 40,260,465 on chromosome 1. For the three probes of IL-1rn, in male mice, there is a significant locus on chromosome 17 which regulates the third probe, which is at distal 3′ UTR located 24,206,409 on chromosome 2. In female mice, there are three regulatory loci that reach to significant level (LOD 3). One is a locus on chromosome 3 that regulates the second probe, ID 1451798. The other two loci are on chromosome 10, one regulate probe #1, ID 1423017, while the other regulate the probe #3, ID 1425663. Thus, the transcriptome regulatory loci in the map show that regulatory mechanism for IL-1rn and its partners are different not only among tissues but also among probes of the same gene.
Fig. 2.
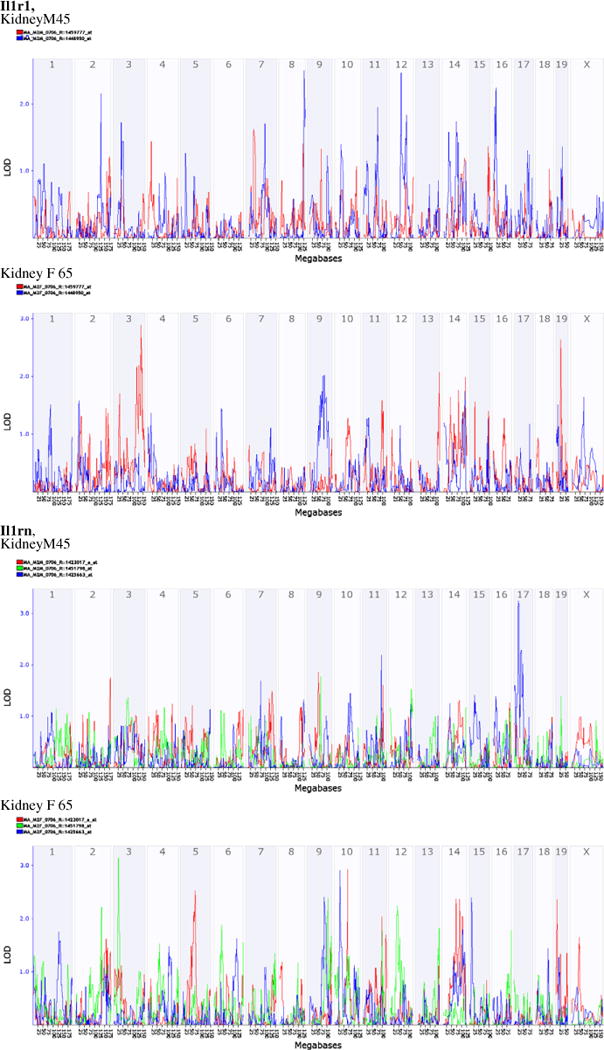
Transcriptome maps of two probes of IL-1r1 and three probes of IL-1rn between female and male kidneys. Probes are from the M430.v2 chip of Affymetrix. The two probes of IL-1r1 are probe ID 1448950 and ID 1459777. The three probes of IL-1rn are ID 1451798, ID 1423017, and ID 1425663. KidneyM45=transcriptome mapping generated from gene expression profiles of kidneys of male mice from 45 BXD strains. Kidney F 65=transcriptome mapping generated from gene expression profiles of kidneys of female mice from 65 BXD strains.
8. Implication from IL-1rn knockout mice
Mice deficient for the production of interleukin 1 receptor antagonist (IL-1Ra) protein due to deletion of the IL-1rn gene has also implicated a complicated story. The mouse model was first described by Horai and coworkers [44] as a model for spontaneous arthritis. At present, it shows that disorders in the deficiency of IL-1rn are influenced by genomic background as well as environment factors. BALB/c mice that are homozygous for the deficiency (BALB−/−) develop inflammation in the hind limbs with an incidence approaching 100% beginning at about 6 weeks of age. However, not all strains of mice are susceptible. Both C57BL and DBA/1 mice are resistant. Furthermore, development of spontaneous arthritis appears to be dependent on exposure to microbial components, because these mice do not develop arthritis in germ-free conditions [45]. Recently, it was reported that BALB−/− mice induced aortic valve inflammation as well [46]; while B6−/− mice promotes Spontaneous Femoral Artery Aneurysm Formation in Mice [47].
