Abstract
Background:
Both individuals with marijuana use and depressive disorders exhibit verbal learning and memory decrements.
Objectives:
This study investigated the interaction between marijuana dependence and depression on learning and memory performance.
Methods:
The California Verbal Learning Test – Second Edition (CVLT-II) was administered to depressed (n=71) and non-depressed (n=131) near-daily marijuana users. The severity of depressive symptoms was measured by the self-rated Beck Depression Inventory (BDI-II) and the clinician-rated Hamilton Depression Rating Scale (HAM-D). Multivariate analyses of covariance statistics (MANCOVA) were employed to analyze group differences in cognitive performance. Pearson’s correlation coefficients were calculated to examine the relative associations between marijuana use, depression and CVLT-II performance. Findings from each group were compared to published normative data.
Results:
Although both groups exhibited decreased CVLT-II performance relative to the test’s normative sample (p<0.05), marijuana-dependent subjects with a depressive disorder did not perform differently than marijuana-dependent subjects without a depressive disorder (p>0.05). Further, poorer CVLT-II performance was modestly associated with increased self-reported daily amount of marijuana use (corrected p<0.002), but was not significantly associated with increased scores on measures of depressive symptoms (corrected p>0.002).
Conclusion:
These findings suggest an inverse association between marijuana use and verbal learning function, but not between depression and verbal learning function in regular marijuana users.
Keywords: Cannabis, cognition, comorbidity, CVLT, depression, Marijuana, neurocognitive, neuropsychological, verbal learning
Introduction
Studies on the neurocognitive functioning of marijuana users have generally found that regular users exhibit decreased performance on tests of verbal learning and memory relative to healthy control participants (1). Verbal learning and memory are most commonly assessed by list-learning tasks such as the California Verbal Learning Test – Second Edition (CVLT-II) (2) or the Rey Auditory Verbal Learning Test (RAVLT) (3). These tasks measure the learning, recall and recognition of a list (16 and 15 words, respectively) that is repeatedly presented by an examiner. Performance on these tasks is correlated in the expected direction with self-reported marijuana use characteristics, such as frequency of use, lifetime-duration of use and abstinence (i.e. greater use is associated with poorer learning performance) (4).
A meta-analysis examining the residual effects of marijuana on neurocognitive functioning (n=704 marijuana users and 484 non-using control participants) demonstrated a significant detrimental influence of long-term marijuana use on learning and memory functions (1). For example, one study examined the role of lifetime duration of marijuana use on neuropsychological function by comparing memory and attention task performance between near-daily marijuana users (n=102) enrolled in treatment to healthy (i.e. non-using) controls (n=33) (5). These authors found that long-term marijuana users (mean of 23.9 years of regular use) exhibited decreased performance relative to both the short-term users (mean of 10.2 years of regular use) and controls on the learning, retention and retrieval trials of the RAVLT (with long-term users recalling significantly fewer words on nearly every trial). However, both the short- and long-term users exhibited decreased attentional performance relative to the controls, with marijuana users underestimating the time taken to complete the Omitted Numbers task. These results, as well as others, suggest that while attentional decrements are associated with the active use of marijuana, verbal learning and memory decrements are specifically associated with long-term marijuana use.
Depressive disorders are highly comorbid with marijuana dependence in clinical settings (6) and they have been shown to be independently associated with mild-to-moderate performance impairment on verbal learning tasks (7), which may be partially attributable to depressive amotivation (8). For example, one study found that participants with recurrent major depressive disorder (n=42) exhibited decreased performance on the CVLT-II learning trials (Trials 4 and 5) relative to healthy controls (n=33) (9). Additionally, participants with elevated depression scores on the Minnesota Multiphasic Personality Inventory (MMPI) (10) have exhibited decreased performance on CVLT-II Trials 1–5 and Short-Delay Free Recall, relative to participants with average depression scores (11). These results suggest an association between depression and decreased functioning in the middle stages of memory (late learning and immediate recall).
