Abstract
The purpose of this study was to use proton magnetic resonance spectroscopy to assess intramuscular lipid and metabolites of lower leg muscles in boys with Duchenne muscular dystrophy (DMD) and determine its relationship with strength and functional ability. Spectroscopic measurements were obtained from four muscles of the lower leg in 25 boys with DMD (9.2±3.1 years) and 10 healthy boys (10.2±2.6 years). Lipid fractions and metabolite concentrations were also determined. Muscle strength, a timed functional test, and the Modified Brooke Lower Extremity Functional Scale were also determined. Lipid fractions were higher (p<0.01) for the DMD group than healthy subjects for all muscles, and lipid fraction was found to be greater in the older DMD boys. The peroneal muscle demonstrated a significant difference in lipid fraction in all DMD age groups. Lipid fractions in all muscles correlated with functional measures (r=0.52–0.70, p<0.001), with smaller inverse correlations with the strength measure (r=−0.36 – −0.56, p<0.05). These findings provide quantifiable information regarding intramuscular lipid and metabolite levels of different muscles across various age groups in boys with DMD and may be used in determining the effect of interventions in future clinical trials.
Keywords: Duchenne muscular dystrophy, lipid fraction, metabolite, spectroscopy
Introduction
Duchenne muscular dystrophy (DMD), the most common form of muscular dystrophy, is an X-linked disease that affects 1 in 3,500 [1] to 6,291 [2] male births. This degenerative neuromuscular disease impacts muscle tissue resulting in weakness and impaired functional abilities. Clinical manifestations of the disease, such as impaired gait, become apparent while the child is 3–5 years old [3–6]. Historically, children with DMD lose the ability to walk by 12 years of age [7]. With the use of glucocorticoid corticosteroids, most children with DMD can increase this time for ambulation[8–11]. However, even most boys taking steroids become non-ambulatory by the age of 15 years [12]. The vast majority of patients with DMD pass away by their mid to late twenties [13, 14], and there is currently no cure for DMD.
Increased lipid infiltration into skeletal muscles of boys with DMD is associated with the progressive weakness and decreased functional abilities [15–17]. Non-invasive measurements using magnetic resonance imaging (MRI) have provided important information concerning this intramuscular lipid infiltration in boys with DMD [18–25]. Some of the previous MRI approaches have primarily focused on qualitative assessments of dystrophic muscle [18–20, 25]. Also, the use of Dixon MRI has been implemented to quantify lipid and water signal in the leg muscles of boys with DMD [13, 26]. MRI measurements that are sensitive to changes to muscle composition and fatty tissue replacement appear to be promising biomarkers to monitor disease progression in this patient population.
In addition to MRI, proton magnetic resonance spectroscopy (1H-MRS) can be utilized to provide further information pertinent to muscle tissue in boys with DMD. 1H-MRS is commonly considered the gold standard for measuring lipid and major muscle metabolites. Some investigators have suggested that an alteration in prominent metabolites [the ratio of trimethyl ammonium (TMA) to total creatine (tCr)] may be linked to cell membrane changes that occur from disease progression in DMD [27–29]. Quantifying intramuscular tCr may also be clinically important in boys with DMD as creatine supplementation is commonly used and has been studied as a potential therapy with varying results in DMD [30, 31].
Furthermore, 1H-MRS provides a means to determine the location of lipid in skeletal muscle [32–35]. The accumulation of intramuscular lipid impacts the physiological function of muscle differently depending on where the lipid is stored. Intramyocellular lipid (IMCL) is found within skeletal muscle fibers while extramyocellular lipid (EMCL) is located outside of the muscle cell. The amount of IMCL has been shown to affect insulin resistance [36–38]) and is important in people with decreased activity levels (such as boys with DMD). 1H-MRS provides a means to differentiate between IMCL and EMCL and quantify the levels of these fats.