These findings indicate that the influence of IL-1rn on disease development is much dependent on individual’s genomic background and environmental factors. In spite of the considerable amount of work on the knockout of IL-1rn in several strains including congenics [48], a systematic investigation of impact of IL-1rn deficiency in all available mouse strains probably will shine some light on the function of IL-1rn in different genomic background, and therefore will be beneficial to the application of IL-1rn in therapeutic treatment for human diseases.
9. Future prospective
Taking the advantages of rapid development of technologies in genomics and protein analyses, future study should be on detailed analysis of the function of IL-1rn isoforms and its interactions with genomic backgrounds and environmental factors. The complexity of therapeutic applications targeting on IL-1rn pathway comes from several levels. First, IL-1rn is expressed in multiple tissues. Second, it interacts differently with different genes or pathways, suggesting it may play different roles in multiple pathways. Third, it has different isoforms that may play different roles in different tissues or pathways. And fourth, it is influenced by genomic background and environmental factors. Because of the different splicing patterns of the IL-1rn gene, it is critical to understand the relationship between each isoforms of the IL-1RN in particular tissues and its function. Furthermore, genetic variability of IL-1rn and in its isoforms, e.g. SNP in human population may result a different response of individuals to the same drug that targets a particular isoform of IL-1rn.
The complex interaction and regulation of IL-1rn and its partners in a variety of tissues and organs suggest that there is a long way to go to elucidate the molecular mechanism of IL-1rn and its partners. It implies that they may be relevant to multiple functions of human body, depending on genomic backgrounds of individuals. In other words, they may relate to multiple diseases or disorders. The key issue is the role each of them plays in a specific disease or disorders is not completely understood. Thus what would be the impact of their expressions on a specific disease or disorder, promotion, aggregation, or suppression? Targeted transgenic and knockout animal models in specific tissue or organs appear valuable tools for this purpose. Further studies of the molecular function of IL-1rn and genome-environment interaction are necessary.
Furthermore, considering the complexity of expression and interaction among IL-1rn and its partners and with genomic background and environmental factors, targeted specific sequences or oligos of Il-1rn might be less toxic and more specific to individual disease. Treatment with antisense oligos, short peptides, siRNAs have been recognized as efficient tools in therapeutic application. Study of specific binding sites or the interaction site and biding domains in the Il-1rn gene and its isoforms of proteins may have great potential in the improvement of the current therapeutic application of anakinra.
Supplementary Material
Acknowledgments
The study was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (R01 AR51190 to WG; R01 AR50785 to JS), National Natural Science Foundation of China (Project 81171679 to YHC) and the Veterans Administration Medical Center in Memphis, TN.
Footnotes
Supplementary materials related to this article can be found online at doi:10.1016/j.intimp.2012.02.014.
Conflict of interest statement
None.
References
- 1.Carter DB, Deibel MR, Jr, Dunn CJ, Tomich CS, Laborde AL, Slightom JL, et al. Purification, cloning, expression and biological characterization of an interleukin-1 receptor antagonist protein. Nature. 1990;344:633–8. doi: 10.1038/344633a0. [DOI] [PubMed] [Google Scholar]
- 2.Schiff MH. Role of interleukin 1 and interleukin 1 receptor antagonist in the mediation of rheumatoid arthritis. Ann Rheum Dis. 2000;59(Suppl. 1):i103–8. doi: 10.1136/ard.59.suppl_1.i103. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blakemore AI, Tarlow JK, Cork MJ, Gordon C, Emery P, Duff GW. Interleukin-1 receptor antagonist gene polymorphism as a disease severity factor in systemic lupus erythematosus. Arthritis Rheum. 1994;37:1380–5. doi: 10.1002/art.1780370917. [DOI] [PubMed] [Google Scholar]
