Abstract
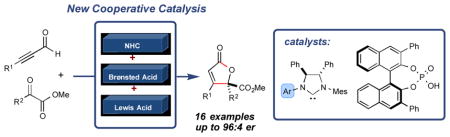
The first highly enantioselective NHC-catalyzed [3+2] annulation reaction with α,β-alkynals and α-ketoesters has been developed. A new mode of cooperative catalysis involving the combination of a chiral Brønsted acid and a C1-symmetric biaryl saturated imidazolium precatalyst was required to generate the desired γ-crotonolactones in high yields and levels of enantioselectivity.
Keywords: cooperative catalysis, asymmetric synthesis, N-heterocyclic carbene
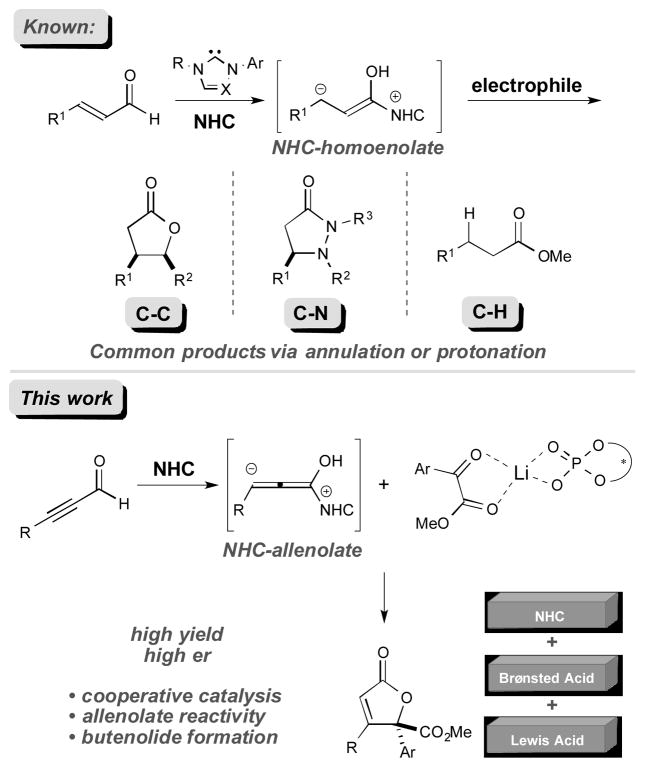
N-heterocyclic carbene (NHC)-catalyzed homoenolate additions to various electrophiles[1] represent an enabling class of transformations that have been employed for the construction of complex hetero- and carbocyclic systems through unconventional Umpolung reactivity.[2] While significant advancements have been achieved through this manifold, a limited number of reports of NHC-catalyzed homoenolate additions involving alkynyl aldehydes have appeared. In 2006, Zeitler reported the NHC-catalyzed redox esterification of alkynal aldehydes[3] and subsequent independent reports by Bode, Xiao, Du and Alexakis all reported NHC-catalyzed oxidative transformations of alkynals.[4] Common to these studies is the protonation of the allenolate intermediate to afford activated, electrophilic α,β-unsaturated carbonyl intermediates. To date, the complimentary reaction of nucleophilic NHC-bound allenolates and electrophiles to forge new C–C bonds at the β-position of the alkynal has received significantly less attention.[5] There have been a few pioneering studies of this type of reaction, however, the development of efficient and highly enantioselective methods has remained elusive. For example, the She group recently extended the NHC-Lewis acid catalysis concept and reported an approach for the relatively unselective [3+2] annulation of alkynals and β,γ-unsaturated α-ketoesters to generate γ-crotonolactones with low stereoinduction.[6] Independently, the Snyder group has shown the potentially powerful application of the allenolate reactivity via NHC-Lewis acid catalysis for diastereoselective annulation reactions toward the securinega family of alkaloids.[5b]
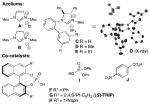
Cooperative NHC catalysis has been employed as a strategy to expand the capabilities of NHC catalysis and has resulted in marked improvements in efficiency and selectivity in several cases. Notably, the use of NHC/Lewis acid or Brønsted acid cooperative catalysis for enhancing selectivity and incorporating previously inactive reaction partners has seen significant success.[7],[8] Despite the demonstrated utility of chiral Brønsted acids (CBAs) for a variety of asymmetric transformations,[9],[10],[11] the combination of NHCs and chiral Brønsted acids represents new opportunity for small molecule activation. Herein we report a general cooperative NHC/chiral Brønsted acid strategy to achieve a highly enantioselective addition of alkynals to α-ketoesters (Figure 1).
