Abstract
Purpose of review
For over 30 years, Delayed Sleep Phase Disorder (DSPD) has been defined as a debilitating sleep condition. Recently there is more awareness of DSPD in young people, yet considerable information is needed to understand its etiology and treatment. This review describes the latest research findings describing the clinical features, etiology and treatment of DSPD.
Recent findings
The prevalence of DSPD in adolescents and young adults ranges from 1 to 16%. The impact on the individual is significant, particularly in the domains of school/work performance and mental health. We propose contributing factors include reduced homeostatic sleep pressure, a lengthened and delayed circadian rhythm, insensitivity to clock-resetting morning light, and heightened cognitive activity. Evening melatonin administration as a sole treatment appears promising, as is a combination of cognitive-behavior therapy plus morning bright light.
Summary
Recent findings suggest clinicians should be aware of the clinical features (i.e., significant daytime sleepiness, anxiety and depression symptoms, potential for school drop out) of DSPD, as several biological features underpinning this disorder are unseen in clinical settings. We advise clinicians to become familiar with exogenous evening melatonin administration, and cognitive and behavioral techniques to simultaneously treat the delayed circadian rhythm and associated sleep-onset insomnia.
Keywords: Delayed Sleep Phase Disorder, Adolescence, Clinical features, Etiology, Treatment
Introduction
The second edition of the International Classification of Sleep Disorders (ICSD-2), and the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) concur that Delayed Sleep Phase Disorder (DSPD) is recognized as normal sleep which is significantly delayed in its timing with respect to the individual's desired sleep onset and rise times [1,2]. The ICSD-2 states that a person experiencing DSPD may fall asleep between 1am to 6am [1], which creates significant difficulty rising for work or school in the morning. Sleep onset times may be moderated by age, culture, and whether the individual is still able to attend morning commitments (e.g., school) [3-5]. Seventeen percent of high school students report difficulty falling asleep before 2am at least 3 times per week, which is symptomatic for DSPD [4]. Many adolescents throughout the world show a delayed sleep pattern [5], but the sleep pattern is considered disordered when it significantly impacts upon an important area of the individual's functioning [1,2]. The most common area of functioning affected is school life, including frequently being late for school, non-school attendance and drop-out, oversleeping on school mornings, excessive sleepiness at school, chronic sleep reduction, and poor school performance [3,4,6-8]. Poor school performance has been more highly correlated with daytime sleepiness than sleep quality and sleep duration [9]. Excessive sleepiness on school days is likely due to school-week sleep being restricted (e.g., range 5.8-7.0 hrs) [3,4] compared to that of their healthy peers (e.g., range 7.3-8.4 hrs) [4,5]. A lengthy sleep latency (69-124 min) may also contribute to this restricted school night sleep [3,4,8]. The adolescent with DSPD will obtain sleep within normal limits (e.g., 9.5 hrs) during free days (i.e., weekends) [1,2,4,10], as their sleep offset time occurs later in the morning [3], and often into the afternoon [4]. It is likely that the negative consequences of DSPD continue after the school years as young adults with DSPD report significant disruptions to aspects of their work, family, and social life [11].
Estimates of DSPD prevalence range from 1% to 16% [1,6] in adolescent populations, with estimates of Delayed Sleep Timing (i.e., delayed sleep pattern without differential diagnosis) lying mid-way in the range (∼8%) [4]. Several behaviors have been identified in adolescents with DSPD, which differ from their good sleeping peers. Associated psychopathology with DSPD includes substance use (i.e., tobacco, alcohol, caffeine), and elevated anxiety and depression symptoms [4,6,7,11,12]. It is possible that the combination of depression symptoms and short sleep is due to a polymorphism of the 5-HTTLPR genotype [13], however it is yet to be confirmed in young people with DSPD. Poor sleep hygiene has been associated with DSPD (i.e., bedroom presence/bedtime use of electronic media, napping after school) [3,7], though direct comparisons have not yet been performed with matched non-clinical samples. Finally, small trends have been found for DSPD adolescents to be less involved in sport, yet undertaking more extra-curricular activities [6]. As sporting matches are often performed on weekend mornings, it may be that sport provides a consistent rise time across the week, disallowing circadian rhythm delays [14], and thus act as a protective factor.