Despite findings that learning and memory performance are decreased in marijuana users and depressed populations, to our knowledge, the interaction between marijuana dependence and depression on verbal learning and memory functioning has not been investigated. Thus, we examined this interaction by comparing CVLT-II performance of marijuana-dependent individuals with and without a comorbid depressive disorder. Based on current literature, we expected that both groups of marijuana users would exhibit decreased performance on the learning and immediate recall trials, relative to the general population, and that these effects would be greater in the depressed marijuana-using group.
Methods
Participants
Participants were drawn from two distinct pharmacotherapy trials for marijuana dependence: one for treatment of psychiatrically-healthy marijuana users and one for treatment of marijuana users with depression (12,13). Both of these studies were approved by the New York State Psychiatric Institute Institutional Review Board and all participants provided informed consent prior to assessment. Both groups were recruited from the general population by advertisements for treatment-seeking marijuana users with and without depression.
All participants were administered the Structured Clinical Interview for DSM-IV Disorders (SCID-I) (14) to derive DSM-IV psychiatric diagnoses (15). A current diagnosis of marijuana dependence was required for all participants prior to study entry. The severity of depressive symptoms was measured convergently by the self-rated Beck Depression Inventory (BDI-II) (16) and the clinician-rated Hamilton Depression Rating Scale (HAM-D) (17). In the non-depressed group (MJ; n=131), no participant met DSM-IV criteria for any depressive disorder. In the depressed marijuana group (MJ+Dep; n=71), all participants met DSM-IV criteria for current major depressive disorder or dysthymic disorder, and had received baseline HAM-D scores>12. Bipolar, psychotic, and primary cognitive disorders were exclusionary, as was dependence on any substance other than nicotine and alcohol. Although, in general, participants who met psychiatric criteria for alcohol dependence were not excluded (MJ, n=9; MJ+Dep, n=8), those who were determined to be physiologically-dependent by study physicians were excluded. Demographic characteristics, and other substance use and psychiatric characteristics were assessed at treatment baseline.
Marijuana use patterns were assessed by the Timeline Follow-Back method (18), using standardized units (SU; 1 blunt=3 joints=3 bowls=$5=1 SU). A negative breathalyzer screening (BrAC=0.00) was required prior to CVLT-II administration. A quantitative urine toxicology test was also conducted to determine the levels of Delta 9-tetrahydrocannabinol (Δ9-THC) metabolites on the day of cognitive assessment (ng/ml). All participants were tested during a placebo lead-in week of clinical trial participation; therefore no participant had received active study medication at the time of testing. Any individual exhibiting obvious indications of acute intoxication from marijuana were excluded. All participants were compensated $25.00 for their participation in cognitive testing.
Demographic and clinical group differences
Demographic information, clinical characteristics and substance use patterns are presented in Table 1. Differences between groups were analyzed with independent sample t-tests for continuous variables and Chi-square tests for categorical variables. Groups were similar in terms of age, education, sex and ethnicity; the average participant tended to be in his/her mid-to-late thirties, well-educated, male and Caucasian. As expected, the MJ+Dep group exhibited greater levels of HAM-D and BDI-II depressive symptoms (moderate to severe) than the MJ group (minimal to moderate; p<0.01). No group differences (p>0.05) were found on self-reported marijuana amount per using day (gm) or urine-THC value at testing (ng/ml). The MJ group reported smoking marijuana about 2 days more frequently during the 30 days prior to testing than the MJ+Dep group (p<0.05), although this is unlikely to be a clinically-significant difference. Groups were also similar in their reported alcohol consumption. Thus, well-matched groups of near-daily marijuana users with mild-to-moderate alcohol consumption, differing mainly on depressive comorbidity, were compared in this study.
Table 1.
