Abstract
Background
Injury due to myocardial infarction (MI) is largely irreversible. Once an infarct has occurred, the clinical goal becomes limiting remodeling, preserving left ventricular (LV) function and preventing heart failure. While traditional approaches (e.g. β-blockers) partially preserve LV function, novel strategies are needed to limit ventricular remodeling post-MI.
Objectives
The aim of this study was to determine the role of glycogen synthase kinase-3α (GSK-3α) in the post- MI remodeling.
Methods
Mice with cardiomyocyte specific conditional deletion of Gsk3α and littermate controls underwent sham or MI surgery. Heart function was assessed using serial M-mode echocardiography.
Results
Gsk3α deletion in the heart markedly limits remodeling and preserves LV function post-MI. This is due, at least in part, to dramatic thinning and expansion of the scar in the control hearts, which was less in the KO. In contrast, the border zone in the KO demonstrated a much thicker scar and there were more viable cardiomyocytes within the scar/border zone. This was associated with less apoptosis and more proliferation of cardiomyocytes in the KO. Mechanistically, reduced apoptosis was due, at least in part, to a marked decrease in the Bax/Bcl-2 ratio, and increased cardiomyocyte proliferation was mediated through cyclin E1 and E2F-1 in the KO hearts.
Conclusions
Taken together, these findings show that reducing GSK-3α expression in the cardiomyocyte limits ventricular remodeling and preserves cardiac function post- MI. Targeting specifically GSK-3α, could be a novel strategy to limit adverse remodeling and heart failure.
Keywords: GSK-3α, Myocardial infarction, Ventricular remodeling, Heart failure, Cardiomyocyte proliferation
Introduction
Myocardial infarction (MI) and its sequellae, in particular heart failure, are the leading cause of death in the developed world and are rapidly becoming a major killer in the developing world (1,2). The neonatal heart has the capability of regeneration through cardiomyocyte proliferation (3), but appears to lose much of this capability early post-birth (4). Various stressors, most importantly ischemic injury, can lead to progressive cardiomyocyte loss. Apoptosis is uncommon in the normal heart, but is frequently seen in ischemic heart (5). The endogenous regenerative capacity of the heart appears insufficient to replenish the lost myocytes, so even relatively low levels of apoptosis can have profound effects on cardiac function (5). This has necessitated employment of strategies such as injection of stem/progenitor cells into the infarcted region (6). While these strategies hold promise, activation of endogenous repair mechanisms within the heart could, in theory, represent a superior strategy.
Glycogen synthase kinase (GSK)-3β has been well-studied in various pathophysiological conditions (7-11), but, the role of GSK-3α in various pathologic settings, including ischemic injury and heart failure, is much less studied and poorly understood. This bias towards GSK-3β arose from early reports using Drosophila that concluded that GSK-3β was the dominant isoform since it was better able to rescue Wnt/wingless pathway defects (12,13). This bias appears to remain, with many more publications focusing on GSK-3β than on GSK-3α. To summarize, although the roles of GSK-3α remain unclear in many settings, it is clear that there are both unique and overlapping functions of the two isoforms. In short, despite the 97% sequence similarity, these kinase have many non-overlapping roles.
Global deletion of Gsk3α is detrimental in the setting of stress, leading to deterioration in cardiac function and increased hypertrophy, cardiac fibrosis and ventricular remodeling (14,15). Surprisingly, a similar phenotype of heart failure and hypertrophy has been reported with a constitutively active (S21A mutant) GSK-3α global knock-in (KI) mouse when challenged with pressure overload (16). Furthermore, cardiac specific over-expression of GSK-3α via transgenesis attenuates cardiac hypertrophy and increases fibrosis and apoptosis post-TAC (17,18). These observations, from three different models, have led to uncertainty as to the roles of GSK-3α in the pathophysiology of heart failure. Moreover, global knock-out (KO) or knock-in (KI) models can lead to compensatory effects that complicate the interpretation of phenotypes (19) and rarely if ever recapitulate the conditional gene deletion due to a variety of reasons.
Herein, for the first time, we employ a cardiomyocyte-specific conditional KO mouse to characterize the rather surprising roles of GSK-3α in regulating the consequences of myocardial infarction. We report that conditional deletion of GSK-3α leads to marked protection post-MI that is due to less cardiomyocyte death, less scar expansion and thinning, and increased cardiomyocyte proliferation in the KO hearts, the latter being driven by alterations in activity of cell cycle regulators. We believe that these studies will bring much more clarity to the true roles played by GSK-3α in the infarcted heart.
Methods
A detailed method is supplied as supplemental materials
Mice
The Gsk3αflox/flox (fl/fl) mouse was generated as previously described (8). Mice expressing α-myosin heavy chain (α-MHC) promoter-driven, tamoxifen (Tam)-inducible heterozygous Mer-Cre-Mer (gift from Dr J. Molkentin, Cincinnati Children’s Hospital, Cincinnati, Ohio) were crossed for two generations with Gsk3αfl/fl mice in order to generate Gsk3αfl/fl Cre mice. Both mouse strains were on the C57BL/6 background. At 12 weeks of age, when physiological development is largely complete, all male mice were placed on a tamoxifen chow diet (400mg/kg) for 15 days followed by regular chow for an additional 15 days (to allow the clearance of tamoxifen from the mice). Gsk3αfl/fl/ Cre+/− /Tam mice were conditional knockout (KO), whereas littermates Gsk3αfl/fl/Tam represented controls (WT). The Institutional Animal Care and Use Committee of Temple University approved all animal procedures and treatments.
