Abstract
One of the greatest challenges that scientists face when studying the neurobiology and/or genetics of alcohol (ethanol) consumption is that most pre-clinical animal models do not voluntarily consume enough ethanol to achieve pharmacologically meaningful blood ethanol concentrations (BECs). Recent rodent models have been developed that promote binge-like levels of ethanol consumption associated with high BECs (i.e., 100 mg/dl or higher). This paper describes procedures for an animal model of binge-like ethanol drinking which has come to be called “drinking in the dark” (DID). The “basic” variation of DID involves replacing the water bottle with a bottle containing 20% ethanol for 2 to 4 hours, beginning 3 hours into the dark cycle, on cages of singly-housed C57BL/6J mice. Using this procedure, mice typically consume enough ethanol to achieve BECs greater than 100 mg/dl and to exhibit behavioral evidence of intoxication. An alternative 2-bottle (ethanol and water) procedure is also described.
Keywords: ethanol, binge-like, drinking-in-the-dark, consumption, limited-access
INTRODUCTION
Historically, pre-clinical alcoholism research has primarily relied on rodent models that involved voluntary consumption of ethanol, in which rats or mice were given 24-h/day access to ethanol solutions and water simultaneously in separate bottles. However, a major limitation associated with voluntary ethanol consumption procedures is that most rodents typically consume small amounts of ethanol that do not generate BECs thought to be pharmacologically meaningful and characteristic of binge drinking patterns. About a decade ago a ‘binge’ was operationally defined by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) as a pattern of drinking that produces BECs greater than 0.08% (80 mg/dl) (NIAAA, 2004). In recent years, animal models of binge-like ethanol drinking have emerged, designed with the primary goal of establishing procedures that cause animals to achieve pharmacologically relevant BECs in a short period of time [in the case of mice, a criterion of 100 mg/dl or greater has been suggested because of their high rate of ethanol metabolism (Crabbe et al., 2011a)]. Here we provide detailed instructions on the use of a mouse model of binge-like ethanol drinking, originally described by one of the current co-authors (Rhodes et al., 2005), which has subsequently come to be called the “drinking in the dark” (DID) procedure. The most commonly employed DID procedure entails giving C57BL/6J mice limited access (2- to 4-h) to a 20% (v/v) ethanol solution, in place of water, beginning 3-h into the dark phase of the circadian cycle. C57BL/6J mice typically exhibit binge-like drinking patterns that are associated with BECs greater than 100 mg/dl. The most commonly used one-bottle “ethanol only” procedure is first described and is followed by a description of an alternate 2-bottle procedure involving concurrent access to a bottle of 20% ethanol and a second bottle of water.
BASIC PROTOCOL FOR DID PROCEDURES
Several studies have extensively characterized DID, in which mice drink significant amounts of an unsweetened 20% ethanol solution if it is presented for 2–4 hours, 3 hours into the dark cycle (Lyons et al., 2008; Moore et al., 2010; Moore et al., 2007; Rhodes et al., 2005; Rhodes et al., 2007). C57BL/6 mice consume large quantities of ethanol (4–8 g ethanol/kg body weight), often reaching BECs of 100 mg ethanol/dl plasma, or greater. Such BECs are physiologically relevant, producing significant motor incoordination on a balance beam motor impairment task (Moore et al., 2007; Rhodes et al., 2007), as well as tolerance to this effect over repeated binge-like ethanol drinking episodes (Linsenbardt et al., 2011). Neuroadaptive changes in the pattern of both ethanol intake and home cage locomotor activity over repeated episodes of binge-like ethanol drinking have also been observed (Linsenbardt and Boehm, in press), as well as increases of subsequent 24-hour voluntary ethanol drinking, an effect that becomes more robust with a more extensive prior history of binge-like intake (Cox et al., 2013). Importantly, with DID procedures mice are not water deprived at any time except during the ethanol access period and are therefore not “artificially” motivated to drink the ethanol solution. Furthermore, lengthy periods of sucrose-fading or ethanol concentration ramping are not required. DID appears to model human binge drinking, or the consumption of ≥5 drinks (for an average male) during a single occasion with BECs reaching 80 mg/dl or greater (NIAAA, 2004). Furthermore, given its simplicity and minimal time requirements, the DID procedure is an excellent high throughput tool for characterization of potential pharmacotherapies in the treatment of binge alcohol intake (Kamdar et al., 2007) as the investigator knows precisely when to administer a drug and assess subsequent effects on drinking. This section describes the most commonly used “basic” one-bottle DID protocol.