We have previously demonstrated substantial replacement of muscle with non-contractile tissue in the thigh muscles of boys with DMD older than 10 years [39]. In the current study, we chose to examine the lower leg musculature. These muscles may have an advantage over more proximal muscles due to the slower disease progression in the lower leg muscles and therefore may be more applicable for use as an outcome measure over a greater range of ages. Torriani et al [16] recently measured lipid fraction using 1H-MRS in the soleus (Sol) and tibialis anterior (TA) muscles of a small group of boys with DMD (n=9). They found greater lipid in both of these muscles relative to healthy controls. They also qualitatively noted that the peroneal (Per) muscle had the greatest degree of lipid amongst the lower leg muscles in DMD. However, the extent of lipid infiltration within the Per was not quantified. Additional investigation using 1H-MRS to quantify muscle fatty infiltration in various lower leg muscles of children with DMD in a larger sample size that allows for stratifying subjects by age and disease severity should provide useful information about how this disease impacts the lower leg muscles and its potential impact on strength and walking.
Therefore, the objectives of this study were to: 1) compare intramuscular lipid using 1H-MRS among four key muscles involved in the primary movements of the lower leg in boys with DMD and age-matched healthy controls, 2) examine the intramuscular lipid levels of boys with DMD in different age groups, as well as the contribution of the IMCL and EMCL levels within the lower leg muscles 3) examine metabolite concentrations (TMA and tCr), and 4) determine the association between intramuscular lipid with strength and functional ability in children with DMD and healthy children.
Materials and Methods
Subjects
Twenty-five boys with a medical diagnosis of DMD confirmed by genetic testing (mean age 9.2 ± 3.1 years) and 10 healthy control (CON) children (mean age 10.2 ± 2.6 years) participated in the study. Demographic data for all subjects are shown in Table 1. Twenty-one of the 25 DMD subjects were still ambulatory, and all 25 were taking glucocorticoid corticosteroids at the time of testing. To examine the lipid levels at different ages for the second objective of this study, the DMD subjects were further divided into three groups based upon age: 5–7 years (n = 10), 8–10 years (n = 9), and 11+ years (n = 6). All aspects of this research project were approved by the Institutional Review Board of the University of Florida.
Table 1.
Subject Demographics
| DMD | CON | |
|---|---|---|
| Number of Subjects | 25 | 10 |
| Number Ambulatory | 21 | 10 |
| Age (years) | 9.2 ± 3.1 | 10.2 ± 2.6 |
| BSA (m2) | 1.2 ± 0.2 | 1.1 ± 0.3 |
| Modified Brooke Lower Extremity Score | 2 (1–9)** | 1 |
Values are mean ± SD except for Modified Brooke Lower Extremity Score demonstrating median and range. DMD, Duchenne muscular dystrophy group; CON, control group. BSA, body surface area.
p < 0.01 significant difference between groups.
1H-MRS Data Acquisition
Spectroscopic measurements were obtained from the Sol, medial gastrocnemius (MG), TA, and Per muscles of the right lower leg. Measurements were performed on either a 1.5T (Signa, GE Medical Systems) or a 3.0T (Achieva, Philips Medical Systems) whole-body scanner. The subjects were positioned supine with their lower leg centered inside either a lower-extremity quadrature coil (1.5T) or an eight channel SENSE receive only extremity coil (3.0T). First, gradient-echo MRI was performed to obtain transaxial, fat suppressed T1 weighted images of the lower leg from the knee to the ankle. The parameters were as follow: 1.5T scanner: TR 18 msec, TE 3.5 ms, flip angle 10°; 3T scanner: TR 24 ms, TE 1.8ms, and 20° flip angle. Parameters for both systems included an acquisition matrix of 224×224 pixels and an optimized field of view (~12×12 cm2). For 1H-MRS measurements, volume localized unsuppressed spectra from the Sol, MG, TA, and Per muscles were acquired using a stimulated echo acquisition mode (STEAM) technique. A large voxel was carefully placed inside the belly of each of the four muscles (Sol 4,500–17,600 mm3, MG 2,400–15,300 mm3, TA 1,800–7,400 mm3, Per 1,600–6,800 mm3) with care taken to avoid visible vasculature, subcutaneous fat, and myofascial layers using the transaxial T1 weighted images (Fig. 1). The following acquisition parameters were used for 1H7 MRS measures for each of the four muscles: 64 averages, echo time 108 ms, repetition time 3,000 ms, 2,084 data points, a spectral width of 2,500 Hz, and 4 phase cycles.