- 4.Mansfield JC, Holden H, Tarlow JK, Di Giovine FS, McDowell TL, Wilson AG, et al. Novel genetic association between ulcerative colitis and the anti-inflammatory cytokine interleukin-1 receptor antagonist. Gastroenterology. 1994;106:637–42. doi: 10.1016/0016-5085(94)90696-3. [DOI] [PubMed] [Google Scholar]
- 5.Tarlow JK, Clay FE, Cork MJ, Blakemore AI, McDonagh AJ, Messenger AG, et al. Severity of alopecia areata is associated with a polymorphism in the interleukin-1 receptor antagonist gene. J Invest Dermatol. 1994;103:387–90. doi: 10.1111/1523-1747.ep12395398. [DOI] [PubMed] [Google Scholar]
- 6.Schrijver HM, Crusius JB, Uitdehaag BM, García González MA, Kostense PJ, Polman CH, et al. Association of interleukin-1beta and interleukin-1 receptor antagonist genes with disease severity in MS. Neurology. 1999;52:595–9. doi: 10.1212/wnl.52.3.595. [DOI] [PubMed] [Google Scholar]
- 7.Blakemore AI, Cox A, Gonzalez AM, Maskil JK, Hughes ME, Wilson RM, et al. Interleukin-1 receptor antagonist allele (IL1RN*2) associated with nephropathy in diabetes mellitus. Hum Genet. 1996;97:369–74. doi: 10.1007/BF02185776. [DOI] [PubMed] [Google Scholar]
- 8.Strandberg L, Lorentzon M, Hellqvist A, Nilsson S, Wallenius V, Ohlsson C, et al. Interleukin-1 system gene polymorphisms are associated with fat mass in young men. J Clin Endocrinol Metab. 2006;91:2749–54. doi: 10.1210/jc.2005-2786. Epub 2006 Apr 24. [DOI] [PubMed] [Google Scholar]
- 9.Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426–37. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy S, Jia S, Geoffrey R, Lorier R, Suchi M, Broeckel U, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438–44. doi: 10.1056/NEJMoa0809568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baradaran-Rahimi H, Radvar M, Arab HR, Tavakol-Afshari J, Ebadian AR. Association of interleukin-1 receptor antagonist gene polymorphisms with generalized aggressive periodontitis in an Iranian population. J Periodontol. 2010;81:1342–6. doi: 10.1902/jop.2010.100073. [DOI] [PubMed] [Google Scholar]
- 12.Schulz Susanne, Stein Jamal M, Altermann Wolfgang, Klapproth Jana, Zimmermann Uta, Reichert Yvonne. Single nucleotide polymorphisms in interleukin-1gene cluster and subgingival colonization with Aggregatibacter actinomycetemcomitans in patients with aggressive periodontitis. Hum Immunol. 2011;72:940–6. doi: 10.1016/j.humimm.2011.05.009. Epub 2011 Jun 1. [DOI] [PubMed] [Google Scholar]
- 13.Naumann L, Feist E, Natusch A, Langen S, Krause A, Buttgereit F, et al. IL1-receptor antagonist anakinra provides long-lasting efficacy in the treatment of refractory adult-onset Still’s disease. Ann Rheum Dis. 2010;69:466–7. doi: 10.1136/ard.2009.108068. [DOI] [PubMed] [Google Scholar]
- 14.Arman A, Soylu O, Yildirim A, Furman A, Ercelen N, Aydogan H, et al. Interleukin-1 receptor antagonist gene VNTR polymorphism is associated with coronary artery disease. Arq Bras Cardiol. 2008;91:293–8. doi: 10.1590/s0066-782x2008001700002. [DOI] [PubMed] [Google Scholar]
- 15.Xu M, He L. Convergent evidence shows a positive association of interleukin-1 gene complex locus with susceptibility to schizophrenia in the Caucasian population. Schizophr Res. 2010;120:131–42. doi: 10.1016/j.schres.2010.02.1031. Epub 2010 Mar 26. [DOI] [PubMed] [Google Scholar]
- 16.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404(6776):398–402. doi: 10.1038/35006081. Erratum in:Nature 2000;412(6842):99. [DOI] [PubMed] [Google Scholar]
- 17.Peleteiro B, Lunet N, Carrilho C, Durães C, Machado JC, La Vecchia C, et al. Association between cytokine gene polymorphisms and gastric precancerous lesions: systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:762–76. doi: 10.1158/1055-9965.EPI-09-0917. [DOI] [PubMed] [Google Scholar]