Antibodies
A detailed antibody list and application is supplied as supplementary material.
Myocardial Infarction
Following baseline echocardiography, permanent occlusion of the proximal left anterior descending (LAD) coronary artery, or sham surgery, was performed in WT versus KO male littermates as described previously (20). After the 8-week echo examination, mice were euthanized for different studies.
Echocardiography
Echocardiography was performed as described previously (15). In brief, transthoracic two-dimensional motion mode-echocardiography was performed at 0, 1, 2, 4 and 8 wks post-MI with a 12-mHz probe (VisualSonics) on mice anesthetized by inhalation of isoflurane (1-1.5%). LV end-systolic interior dimension (LVID;s), enddiastolic interior dimension (LVID;d), ejection fraction (EF) and fractional shortening (FS) values were analyzed using the Vevo770 program.
Histochemistry/ Immunohistochemistry
A detail protocol is provided as supplemental material. In brief, post 3 and 8 week MI, whole heart was excised from anesthetized mice and fixed in 4% paraformaldehyde, dehydrated through increasing concentrations of ethanol, and then embedded in paraffin (9). Heart sections (5μm) were stained using Masson trichrome (Sigma-Aldrich) or immunofluorescence antibodies. A Nikon Eclipse 80i fluorescent microscope and NIS Elements software were used to capture the images and analysis.
Statistics
Differences between data groups were evaluated for significance using unpaired t-test (Graph Pad Prism Software Inc., San Diego, CA). Data are expressed as mean±SEM. For all tests, a P value <0.05 was considered for statistical significance.
Results
GSK-3α activity in ischemic heart and characterization of cardiomyocyte specific Gsk3α KO mice
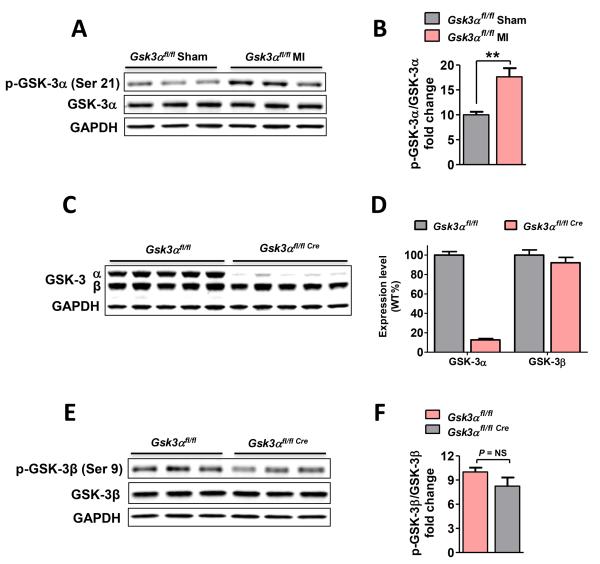
To test our hypothesis that GSK-3α plays a critical role in MI-induced remodeling, we first determined the effect of ischemic injury on GSK-3α activity as assessed by phosphorylation. WT mice were subjected to MI surgery and three weeks later LV lysates were analyzed for GSK-3α phosphorylation (on Ser21). Phosphorylation of GSK-3α was significantly increased, confirming that chronic MI leads to inhibition of GSK-3α (Fig. 1A-B & Suppl Fig. 1A-B). Therefore, we asked if GSK-3α might have a role in MI-induced ventricular remodeling. We employed the tamoxifen-inducible Mer-Cre-Mer system to delete Gsk3α specifically in cardiomyocytes. The progeny were viable, fertile, and showed no overt pathologic phenotype. After tamoxifen treatment, expression level of GSK-3α and GSK-3β was determined by immunoblotting. GSK-3α protein expression in the KO was reduced to 12% of baseline and GSK-3β expression and activity were comparable (Fig. 1C-F).
Figure 1. GSK-3α activity in ischemic heart and characterization of conditional Gsk3α KO mice.
(A) Immunoblot of sham and 3 wk post-MI LV lysates, and quantification (B) shows significantly increased phosphorylation (inhibition) of GSK-3α at Ser21 post-MI. (C) GSK-3α and GSK-3β protein expression was analyzed by immunoblotting of LV lysates. Immunoblot panel shows protein expression in KO and littermate controls, n=13 for each group. (D) Immunoblot quantification confirms an 88.5±6.2% reduction of GSK-3α expression in KO vs WT. GSK-3β expression was unchanged. (E) Immunoblotting using 3 wk post-MI LV lysates and (F) Blot quantification shows comparable phosphorylation of GSK-3β in WT and KO post-MI. n= 4-5 for WT and KO. Data are presented as mean±SEM, ** P<0.01.