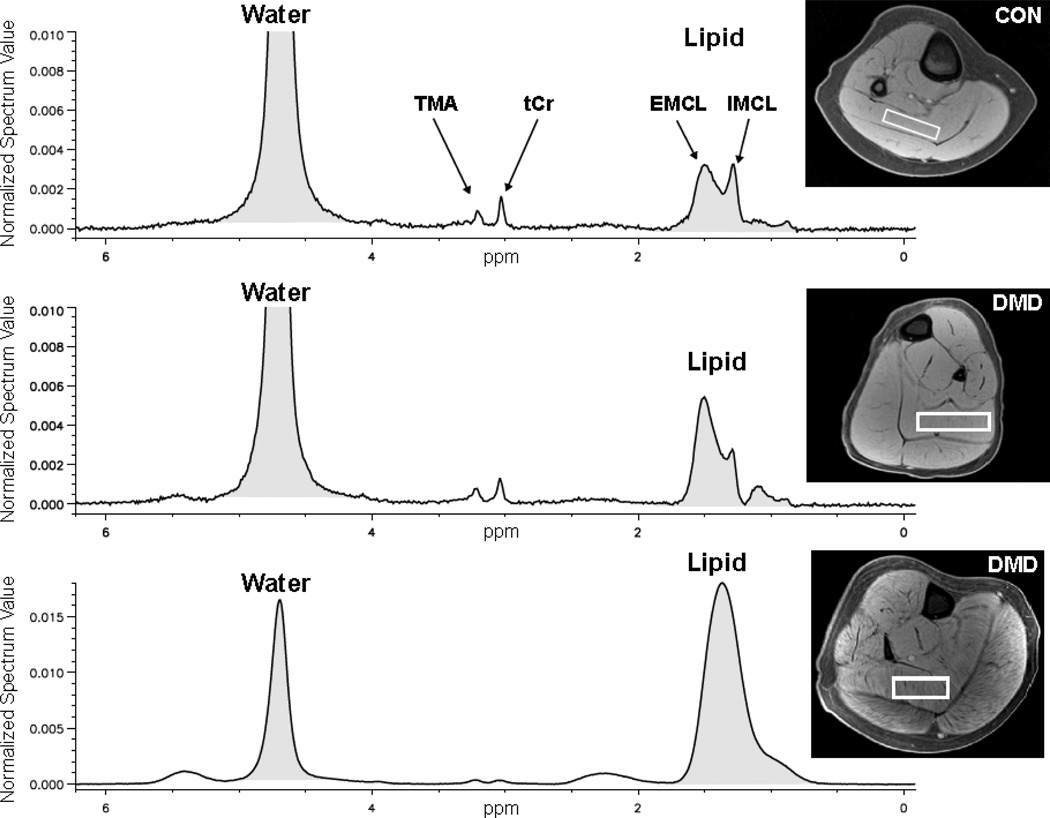
Figure 1.
Representative transaxial slices from T1 weighted MRI of the lower leg in a healthy control (CON) (top), boy with DMD demonstrating minimal lipid infiltration (middle), and a boy with DMD with more advanced lipid infiltration (bottom). The voxel placement for the 1H-MRS is outlined in white in the soleus muscles in each of the MRI’s. The corresponding spectra demonstrate the metabolites (TMA and tCr) as well as the decreasing split resolution between EMCL and IMCL with increasing lipid infiltration.