Demographic information, clinical characteristics and substance use patterns.
| MJ (n = 131) |
MJ + Dep (n = 71) |
||||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | Test value | df | p a | |
| Age (years) | 37.0 | 10.5 | 35.3 | 9.9 | t = 1.09 | 200 | 0.28 |
| Education (years) | 14.3 | 2.5 | 14.7 | 2.4 | t = −1.20 | 197 | 0.23 |
| HAM-D | 5.4 | 3.8 | 18.5 | 4.4 | t = −21.92 | 198 | 0.00 |
| BDI-II | 13.1 | 9.0 | 27.9 | 10.8 | t = −9.78 | 184 | 0.00 |
| Marijuana | |||||||
| Days of use/past 30 days | 28.4 | 3.2 | 26.7 | 6.5 | t = 2.52 | 196 | 0.01 |
| Amount/using day (gm) | 2.5 | 2.8 | 2.4 | 2.2 | t = 0.10 | 196 | 0.92 |
| Urine-THC value (ng/ml) | 1853.1 | 2971.9 | 1282.7 | 1452.6 | t = 1.50 | 191 | 0.14 |
| Alcohol | |||||||
| Days of use/past 30 days | 8.0 | 8.0 | 8.1 | 8.9 | −0.09 | 181 | 0.93 |
| Amount/using day (SDUs)b | 2.4 | 2.2 | 3.0 | 2.4 | −1.75 | 180 | 0.08 |
| Sex | |||||||
| Male | 81% | n = 106 | 73% | n = 52 | χ2 = 1.59 | 1 | 0.21 |
| Female | 19% | n = 25 | 27% | n = 19 | |||
| Ethnicity | |||||||
| Caucasian | 46% | n = 61 | 41% | n = 29 | χ2 = 0.64 | 4 | 0.97 |
| African American | 24% | n = 31 | 25% | n = 18 | |||
| Hispanic | 24% | n = 31 | 27% | n = 19 | |||
| Other | 6% | n = 8 | 7% | n = 5 | |||
HAM-D, Hamilton Depression Rating Scale. BDI, Beck Depression Inventory.
Bold indicates a significant group difference (p < 0.05 level);
Standard drink units (i.e. 12-oz beer, 4.5-oz wine, 1.25-oz liquor).
California Verbal Learning Test – Second Edition (CVLT-II)
The CVLT-II was administered by trained research assistants. In this task, the participant is orally presented 16 words (List A) from 4 semantic categories (food, furniture, means of transportation and clothing) five times. After each presentation, the participant is required to reproduce as many words as possible (Learning Trials 1–5). Following these trials, the participant is presented a new list of 16 words (List B) from four different thematic categories (A, B, C & D) and required to reproduce as many words as possible. Following both 5- and 20-minute delays (Short- and Long-Delay), the participant is asked to recall as many words as possible from List A, without and with cues from the examiner (Free and Cued Recall). Finally, the participant is presented a 40-word list, which includes the 16 List A words interleaved among 24 distracter words, and asked to indicate which words were from List A. Primary learning measures consisted of the number of words reproduced on Trials 1 and 5, while primary recall measures consisted of the number of words reproduced on the Short-Delay Free Recall (SDFR) and Long-Delay Free Recall (LDFR) trials. Secondary measures consisted of the number of words produced on all learning trials (1–5; Total Learning) and on the Short- and Long-Delay Cued Recall trials (SDCR and LDCR, respectively), as well as the number of words correctly and incorrectly recognized (Recognition Hits and False Positives) and the number of learning/recall errors (Repetition and Intrusions, respectively).