Figure 1.
NHC-Catalyzed Annulation of Alkynals.
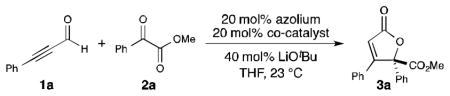
We postulated that the dearth of reports detailing NHC-catalyzed reactions with alkynals in new C–C bond forming processes might be explained by the nature of the allenolate intermediate,[12] wherein this transient species is rapidly protonated rather than productively interact with a more complex electrophile. We envisioned that under a more efficient mode of activation, α-ketoesters could be a suitable class of allenolate acceptors. Hence, the combination of alkynal 1a with α-ketoester 2a in the presence of lithium tert-butoxide and IMes•Cl precatalyst was explored (A, Table 1, entry 1). These conditions did not produce any of the desired product. However, the use of saturated imidazolium (SImes•Cl) B afforded trace amounts of butenolide 3a (entry 2). Guided by our previous NHC/Lewis acid studies, a slightly improved yield was observed with 1 equiv. of lithium chloride (entry 3).[7f] Even though the reaction yield was poor, this result indicated that lithium cations may be activating methyl benzoylformate toward addition. The introduction of chiral Brønsted acid F (20 mol %) with achiral NHC from B produced a significant increase in yield (54%), but the annulation product was effectively racemic (entry 4). We then turned our attention toward exploring the use of a chiral NHC in combination with a chiral Brønsted acid (CBA). While established chiral triazolium-based NHC catalysts are ineffective for this transformation (see Supp. Info.), it became apparent that C1-symmetric biaryl saturated imidazolium-derived NHC catalyst C, originally disclosed by the Hoveyda group,[13],[14] provided the desired product in excellent yield (entry 6). Indeed, the use of these 2,3-dihydroimidazole-2-ylinene structures in carbene catalysis is far less developed than their imidazolium and triazolium derived counterparts. Interestingly, conducting the reaction under more dilute conditions in the presence of molecular sieves resulted in a further enhancement in enantioselectivity (from 79:21 to 90:10 er, entry 7). With these intriguing results in hand, we investigated various C1-symmetric biaryl saturated imidazolium precatalysts, with the highest yield and enantioselectivity observed with catalyst D (entry 9). The introduction of more sterically demanding aryl or naphthyl groups on the chiral phosphoric acid, such as G (i.e., (S)-TRIP) and H, or on the imidazolium salt, such as E, resulted in decreased reactivity and enantioselectivity (entries 10–12). Therefore, precatalyst D and chiral phosphoric acid F were chosen as our optimized catalysts for further study (entry 9). The use of the (R)-phosphoric acid instead of its (S)-enantiomer did not diminish catalyst reactivity and enantiomeric ratio, thereby indicating a lack of an expected match/mismatch relationship between the phosphoric acid chirality and the NHC was observed (entry 13). However, racemic phosphoric acid F provides the desired product with slightly decreased yield and enantioselectivity (entry 14). At our current level of understanding of this complex reaction, we assume that unexpected inactive species might be formed between the lithium ion and (R)-and (S)-phosphate that generates product in lower yield and slightly decreased enantiomeric ratio. Clearly, this is a multivariable system and further investigations to delineate more fully the specific roles of the lithium cation, NHC and Brønsted acid/chiral phosphate are on going. In addition, achiral phosphoric acid I can also serve as a co-catalyst, albeit with diminished yield and selectivity (Table 1, entry 15). However, other Brønsted acids, such as 4-nitrobenzoic acid, did not provide any desired product (Table 1, entry 19).
Table 1.