Deletion of Gsk3α limits post-MI cardiac remodeling and preserves contractile function
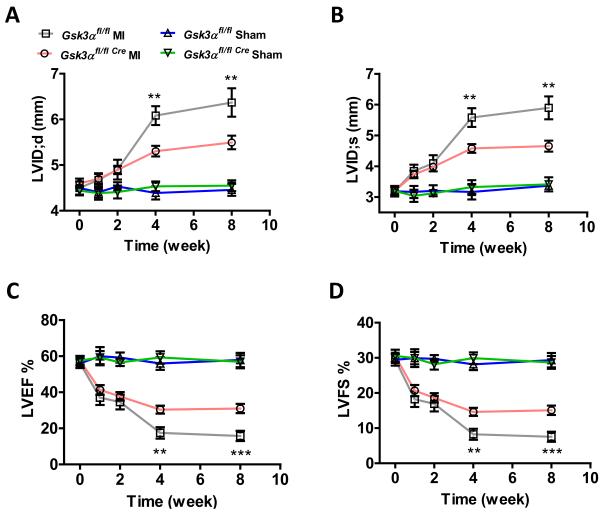
Gsk3α KO and littermate control mice underwent sham or MI surgery and cardiac function was determined using serial two-dimensional motion mode-echocardiography at various time points up to 8 weeks (wks). Although post-MI infarct size, hypertrophy, fibrosis and inflammation (as assessed by NF-kB activation) were comparable in WT vs KO (Suppl Fig. 1-4), ventricular chamber dilatation, both in systole and diastole, was significantly less in the KO consistent with reduced remodeling (Fig. 2A-B). The preserved chamber dimensions in the KO were associated with significantly better LV function as reflected by preserved ejection fraction and fractional shortening (Fig. 2C-D). The smaller LV internal dimension and the preserved cardiac function in the KO were consistent and remained significantly better up to the end of the study (8 wk post-MI). Although, global Gsk3α deletion leads to detrimental phenotypes due to upregulation of m-TORC1 (14,21), this was not the case in the cardiac-specific conditional KO (Suppl Fig. 5A-C). These observations suggest that inhibition of GSK-3α could be a novel strategy to limit adverse ventricular remodeling and dysfunction post-MI.
Figure 2. Deletion of Gsk3α preserves post-MI cardiac function and limits LV remodeling.
Following MI or sham surgery, cardiac function was assessed by transthoracic two-dimensional M-mode echocardiography at various time points shown over a period of 8 weeks. Left ventricle end-diastolic dimension (LVID;d) (A) and endsystolic dimension (LVID;s) (B) were significantly less in the KO at 4 and 8 wks post-MI. (C) LV ejection fraction (LVEF) and (D) LV fractional shortening (LVFS) were significantly greater in KO at 4 and 8 wk post-MI. n=16-22 for MI and n=4 for sham group. Values are presented as mean±SEM and P values shown are for the comparison between WT and KO mice subjected to MI, **P <0.01, ***P <0.001.
Deletion of Gsk3α attenuates post-MI myocyte death and scar expansion
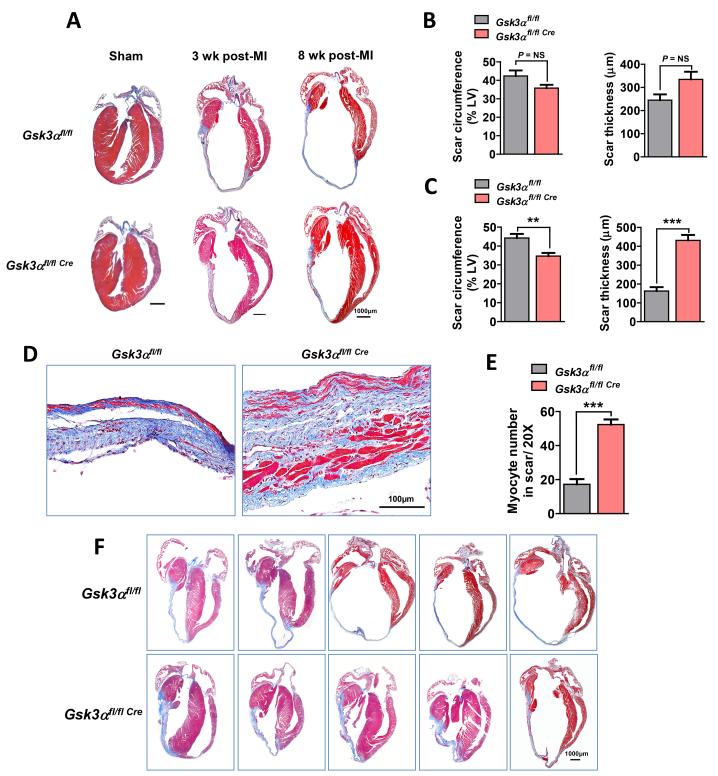
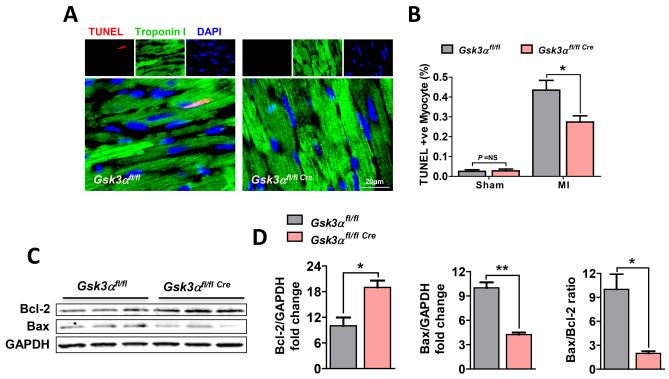
The most striking finding in our studies was a marked difference in scar expansion and content of the scar. In the WT, the scar was markedly thinned and comprised a much larger percent circumference of LV than in the KO (Fig. 3A, B, C, F). Furthermore, there were very few viable myocytes within the scar area in the WT. In contrast, the scar in the Gsk3α KO hearts was much thicker and importantly contained significantly greater numbers of viable cardiomyocytes (Fig. 3D, E). To determine the mechanism of the increased thickness and reduced scar circumference in the KO hearts, we performed TUNEL staining using 3wk post-MI heart sections. TUNEL positive cardiomyocytes were significantly increased post-MI irrespective of genotype. However, the number of apoptotic cells was significantly less in KO hearts (Fig. 4A, B). The KO also showed increased expression of the anti-apoptotic factor Bcl-2 and decreased expression of pro-apoptotic Bcl-2 associated X (Bax) protein expression post-MI (Fig. 4C, D). Thus the ratio of Bax to Bcl-2, an important indicator of caspase-3 activation and apoptosis, was significantly lower in the KO hearts (Fig. 4D). These findings suggest that GSK-3α promotes MI-induced cardiomyocyte death, which leads to scar expansion and thinning and adverse remodeling.