Data analyses
All data analyses were conducted using the Statistical Package for the Social Sciences – Version 18. First, mean raw and standard (z) scores were calculated for each group on all of the CVLT-II measures described above. Because there was no healthy control group in this study, CVLT-II performance (age/sex-corrected z-scores)1 for each group was first compared to published normative data with a series of three-group one-way analyses of variance (ANOVA). Significant differences on these ANOVAs (p<0.05) were then probed with single sample t-tests. However, due to the relative lenience of this statistical approach, only comparisons between normative data and each MJ group were reported. Next, a more rigorous multivariate analysis of covariance (MANCOVA) was employed to analyze overall differences on CVLT-II performance between the two MJ groups. Group was the between-subjects variable, marijuana use frequency was the covariate, and CVLT-II raw scores were the dependent variables. To account for the potential influence of alcohol use on these results, we removed all participants diagnosed with alcohol dependence and conducted these analyses again. In addition, to examine the relative associations between marijuana use, depression and CVLT-II performance, Pearson’s correlation coefficients were calculated between measures of these variables (mean self-reported days and amounts of use and mean baseline urine-THC values; mean BDI-II and HAM-D scores) and CVLT-II indices. To account for potential Type 2 error resulting from the multiple correlations that were computed, all correlational results were referenced against a Bonferroni-corrected p value of 0.002.
Results
Comparison to control normative data
The mean data for all of the CVLT-II indices, along with respective comparisons to the estimated population data, are presented in Table 2. With the exception of total Repetition Errors, three-group one-way analyses of variance (ANOVA) revealed overall group differences on all performance indices (p<0.05). Follow-up t-tests revealed decreased performance in both MJ groups on all indices relative to the normative sample (p<0.05), except for Trials 1–5 Total (p>0.05) and number of Repetition Errors (MJ+Dep group only; p>0.05). Scores for nearly every index for both groups were≥1.5 SD below the normative sample, indicative at least a moderate impairment; exceptions were Trial 1–5 Total (both groups), Short Delay Cued Recall (MJ+Dep group only), Recognition Hits (both groups), Repetition Errors (both groups) and Intrusion errors (MJ+Dep group only). Thus, signs of relative impairment in both groups of marijuana users were found on measures of auditory attention and delayed recall, and delayed recognition for the non-depressed marijuana users.
Table 2.
CVLT-II results; comparison to normative data and between-subjects effects.
| MJ |
MJ + Dep |
MJ vs. MJ + Dep vs. Normsc |
|||||
|---|---|---|---|---|---|---|---|
| Index | M (SD) | %ileb | M (SD) | %ileb | F | df | pa |
| Trial 1 | 5.08 (1.9) | 16 | 5.51 (2.1) | 16 | 47.62 | 2 | 0.00 |
| Trial 5 | 10.69 (2.9) | 31 | 11.0 (2.6) | 31 | 22.55 | 2 | 0.00 |
| Trials 1–5 Total | 42.72 (11.5) | 50 | 44.69 (10.3) | 50 | 15.08 | 2 | 0.00 |
| Trial B | 4.47 (1.9) | 16 | 5.13 (2.0) | 16 | 39.62 | 2 | 0.00 |
| Short Delay Free Recall | 8.79 (3.5) | 31 | 9.77 (3.5) | 31 | 20.83 | 2 | 0.00 |
| Long Delay Free Recall | 9.18 (3.6) | 16 | 10.10 (3.0) | 31 | 25.24 | 2 | 0.00 |
| Short Delay Cued Recall | 10.21 (3.4) | 31 | 11.17 (2.9) | 31 | 13.81 | 2 | 0.00 |
| Long Delay Cued Recall | 10.22 (3.2) | 31 | 11.13 (2.9) | 31 | 18.67 | 2 | 0.00 |
| Hits | 14.15 (2.0) | 16 | 14.45 (1.8) | 16 | 14.58 | 2 | 0.00 |
| False Positives | 3.72 (4.4) | 84 | 3.23 (4.5) | 69 | 14.45 | 2 | 0.00 |
| Repetition | 5.43 (4.0) | 69 | 4.80 (4.1) | 69 | 2.86 | 2 | 0.06 |
| Intrusions | 4.26 (4.4) | 69 | 3.40 (3.9) | 50 | 3.86 | 2 | 0.02 |
Bold indicates a significant group difference at thep< 0.05 level;
Percentiles are based on males and females aged 30–44 (2);
Results for three-group one-way analyses of variance (ANOVA).