Optimization of Reaction Conditions.[a]
| ||||
|---|---|---|---|---|
| entry | azolium | co-catalyst | % yield[b] | er[c] |
| 1 | A | - | no rxn | - |
| 2 | B | - | trace | - |
| 3 | B | LiCl (1 equiv) | 24 | - |
| 4 | B | F | 54 | 52:48 |
| 5 | C | - | trace | n.d. |
| 6 | C | F | 90 | 79:21 |
| 7[d] | C | F | 85 | 90:10 |
| 8[d] | D | LiCl (1 equiv) | 34 | 89:11 |
| 9[d] | D | F | 85 | 93:7 |
| 10[d] | D | G | 85 | 91:9 |
| 11[d] | D | H | trace | n.d. |
| 12[d] | E | F | 52 | 92:8 |
| 13[d] | D | ent-F | 84 | 93:7 |
| 14[d] | D | rac-F | 74 | 91:9 |
| 15[d] | D | I | 77 | 89:11 |
| 16[d,e] | C | F | trace | n.d. |
| 17[d,f] | C | F | no rxn | - |
| 18[d,g] | C | F | no rxn | - |
| 19[d] | C | J | no rxn | - |
|
| ||||
| ||||
Conditions: 1a (0.05 mmol, 1 equiv), 2a (1.5 equiv), azolium (0.2 equiv), phosphoric acid (0.2 equiv), LiOtBu (0.4 equiv) in THF (0.15 M) at 23 °C for 48 h.
Determined by GC-MS with n-dodecane as internal standard.
Determined by HPLC analysis.
4Å MS were used at 0.014 M concentration in THF.
20 mol% LiOtBu was used.
NaOtBu was used as the base instead of LiOtBu.
Mg(OtBu)2 was used as the base instead of LiOtBu.
To gain further information about the role of the CBA, the reaction was performed using a decreased amount of base (20 mol%, entry 16). This modification provided only trace amounts of the product. Considering the pKa values of the azolium and Brønsted acids,[15],[16] it is likely under these conditions that phosphoric acid F would be deprotonated initial, and the NHC might not be generated in high enough concentrations from the azolium salt precatalyst. A second aspect is whether the lithium cation is involved in organizing the transition state[7f],[17] or if lithium tert-butoxide was simply acting as a base. To probe this possibility, sodium tert-butoxide or magnesium di-tert-butoxide was employed as the base, no product was obtained, suggesting the involvement of lithium cation in the reaction mechanism (entries 17 and 18). Furthermore, the 31P NMR spectroscopy was employed to probe the state of the phosphoric acid/phosphate under the reaction conditions, we observed a noticeable change in chemical shift of phosphoric acid F in the presence of LiOtBu (see Supp. Info. for details). Notably, alkali or alkaline salts of BINOL-derived phosphates are known as efficient catalysts,[11a, 18] and in some cases, the counterion plays a significant role.[18b, 19] Based on this precedent, our working hypothesis is that the lithium ion coordinates the phosphate and α-ketoester, and could be a part of activation system under the reaction conditions (see Figure 1).
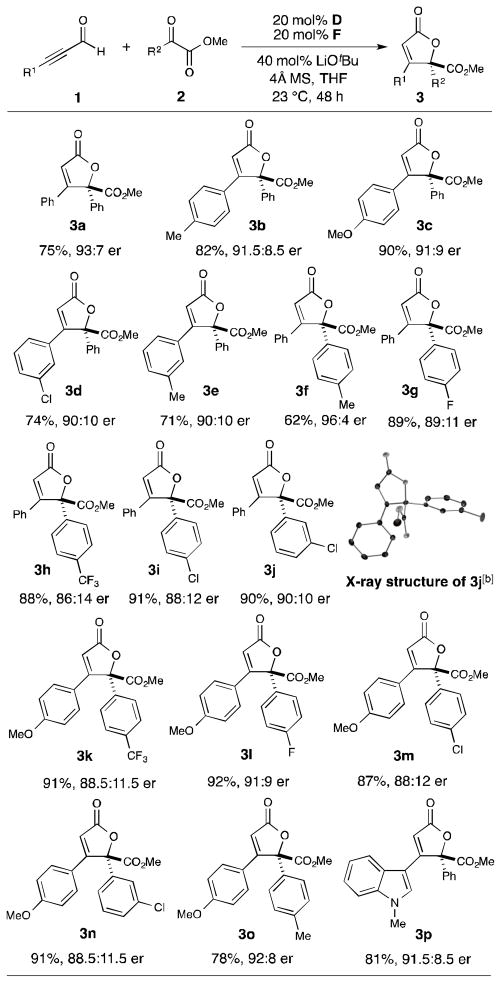
Subsequent to these initial mechanistic probe experiments, the scope of this formal [3+2] annulation was explored (Table 2). The reaction is tolerant of both electron-deficient and electron-rich aromatic alkynals and α-ketoesters. The desired products are obtained in good to excellent yield (62–92%) with high enantioselectivities (up to 96:4 er). In particular, alkynals possessing meta- or para-substituents on the aromatic structure perform well, (3b-3e) however, ortho-substituted aromatic alkynals are not suitable substrates under the current optimized conditions.[20] The variation of the α-ketoester was also explored. The α-ketoester bearing a para-methyl group provides the butenolide product (3f) with excellent enantioselectivity (96:4 er). α-Ketoesters possessing an electron-withdrawing group afford the products in excellent yield (3g–3j), but with a slight decrease in enantioselectivity (up to 90:10 er). The combination of substituted alkynals with α-ketoesters enabled the preparation of various highly functionalized butenolides (3k–3o). In addition, the indole-derived alkynal produces indole-substituted lactone 3p in good yield and excellent enantioselectivity.