- 18.Bodar EJ, Kuijk LM, Drenth JP, van der Meer JW, Simon A, Frenkel J. On-demand anakinra treatment is effective in mevalonate kinase deficiency. Ann Rheum Dis. 2011;70:2155–8. doi: 10.1136/ard.2011.149922. Epub 2011 Aug 21. [DOI] [PubMed] [Google Scholar]
- 19.Sikora KA, Grom AA. Update on the pathogenesis and treatment of systemic idiopathic arthritis. Curr Opin Pediatr. 2010;23(6):640–6. doi: 10.1097/MOP.0b013e32834cba24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nigrovic PA, Mannion M, Prince FH, Zeft A, Rabinovich CE, van Rossum MA, et al. Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis: report of forty-six patients from an international multicenter series. Arthritis Rheum. 2011;63:545–55. doi: 10.1002/art.30128. [DOI] [PubMed] [Google Scholar]
- 21.Bruck N, Suttorp M, Kabus M, Heubner G, Gahr M, Pessler F. Rapid and sustained remission of systemic juvenile idiopathic arthritis-associated macrophage activation syndrome through treatment with anakinra and corticosteroids. J Clin Rheumatol. 2011;17:23–7. doi: 10.1097/RHU.0b013e318205092d. [DOI] [PubMed] [Google Scholar]
- 22.Tsukamoto H, Ueda N, Horiuchi T. Progress in classification and treatment for TNF receptor-associated periodic syndrome. Nihon Rinsho Meneki Gakkai Kaishi. 2011;34(5):361–8. doi: 10.2177/jsci.34.361. [DOI] [PubMed] [Google Scholar]
- 23.Tran AP, Edelman J. Interleukin-1 inhibition by anakinra in refractory chronic tophaceous gout. Int J Rheum Dis. 2011;14:e33–7. doi: 10.1111/j.1756-185X.2011.01629. [DOI] [PubMed] [Google Scholar]
- 24.Vandenhende MA, Bentaberry F, Morlat P, Bonnet F. Anakinra: an effective treatment in the Schnitzler syndrome. Joint Bone Spine. 2011;78(6):636–7. doi: 10.1016/j.jbspin.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Terrada C, Neven B, Boddaert N, Souied EH, Prieur AM, Quartier P, et al. Ocular modifications in a young girl with cryopyrin-associated periodic syndromes responding to interleukin-1 receptor antagonist anakinra. J Ophthalmic Inflamm Infect. 2011;1:133–6. doi: 10.1007/s12348-010-0018-2. Epub 2011 Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kluger N, Gil-Bistes D, Guillot B, Bessis D. Efficacy of anti-interleukin-1 receptor antagonist anakinra (Kineret®) in a case of refractory Sweet’s syndrome. Dermatology. 2011;222:123–7. doi: 10.1159/000326112. Epub 2011 Apr 5. [DOI] [PubMed] [Google Scholar]
- 27.Kuemmerle-Deschner JB, Tyrrell PN, Koetter I, Wittkowski H, Bialkowski A, Tzaribachev N, et al. Efficacy and safety of anakinra therapy in pediatric and adult patients with the autoinflammatory Muckle–Wells syndrome. Arthritis Rheum. 2011;63:840–9. doi: 10.1002/art.30149. [DOI] [PubMed] [Google Scholar]
- 28.Brown CA, Toth AP, Magnussen B. Clinical benefits of intra-articular anakinra for arthrofibrosis. Orthopedics. 2010;33:877. doi: 10.3928/01477447-20101021-09. [DOI] [PubMed] [Google Scholar]
- 29.Stankovic Stojanovic K, Delmas Y, Ureña Torres P, Peltier J, Pelle G, Jéru I, et al. Dramatic beneficial effect of interleukin-1 inhibitor treatment in patients with familial Mediterranean fever complicated with amyloidosis and renal failure. Nephrol Dial Transplant. 2011;29 doi: 10.1093/ndt/gfr528. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.El-Osta H, Janku F, Kurzrock R. Successful treatment of Castleman’s disease with interleukin-1 receptor antagonist (Anakinra) Mol Cancer Ther. 2010;9:1485–8. doi: 10.1158/1535-7163.MCT-10-0156. Epub 2010 May 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown C, Toth A, Magnussen R. Clinical benefits of intra-articular anakinra for persistent knee effusion. J Knee Surg. 2011;24:61–5. doi: 10.1055/s-0031-1275398. [DOI] [PubMed] [Google Scholar]
- 32.Rigante D, Leone A, Marrocco R, Laino ME, Stabile A. Long-term response after 6-year treatment with anakinra and onset of focal bone erosion in neonatal-onset multisystem inflammatory disease (NOMID/CINCA) Rheumatol Int. 2011;31:1661–4. doi: 10.1007/s00296-010-1787-5. Epub 2011 Jan 15. [DOI] [PubMed] [Google Scholar]
- 33.Lin Z, Hegarty JP, Lin T, Ostrov B, Wang Y, Yu W, et al. Failure of anakinra treatment of pyoderma gangrenosum in an IBD patient and relevance to the PSTPIP1 gene. Inflamm Bowel Dis. 2011;17:E41–2. doi: 10.1002/ibd.21684. Epub 2011 Mar 15. [DOI] [PubMed] [Google Scholar]