Materials
Adult (8 week old, male or female depending on experimental requirements) C57BL/6J mice (obtained from the Jackson Laboratory, Bar Harbor, ME or Sacramento, CA)
Standard shoebox mouse cage made of clear polycarbonate (dimensions: 10.5in L x 6inW x 5in H)
Electropolished stainless steel wire bar lid (dimensions: 10.5in L x 6in W) with food hopper
Water bottle with sipper tube
Constructed ball-bearing sipper tubes (see Supporting Procedures)
Balance/scale
Binder clip (Acco brand medium binder clips, 1.587 cm capacity; Lincolnshire, IL)
Flashlight with red lens cover
20% (v/v) ethanol solution (see Supporting Procedures)
Heparin coated capillary tubes and associated supplies for blood sampling (see Supporting Procedures for determination of blood ethanol concentration)
Protocol Steps
The four-day DID procedure was developed and optimized for adult C57BL/6J inbred mice.1 C57BL/6J mice may be purchased from the Jackson Laboratory or bred in-house. However, if bred in house, the breeders should be obtained from the Jackson Laboratory. Mice can be group housed until the time of testing.
At least one week prior to initiation of testing, each mouse must be single housed in a standard shoebox mouse cage with woodchip or other type mouse bedding. Standard mouse chow and water is provided ad libitum. Importantly, mice should be housed in the same room, with the same ambient temperature (21±1°C) and 12-hour light/dark cycle that they will ultimately be tested in.2 This will allow mice to sufficiently acclimate to DID housing conditions prior to assessment of drinking.
Prior to daily assessment of DID, each mouse must be weighed using a scale/balance. The weight of each mouse must be known if the researcher is to ultimately determine grams of ethanol consumed per kilogram of body weight (g/kg). However, it is also important to avoid disturbing the mice as this could alter subsequent behavior. Thus, the physical act of taking mouse weights is best accomplished before lights out. This will ensure that each mouse has had adequate time to settle down again prior to the introduction of the alcohol solution three hours after lights out. Additionally, under normal conditions mouse body weights should remain relatively stable over the 4 day test period, and thus the collection of body weights could be limited (e.g., to the first day of the testing procedure).
Before the ethanol drinking period begins, the ball-bearing DID sipper tubes must be filled with the ethanol solution (see Supporting Procedures). This is most easily accomplished in the period between lights out and the beginning of the ethanol access period 3 hours later. See Supporting Procedures for details on the construction of the DID sipper tubes.
Three hours into the dark cycle, the ethanol-filled ball-bearing sipper tubes are introduced to the mice. Our cages have a stainless steel wire bar tops with a triangular shaped indentation down into the cage for food pellets and a water bottle (see Figure 1). The bottle area is separated from the food area by a flat stainless steel partition. There is a flat ring, similar to a washer, on the water bottle side of the triangular indentation through which the sipper tube associated with the regular water bottle is passed. The regular water bottle is withdrawn, and the DID sipper tube is inserted through this ring and allowed to rest at the 90° angle of the wire cage top triangular indentation (same angle and position as the regular water bottle). Mice will sometimes climb on and otherwise play with the DID sipper tube, increasing the likelihood of unwanted leakage. To avoid such leakage, an Acco binder clip can be used to secure the sipper tube to the wire cage top.3 Immediately after securing each DID sipper tube, read and record its fluid level by reading the meniscus; a flashlight with a red lens cover can be used for this purpose. All subsequent timing is in reference to reading the meniscus level of the first DID sipper tube (T0). Figure 1 shows the typical setup of a mouse cage during DID procedures.
Mice are allowed access to the ethanol-filled ball-bearing DID sipper tubes for 2 hours, at which point the amount of fluid consumed is read and recorded, beginning with the first DID sipper tube introduced from step 5 above. As each DID sipper tube is read and fluid intake recorded, the tube is withdrawn and the regular water bottle reintroduced. We actually read the entire experiment’s sipper tube volumes, and then go back and exchange bottles to avoid clanking noises. The mice are then left undisturbed until day 2 (and then day 3) when steps 1–6 above are repeated.
On day 4 of the procedure, steps 1–6 are again repeated with the exception that the ethanol-filled ball-bearing DID sipper tube is generally left in place for 4 hours before fluid volumes are read and recorded.4
Immediately upon withdrawal of each DID sipper tube, a blood sample is taken for later determination of blood ethanol content.5 See Supporting Procedures for details on blood sampling and subsequent determination of blood ethanol concentration.
The DID ball-bearing sipper tubes and associated silicone stoppers are cleaned after each use by dumping out any remaining ethanol solution, soaking the sipper tubes and stoppers for at least 20 minutes in a 10% bleach solution, and then thoroughly rinsing in tap water. The sipper tubes and stoppers are then left to air dry.
Figure 1.