Table 2.
Substrate Scope.[a]
|
See Supporting Information for details. Yields are of isolated product after column chromatography. Enantiomeric ratio was determined by HPLC analysis.
Absolute configuration was determined by X-ray crystallography.
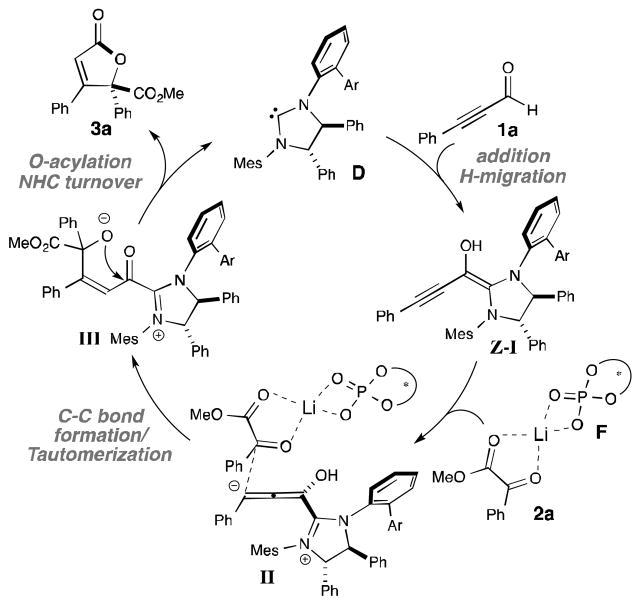
The proposed reaction pathway for this annulation reaction is shown in Scheme 1. The initial addition of catalyst D to alkynyl aldehyde 1a and subsequent formal 1,2-H migration generates Breslow intermediate Z-I.[21] The NHC-bound allenolate may undergo addition to the α-ketoester activated by the chiral Brønsted acid derived co-catalyst (II). A proton/hydrogen atom or lithium ion is involved in this activation mode via a deprotonation-protonation process. Carbon-carbon bond formation and subsequent tautomerization forms acyl azolium intermediate III, which then undergoes O-acylation to afford the lactone product (3a) and turnover the NHC catalyst.
Scheme 1.
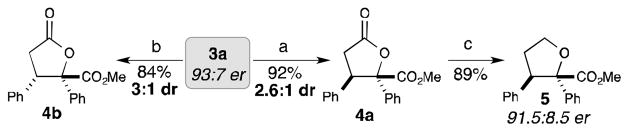
The butenolide products can be converted into enantioenriched γ-butyrolactones by selective hydrogenation reactions (Scheme 2). The use of Pearlman’s catalyst furnished cis-γ-butyrolactone 4a as the major product (2.6:1 dr) in 92% yield. An ester-directed homogeneous hydrogenation with Crabtree’s catalyst afforded trans-γ-butyrolactone 4b as the major product (3.1:1 dr).[22] In the presence of catalytic indium(III) bromide and triethylsilane, γ-butyrolactone 4a can also be selectively reduced to provide the corresponding multi-substituted tetrahydrofuran (5)[23], a scaffold found in numerous biologically active compounds.[24]
Scheme 2.
Synthetic Transformations.[a]
[a] Conditions: a) Pd(OH)2/C, H2, EtOAc. b) Crabtree’s catalyst, H2, CH2Cl2. c) 10 mol% InBr3, Et3SiH, CHCl3.