After the removal of alcohol-dependent participants, all scores as noted above remained ≥1.5 SD below the normative sample. Additionally, all overall and group differences remained statistically significant, with the exception of the depressed group’s number of Intrusion Errors as compared to the normative sample; this difference was no longer significant (p>0.05).
Between- MJ-group comparisons
MANCOVA results (MJ group n=121; MJ+Dep group n=57) revealed no overall difference between groups on CVLT-II performance scores (F12, 164=0.99, p>0.05). Therefore, group differences on individual CVLT indices were not statistically examined. Removal of alcohol-dependent participants (MJ group n=112; MJ+Dep group n=49) did not change this pattern of significance.
Association between severity of depressive symptoms, marijuana use frequency, and CVLT-II performance
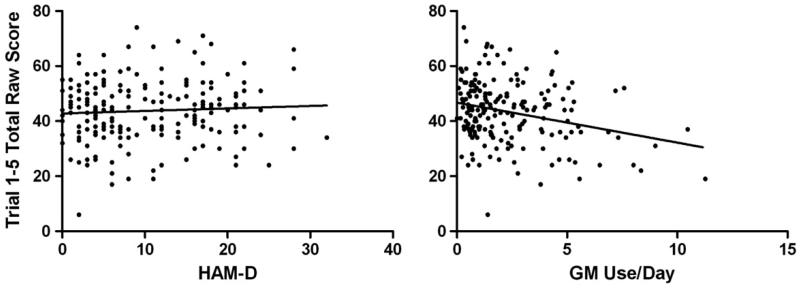
Correlations between CVLT-II performance scores and depression/marijuana use indicators across the total sample are presented in Table 3. No correlations were found between CVLT-II scores and either scores on the HAM-D or the BDI-II (p>0.05), suggesting that there was no overall relationship between severity of depressive symptoms and learning and memory performance. However, mild inverse correlations were found between reported amount of marijuana use (GM/day) and CVLT-II Trial 5, Trials 1–5 Total, Short Delay Free Recall and Long Delay Cued Recall scores (p<0.002). Reported frequency of marijuana use (days/month) and urine-THC values were uncorrelated with all CVLT-II indices (p>0.002). These results suggested that increased reported daily amounts of marijuana use, but not increased level of depressive symptoms, was associated with poorer performance on primary measures of verbal learning and recall, and vice-versa. This differing pattern of association is represented in Figure 12, which displays the scatterplots for the correlations between CVLT-II Trial 1–5 Total mean scores and HAM-D scores and reported GM use/day.
Table 3.
Correlations across the entire sample between CVLT-II indices, frequency of marijuana use and depression measures.
| Marijuana use |
Depression |
||||
|---|---|---|---|---|---|
| Index | # Daysa | GM/Dayb | THCc | HAM-D | BDI |
| Trial 1 | −0.04 | − 0.14 | − 0.17 | 0.04 | 0.06 |
| Trial 5 | −0.06 | − 0.24* | − 0.19 | 0.04 | 0.01 |
| Trials 1–5 | −0.07 | − 0.24* | − 0.16 | 0.06 | 0.06 |
| Trial B | −0.11 | − 0.18 | − 0.18 | 0.07 | 0.06 |
| Short Delay Free Recall | −0.12 | − 0.25* | −0.13 | 0.09 | 0.09 |
| Long Delay Free Recall | 0.00 | − 0.14 | −0.08 | 0.11 | 0.10 |
| Short Delay Cued Recall | −0.08 | − 0.19 | − 0.16 | 0.14 | 0.09 |
| Long Delay Cued Recall | −0.08 | − 0.22* | −0.12 | 0.09 | 0.10 |
| Hits | −0.07 | −0.03 | −0.01 | 0.08 | 0.08 |
| False Pos. | 0.03 | 0.14 | 0.19 | 0.03 | 0.07 |
| Repetitions | −0.08 | −0.05 | −0.03 | −0.07 | −0.01 |
| Intrusions | 0.86 | 0.11 | 0.07 | −0.03 | −0.04 |
HAM-D, Hamilton Depression Rating Scale. BDI, Beck Depression Inventory. Bold indicates a significant correlation at the p < 0.05 level;
indicates that significance was retained with Bonferroni-corrected value (p < 0.002);
days of use/past 30 days;
amount/using day (gm);
urine-THC value on the day of assessment (ng/ml).