Figure 3. Better-preserved wall thickness and reduced scar circumference in the KO hearts.
(A) Masson’s Trichrome-stained images show sham, 3 wk and 8 wk post-MI KO and control hearts. (B) Scar circumference was measured and expressed as a percentage of the total area of LV myocardium. Scar circumference and scar thickness were comparable in 3 wk post-MI KO and WT, n=4-8 for WT and KO. (C) Scar thickness measurement in both WT and KO revealed a significantly thicker and less expansive scar in 8 wk post-MI KO hearts, n=8-13 for WT and KO. (D) Representative images of scars at higher magnification show a thicker scar with increased numbers of viable cardiomyocytes (red cells) in 8 wk post-MI KO heart. (E) Quantification of viable myocytes in the scar area revealed a significantly increased number in the KO hearts, n=7 hearts for each group. (F) Images of 8 wk post-MI hearts from KO and littermate controls show less extensive and thicker scar in the KO. Results are expressed as mean±SEM, ** P<0.01; *** P<0.0001.
Figure 4. Gsk3α deletion attenuates post-MI cardiomyocyte death.
(A) Representative images show TUNEL-positive cardiomyocytes from the WT and Gsk3α KO hearts. (B) Quantification shows significantly decreased numbers of TUNEL-positive myocytes in 3 wks post-MI KO LV. n=4 for sham and n=7 for MI group. (C) Representative immunoblot and (D) quantification shows increased Bcl-2 expression, decreased expression of Bax and decreased Bax/Bcl-2 ratio in the KO LV lysates, n=4-5 for WT and KO. Data are presented as mean±SEM. * P<0.05; ** P<0.001.
Deletion of Gsk3α promotes post-MI cardiomyocyte proliferation
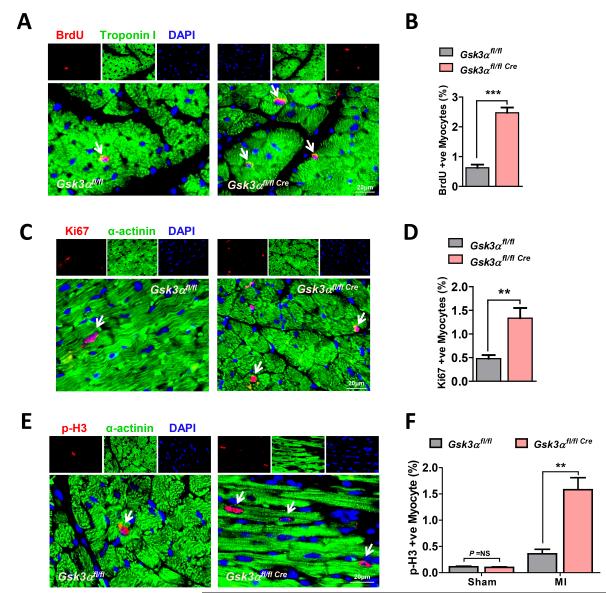
The increased number of viable cardiomyocytes in the KO hearts also raised the possibility that deletion of Gsk3α might drive cardiomyocyte proliferation. The role of GSK-3α in cardiomyocyte proliferation post-MI is unknown. To determine if GSK-3α regulates this process, WT and KO animals were subjected to MI surgery and then 5-bromo-2-deoxyuridine (BrdU) was injected starting from the 3rd week and continuing for five days. Immunostaining for BrdU and cardiomyocyte-specific marker Troponin I was performed using 3 wk post-MI LV sections. Only cells that were both BrdU and Troponin I positive, with BrdU co-localizing with 4,6-diamidino- 2-phenylindole (DAPI), were counted as proliferating cardiomyocytes. Indeed, the number of proliferating cardiomyocytes was significantly increased in the KO hearts (Fig. 5A, B). Immunostaining for another DNA synthesis marker, Ki67, also showed increased numbers of Ki67 positive cardiomyocytes in the KO heart (Fig. 5C, D). To further investigate the role of GSK-3α on MI-induced cardiomyocyte proliferation, the mitosis marker, phospho-Histone H3 (p-H3) (Ser10), was examined by immunostaining. Consistent with the above findings, an increased number of p-H3-positive cardiomyocytes were observed in the KO hearts (Fig. 5E, F). These results confirm robust post-MI cardiomyocyte proliferation in the KO hearts and suggest that GSK-3α is a potent regulator of cardiomyocyte proliferation in the adult ischemic heart.