In conclusion, a highly efficient asymmetric [3+2] annulation reaction of alkynyl aldehydes with α-ketoesters via NHC/chiral phosphate cooperative catalysis has been developed. Alkynyl aldehydes can be converted into the corresponding enantiomerically enriched substituted butenolides in good yield and enantioselectivity. This challenging transformation features a new mode of cooperative catalysis utilizing the combination of an underexplored saturated imidazolium catalyst and chiral phosphoric acid, which was required to achieve the observed enhanced yields and enantioselectivities. Continuing investigations involving the use of these C1-symmetric catalysts and this new mode of cooperative catalysis are ongoing.
Supplementary Material
Acknowledgments
Financial support has been generously provided by the NIH (R01-GM073072). We thank Prof. Paul Ha-Yeon Cheong and Ryne C. Johnston (Oregon State Univeristy), for assistance with DFT Studies and Dr. Paul W. Siu (NU) and Dr. Ki Po Jang (NU) for providing X-ray crystallography support (NU).
Footnotes
Supporting information for this article is available on the Web under http://www.angewandte.org or from the author.
References
- 1.For a recent review on NHC derived homoenolate additions, see: Nair V, Menon RS, Biju AT, Sinu CR, Paul RR, Jose A, Sreekumar V. Chem Soc Rev. 2011;40:5336–5346. doi: 10.1039/c1cs15139h.For the first reports on NHC catalyzed homoenolate reactions, see: Burstein C, Glorius F. Angew Chem. 2004;116:6331–6208. doi: 10.1002/anie.200461572.Angew Chem Int Ed. 2004;43:6205–6208. doi: 10.1002/anie.200461572.Sohn SS, Rosen EL, Bode JW. J Am Chem Soc. 2004;126:14370–14371. doi: 10.1021/ja044714b.
- 2.For selected reviews on NHC organocatalysis, see: Enders D, Niemeier O, Henseler A. Chem Rev. 2007;107:5606–5655. doi: 10.1021/cr068372z.Phillips EM, Chan A, Scheidt KA. Aldrichimica Acta. 2009;42:55–66.Bugaut X, Glorius F. Chem Soc Rev. 2012;41:3511–3522. doi: 10.1039/c2cs15333e.Vora HU, Sheeler P, Rovis T. Adv Synth Catal. 2012;354:1617–1639. doi: 10.1002/adsc.201200031.
- 3.Zeitler K. Org Lett. 2006;8:637–640. doi: 10.1021/ol052826h. [DOI] [PubMed] [Google Scholar]
- 4.a) Kaeobamrung J, Manattananchai J, Zheng P, Bode JW. J Am Chem Soc. 2010;132:8810–8812. doi: 10.1021/ja103631u. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhu ZQ, Xiao JC. Adv Synth Catal. 2010;352:2455–2458. [Google Scholar]; c) Zhu ZQ, Zheng XL, Jiang NF, Wan X, Xiao JC. Chem Commun. 2011;47:8670–8672. doi: 10.1039/c1cc12778k. [DOI] [PubMed] [Google Scholar]; d) Du D, Hu Z, Jin J, Lu Y, Tang W, Wang B, Lu T. Org Lett. 2012;14:1274–1277. doi: 10.1021/ol300148f. [DOI] [PubMed] [Google Scholar]; e) Romanov-Michailidis F, Besnard C, Alexakis A. Org Lett. 2012;14:4906–4909. doi: 10.1021/ol3022287. [DOI] [PubMed] [Google Scholar]
- 5.a) Zhao YM, Tam Y, Wang YJ, Li Z, Sun J. Org Lett. 2012;14:1398–1401. doi: 10.1021/ol300111m. [DOI] [PubMed] [Google Scholar]; b) ElSohly AM, Wespe DA, Poore TJ, Snyder SA. Angew Chem. 2013;125:5901–5906. doi: 10.1002/anie.201301849. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52:5789–5794. doi: 10.1002/anie.201301849. [DOI] [PubMed] [Google Scholar]; c) Han R, Qi J, Gu J, Ma D, Xie X, She X. ACS Catal. 2013;3:2705–2709. [Google Scholar]
- 6.Qi J, Xie X, Han R, Ma D, Yang J, She X. Chem Eur J. 2013;19:4146–4150. doi: 10.1002/chem.201204386. Only moderate enantioselectivities (up to 53% ee) were obtained for this reaction. [DOI] [PubMed] [Google Scholar]