The Etiological Bases for Delayed Sleep Phase Disorder
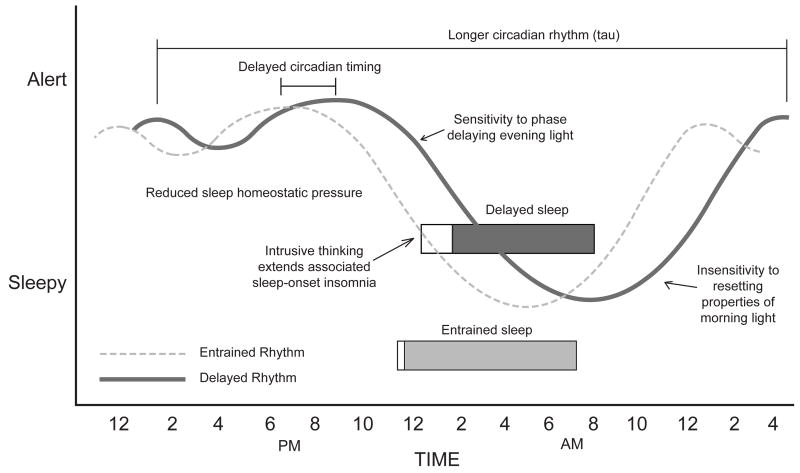
We now turn our attention to the etiological bases for Delayed Sleep Phase Disorder before discussing evidence-based treatments. Figure 1 provides an understanding of the possible contributing factors to Delayed Sleep Phase Disorder.
Figure 1.
Contributing factors to Delayed Sleep Phase Disorder.
Reduced Homeostatic Sleep Pressure
A potentially important and overlooked contributor to DSPD is reduced sleep homeostatic pressure leading to greater evening alertness [10]. Recent longitudinal analyses of adolescents' EEG have demonstrated reductions of slow-wave sleep that are associated with reduced sleep homeostasis [15,16]. Recent pioneering research in younger and older good-sleeping adolescents shows a longer latency to sleep onset in older adolescents after 14.5-18.5 hours awake [17]. This results in a longer latency to sleep onset from 9:30pm till 1:30am for older adolescents who rises at approximately 7:00am [5]. This sleep onset time lies at the beginning of the 1:00am-6:00am sleep-onset range for DSPD [1]. We speculate that adolescents with DSPD may have significantly reduced homeostatic sleep pressure during the day (compared to their good sleeping peers) that would push their sleep onset even later. However, no data to support this hypothesis exist yet.
Circadian Rhythm Abnormalities
Two propositions for circadian abnormalities in DSPD have been provided. The most established idea is that circadian rhythm timing is delayed in individuals with DSPD. Saxvig and colleagues reported a 3-hour delay in the timing of dim light melatonin onset (DLMO; 2:00am) and polysomnography (PSG)-defined sleep onset (3:08am) in a group of 35 adolescents and young adults diagnosed with DSPD (relative to 19 matched healthy controls; DLMO at 11:00pm; PSG-defined sleep onset at 12:07am) [10]. A similar circadian delay has also been demonstrated for core body temperature minimum (Good sleepers=2:43am vs. DSPD =6:38am) [18] and peak melatonin levels (Good sleepers=1:30am vs. DSPD=5:30am) [11].
The second proposition is that the length of the circadian period (tau) is longer in individuals with DSPD compared to good sleepers [19]. A case study of a 30-year old male in 2007 found a core temperature tau of 25.38 hrs [20]. Recent data of 6 young adults (age=22.0±3.3yrs) with DSPD showed a mean core temperature tau of 24.90 hrs, which was significantly longer than matched good sleepers (tau=24.48 hrs) [18]. A tau near 25.0 hrs, or over, creates significant difficulty advancing sleep timing to the 24-hr day [4].
Sensitivity to evening light and/or insensitivity to morning light
The etiology of DSPD may also be explained by an altered response to photic stimuli that is necessary for entrainment to the 24-h day. Because tau is slightly different from 24 hours in all humans [19], adjustment is necessary to synchronize the internal circadian clock to the external 24-h clock. The primary synchronizing stimulus to the circadian system is the daily variation of light and dark. A Phase Response Curve (PRC) to light can explain circadian system shifts, where delays occur when light is presented in the evening (beginning of sleep), and advances when light is presented in the morning (end of sleep). There are no published light PRCs for adolescents, though this is the primary aim of one ongoing study [21], and it remains unknown whether the PRC to light is similar for those with DSPD. Aoki and colleagues [22] continue to be the only group that has tested light sensitivity in patients with DSPD. Their melatonin suppression data suggested that DSPD patients are more sensitive to phase delaying light. Systematic tests of phase shifting in response to light, however, are still needed in this patient population.