Figure 1.
Correlations between CVLT-II Total Learning (Trials 1–5) and Hamilton Depression Rating Scale (HAM-D) raw scores (left panel; r=0.06; p>0.05) and GM use/day (right panel; r=−0.24; p<0.001); one outlier on reported daily MJ use (i.e. 20.9 GM/day) was removed from the GM use/day scatterplot for visual clarity (but was included in the analysis).
Discussion
The present findings indicated that: (i) marijuana-dependent participants exhibited decreased performance on the CVLT-II, a measure of verbal learning and recall, relative to the test’s normative sample; (ii) marijuana-dependent participants with a depressive disorder did not perform differently than marijuana-dependent subjects without a depressive disorder; and (iii) CVLT-II performance was correlated with self-reported daily amount of marijuana use, but uncorrelated with measures of depressive symptoms. Collectively, these findings suggest an inverse association between marijuana use and verbal learning function, but not between depression and verbal learning function among regular marijuana users. To the best of our knowledge, this is the first investigation of the influence of depression on verbal learning function in marijuana-dependent subjects.
The finding that verbal learning decrements were modestly associated with marijuana use is consistent with a considerable amount of literature demonstrating that: (i) verbal learning performance is decreased in active marijuana users (1,19,20), (ii) verbal learning performance is inversely correlated with severity measures of use (4,21), and (iii) verbal learning performance improves with increasing abstinence (22–24). However, the finding that verbal learning was not further decreased in participants with a comorbid depressive disorder was unexpected, considering that some studies have found that verbal memory function is decreased in non-marijuana-using depressed individuals (7,9,11). Generally, deficits among depressed participants on the CVLT-II include decreased recall over the five learning trials and measures of delayed recall (2). Although the depressed sample in the current study exhibited decreased performance on these indices compared to the normative sample, it was equivalent to the performance of the non-depressed group. Therefore, there was no additive effect of depression on learning performance in marijuana users. Given the lack of consistent evidence for verbal learning decrements in depressed individuals (25,26), as opposed to findings for marijuana use (1), depression may not have as strong an influence on verbal learning as marijuana. In line with this notion, an investigation from an earlier subset (n=108) of the participants used in the current study (27) found no evidence of other neurocognitive decrements in the depressed participants relative to the non-depressed participants. Thus, our results suggest that depression does not interact with marijuana use to further decrease verbal learning and other neurocognitive functions.
Consistent with previous research (4,21) and regardless of mood diagnosis, the self-reported daily amount of marijuana use (gram values) in the entire sample was mildly and inversely correlated with a number of primary measures of CVLT-II performance, indicating a modest relationship between marijuana use and verbal learning difficulty. Surprisingly, reported frequency of marijuana use (days/month) and urine-THC values were uncorrelated with CVLT-II performance, suggesting a dissociation between different measures of marijuana use in terms of their relationships to learning and memory performance in this sample. In contrast to marijuana use, no measures of depression severity (either self- or clinician-rated) were correlated with CVLT-II performance, indicating an overall lack of relationship between depressive symptom severity and verbal learning and memory performance.