- 7.a) Cardinal-David B, Raup DEA, Scheidt KA. J Am Chem Soc. 2010;132:5345–5347. doi: 10.1021/ja910666n. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Phillips EM, Riedrich M, Scheidt KA. J Am Chem Soc. 2010;132:13179–13181. doi: 10.1021/ja1061196. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Raup DEA, Cardinal-David B, Holte D, Scheidt KA. Nature Chem. 2010;2:766–770. doi: 10.1038/nchem.727. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Cohen DT, Cardinal-David B, Roberts JM, Sarjeant AA, Scheidt KA. Org Lett. 2011;13:1068–1071. doi: 10.1021/ol103112v. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Cohen DT, Cardinal-David B, Scheidt KA. Angew Chem. 2011;123:1716–1720. doi: 10.1002/anie.201005908. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:1678–1682. doi: 10.1002/anie.201005908. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Dugal-Tessier J, O’Bryan EA, Schroeder TBH, Cohen DT, Scheidt KA. Angew Chem. 2012;124:5047–5051. doi: 10.1002/anie.201201643. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2012;51:4963–4967. doi: 10.1002/anie.201201643. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Mo J, Chen X, Chi YR. J Am Chem Soc. 2012;134:8810–8813. doi: 10.1021/ja303618z. [DOI] [PubMed] [Google Scholar]
- 8.a) Zhao X, DiRocco DA, Rovis T. J Am Chem Soc. 2011;133:12466–12469. doi: 10.1021/ja205714g. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu G, Wilkerson PD, Toth CA, Xu H. Org Lett. 2012;14:858–861. doi: 10.1021/ol203375y. [DOI] [PubMed] [Google Scholar]; c) Fu Z, Sun H, Chen S, Tiwari B, Li G, Chi YR. Chem Commun. 2013;49:261–263. doi: 10.1039/c2cc36564b. [DOI] [PubMed] [Google Scholar]; d) McCusker EO, Scheidt KA. Angew Chem. 2013;125:13861–13865. [Google Scholar]; Angew Chem Int Ed. 2013;52:13616–13620. doi: 10.1002/anie.201307292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akiyama T, Itoh J, Fuchibe K. Adv Synth Catal. 2006;348:999–1010.Connon SJ. Angew Chem. 2006;118:4013–4016.Angew Chem Int Ed. 2006;45:3909–3912. doi: 10.1002/anie.200600529.Akiyama T. Chem Rev. 2007;107:5744–5758. doi: 10.1021/cr068374j.Doyle AG, Jacobsen EN. Chem Rev. 2007;107:5713–5743. doi: 10.1021/cr068373r.List B. Chem Rev. 2007;107:5413–5415. doi: 10.1021/cr0684016.Connon SJ. Chem Commun. 2008:2499–2510. doi: 10.1039/b719249e.Dondoni A, Massi A. Angew Chem. 2008;120:4716–4739.Angew Chem Int Ed. 2008;47:4638–4660. doi: 10.1002/anie.200704684.MacMillan DWC. Nature. 2008;455:304–308. doi: 10.1038/nature07367.Bartoli G, Bencivenni G, Dalpozzo R. Chem Soc Rev. 2010;39:4449–4465. doi: 10.1039/b923063g.Kampen D, Reisinger CM, List B. Top Curr Chem. 2010;291:395–456. doi: 10.1007/978-3-642-02815-1_1.Terada M. Synthesis. 2010:1929–1982.Rueping M, Kuenkel A, Atodiresei I. Chem Soc Rev. 2011;40:4539–4549. doi: 10.1039/c1cs15087a.Brak K, Jacobsen EN. Angew Chem. 2013;125:558–588. doi: 10.1002/anie.201205449.Angew Chem Int Ed. 2013;52:534–561. doi: 10.1002/anie.201205449.Lv J, Luo SZ. Chem Commun. 2013;49:847–858. doi: 10.1039/c2cc34288j.Mahlau M, List B. Angew Chem. 2013;125:558–588. doi: 10.1002/anie.201205343.Angew Chem Int Ed. 2013;52:518–533. doi: 10.1002/anie.201205343.For a review on super Brønsted acid catalysis, see: Cheon CH, Yamamoto H. Chem Commun. 2011;47:3043–3056. doi: 10.1039/c0cc04867d.For selected examples of super Brønsted acid catalysis, see: Nakashima D, Yamamoto H. J Am Chem Soc. 2006;128:9626–9627. doi: 10.1021/ja062508t.Cheon CH, Yamamoto H. J Am Chem Soc. 2008;130:9246–9247. doi: 10.1021/ja8041542.