Figure 5. Deletion of Gsk3α promotes post-MI cardiomyocyte proliferation.
(A) Representative images show BrdU-positive cardiomyocytes from the WT and Gsk3α KO hearts. (B) Quantification shows significantly increased numbers of BrdU-positive myocytes in 3 wk post-MI KO hearts, n=6 for each group. (C) Images show Ki67 positive cardiomyocytes. (D) Quantification shows an increased number of Ki67-positive cardiomyocytes in Gsk3α KO hearts, n=4-5. (E) Representative images show p-H3 (Ser10)-positive myocytes in 3 wk post-MI hearts. (F) Quantification shows an increased number of p-H3 positive myocytes in the KO hearts, n=4 for sham and n=6 for MI group. Results are expressed as mean±SEM. ** P<0.01, *** P<0.0001.
Gsk3α deletion promotes post-MI cyclin E1 and E2F-1 recruitment
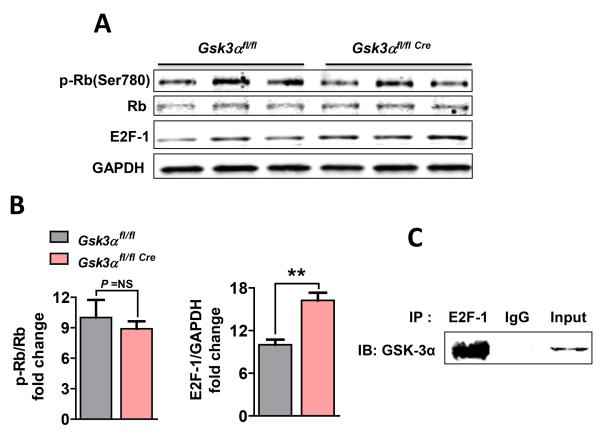
To determine how GSK-3α might regulate cardiomyocyte proliferation, we examined pro-proliferative factors that might be responsible including the Wnt/β-catenin pathway, cyclin-dependent kinases (Cdks), cyclins and the E2F-1 transcription factor (Suppl Fig. 6A-C). From this list we identified two candidates, cyclin E1 and E2F-1, in post-MI KO hearts. Protein level of the transcription factor E2F-1 was significantly increased in the KO heart post-MI (Fig. 6A, B). This was not regulated through retinoblastoma protein (Rb), since phosphorylation (Ser780) of Rb was comparable between WT and KO (Fig. 6A, B). To further dissect molecular mechanisms responsible for GSK-3α-mediated E2F-1 signaling, we examined the possibility of direct interaction between these proteins by immunoprecipitation (IP) assay using neonatal rat ventricular myocyte (NRVM) lysates. We observed a strong interaction between GSK-3α and E2F-1 (Fig. 6C), suggesting that GSK-3α directly regulates E2F-1 levels in cardiomyocytes independent of Rb phosphorylation.
Figure 6. Gsk3α deletion promotes E2F-1 recruitment in vivo.
(A) Representative immunoblots show phospho-Retinoblastoma protein (pRb) (Ser790) and E2F-1 from 3 wk post-MI LV lysates. (B) Quantification shows comparable pRb and significantly increased levels of E2F-1 in the post-MI KO hearts, n=4-5. Results are expressed as mean±SEM, ** P<0.01. (C) Co-immunoprecipitation using an antibody against E2F-1 followed by immunoblotting for GSK-3α in neonatal rat ventricular myocytes (NRVM) confirms a physical interaction between these molecules.
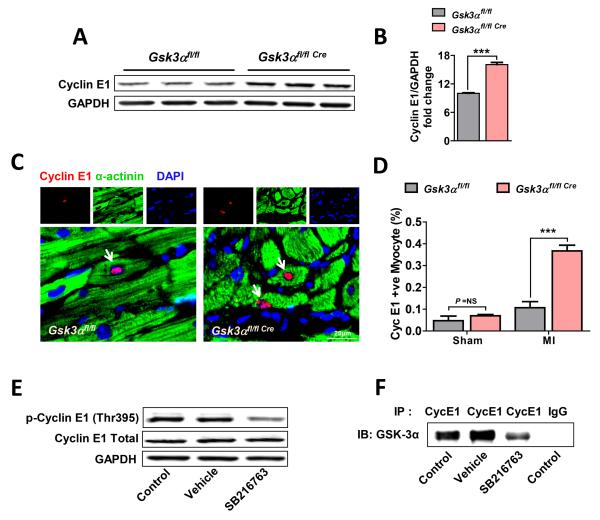
Cyclin E1 is an important G1 to S transition phase marker and plays an important role in cell proliferation. A significant upregulation of cyclin E1 was observed in the KO hearts post-MI (Fig. 7A, B). To examine cyclin E1 specifically in cardiomyocytes, immunostaining was performed on 3 wk post-MI heart sections. Only double positive cells with cyclin E1 and α-actinin (cardiomyocyte marker), with cyclin E1 co-localizing with DAPI, were counted as proliferating cardiomyocytes. The number of cyclin E1-positive cardiomyocytes was significantly increased in the KO (Fig. 7C, D). To study the mechanism responsible for GSK-3α mediated cyclin E1 regulation, NRVMs were treated with the GSK-3 inhibitor (SB216763) for 1.5 hours and cyclin E1 phosphorylation was determined. Phosphorylation of cyclin E1 (Thr395) was significantly decreased by GSK-3 inhibition (Fig. 7E). To determine if GSK-3α and cyclin E1 directly interact, we performed co-IP assays using cyclin E1 antibody, which suggested a direct interaction between GSK-3α and cyclin E1. To examine if this interaction is dependent on GSK-3α kinase activity, co-IP assay was performed in the presence of GSK-3 inhibitor (SB216763). Indeed, the interaction between cyclin E1 and GSK-3α was reduced in the presence of GSK-3 inhibitor (Fig. 7F) as reflected by a lower level of pull down of co-IP’d protein. These data suggest that GSK-3α directly interacts and phosphorylates cyclin E1 in cardiomyocytes. To our knowledge, this is the first report of direct regulation of cyclin E1 by GSK-3α. Taken together, these findings suggest that deletion of GSK-3α promotes post-MI E2F-1 and cyclin E1 recruitment and induces the re-entry of adult cardiomyocytes into the cell cycle.