1H-MRS Data Analysis
Concentrations of lipid and water were determined for each of the four muscles with a custom-written software application in Interactive Data Language (IDL version 7.1.1, ITT Visual Information Systems). After a zero order phase correction, water and lipid concentrations were quantified via area integration. To assess the lipids, we included all lipid peaks from 0.5–2.75 ppm, and the water peak included values between 4.30–5.10 ppm. A lipid fraction ratio (Lipid:Lipid+Water) was then determined without correcting for the effects of T1 and T2.
Contents of TMA, tCr, EMCL, and IMCL were also determined using Magnetic Resonance User Interface for Java (jMRUI) software. Water amplitude was determined with AMARES. An HLSVD filter was used to then remove the water peak so that further metabolite and lipid concentrations could be determined using prior knowledge and starting values (at 5.45, 3.91, 3.2, 3.02, 2.35, 1.5, 1.3, 1.1, and 0.9 ppm). Spectra were only analyzed for IMCL and EMCL if the split resolution could be readily indentified [40]. IMCL and EMCL were analyzed for 9 DMD and 10 CON subjects for the Sol, 11 DMD and 10 CON subjects for the MG, 16 DMD and 10 CON subjects for the TA, and 6 DMD and 7 CON subjects for the Per. TMA and tCr peaks were able to be detected and differentiated from baseline noise for the following muscles: Sol (DMD n=19, CON n=10), MG (DMD n=12, CON n=5), TA (DMD n=8, CON n=5), and Per (DMD n=5, CON n=7). Water, lipid, and the metabolite concentrations were corrected for T1 and T2 relaxation using values from the literature [41]. Concentrations of TMA, tCr, EMCL, and IMCL were each determined relative to water, and ratios of TMA/tCr and IMCL/EMCL were also calculated.
Isometric Muscle Strength Testing
Isometric peak torque of the plantarflexor (PF) muscle group was assessed for the right leg using a Biodex dynamometer as previously described [42]. The knee was placed between 0° to 10° of flexion and the ankle was placed in neutral. The subject was instructed to push as hard as possible for 5 seconds, followed by a 1-minute rest. Five trials were performed, and the highest torque value was used for analysis (peak torque). This protocol has been shown to be reliable in boys with DMD [42]. Peak torque values were normalized to body surface area (BSA) [42].
Functional Assessment
To assess functional abilities, ambulatory subjects performed the time to walk 30 feet (30ft Walk) [16, 42–45]. Subjects performed the task three times, and the fastest time was recorded for analysis. The functional ability for all of the subjects with DMD was also ranked by a physical therapist using the Modified Brooke Lower Extremity Functional Scale [17, 39, 42, 46–49]. This scale ranges from a grade of 1 (able to walk and climb stairs independently) to a grade of 10 (confined to bed).
Statistical Analyses
All statistical analyses were done with SPSS statistical software (version 17.0). Means and standard deviations were calculated for all variables for both groups of participants with the exception of the Modified Brooke Score for which a median and range were determined. Mann-Whitney tests were used to examine differences between the DMD and CON groups. Kruskal Wallis testing was used to determine differences in lipid fraction among the three age groups of boys with DMD for all four muscles. Relationships between functional tests with lipid fraction of the four individual muscles and the Composite (sum) of all four muscles together were examined using Spearman Rho correlation coefficients. Pearson correlation coefficients were used to examine the relationships between muscle strength and lipid fraction. Alpha level was set at 0.05 for all statistical testing.
Results
The demographics for both the DMD and CON groups are shown in Table 1. For the DMD group, the median Modified Brooke Score was 2 ranging from grade 1 to 9. This scale indicates that the majority of patients in this group were ambulating with minimal to moderate difficulty, except for four subjects who were non-ambulatory (grade 9) at the time of testing.