A side (top) and aerial (bottom) view of the mouse cage set up for the basic one-bottle DID procedure. Note the Acco clip used to secure the ball-bearing sipper tube to the wire cage lid. Calibrations on the sipper tubes are aimed upwards so that the experimenter can record measures over the course of ethanol access.
Step Annotations
DID procedures have been used to assess limited-access ethanol intake in other mouse genotypes, with varying results. The reader is referred to (Crabbe et al., 2012) for assessment of DID in a panel of 23 inbred mouse strains and (Blednov et al., 2012) for studies of certain null mutants in the DID procedures.
DID takes advantage of the nocturnal nature of mice and is therefore assessed early during the dark phase of the light/dark cycle, when rodents do the majority of their feeding and drinking for the 24 hr period. Often this involves first housing mice in a room on a reverse 12-hour light/dark cycle (i.e., with lights off in the morning, and lights coming back on again 12 hours later in the late afternoon or early evening) so that assessment of drinking can be conducted during the normal workday for lab personnel. If mice are first shifted from a standard light/dark cycle to a reverse cycle, one to two weeks of adaptation is required to insure that they are synchronized to the new cycle.
The experimenter may also wish to set up a series of empty cages in which ethanol-filled ball-bearing DID sipper tubes are introduced. Such empty cages allow the experimenter a means by which to assess leakage associated with insertion and withdrawal of the DID sipper tubes or inadvertent disturbance of the cage rack.
Alternative periods of ethanol intake can be employed. For example, in cases where a pharmacological treatment is given prior to the alcohol intake period, it may be advantageous to assess intake in a shorter access period, particularly if the drug effect might be expected to wane before the end of a longer 4-hour access period. Similarly, it may be of interest to assess intake at multiple intervals within the 4-hour access period. The flashlight with the red lens can be used for this purpose. The potential benefits of more frequent readings of intake should be weighed against the potential for disruption of drinking behavior by disturbing or distracting the mice.
In some experiments, the experimenter may wish to repeat the 4-day cycle of DID described above. In such cases, he/she might consider the disruption to subsequent alcohol intake or other subsequent experimental manipulation that blood sampling may induce, and opt to omit this step, or delay it until the end of the final cycle of DID.
VARIATIONS OF THE DID PROCEDURE
Two-Bottle Choice Preference Drinking-in-the-Dark (DID)
While the basic one-bottle DID procedure described above is the most commonly used procedure in the literature, a two-bottle variation of DID has been described (Blednov et al., 2012; Giardino and Ryabinin, 2013; Kaur and Ryabinin, 2010; Rhodes et al., 2007). Rather than replacing the cage water bottle with an ethanol-filled sipper tube, the cage water bottle is removed and both ethanol- and water-filled sipper tubes are provided. This procedure offers the advantage of allowing the experimenter to calculate ethanol preference [volume of ethanol solution consumed/total fluid consumption (volume of ethanol solution consumed + volume of water consumed)]. Additionally, one can simultaneously assess the effects of pharmacological or genetic manipulations of ethanol and water intake. However, it has been demonstrated that mice achieve lower BECs using the two-bottle variation relative to the one-bottle approach (Crabbe et al., 2009; Rhodes et al., 2007), limiting the potential utility of the two-bottle DID procedure in modeling binge-like ethanol drinking. These factors must be weighed when choosing between the basic one-bottle versus two-bottle variation of the DID procedure.
Materials
Materials are identical to the basic DID procedure described above, except that one additional ball-bearing sipper tube (see Supporting Procedures) per cage will be needed. Some modification of the cage lid may be adopted to accomodate the extra bottle.
Protocol Steps
-
1–3
Steps 1 through 3 are the same as with the basic protocol above with the exception that mice will need to be habituated to two-bottle drinking. At least one week before DID procedures are initiated, mice should be singly housed and given access to 2 ball-bearing sipper tubes filled with water at the same time that DID procedures are to be performed. Thus, beginning 3 hours into the dark cycle, remove the home cages water bottle and insert 2 ball-bearing sipper tubes, filled with fresh water (tap or bottle spring water), into the wire cage. Because 2 bottles are used, food must be removed from the cage lid1. One ball-bearing sipper tube is inserted into the ring that normally houses the cage water bottle, and the second ball-bearing sipper tube is carefully inserted between the bars on the side of the cage where food is normally placed. Attach the sipper tubes to the wire cage top using Acco binder clips. Two hours later, remove the 2 sipper tubes and replace the original home cage drinking bottle and food.
-
4
On the day of ethanol introduction, one ball-bearing DID sipper tube must be filled with the ethanol solution (see Supporting Procedures) and the other with water, prior to the start of the drinking period. As noted above, this is most easily accomplished in the period between lights out, and the beginning of the ethanol access period 3 hours later.