There are several limitations to the present study. First, we did not incorporate a healthy control group to directly measure the degree of impairment exhibited by the patient groups. Our comparison of participants to the normative sample’s mean scores only provides a rough sense of performance impairment, since many of the demographic and clinical characteristics of the normative sample are unknown. Second, the CVLT-II was the only instrument used to measure learning and recall; additional measures (e.g. visual learning) would have provided a more extensive picture of the relationship between marijuana use, depression and learning/memory. Third, we could not fully account for the potential intoxicating effects of marijuana, nor did we consider the role of task effort, acutely on cognitive performance; our findings may in part reflect these factors. Fourth, we did not have rigorous data on other marijuana use variables, including lifetime duration, periods of abstinence and the recency of last use. These factors are relevant influences on cognitive functioning and should be incorporated into future research. Finally, although the pattern of results did not substantially change when we removed those participants with alcohol dependence, we could not fully account for the influence of alcohol use on performance. These factors remain the subject of future research.
In summary, our results suggest that while marijuana users exhibited significant verbal learning decrements on the CVLT-II, depression had no additive effect on these decrements. Furthermore, while an indicator of marijuana use severity was associated with learning and recall performance across the entire sample, indicators of depression severity were not. Thus, in regular marijuana users, marijuana use may be more associated with verbal learning impairment than depressive symptomatology. Regarding possible treatment implications, these results imply that among marijuana patients, memory deficit is more likely to resolve following marijuana-use reduction and abstinence, but not necessarily throughout depression remission. It is important to consider these implications, particularly where cognitive therapies are utilized in treatment-seeking populations. Further research is needed to expand on the breadth and clinical relevance of these findings.
Acknowledgements
The authors gratefully acknowledge the research staff at the Substance Treatment and Research Service (STARS) of Columbia University Medical Center/New York State Psychiatric Institute for their assistance in data collection. Funding for this research was provided by NIDA grants R01 DA15451, p<0 DA09236 and K24 DA029647.
Footnotes
Portions of this research were presented at the 2010 meeting of the College on Problems of Drug Dependence.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Z-scores were employed for this comparison since the CVLT-II manual only provides means and SDs for z-scores for the normative population.
One MJ+Dep participant who reported an extraordinarily high amount of daily marijuana use (20.9 GM/day) was deemed an outlier, and thus removed from the GM use/day-CVLT-II Total Learning scatterplot for visual clarity; the correlation was significant with and without this outlying data (p<0.002).
References
- 1.Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute neurocognitive effects of marijuana use: a meta-analytic study. J Int Neuropsycholog Soc. 2003;9:679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- 2.Delis DC, Kramer JH, Kaplan E, Ober BA. CVLT-II California Verbal Learning Test. 2nd. Psychological Corporation; San Antonio, TX: 2000. Adult Version. [Google Scholar]
- 3.Schmidt M. Rey Auditory and Verbal Learning Test: a handbook. Western Psychological Services; Los Angeles, CA: 1996. [Google Scholar]
- 4.Solowij N, Battisti R. The chronic effects of marijuana on memory in humans: a review. Curr Drug Abuse Rev. 2008;1:81–98. doi: 10.2174/1874473710801010081. [DOI] [PubMed] [Google Scholar]
- 5.Solowij N, Stephans RS, Roffman RA, Babor T, Kadden R, Miller M, et al. Cognitive functioning of long-term heavy marijuana users seeking treatment. JAMA. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- 6.Mathews RR, Hall WD, Gartner CE. Depression and psychological distress in tobacco smokers and people with marijuana dependence in the National Survey of Mental Health and Wellbeing. Med J Australia. 2011;195:S12–15. doi: 10.5694/j.1326-5377.2011.tb03259.x. [DOI] [PubMed] [Google Scholar]