Figure 7. GSK-3α regulates post-MI cyclin E1.
(A) Immunoblot of LV lysates and (B) quantification show increased levels of cyclin E1 in 3 wk post-MI Gsk3α KO hearts, n=4-5. (C) Representative images show cyclin E1-positive cardiomyocytes in 3 wk post-MI WT and KO heart. (D) Quantification shows significantly increased numbers of cyclin E1-positive cardiomyocytes in the post-MI KO hearts, n=5-6 for WT and KO hearts. Results are expressed as mean±SEM, *** P<0.0001. (E) NRVMs were exposed to GSK-3 inhibitor SB216763 (10μM) for 1.5 hours followed by immunoblotting for phospho-cyclin E1 (Thr395). Immunoblot shows a low level of phosphorylation of cyclin E1 in drug-treated NRVMs in comparison to control and vehicle-treated groups. (F) NRVMs were similarly treated as above with SB216763, and co-immunoprecipitation was performed using lysate and antibody against cyclin E1 followed by immunoblotting for GSK-3α from co-IP’d protein. Blot shows a strong interaction between GSK-3α and cyclin E1. However, in contrast, diminished binding was observed when the kinase activity of GSK-3α was inhibited with SB216763.
Discussion
Following an MI, the heart undergoes the process of remodeling, which consists of LV dilatation and contractile dysfunction, often leading to heart failure. Strategies to prevent remodeling largely center around neuro-hormonal blockade (e.g. β blockers and angiotensin converting enzyme inhibitors) and while beneficial to some degree, often cannot prevent heart failure in many patients, especially those with larger infarcts. Herein, we identify the α isoform of GSK-3 as a novel and potent promoter of remodeling post MI, in part driven by profound scar expansion, and leading to marked LV dysfunction. In stark contrast, deletion of GSK-3α leads to much less dilatative remodeling and better-preserved LV function. The diminished remodeling post-MI in the KO is accompanied by a thicker scar and markedly increased numbers of viable cardiomyocytes within the scar area of KO hearts compared to WT. The profound reduction in ventricular dilatation and scar expansion in the KO was supported by both less apoptosis and new myocyte formation post-MI (Central Illustration).
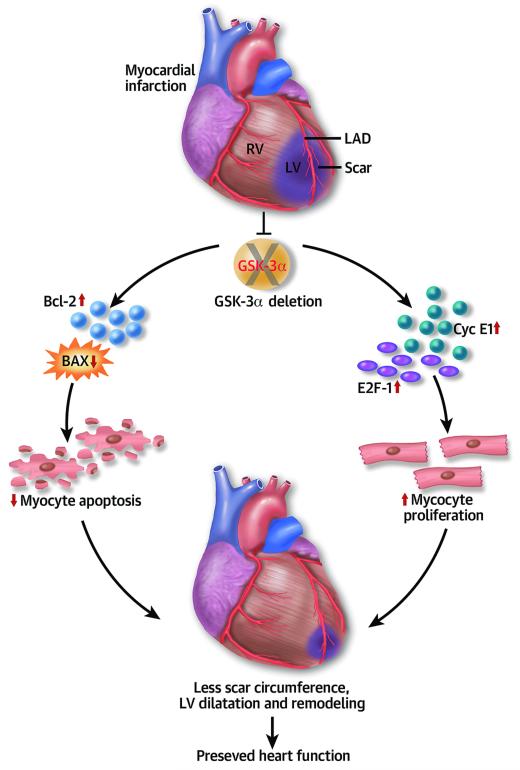
Central illustration. Schematic representation shows pathogenesis of post-MI cardiac remodeling in the GSK3α KO heart.
In resting cells, GSK-3α is in an active state and myocardial infarction leads to it inhibition. Deletion of Gsk3α in cardiomyocytes induces up-regulation of cyclin E1 and the E2F-1 transcription factor that prmotes cardiomyocyte proliferation post-MI. Cardiac specific deletion of GSK-3α also up-regulates Bcl-2 and down-regulates BAX expression post-MI, resulting in attenuated myocyte apoptosis in the KO hearts. Taken together, increased myocyte proliferation and less apoptosis lead to profound cardiac protection as reflected by preserved LV function and reduced ventricular remodeling in the KO post-MI.