-
5
Three hours into the dark cycle, food is removed from the cage and the ethanol- and water-filled ball-bearing sipper tubes are introduced to the mice. Immediately after securing each DID sipper tube with Accro binder clips, read and record its fluid level by reading the meniscus; a flashlight with a red lens cover can be used for this purpose. All subsequent timing is in reference to reading the meniscus level of the first DID sipper tube (T0).
-
6
Mice are allowed access to the ethanol- and water-filled ball-bearing DID sipper tubes for 2 hours, at which point the amount of fluid consumed is read and recorded, beginning with the first cage that received sipper tubes from step 5 above. As each DID sipper tube is read and fluid intake recorded, the tubes are withdrawn and the regular water bottle reintroduced. As noted above, the entire experiment’s sipper tube volumes may first be recorded and they may then be exchanged for water bottles to avoid noise-induced disturbance of drinking. Food is returned to the cage lid. The mice are then left undisturbed until day 2 (and then day 3) when steps 4–6 above are repeated.2
-
7
On day 4 of the procedure steps 4–6 are again repeated with the exception that the ethanol- and water-filled ball-bearing DID sipper tubes are generally left in place for 4 hours before fluid volumes are read and recorded.
-
8
Immediately upon withdrawal of each DID sipper tubes, a blood sample is taken for later determination of blood ethanol content. See Supporting Procedures for details on blood sampling and subsequent determination of blood ethanol concentration.
-
9
The DID ball-bearing sipper tubes and associated silicone stoppers are cleaned after each use by dumping out any remaining ethanol solution or water, soaking the sipper tubes and stoppers for at least 20 minutes in a 10% bleach solution, and then thoroughly rinsing in tap water. The sipper tubes and stoppers are then left to air dry.
Step Annotations
Because food may have been removed from the cage lid to accommodate 2-bottle DID, the experimenter must decide whether food should be provided or remain absent during DID procedures. If keeping food available is desired, a few food pellets can be placed in the bottom of the mouse cage.
There is often concern that mice may develop place/bottle preferences with two-bottle procedures, regardless of the drinking solution in specific bottles. To avoid this potential confound, alternate the position (left side versus right side) that the ethanol bottle is placed each day or every other day.
SUPPORTING PROCEDURES
Construction of the Sipper Tubes
Each sipper tube used to assess Drinking-in-the-Dark is made from a 10 ml serological pipet, a ball-bearing sipper, a section of heat shrink tubing, and a silicone stopper. The goal is to construct sipper tubes that can measure 2–4 hour fluid intake to the nearest 0.05 ml.
Materials
10 ml disposable clear polystyrene serological pipets (Fisher Scientific)
Sterile utility knife blade
Stainless steel bearing sipper tubes (2.5 inch length, 5/16 inch diameter; Ancare Corp.)
Heat shrink tubing (3/8 inch diameter; 3M)
Heat gun (Master Heat Gun, model HG-301A; Master Appliance Corp.)
Silicone stoppers (Fisher, European size 10D)
Protocol Steps
Cut off both ends of the disposable serological pipet with the utility knife blade, leaving the cylinder with the original graduated markings from about 2 ml to 9 ml.
Forcibly insert a ball-bearing sipper tube into one end of the cut serological pipet, resulting in a tight fit. Sipper tubes with ball-bearings are used because higher alcohol concentrations are likely to leak from sipper tubes without ball-bearings.
Seal the junction between the cut serological pipet and the inserted ball-bearing sipper tube by slipping a 1 inch long section of heat shrink tubing over it, and using the heat gun to shrink the tubing down to a snug 5/16 inch fit.
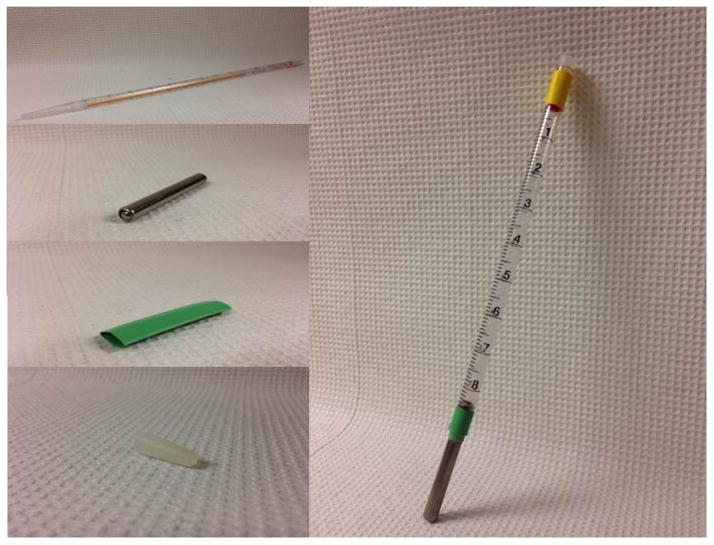
Close the other end of the serological pipet by inserting a silicone stopper. The end of the stopper extends about 1 ml into the pipet, leaving a total of about 5 ml of total fluid space in the fully constructed sipper tube. Figure 2 shows a constructed sipper tube and supplies needed to construct the tube.