- 34.Mahamid M, Paz K, Reuven M, Safadi R. Hepatotoxicity due to tocilizumab and anakinra in rheumatoid arthritis: two case reports. Int J Gen Med. 2011;4:657–60. doi: 10.2147/IJGM.S23920. Epub 2011 Sep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Asseldonk EJ, Stienstra R, Koenen TB, Joosten LA, Netea MG, Tack CJ. Treatment with Anakinra improves disposition index but not insulin sensitivity in nondiabetic subjects with the metabolic syndrome: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2011;96:2119–26. doi: 10.1210/jc.2010-2992. Epub 2011 Apr 20. [DOI] [PubMed] [Google Scholar]
- 36.Luheshi NM, Rothwell NJ, Brough D. Dual functionality of interleukin-1 family cytokines: implications for anti-interleukin-1 therapy. Br J Pharmacol. 2009;157(8):1318–29. doi: 10.1111/j.1476-5381.2009.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chesler EJ, Lu L, Wang J, Williams RW, Manly KF. WebQTL: rapid exploratory analysis of gene expression and genetic networks for brain and behavior. Nat Neurosci. 2004;7:485–6. doi: 10.1038/nn0504-485. [DOI] [PubMed] [Google Scholar]
- 38.Peirce JL, Lu L, Gu J, Silver LM, Williams RW. A new set of BXD recombinant inbred lines from advanced intercross populations in mice. BMC Genet. 2004;29:5–7. doi: 10.1186/1471-2156-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crow James F. Haldane, Bailey, Taylor and recombinant-inbred lines. Genetics. 2007;176:729–32. doi: 10.1093/genetics/176.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao H, Wu J, Lam S, Duan R, Newnham C, Molday RS, et al. Temporal and tissue specific regulation of RP-associated splicing factor genes PRPF3, PRPF31 and PRPC8—implications in the pathogenesis of RP. PLoS One. 2011;6(1):e15860. doi: 10.1371/journal.pone.0015860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linder B, Dill H, Hirmer A, Brocher J, Lee GP, Mathavan S, et al. Systemic splicing factor deficiency causes tissue-specific defects: a zebrafish model for retinitis pigmentosa. Hum Mol Genet. 2011;20(2):368–77. doi: 10.1093/hmg/ddq473. Epub 2010 Nov 3. [DOI] [PubMed] [Google Scholar]
- 42.de Navas LF, Reed H, Akam M, Barrio R, Alonso CR, Sánchez-Herrero E. Integration of RNA processing and expression level control modulates the function of the Drosophila Hox gene Ultrabithorax during adult development. Development. 2011;138(1):107–16. doi: 10.1242/dev.051409. Epub 2010 Nov 29. [DOI] [PubMed] [Google Scholar]
- 43.Evans I, Dower SK, Francis SE, Crossman DC, Wilson HL. Action of intracellular IL-1Ra (Type 1) is independent of the IL-1 intracellular signalling pathway. Cytokine. 2006;33(5):274–80. doi: 10.1016/j.cyto.2006.02.003. Epub 2006 Mar 27. [DOI] [PubMed] [Google Scholar]
- 44.Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000;191:313–20. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou F, He X, Iwakura Y, Horai R, Stuart JM. Arthritis in mice that are deficient in interleukin-1 receptor antagonist is dependent on genetic background. Arthritis Rheum. 2005;52:3731–8. doi: 10.1002/art.21481. [DOI] [PubMed] [Google Scholar]
- 46.Isoda K, Matsuki T, Kondo H, Iwakura Y, Ohsuzu F. Deficiency of interleukin-1 receptor antagonist induces aortic valve disease in BALB/c mice. Arterioscler Thromb Vasc Biol. 2010;30:708–15. doi: 10.1161/ATVBAHA.109.201749. Epub 2010 Jan 28. [DOI] [PubMed] [Google Scholar]
- 47.Isoda K, Kitagaki M, Niida T, Kondo H, Matsubara O, Kikuchi M, et al. Deficiency of interleukin-1 receptor antagonist promotes spontaneous femoral artery aneurysm formation in mice. Am J Pathol. 2012 Jan 11; doi: 10.1016/j.ajpath.2011.11.028. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Wolf G, Livshits D, Beilin B, Yirmiya R, Shavit Y. Interleukin-1 signaling is required for induction and maintenance of postoperative incisional pain: genetic and pharmacological studies in mice. Brain Behav Immun. 2008;22(7):1072–7. doi: 10.1016/j.bbi.2008.03.005. Epub 2008 Apr 28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