- 7.Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression. Br J Psychiat. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- 8.Benitez A, Horner MD, Bachman D. Intact cognition in depressed elderly veterans providing adequate effort. Arch Clin Neuropsychol. 2011;26:184–193. doi: 10.1093/arclin/acr001. [DOI] [PubMed] [Google Scholar]
- 9.Smith DJ, Muir WJ, Blackwood DH. Neurocognitive impairment in euthymic young adults with bipolar spectrum disorder and recurrent major depressive disorder. Bipolar Disorders. 2006;8:40–46. doi: 10.1111/j.1399-5618.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- 10.Dahlstrom WG, Welsh GS, Dahlstrom LE. An MMPI handbook: research applications. rev. Vol. 2. University of Minnesota Press; Minneapolis: 1989. [Google Scholar]
- 11.Kizilbash AH, Vanderploeg RD, Curtiss G. The effects of depression and anxiety on memory performance. Arch Clin Neuropsychol. 2002;17:57–67. [PubMed] [Google Scholar]
- 12.Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116:142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Nunes EV, Agosti V, Bisaga A, et al. A randomized double-blind, placebo-controlled trial of venlafaxine-extended release for co-occurring cannabis dependence and depressive disorders. Addiction. 2013;108:1084–1094. doi: 10.1111/add.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.First MB, Spitzer RL, Gibbon M, William JBW. Structured clinical interview for DSM-IV Axis I Disorders, Research Version, Patient Edition (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- 15.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Publishing; Washington, DC: text rev. [Google Scholar]
- 16.Beck AT, Steer R, Brown G. BDI-II Beck Depression Inventory manual. 2nd Harcourt Brace & Company; San Antonio, TX: 1996. [Google Scholar]
- 17.Hamilton M. A rating scale for depression. J Neurol, Neurosurg Psychiat. 1960;23:56–61. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobell LC, Sobell MB, Buchan G, Clenand PA, Fedoroff I, Leo GI. The reliability of the Timeline Followback method applied to drug, cigarette, and marijuana use; Presented at the 30th Annual Meeting of the Association for Advancement of Behavior Therapy; New York, NY, USA. [Google Scholar]
- 19.Crean RD, Tapert SF, Minassian A, MacDonald K, Crane NA, Mason BJ. Effects of chronic, heavy cannabis use on executive functions. J Addiction Med. 2011;5:9–15. doi: 10.1097/ADM.0b013e31820cdd57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messinis L, Kyprianidou A, Malefaki S, Papathanasopoulos P. Neuropsychological deficits in long-term frequent cannabis users. Neurology. 2006;66:737–739. doi: 10.1212/01.wnl.0000201279.83203.c6. [DOI] [PubMed] [Google Scholar]
- 21.Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- 22.Pope HG, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA. 1996;275:521–527. [PubMed] [Google Scholar]
- 23.Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term marijuana users. Arch Gen Psychiat. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- 24.Vadhan NP, van Gorp WG, Levin FR. Specificity of verbal learning impairment and recovery in a marijuana-dependent male: the effects of sustained marijuana abstinence. Cognit Neuropsychiat. 2011;16:158–173. doi: 10.1080/13546805.2010.524494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castaneda AE, Suvisaari J, Marttunen M, Perala J, Saarni SI, Aalto-Setala T, Aro H, et al. Cognitive functioning in a population-based sample of young adults with a history of non-psychotic unipolar depressive disorders without psychiatric comorbidity. J Affect Disord. 2008;110:36–45. doi: 10.1016/j.jad.2007.12.239. [DOI] [PubMed] [Google Scholar]
- 26.Wang CE, Halvorson M, Sundet K, Steffensen AL, Holte A, Waterloo K. Verbal memory performance of mildly to moderately depressed outpatient younger adults. J Affect Disord. 2006;92:283–286. doi: 10.1016/j.jad.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Secora AM, Eddie D, Wyman BJ, Brooks DJ, Mariani JJ, Levin FR. A comparison of psychosocial and cognitive functioning between depressed and non-depressed patients with marijuana dependence. J Addictive Dis. 2010;29:325–337. doi: 10.1080/10550887.2010.489444. [DOI] [PMC free article] [PubMed] [Google Scholar]