Figure 2.
Components needed to construct a ball-bearing sipper tube (left side) and constructed sipper tube (right side). Components shown, from top to bottom (left side) are a 10 ml disposable clear polystyrene serological pipet, a stainless steel ball-bearing sipper tube, heat shrink tubing, and a silicone stopper. Please see Supporting Procedures for details on the construction process.
Preparing the Ethanol Drinking Solution
A 20% (v/v) ethanol solution is most commonly used with DID procedures, though the original characterization of the DID procedure found that concentrations of ethanol ranging from 10–30% led to similar consumption and BEC levels (Rhodes et al., 2005). It is important to keep in mind that using concentrations of ethanol that are too weak (i.e., below 10%) may prevent mice from consuming enough ethanol in the limited 2- to 4-h period of time to achieve binge-like BEC levels.
Materials
190 proof ethanol (Pharmco Inc., Brookfield, CT)
Tap water
200 ml graduated cylinder
500 ml graduated cylinder
Protocol steps
The 20% ethanol drinking solutions should be made fresh daily.1
The 190 proof grain ethanol is composed of approximately 95% ethanol, and 5% water. To make a 20% ethanol solution, measure out 105.3 ml of 190 proof ethanol in a 200 ml graduated cylinder.
Pour the ethanol in the 200 ml graduated cylinder into a 500 ml graduated cylinder, and then bring the total volume up to 500 ml by adding tap water2.
Using the mixed 20% ethanol solution, fill each sipper tube completely from the open end of the tube, closing the open end of the tube with a silicone stopper. Make sure that the silicone stopper fits snugly to prevent spillage from the open end, and excessive leakage from the ball-bearing sipper end, of the sipper tube.
Step Annotations
Depending of the goals of the experiment, and the mouse genotype tested, the experimenter may wish to try higher or lower ethanol concentrations. However, concentrations greater than 20% have an increasing tendency to leak. For a discussion of the various ethanol preparations available, see (Crabbe et al., 2008).
The specific mineral content of local tap water may alter the taste quality of the ethanol solution. Presumably the mice will have adapted to the taste of the local tap water prior to introduction of the alcohol solution. However, if the taste of the ethanol solution becomes a concern, one option is to try bottled spring water.
Determination of Blood Ethanol Concentration
Immediately after the final ethanol access period, blood should be sampled for determination of blood ethanol concentration. Changes in the recorded volume of the ethanol solution upon conclusion of the ethanol access period likely reflect ethanol intake. However leakage, as well as simple jostling of the DID sipper tube by a mouse, can lead to inaccurate intake readings. Assessment of blood ethanol concentration confirms that a particular mouse has actually consumed a physiologically relevant amount of ethanol.
Materials
Sterile surgical grade razor blades
20–50 microliter heparinized capillary tubes
Sterile gauze
5ml microcentrofuge tubes
-
Analox Alcohol Analyzer (Analox Instruments, Lunenberg, MA)
Analox reagent (Analox Instruments, Lunenberg, MA)
Analox ethanol standard (Analox Instruments, Lunenberg, MA)
or
Gas chromatograph
Protocol Steps
Immediately upon conclusion of the final ethanol access period, fluid volumes should be read and recorded, and a blood sample taken for assessment of blood ethanol concentration.
Beginning with the first DID ball-bearing sipper tube (T0), record the final fluid volume, and immediately move the associated cage to another room.
Using a 20–50 microliter heparinized capillary tube, sample blood from either the peri-orbital sinus or tail tip. For peri-orbital sinus blood sampling, the heparinized capillary tube is used to gently puncture the peri-orbital membrane prior to the blood draw. For tail tip sampling the researcher must first nick the tail tip using a sterile surgical grade razor blade prior to the blood draw. In either case, sterile gauze is used to stop the bleeding. See (Parasuraman et al., 2010) for details on peri-orbital sinus and tail tip blood sampling.1 Continue sampling blood from mice in order of sipper tube introduction, with the help of multiple technicians if necessary to keep the final fluid readings and blood samplings synchronized. Regardless of blood sampling method employed, be sure to get proper prior approval from your institution’s animal care and use committee for these invasive measures as well as all other procedures outlined here.