- 10.For selected reviews on metal-Brønsted acid combined catalysis, see: Shao Z, Zhang H. Chem Soc Rev. 2009;38:2745–2755. doi: 10.1039/b901258n.Rueping M, Koenigs RM, Atodiresei I. Chem Eur J. 2010;16:9350–9365. doi: 10.1002/chem.201001140.Zhong C, Xhi X. Eur J Org Chem. 2010:2999–3025.Lv J, Luo S. Chem Commun. 2013;49:847–858. doi: 10.1039/c2cc34288j.
- 11.For selected examples of metal-Brønsted acid combined catalysis, see: Shen K, Liu X, Cai Y, Lin L, Feng X. Chem Eur J. 2009;15:6008–6014. doi: 10.1002/chem.200900210.Yue T, Wang MX, Wang DX, Masson G, Zhu J. J Org Chem. 2009;74:8396–8399. doi: 10.1021/jo9017765.Zhang QW, Fan CA, Zhang HJ, Tu YQ, Zhao YM, Gu P, Chen ZM. Angew Chem. 2009;121:8724–8726. doi: 10.1002/anie.200904565.Angew Chem Int Ed. 2009;48:8572–8574. doi: 10.1002/anie.200904565.Zhao B, Du H, Shi Y. J Org Chem. 2009;74:8392–8395. doi: 10.1021/jo901685c.Hatano M, Moriyama K, Maki T, Ishihara K. Angew Chem. 2010;122:3911–3914. doi: 10.1002/anie.201000824.Angew Chem Int Ed. 2010;49:3823–3826. doi: 10.1002/anie.201000824.Drouet F, Lalli C, Liu H, Masson G, Zhu J. Org Lett. 2011;13:94–97. doi: 10.1021/ol102625s.Ingle GK, Liang Y, Mormino MG, Li G, Frondzek FR, Antilla JC. Org Lett. 2011;13:2054–2057. doi: 10.1021/ol200456y.Zhang Z, Zheng W, Antilla JC. Angew Chem. 2011;123:1167–1170. doi: 10.1002/anie.201006595.Angew Chem Int Ed. 2011;50:1135–1138. doi: 10.1002/anie.201006595.Zheng W, Zhang Z, Kaplan MJ, Antilla JC. J Am Chem Soc. 2011;133:3339–3341. doi: 10.1021/ja109824x.
- 12.For a mechanistic investigation involving NHC catalysis with alkynals, see: Mahatthananchai J, Zheng P, Bode JW. Angew Chem. 2011;123:1711–1715. doi: 10.1002/anie.201005352.Angew Chem Int Ed. 2011;50:1673–1677. doi: 10.1002/anie.201005352.
- 13.For selected examples, see: Veldhuizen JJV, Garber SB, Kingsbury JS, Hoveyda AH. J Am Chem Soc. 2002;124:4954–4955. doi: 10.1021/ja020259c.Veldhuizen JJV, Gillingham DG, Barber SB, Kataoka O, Hoveyda AH. J Am Chem Soc. 2003;125:12502–12508. doi: 10.1021/ja0302228.Hoveyda AH, Gillingham DG, Veldhuizen JJV, Kataoka O, Garber SB, Kingsbury JS, Harrity JPA. Org Biomol Chem. 2004;2:8–23. doi: 10.1039/b311496c.Lee K-s, Brown MK, Hird AW, Hoveyda AH. J Am Chem Soc. 2006;128:7182–7184. doi: 10.1021/ja062061o.Brown MK, May TL, Baxter CA, Hoveyda AH. Angew Chem. 2007;119:1115–1118. doi: 10.1002/anie.200604511.Angew Chem Int Ed. 2007;46:1097–1100. doi: 10.1002/anie.200604511.Lee Y, Akiyama K, Gillingham DG, Brown MK, Hoveyda AH. J Am Chem Soc. 2008;130:446–447. doi: 10.1021/ja0782192.Wu H, Radomkit S, O’Brien JM, Hoveyda AH. J Am Chem Soc. 2012;134:8277–8285. doi: 10.1021/ja302929d.