DSPD may be an extreme manifestation of the normal delay of sleep and circadian rhythms that emerges during adolescence. One hypothesis is that pubertal adolescents have larger phase delay and smaller phase advance responses compared to adults or younger pre-pubertal children. If this hypothesis is correct, the adolescent circadian system would shift later more easily, and it would be more difficult to synchronize the endogenous circadian clock to the external 24-h day length. Animal researchers have measured phase shifts in response to a 15-min 150-lux light pulse in pubertal (49 days) and adult (140 days) female mice [23]. These data have been replotted as a PRC separately by age [24], and illustrated that the phase delay portion of the PRC was larger for the pubertal compared to adult mice. Data from healthy adolescents also provide preliminary support for a reduced circadian response to morning light [25,26]. In a more recent study with young adults (ages 18–30 yrs) who were self-described “struggling night owls” (and who may have fit some criteria for DSPD) [27], participants shifted their sleep earlier for 6 days. One group was instructed to receive short wavelength light exposure in the mornings shortly after waking while the other group remained in normal room lighting. Both groups of night owls advanced by about 1.5 hr, however, the light group did not advance more than the control group. These data suggest morning advancing light was ineffective in these night owls and/or that earlier sleep times and reducing the amount of evening (delaying) light may have been more effective than adding morning short wavelength light.
The mechanism by which light sensitivity may change during adolescence, and in particular during puberty, may be driven by gonadal (sex) hormones. Evidence from animal studies demonstrates that gonadal hormones (i.e., androgens and estrogens) can modulate photic sensitivity either through alterations in glutamatergic signaling from the retinhypothalamic tract (RHT) or by modulating the indirect pathways to the central circadian clock, the suprachiasmatic nuclei (SCN) [28]. Gonadal hormones may also act directly on the SCN to modulate responses to photic stimuli, or these hormones may act downstream. No studies to date have examined gonadal hormone levels in DSPD patients, however, one recent study of adult humans reported higher testosterone levels in males who also endorse a later chronotype (i.e., more of an evening type) [29]. Finally, adolescents diagnosed with DSPD have been found to be exposed to more ambient light between 10:00pm-2:00am and less light between 8:00-9:00am compared to healthy adolescents [30]. These differences were likely tracking later sleep times in the patients, however, light exposure was similar in the 4 hours before sleep onset and 4 hours after sleep offset in both groups. Daily exposure to light and dark relative to a circadian phase marker (i.e., DLMO, core body temperature minimum) may be more informative to test this hypothesis.
Pre-sleep cognitive activity
Researchers and clinicians have noted an overlap between DSPD and sleep-onset insomnia. With sleep latency typically extended in youth with DSPD (e.g., (69-124 min) [3,4,8], this provides an opportune time for intrusive unhelpful thinking. Most adolescents seeking treatment for DSPD report racing thoughts in bed whilst attempting sleep (∼90%) [3], and data from healthy sleep-deprived adolescents show peak intrusive thinking occurs between 4:30pm-8:30pm [31]. This timing likely corresponds with peak maintenance of alertness, which would occur later for adolescents with delayed sleep timing. Catastrophic thinking themes in this population are primarily concerns about their ability to function academically, followed by the effect of poor sleep on their ability to attend school and consequences from their peers/teachers (e.g., shame, embarrassment) [32]. The greater the number of catastrophizing thoughts is indirectly associated with sleep latency via self-reported anxiety [32], suggesting a cognitive-emotional-sleeplessness chain of events. These emerging findings suggest that the role of cognition should be considered when understanding, and treating, DSPD in young people. We now review recent clinical trials, beginning with a single treatment component trial designed to phase advance adolescents' delayed circadian rhythm.