-
Blood samples are processed differently depending on the method of blood alcohol determination.
If samples are assayed using the Analox Alcohol Analyzer, blood samples should be placed immediately into a 5 ml microcentrofuge tube at room temperature, spun down, and stored at 20° C until the time of assay by Analox Alcohol Analyzer. The 100 mg/ml standard provided by Analox is generally recommended to determine blood ethanol concentration. The interested reader is referred to the literature accompanying the Analox Alcohol Analyzer for a detailed description of the methods used to determine blood ethanol concentration using this device.
If samples are assayed using a gas chromatograph, blood samples should be placed immediately into a 5 ml microcentrofuge tube with 50 microliters ice cold ZnSO4. Samples should be kept cold until they are spun down, supernatant decanted, placed into a new 5 ml microcentrofuge tube, and frozen at 20° C until time of assay by gas chromatography. The standard curve to which samples are compared to determine blood ethanol concentration are 0.2367, 0.4734, 0.9468, 1.578, 1.9725, 2.959, 3.945, and 4.932 mg/ml ethanol. The interested reader is referred to (Barkley-Levenson and Crabbe, 2012) for a description of the apparatus and methods used to determine blood ethanol concentration by gas chromatography.
Step Annotations
Other methods of blood sampling may also be employed. For example, a method that has been increasingly suggested by staff veterinarians is blood sampling via the saphenous vein. However, we have found that the peri-orbital sinus and tail tip approaches are easier to conduct under the low light conditions employed in the assessment of DID, particularly when working with mice with dark colored fur such as C57BL/6J mice. The peri-orbital sinus site has the advantages of providing both a more rapid and a more accurate reflection of the brain ethanol concentration than other peripheral sites.
COMMENTARY
Background Information
A discussion of the development of the DID procedure from its antecedents has been presented elsewhere (Crabbe et al., 2011a). That review presents several advantages and disadvantages of the method, and discusses studies that document its basic parameters. For example, mice do not appear to ingest ethanol in the DID setting for its caloric content (Lyons et al., 2008). However, whether their primary motivation is to seek the pharmacological effects of ethanol or its taste has not been established (in this, the DID test resembles all other oral ethanol self-administration procedures). The microstructure of ethanol DID was compared with that of drinking water in C57BL/6J mice, and several features of drinking distinguished the two fluids, including number and size of bouts of drinking and rate of drinking (Crabbe et al., in press; Linsenbardt and Boehm, in press; Rhodes et al., 2005).
Because most studies characterizing DID have been performed in C57BL/6J mice, it should be noted that we can’t be certain how the features of binge-like drinking in this genotype may apply more generally to other mice. C57BL/6J have become the ‘reference genome” for mouse studies, but they have many idiosyncracies that distinguish them from most murine genotypes (Didion and de Villena, 2013). Genetic contributions are important determinants of DID avidity. Inbred strains of the C57/C58 lineage reach binge-level BECs in the DID test, but other genotypes mostly reach BECs well below 80 mg/dl (Crabbe et al., 2012). An exception is the lines of High Drinking in the Dark (HDID) mice, which were selectively bred specifically for high BEC after a 2-day DID exposure. These lines now reach BECs that average 140 mg/dl (HDID-1) and 120 mg/dl (HDID-2), respectively (Crabbe et al., in press). Studies performed with these lines have recently been reviewed (Barkley-Levenson and Crabbe, in press).
Critical Parameters
Note that the DID procedure is executed in a darkened colony room. However, a dim red light (2 lumen/ft2, about 21.5 lux) may be used to allow the experimenter to navigate the room. It is advisable to leave this red light on throughout the experiment so that anyone entering the colony room can see well enough to move about safely, and to avoid unnecessarily disturbing the mice by turning the red light on when entering the room.
The standard DID procedure is routinely conducted over 4 days. There are several reasons for this. First, as reported by Rhodes et al. (Rhodes et al., 2005) and from a good deal of unpublished data, we have found that intake on day 1 does not correlate very substantially with intake on day 2. However, intake on day 2 correlates well with intake on days 3 and 4, and up to day 12. (The data supporting this generalization are from individual differences in drinking assessed in C57BL/6J mice. Because they are inbred, these individual differences are not allelic, but rather must represent other environmental influences and/or differences in gene expression. Second, if a treatment is to be applied, or if multiple groups are being tested, intake on days 2 and 3 allows the experimenter to match subjects for baseline intake. Third, a four day procedure fits easily into a standard work week. Fourth, we have observed that extra days of alcohol experience, particularly if the daily procedure is repeated for a week or more, tends to produce higher average alcohol intake, although these effects are modest. Finally, even a single binge-like drinking episode results in substantial changes in brain state as indicated by patterns of gene expression that differ from water-drinking mice (Mulligan et al., 2011).