- 14.Jang KP, Hutson GE, Johnston RC, McCusker EO, Cheong PUY, Scheidt KA. J Am Chem Soc. 2014;136:76–79. doi: 10.1021/ja410932t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.For examples of pka value determination of NHC, see: Alder RW, Allen PR, Williams SJ. Chem Commun. 1995:1267–1268.Kim YJ, Streitweiser A. J Am Chem Soc. 2002;124:5757–5761. doi: 10.1021/ja025628j.Amyes TL, Diver ST, Richard JP, Rivas FM, Toth K. J Am Chem Soc. 2004;126:4366–4374. doi: 10.1021/ja039890j.Magill AM, Cavell KJ, Yates BF. J Am Chem Soc. 2004;126:8717–8724. doi: 10.1021/ja038973x.Chu Y, Deng H, Cheng JP. J Org Chem. 2007;72:7790–7793. doi: 10.1021/jo070973i.Higgins EM, Sherwood JA, Lindsay AG, Armstrong J, Massey RS, Alder RW, O’Donoghue AC. Chem Commun. 2011;47:1559–1561. doi: 10.1039/c0cc03367g.
- 16.For examples of pKa value determination of Brønsted acid, see: Christ P, Lindsay AG, Vormittag SS, Nuudoerfl JM, Berkessel A, O’Donoghue AC. Chem Eur J. 2011;17:8524–8528. doi: 10.1002/chem.201101157.Seebach D, Beck AK, Bichsel H, Pichota A, Sparr C, Wünsch R, Schweizer WB. Helv Chim Acta. 2012;95:1303–1324.Yang C, Xue XS, Jin JL, Li X, Cheng JP. J Org Chem. 2013;78:7076–7085. doi: 10.1021/jo400915f.
- 17.a) Rong ZQ, Jia MQ, You SL. Org Lett. 2011;13:4080–4083. doi: 10.1021/ol201595f. [DOI] [PubMed] [Google Scholar]; b) Qi J, Xie X, Han R, Ma D, Yang J, She X. Chem Eur J. 2013;19:4146–4150. doi: 10.1002/chem.201204386. [DOI] [PubMed] [Google Scholar]
- 18.Hatano M, Ikeno T, Matsumura T, Torii S, Ishihara K. Adv Synth Catal. 2008;350:1776–1780. [Google Scholar]
- 19.a) Xu S, Wang Z, Zhang X, Zhang X, Ding K. Angew Chem. 2008;120:2882–2885. [Google Scholar]; Angew Chem Int Ed. 2008;47:2840–2843. doi: 10.1002/anie.200705932. [DOI] [PubMed] [Google Scholar]; b) Rueping M, Theissmann TM, Kuenkel A, Koenigs RM. Angew Chem. 2008;120:6903–6906. [Google Scholar]; Angew Chem Int Ed. 2008;47:6798–6801. doi: 10.1002/anie.200802139. [DOI] [PubMed] [Google Scholar]
- 20.We also tested aliphatic alkynals, however, the reaction provided numerous peaks by UPLC and no desired product was observed. Current studies to engage this substrate class is ongoing.
- 21.A computational study at the M06-2X/6-31+G(2df,p)/PCM(THF)//M06-2X/6-31G(d) level of theory indicates that the Z-alkene isomer is more stable by ~0.6 kcal/mol compared to the E-alkene isomer due to minimization of steric interactions.
- 22.For a comprehensive review on substrate-directed reactions, see: Hoveyda AH, Evans DA, Fu GC. Chem Rev. 1993;93:1307–1370.
- 23.a) Sakai N, Moriya T, Konakahara T. J Org Chem. 2007;72:5920–5922. doi: 10.1021/jo070814z. [DOI] [PubMed] [Google Scholar]; b) Sakai N, Moriya T, Fujii K, Konakahara T. Synthesis. 2008:3533–3536. [Google Scholar]
- 24.a) Dean FM. Adv Heterocycl Chem. 1982;30:167–238. [Google Scholar]; b) Zhu Y, Zhai C, Yang L, Hu W. Chem Commun. 2010;46:2865–2867. doi: 10.1039/b924845e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.