Treatment of DSPD in Youth
Van Maanen et al. [8] provided evening melatonin administration (range=0.1-10mg, mean=2.7mg) to adolescents with DSPD (N=53). The timing and dose was individually tailored, based on pre-treatment DLMO (mean = 22:32±1:05h). Treatment began with 1mg and increased if no changes were observed after 1 week. After 10 weeks of treatment, adolescents reported significant decreases in chronic sleep reduction, with post-treatment scores moderately lower (d=0.50) than those of good-sleeping adolescents. No sleep data were reported, nor any follow-up data, so it is unknown how long this effect lasted, as well as the rate of relapse. Although no control was used for melatonin administration (i.e., placebo), this study possessed good ecological validity, and represents a model for sleep disorder clinics. Future controlled studies are needed.
Other clinical trials have used a mixture of treatment components for adolescent's delayed sleep patterns. Abu-Salah and Auger [7] recently reported on the treatments used for 60 adolescent patients (age=10-20 yrs) within a 4-year timespan at a sleep medicine clinic. The most common treatment component was ‘behavioral sleep interventions’ (72%), though it is not known exactly what techniques were used. Evening melatonin administration (65%), and morning bright light therapy (42%) were common, and would assist in phase advancing adolescents' circadian rhythm timing. Fifteen percent received zolpidem, likely to address the associated sleep-onset insomnia. Of 22 adolescents at follow-up, 8 patients met treatment goals, 4 received partial success and 10 a poor treatment outcome. Unfortunately, no information is available yet of changes in sleep and circadian timing.
Using a randomized controlled design, Gradisar and colleagues [3] evaluated cognitive-behavior therapy plus bright light therapy to target the sleep-onset insomnia and delayed circadian rhythm, respectively, in a group of 49 adolescents (age=11-18 yrs) diagnosed with DSPD. Cognitive behavior therapy included sleep hygiene (i.e., reducing evening stimulation [e.g., caffeine intake, exercise, technology use]), stimulus control therapy instructions (i.e., go to bed when feeling sleepy), and cognitive therapy (i.e., identifying and modifying unhelpful nighttime thoughts). Bright light therapy consisted of the adolescent scheduling natural sunlight and/or broad-spectrum white light (∼1,000 lux) from a specialized lamp at their usual wake-up time, and advancing light exposure by 30 min each day. Six 50-minute sessions were scheduled over 8 weeks. Compared to the waitlist control group, the treatment group showed large improvements (d >0.80) in various school-night sleep parameters, including sleep onset latency, sleep onset time, total sleep time, wake after sleep onset, and rise time. Near large effects (d=0.71-0.79) were found for daytime functioning, including daytime sleepiness, fatigue, and depression symptoms. At a 6-month follow-up, 15 of the 23 adolescents who underwent treatment still showed improved school-night sleep patterns (d=0.24-0.72 when comparing pre-treatment to 6-month follow-up). Interestingly, daytime functioning effects at the 6-month follow-up had continued to improve, with large effects exhibited (d=0.86-1.31). Worth noting is that 12% of adolescents withdrew from treatment, and 9 could not commit to beginning treatment. Although the prevalence of DSPD is modest, the impact is significant, suggesting interventions that retain as many adolescents as possible are needed.
In summary, melatonin administration appears to be an effective and simple treatment for adolescent DSPD. The more complex combination of CBT and bright light therapy is also effective at post-treatment, and appears to produce long-term benefits. We eagerly await the findings from future clinical trials for DSPD in young people, including a randomized controlled trial of melatonin and light therapy performed on a sample of 60 Norwegian adolescents and young adults (ClinicalTrials.gov NCT00834886) [10], so that the field may better inform clinicians worldwide of the best-practice treatments for DSPD in young people.
Conclusion
More data are accumulating to form a clinical presentation of DSPD in adolescents and young adults. The etiology of DSPD is becoming better understood, though more evidence is needed for sensitivities to evening and morning light. Melatonin administration as a single treatment, and a combination treatment of CBT plus morning bright light therapy, are both recommended for alleviating the impact of DSPD. Many more clinical trials are needed though to build an evidence base for the treatment of DSPD in youth. This not only includes independent and controlled trials of melatonin, light therapy, and CBT (alone and in combination), but also evaluations of other behavioral (e.g., chronotherapy; scheduled sleep timing) and pharmacological (e.g., methylcobalamin [vitamin B12], zolpidem, triazolam, trazadone) therapies.