DID drinking promotes binge-like BECs because a single bottle of fluid is offered. When we have compared C57BL/6J mice (or HDID-1 mice) in a DID variant where a bottle of water is also offered, the mice ingest less ethanol solution, and reach lower BECs. Although some mice exceed the 80 mg/dl criterion, most do not under these circumstances (Crabbe et al., 2009; Rhodes et al., 2005). Many investigators use some features of the DID procedure (C57BL/6J mice, access limited to 2 or 4 hr early during the circadian dark phase) but also offer water, yet describe the procedure as the “the Drinking in the Dark test.” It should be noted that although mice may ingest substantial amounts of ethanol with water available, they very likely do not drink enough to reach a state of behavioral intoxication.
In all DID tests reported to date, food has been freely available, and Lyons et al. (Lyons et al., 2008) found that relative degree of mild food deprivation did not affect DID intakes. The rationale for studying the early hours of the circadian dark period was based on knowledge that most of the day‘s eating and drinking occurs then. Furthermore, much of normal water ingestion, at least in rats, is prandial, i.e., coincident with meal ingestion (Zorrilla et al., 2005). Thus, it is to be expected that manipulations related to food access or palatability would influence ethanol DID. Similarly, simultaneous access to other rewarding substances such as sweet solutions has not been explored in the DID setting.
The choice of a 4 hr access on the final day of DID exposure was also derived from parametric testing in C57BL/6J mice (Rhodes et al., 2005). However, we recently compared the BEC at the end of 1, 2, 3, or 4 hr of drinking in HDID-1 and HDID-2 mice. We found that BEC may reach peak levels after 3 hr of drinking (Crabbe et al., in press). Thus, the specific test parameters that maximize intake and BEC may vary across genotypes.
Ethanol DID leads to mildly intoxicating BECs in C57BL/6J mice, manifested as mild ataxia-like behaviors (Moore et al., 2007; Rhodes et al., 2005) and activity changes (Linsenbardt and Boehm, in press; Linsenbardt et al., 2011). However, likely due to the higher BECs they attain, HDID mice also show mild signs of ethanol withdrawal convulsions indicating that they have attained mild physical dependence (Crabbe et al., in press). These behavioral consequences of ethanol ingestion may compete with further ingestive behavior, limiting the BEC that can be attained during the DID test.
Troubleshooting
An initially puzzling finding from our DID studies was the occasional mismatch between recorded volume of intake and resulting BEC. For example, there is no physiologically possible way that a 30 gram mouse can ingest 7 ml of 20% ethanol in 4 hr and have a BEC that is unmeasurably low. Tube leakage is one source of this kind of mismatch, and is both unavoidable and unpredictable. Another is the fact that mice apparently sometimes like to manipulate the drinking spout, perhaps as a form of play. We deal with this sort of data by establishing (through regression) predicted BECs from intake volumes and eliminate any mouse’s data where intake radically deviates from the regression (for discussion, see Crabbe et al (Crabbe et al., 2011b), and http://www.scripps.edu/california/research/inia/methodology.html).
Anticipated Results
With DID procedures mice drink enough ethanol in a limited period of time (generally 2–4h) to achieve pharmacologically meaningful BECs (100 mg/dl or higher). Though the BECs achieved on the final test day can vary between experiments, it is commonly reported that C57BL/6J mice, on average, attain BECs of 100 mg/dl but some reports have described BECs greater than 120 mg/dl. Thus, DID procedures are useful when high BECs are the desired end-point and for studies aimed at studying the neurobiology and genetics underlying binge-like ethanol drinking in pre-clinical animal models. Published data depicting ethanol consumption and BECs achieved with DID procedures can be seen in Rhodes et al. (2005) as well as the numerous other publications that are cited and listed in the reference section.
Time Considerations
As noted above, DID is a high throughput procedure that can be completed in a 4-day period. The first 3 days of the procedure typically involve a 2-h ethanol access/data collection period, and the 4th day of the standard DID procedure involves 4-h of ethanol access/data collection. Beyond the time of ethanol access, additional time will be required for preparation (e.g., mixing ethanol solution, weighing mice, etc.) but we estimate that this would require no more than an additional hour each day on average.
Acknowledgments
This work was supported by National Institute of Health grants AA022048, AA013573 and AA015148 to TET, AA10760, AA13519, AA020245 and grants from the US Departments of Veterans Affairs and Defense to JCC, and AA016789 to SLB.
Footnotes
INTERNET RESOURCES
The standard operating procedure (SOP) for DID can also be viewed at the “Methodologies” section of the Integrative Neuroscience Initiative on Alcoholism (INIA) website: http://www.scripps.edu/california/research/inia/methodology.html
Contributor Information
Todd E. Thiele, Email: thiele@unc.edu.