Assessment of Intramuscular Lipid Fraction
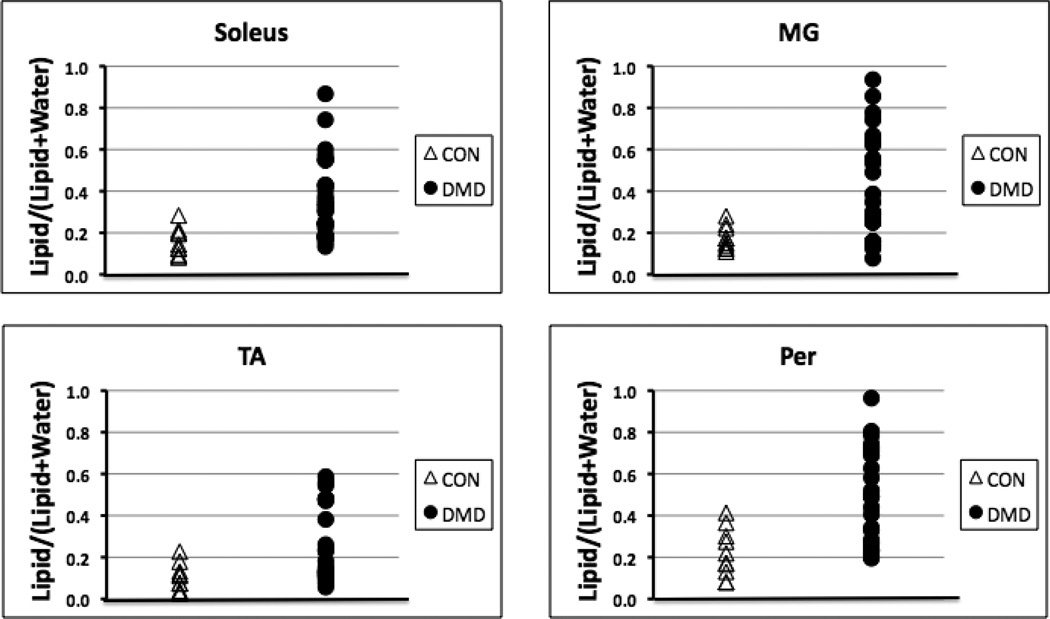
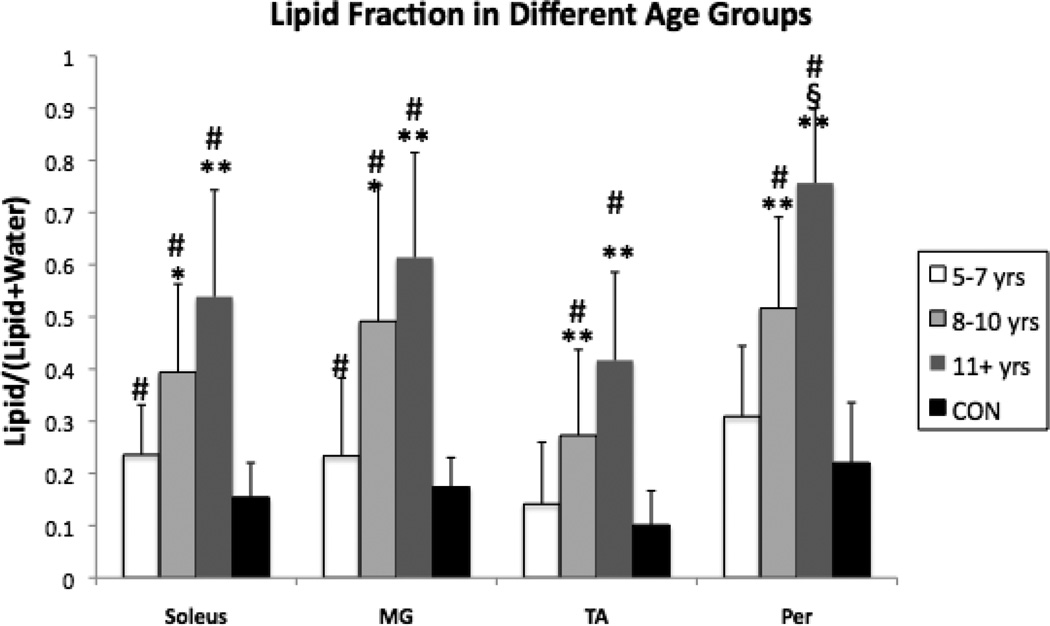
Spectra were successfully obtained in all 25 subjects with DMD and the 10 control subjects. Lipid fraction values for the CON boys were lower than the values for the boys with DMD for all four muscles examined in the lower leg (p < 0.01; Fig. 2). On average, CON lipid fraction values were only 40–45% of that measured in the DMD group. The 4 non-ambulatory subjects demonstrated a substantially higher intramuscular fat fraction for all four lower leg muscles relative to the other 21 subjects with DMD (increases of: Soleus 68%, MG 86%, TA 113%, Per 65%). When the DMD subjects were stratified into the three age groups (5–7 years, 8–10 years, and 11+ years), greater lipid fraction values were found in the older boys with DMD (Fig. 3). Specifically, higher lipid fractions were noted for both the 8–10 year olds and the 11+ year olds compared to the 5–7 year olds for all four muscles. However, the only significant difference in lipid fraction for the 11+ years group relative to the 8–10 year olds was found in the Per muscle (Fig. 3).
Figure 2.
Plots demonstrating individual levels of lipid fraction for both healthy boys (CON) and boys with DMD for all four lower leg muscles.
Figure 3.
Lipid fraction for all four muscles of boys with DMD with the subjects divided into three age groups: 5–7 yrs n = 10, 8–10 yrs n = 9, and 11+ yrs n = 6. Red horizontal line indicates mean lipid fraction from healthy control boys (CON, n = 10). Differences were noted for most of the DMD relative to the age-matched CON groups (#p < 0.05). Differences were noted among DMD groups from 5–7 yrs group (*p < 0.05; **p < 0.01) and from the 8–10 yrs group (§p < 0.05).