Key points.
The most commonly reported consequences from DSPD include daytime sleepiness that appears to affect school/work performance.
The circadian rhythm length (tau) is significantly longer in patients with DSPD.
Heightened cognitive activity whilst attempting sleep can be targeted with cognitive-behavior therapy.
Melatonin in the evening and bright light therapy in the morning will help phase advance the delayed circadian rhythm
Acknowledgments
Conflicts of Interest and Source of Funding Acknowledgements: Michael Gradisar has received honoraria from the Australian Psychological Society and the Australian Association of Cognitive Behaviour Therapy for the provision of workshops, and from the Raising Children's Network and Parenting Research Centre as a quality assurance assessor. NIH grant R01HL105395 has been awarded to Stephanie Crowley. The content is solely the responsibility of the authors and does not necessarily represent the official views of the above-named organizations.
Funding disclosure: NIH grant R01HL105395 awarded to S. Crowley. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Bibliography
- 1.American Academy of Sleep Medicine. Diagnostic and coding manual. 2nd. Westchester, IL: American Academy of Sleep Medicine; 2005. The international classification of sleep disorders. [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. American Psychiatric Association; 2013. [Google Scholar]
- 3*.Gradisar M, Dohnt H, Gardner G, et al. A randomized controlled trial of cognitive-behavior therapy plus bright light therapy for adolescent Delayed Sleep Phase Disorder. Sleep. 2011;34:1671–1680. doi: 10.5665/sleep.1432. This recent controlled clinical trial demonstrated large improvements in sleep that were maintained in a subsample of adolescents at a 6-month follow-up. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4*.Saxvig IW, Pallesen S, Wilhelmsen-Langeland A, Molde H, Bjorvatn B. Prevalence and correlates of delayed sleep phase in high school students. Sleep Med. 2012;13:193–199. doi: 10.1016/j.sleep.2011.10.024. This study reports an 8.4% prevalence rate of delayed sleep timing in a sample of school-attending adolescents. Adolescents with delayed sleep timing reported more emotional symptoms and poor sleep hygiene. [DOI] [PubMed] [Google Scholar]
- 5.Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12:110–118. doi: 10.1016/j.sleep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Lovato N, Gradisar M, Short M, Dohnt H, Micic G. Delayed Sleep Phase Disorder in an Australian school-based sample of adolescents. J Clin Sleep Med. doi: 10.5664/jcsm.2998. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu-Salah TM, Auger R. Adolescent delayed sleep phase disorder: clinical features and response to treatment. Sleep. 2013;36:A189–A190. [Google Scholar]
- 8*.Van Maanen A, Dewald-Kaufmann JF, Smits MG, Oort FJ, Meijer AM. Chronic sleep reduction in adolescents with Delayed Sleep Phase Disorder and effects of melatonin treatment. Sleep Biol Rhythms. 2013;11:99–104. This clinical trial used a simple administration of evening melatonin to good effect on adolescents' self-reported chronic sleep reduction. [Google Scholar]
- 9.Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bogels SM. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: a meta-analytic review. Sleep Med. 2010;14:179–89. doi: 10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 10*.Saxvig IW, Wilhelmsen-Langeland A, Pallesen S, et al. Objective measures of sleep and dim light melatonin onset in adolescents and young adults with delayed sleep phase disorder compared to healthy controls. J Sleep Res. doi: 10.1111/jsr.12030. in press. 1.1111/jsr.12030. This study objectively supported the diagnostic criterion that the sleep of those with DSPD is normal when allowed to choose when to sleep. [DOI] [PubMed] [Google Scholar]
- 11.Micic G, Lovato N, Gradisar M, Ferguson S, Burgess HJ, Lack L. Melatonin profiles of individuals with Delayed Sleep Phase Disorder and good sleepers. preparation [Google Scholar]