The traditional strategy i.e. total somatic (global) gene deletion, can lead to secondary and compensatory effects that greatly complicate the interpretation of phenotypes (19). Global gene deletion can result in embryonic lethality if the gene is required for development. Moreover, global deletion induces compensatory changes in other genes as a means of preventing lethality. One of the best examples is from the GSK-3 family i.e., global deletion of Gsk3β is lethal due to hyper-proliferation of cardiomyoblast and congenital heart defects (9). However, cardiac-specific conditional deletion has no overt effect and is protective post-MI in adults (11). Conversely, germline deletion of Gsk3α does not affect embryonic development and pathophysiology of adult mice up to 4 months post-birth but leads to functional deterioration with aging (15,21). Given these complications of global gene targeting, tissue-specific approaches have become more widely adopted to bypass the confounding phenotypes observe in global gene targeting strategies.
Both global deletion and constitutive activation (CA) of GSK-3α, under a variety of stresses have been shown to induce detrimental phenotypes of cardiac hypertrophy and remote fibrosis (15,16). Moreover, germline global deletion of Gsk3α has been shown to induce progressive cardiac hypertrophy and contractile dysfunction even in the absence of a cardiac-specific stress (14,21). Importantly, our findings, which show no hypertrophy or fibrosis post-MI, provide a stark contrast from previous reports and suggest that GSK-3α likely plays little or no direct role in stress-induced cardiac hypertrophy and remote fibrosis. In summary, the previously reported role of GSK-3α in various stress settings, are likely secondary effects of global gene deletion and /or transgenesis.
Cardiomyocyte death, even at very low rates, can lead to significant collective loss of cardiomyocytes over time and is reported to cause post-MI cardiac dysfunction and heart failure (22). Our findings show that GSK-3α is a potent promoter of cardiomyocyte death and apoptosis in the ischemic heart by inducing Bcl-2 and inhibiting Bax activity. Bcl-2 is a known anti-apoptotic factor (23) that protects against oxidative stress-induced apoptosis and preserves cardiomyocyte viability and LV function in ischemic heart when over-expressed (24). Our findings suggest that cardiomyocyte-specific deletion of Gsk3α leads to increased expression of Bcl-2 and this limits cardiomyocyte death in chronic MI.
We also report robust cardiomyocyte proliferation in the Gsk3α KO hearts as evidenced by increased Ki67 and BrdU-positive cardiomyocytes. Mechanistically, we found that deletion of Gsk3α increases E2F-1 recruitment in the KO hearts post-MI. Although a previous study showed that GSK-3α regulates E2F through cyclin D1 post-TAC (16), we observed that cyclin D1 played no role in the cardiomyocyte-specific KO post-MI (Suppl Fig. 6A-C). These observations suggest that GSK-3α might regulate E2F-1 in a stress-dependent manner as cyclin D1 was involved in TAC but not in MI. Several studies have shown that activation of Rb regulates E2F-1 (25,26), however this was not a mechanism in the current study, as phosphorylation of Rb was comparable between WT and KO. Further, we found that GSK-3α directly interacts with E2F-1 in the cardiomyocyte. These findings suggest that GSK-3α is a direct regulator of E2F-1 independent of cyclin D1 and Rb. Similarly, an increased level of the G1 / S transition phase marker cyclin E1 was observed in the KO hearts. Our data suggest that GSK-3α phosphorylates and regulates cyclin E1 levels and emphasize the fact that GSK-3α is a key and novel regulator of cyclin E1 in the cardiomyocyte. The elevated levels of E2F-1 and cyclin E1 in the KO appear to be the central mechanism for cardiomyocyte proliferation.
Herein we propose that selective inhibition of GSK-3α could significantly improve outcomes in patients with ischemic injury. Selective inhibition of GSK-3α has not, to our knowledge, been achieved with available drugs. In fact, inhibition of GSK-3β can have adverse effects on the heart. The ideal scenario would be to have a compound that selectively inhibits GSK-3α (and not the other isoform, GSK-3β). Eventually this hurdle will be conquered and small molecules will be developed that selectively target GSK-3α. These agents could be very beneficial in patients with ischemic disease.
In summary, we present findings that reveal the potential translational value of GSK-3α inhibition in the chronic MI setting. Cardiomyocyte-specific conditional deletion of Gsk3α preserves cardiac function, limits ventricular remodeling, attenuates cardiomyocyte death, restricts scar expansion and thinning, and promotes cardiomyocyte proliferation post-MI. Taken together, these observations suggest that selective inhibition of GSK-3α could be a therapeutic strategy to limit MI-induced cardiac remodeling and heart failure.
Supplementary Material
Perspectives.
Competency in Medical Knowledge
In mouse models of left ventricular function following myocardial infarction, genetic expression of glycogen synthase kinase-3α (GSK-3α) is associated with more severe ventricular dilatation, adverse remodeling, and heart failure.
Translational Outlook
All currently available GSK-3 inhibitors target both the α and β isoforms, but development of new drugs that specifically inhibit GSK-3α could be promising agents for study in large animal models of myocardial infarction.
Acknowledgments
Sources of Funding; This work was supported by grants HL061688 and HL108178 from the U.S. National Heart Lung and Blood Institute (to T.F.) and CIHR MOP74711 (to J.R.W.).