John C. Crabbe, Email: crabbe@ohsu.edu.
Stephen L. Boehm, II, Email: slboehm@iupui.edu.
LITERATURE CITED
- Barkley-Levenson AM, Crabbe JC. Ethanol drinking microstructure of a high drinking in the dark selected mouse line. Alcohol Clin Exp Res. 2012;36:1330–1339. doi: 10.1111/j.1530-0277.2012.01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley-Levenson AM, Crabbe JC. High Drinking in the Dark mice: A genetic model of drinking to intoxication. Alcohol. doi: 10.1016/j.alcohol.2013.10.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 2012;17:108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BR, Olney JJ, Lowery-Gionta EG, Sprow GM, Rinker RA, Navarro M, Kash TL, Thiele TE. Repeated cycles of binge-like ethanol drinking in male C57BL/6J mice augments subsquent voluntary ethanol intake but not other dependence-like phenotypes. Alcohol Clin Exp Res. 2013;37:1688–1695. doi: 10.1111/acer.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Cameron AJ, Munn E, Bunning M, Wahlsten D. Overview of mouse assays of ethanol intoxication. Curr Protoc Neurosci. 2008;Chapter 9(Unit 9):26. doi: 10.1002/0471142301.ns0926s42. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Harris RA, Koob GF. Preclinical studies of alcohol binge drinking. Ann N Y Acad Sci. 2011a;1216:24–40. doi: 10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Belknap JK, Spence SE, Cameron AJ, Schlumbohm JP, Huang LC, Barkley-Levenson AM, Ford MM, Phillips TJ. Progress in a replicated selection for elevated blood ethanol concentrations in HDID mice. Genes Brain Behav. doi: 10.1111/gbb.12105. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Huang LC, Schlumbohm JP, Spence SE, Barkley-Levenson AM, Finn DA, Rhodes JS, cameron AJ. Ethanol withdrawal-associated drinking in the dark: Common and discrete genetic contributions. Add Gen. 2012;1:3–11. doi: 10.2478/addge-2012-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Rhodes JS, Yu CH, Brown LL, Phillips TJ, Finn DA. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biol Psychiatry. 2009;65:662–670. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Spence SE, Brown LL, Metten P. Alcohol preference drinking in a mouse line selectively bred for high drinking in the dark. Alcohol. 2011b;45:427–440. doi: 10.1016/j.alcohol.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didion JP, de Villena FP. Deconstructing Mus gemischus: advances in understanding ancestry, structure, and variation in the genome of the laboratory mouse. Mamm Genome. 2013;24:1–20. doi: 10.1007/s00335-012-9441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Ryabinin AE. CRF1 Receptor Signaling Regulates Food and Fluid Intake in the Drinking-in-the-Dark Model of Binge Alcohol Consumption. Alcohol Clin Exp Res. 2013;37:1161–1170. doi: 10.1111/acer.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology (Berl) 2007;192:207–217. doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Kaur S, Ryabinin AE. Ghrelin receptor antagonism decreases alcohol consumption and activation of perioculomotor urocortin-containing neurons. Alcohol Clin Exp Res. 2010;34:1525–1534. doi: 10.1111/j.1530-0277.2010.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL., 2nd Alterations in the rate of binge ethanol consumption: implications for preclinical studies in mice. Addict Biol. doi: 10.1111/adb.12052. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Griffin KD, Gigante ED, Boehm SL., 2nd Tolerance to ethanol’s ataxic effects and alterations in ethanol-induced locomotion following repeated binge-like ethanol intake using the DID model. Alcohol Clin Exp Res. 2011;35:1246–1255. doi: 10.1111/j.1530-0277.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons AM, Lowery EG, Sparta DR, Thiele TE. Effects of food availability and administration of orexigenic and anorectic agents on elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008;32:1962–1968. doi: 10.1111/j.1530-0277.2008.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Mariani JN, Linsenbardt DN, Melon LC, Boehm SL., 2nd Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. Alcohol Clin Exp Res. 2010;34:734–742. doi: 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL., 2nd GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacol Biochem Behav. 2007;88:105–113. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan MK, Rhodes JS, Crabbe JC, Mayfield RD, Harris RA, Ponomarev I. Molecular profiles of drinking alcohol to intoxication in C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:659–670. doi: 10.1111/j.1530-0277.2010.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA. National Institute on Alcohol Abuse and Alcoholism Council approves definition of binge drinking 2004 [Google Scholar]
- Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 2010;1:87–93. doi: 10.4103/0976-500X.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1450–1467. doi: 10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]
KEY REFERENCE
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]