Muscle Strength
Peak torque normalized to BSA was higher for both the KE and PF muscle groups in the CON group compared to the boys with DMD. KE peak torque values were 73.5 ± 25.4 N/m for the CON group and 21.6 ± 10.8 N/m for the boys with DMD. PF peak torque values were 80.1 ± 19.8 N/m CON and 30.8 ± 13.4 N/m DMD.
Functional Ability
The DMD group required more time to walk 30 ft relative to the CON group. Four of the 25 DMD subjects were unable to complete the 30ft Walk. Times for the CON and DMD groups of subjects who completed the 30ft Walk were as follows: 4.3 ± 0.6 s vs. 6.3 ± 1.1 s.
Relationships between Lipid Fraction and Functional Measures
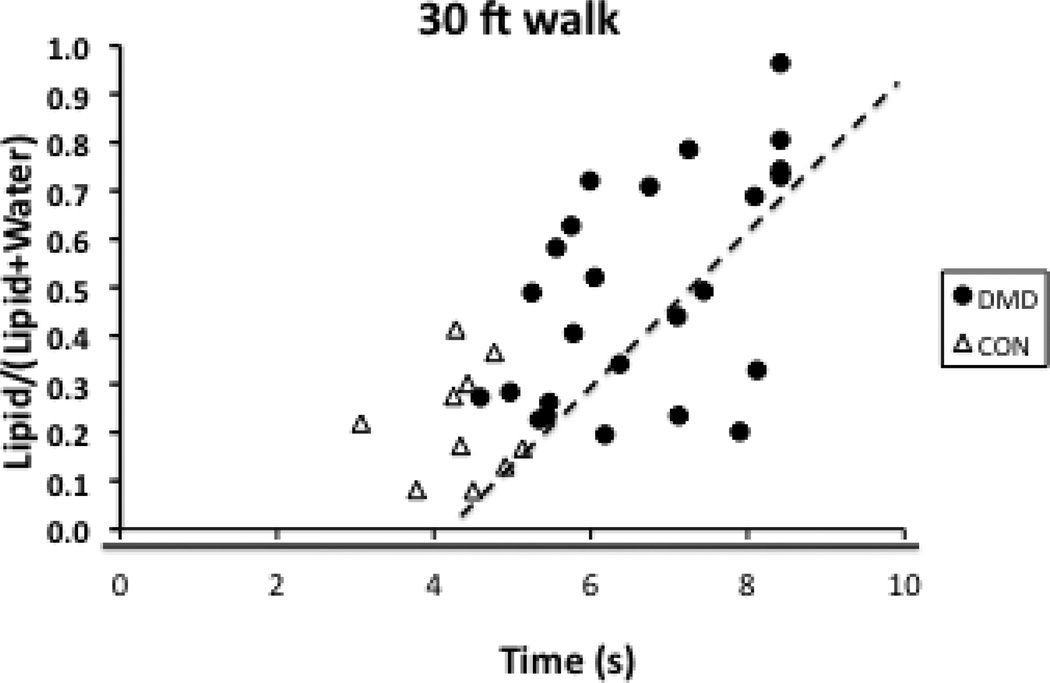
Strong to moderate relationships were noted between the lipid fraction and the Modified Brooke Scale as well as the 30ft Walk (Table 2). Correlation coefficients ranged from 0.52–0.70 with the higher correlations found for the Sol and Per muscles and the lowest for the TA. A scatter plot for the Per muscle is shown in Fig. 4. As a post-hoc analysis, we made a Composite lipid fraction by summing the lipid fractions for all four muscles for each subject. The relationship between this Composite lipid fraction was slightly greater (correlation coefficients = 0.63–0.74) than what was found for the Sol and Per muscles. Significant correlations were also found between peak torque (strength) and the lipid fractions, although these moderate correlations were not as strong as those found with the Modified Brooke Scale and the 30ft Walk (Table 2).
Table 2.