Abbreviations
- α-MHC
α-myosin heavy chain
- BrdU
5-bromo-2′-deoxyuridine
- GSK
Glycogen synthase kinase
- Gsk3αfl/fl
Homozygous Gsk3α floxed mouse
- Gsk3αfl/fl Cre
Homozygous Gsk3α floxed mouse with heterozygous Mer-Cre-Mer
- LAD
Left anterior descending coronary artery
- LVEF
Left ventricular ejection fraction
- LVFS
Left ventricular fractional shortening
- MI
Myocardial infarction
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
References
- 1.Velagaleti RS, Pencina MJ, Murabito JM, et al. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057–62. doi: 10.1161/CIRCULATIONAHA.108.784215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kenneth DJX, Sherry LM, Arialdi MM, Hsiang-Ching K. Death: Preliminray data for 2009. National vital statistics report. 2011;59:1–51. [PubMed] [Google Scholar]
- 3.Porrello ER, Mahmoud AI, Simpson E, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–80. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res. 2002;90:1044–54. doi: 10.1161/01.res.0000020201.44772.67. [DOI] [PubMed] [Google Scholar]
- 5.Chiong M, Wang ZV, Pedrozo Z, et al. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. 2011;2:e244. doi: 10.1038/cddis.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–57. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Haq S, Michael A, Andreucci M, et al. Stabilization of b-catenin by a Wntindependent mechanism regulates cardiomyocyte growth. Proc Nat Acad Sci. 2003;100:4610–4615. doi: 10.1073/pnas.0835895100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12:957–71. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerkela R, Kockeritz L, Macaulay K, et al. Deletion of GSK-3beta in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation. J Clin Invest. 2008;118:3609–18. doi: 10.1172/JCI36245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doble BW, Woodgett JR. Exploring pluripotency with chemical genetics. Cell Stem Cell. 2009;4:98–100. doi: 10.1016/j.stem.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woulfe KC, Gao E, Lal H, Harris D, Fan Q, Vagnozzi R, DeCaul M, Shang X, Patel S, Woodgett JR, Force T, Zhou J. Glycogen synthase kinase-3beta regulates post-myocardial infarction remodeling and stress-induced cardiomyocyte proliferation in vivo. Circ Res. 2010;106:1635–1645. doi: 10.1161/CIRCRESAHA.109.211482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruel L, Bourouis M, Heitzler P, Pantesco V, Simpson P. Drosophila shaggy kinase and rat glycogen synthase kinase-3 have conserved activities and act downstream of Notch. Nature. 1993;362:557–60. doi: 10.1038/362557a0. [DOI] [PubMed] [Google Scholar]
- 13.Siegfried E, Chou TB, Perrimon N. Wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell. 1992;71:1167–1179. doi: 10.1016/s0092-8674(05)80065-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Lal H, Chen X, Shang X, Song J, Li Y, Kerkela R, Doble BW, MacAulay K, DeCaul M, Koch WJ, Farber J, Woodgett J, Gao E, Force T. GSK-3alpha directly regulates beta-adrenergic signaling and the response of the heart to hemodynamic stress in mice. J Clin Invest. 2010;120:2280–91. doi: 10.1172/JCI41407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lal H, Zhou J, Ahmad F, et al. Glycogen synthase kinase-3alpha limits ischemic injury, cardiac rupture, post-myocardial infarction remodeling and death. Circulation. 2012;125:65–75. doi: 10.1161/CIRCULATIONAHA.111.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuda T, Zhai P, Maejima Y, et al. Distinct roles of GSK-3alpha and GSK-3beta phosphorylation in the heart under pressure overload. Proc Natl Acad Sci U S A. 2008;105:20900–5. doi: 10.1073/pnas.0808315106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhai P, Gao S, Holle E, et al. Glycogen synthase kinase-3alpha reduces cardiac growth and pressure overload-induced cardiac hypertrophy by inhibition of extracellular signal-regulated kinases. J Biol Chem. 2007;282:33181–91. doi: 10.1074/jbc.M705133200. [DOI] [PubMed] [Google Scholar]
- 18.Maejima Y, Galeotti J, Molkentin JD, Sadoshima J, Zhai P. Constitutively active MEK1 rescues cardiac dysfunction caused by overexpressed GSK-3alpha during aging and hemodynamic pressure overload. Am J Physiol Heart Circ Physiol. 2012;303:H979–88. doi: 10.1152/ajpheart.00415.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis J, Maillet M, Miano JM, Molkentin JD. Lost in transgenesis: a user’s guide for genetically manipulating the mouse in cardiac research. Circ Res. 2012;111:761–77. doi: 10.1161/CIRCRESAHA.111.262717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107:1445–53. doi: 10.1161/CIRCRESAHA.110.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J, Freeman TA, Ahmad F, et al. GSK-3alpha is a central regulator of agerelated pathologies in mice. J Clin Invest. 2013;123:1821–32. doi: 10.1172/JCI64398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kung G, Konstantinidis K, Kitsis RN. Programmed necrosis, not apoptosis, in the heart. Circ Res. 2011;108:1017–36. doi: 10.1161/CIRCRESAHA.110.225730. [DOI] [PubMed] [Google Scholar]
- 23.Hockenbery DM, Oltval ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 24.Chatterjee S, Stewart AS, Bish LT, et al. Viral gene transfer of the antiapoptotic factor Bcl-2 protects against chronic postischemic heart failure. Circulation. 2002;106:I212–7. [PubMed] [Google Scholar]
- 25.Harbour JW, Dean DC. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 2000;14:2393–409. doi: 10.1101/gad.813200. [DOI] [PubMed] [Google Scholar]
- 26.Nevins JR. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–9. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.