- 12.Inoue Y, Komada Y, Abe T. Relation between morningness-eveningness score and depressive symptoms among patients with delayed sleep phase syndrome. Sleep. 2013;36:A183. doi: 10.1016/j.sleep.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Carskadon MA, Sharkey KM, Knopik VS, McGeary JE. Short sleep as an environmental exposure: a preliminary study associating 5-HTTLPR genotype to self-reported sleep duration and depressed mood in first-year university students. Sleep. 2012;35:791–796. doi: 10.5665/sleep.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowley SJ, Carskadon MA. Modifications to weekend recovery sleep delay circadian phase in older adolescents. Chronobiol Int. 2010;27:1469–1492. doi: 10.3109/07420528.2010.503293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell IG, Darchia N, Higgins LM, Dykan IV, Davis NM, de Bie E, Feinberg I. Adolescent changes in homeostatic regulation of EEG activity in the delta and theta frequency bands during NREM sleep. Sleep. 2011;34:83–91. doi: 10.1093/sleep/34.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarokh L, Van Reen E, LeBourgeois M, Seifer R, Carskadon MA. Sleep EEG provides evidence that cortical changes persist into late adolescence. Sleep. 2011;34:1385–1393. doi: 10.5665/SLEEP.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor DJ, Jenni OG, Acebo C, Carskadon MA. Sleep tendency during extended wakefulness: insights into adolescent sleep regulation and behaviour. J Sleep Res. 2005;14:239–244. doi: 10.1111/j.1365-2869.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- 18*.Micic G, de Bruyn A, Lovato N, Wright H, Gradisar M, Ferguson S, Burgess HJ, Lack L. The endogenous circadian temperature period length (tau) in Delayed Sleep Phase Disorder compared to good sleepers. J Sleep Res. doi: 10.1111/jsr.12072. in press. This paper provides evidence for a significantly lengthened core body temperature tau in young adults with DSPD. [DOI] [PubMed] [Google Scholar]
- 19.Duffy JF, Wright KP. Entrainment of the human circadian system by light. J Biol Rhythms. 2005;20:326–338. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- 20.Campbell SS, Murphy PJ. Delayed Sleep Phase Disorder in temporal isolation. Sleep. 2007;30:1225–1228. doi: 10.1093/sleep/30.9.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crowley SJ, Eastman CI. Shedding light on the adolescent phase response curve (PRC) Sleep. 2012;35:A64. [Google Scholar]
- 22.Aoki H, Ozeki Y, Yamada N. Hypersensitivity of melatonin suppression in response to light in patients with delayed sleep phase syndrome. Chronobiol Int. 2001;18:263–71. doi: 10.1081/cbi-100103190. [DOI] [PubMed] [Google Scholar]
- 23.Weinert D, Kompauerova V. Light-induced phase and period responses of circadian activity rhythms in laboratory mice of different age. Zoology. 1998;101:45–52. [Google Scholar]
- 24.Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31:276–284. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: Implications for behavior. Ann NY Acad Sci. 2004;1021:276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- 26.Crowley SJ, Carskadon MA. Modifications to weekend recovery sleep delay circadian phase in older adolescents. Chronobiol Int. 2010;27:1469–1492. doi: 10.3109/07420528.2010.503293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharkey KM, Carskadon MA, Figueiro MG, Zhu Y, Rea MS. Effects of an advanced sleep schedule and morning short wavelength light exposure on circadian phase in young adults with late sleep schedules. Sleep Med. 2011;12:685–692. doi: 10.1016/j.sleep.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagenauer MH, Lee TM. The neuroendocrine control of the circadian system: adolescent chronotype. Front Neuroendocrinol. 2012;33:211–229. doi: 10.1016/j.yfrne.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Randler C, Ebenhoh N, Fischer A, et al. Chronotype but not sleep length is related to salivary testosterone in young adult men. Psychoneuroendocrinology. 2012;37:1740–1744. doi: 10.1016/j.psyneuen.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Auger RR, Burgess HJ, Dierkhising RA, Sharma RG, Slocumb NL. Light exposure among adolescents with Delayed Sleep Phase Disorder: A prospective cohort study. Chronobiol Int. 2011;28:911–920. doi: 10.3109/07420528.2011.619906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coles ME, Sharkey KM, Carskadon MA, Nota JA, Schubert JR. Obsessive compulsive symptoms during prolonged wakefulness in adolescents. Sleep. 2013;36:A317. [Google Scholar]
- 32.Hiller R, Lovato N, Gradisar M, Oliver M, Slater A. Trying to fall asleep whilst catastrophizing: what sleep-disordered adolescents think. Submitted. doi: 10.1016/j.sleep.2013.09.014. [DOI] [PubMed] [Google Scholar]