Spearman Rho correlation coefficients were used to determine the relationships between Function (Brooke score and 30ft walk) with levels of lipid. Pearson correlation coefficients were used to determine the associations between PF strength values and lipid levels.
| Modified Brooke | 30ft Walk | PF Strength | |
|---|---|---|---|
| Sol | 0.70** | 0.64** | −0.55** |
| MG | 0.67** | 0.58** | −0.50** |
| TA | 0.63** | 0.52** | −0.56** |
| Per | 0.70** | 0.64** | −0.36* |
| Composite of 4 muscles | 0.74** | 0.66** | −0.55* |
Sol, soleus; MG, medial gastrocnemius; TA, tibialis anterior; Per, peroneal muscles; KE, knee extensors; PF, plantarflexors.
p < 0.05;
p < 0.001.
Figure 4.
Scatterplot demonstrating relationships between lipid fraction of the peroneal muscles and the 30ft Walk for boys with DMD and healthy controls.
Metabolites, IMCL, and EMCL concentrations
Approximately 50% of the spectra acquired in the subjects with DMD demonstrated a clear distinction in IMCL and EMCL and were deemed appropriate for these peaks to be reliably resolved [40]. All of the spectra from the 10 controls were reliably analyzed for IMCL and EMCL for all the muscles except for the Per muscle, which had usable spectra in 6 controls. Table 3 demonstrates metabolite, IMCL, and EMCL concentrations from the four lower leg muscles. The only significant differences between the DMD and control groups were the TMA/H20 and tCr/H20 levels in the MG muscle with the DMD group having lower levels for both variables relative to the CON group.
Table 3.
Metabolite, IMCL, and EMCL rations of concentrations from four lower leg muscles as determined with jMRUI for both the DMD and CON groups.
| Table 3 | Sol | MG | TA | Per | ||||
|---|---|---|---|---|---|---|---|---|
| DMD | CON | DMD | CON | DMD | CON | DMD | CON | |
| IMCL/H2O*100 | 1.44±1.02 | 0.52±0.14 | 0.32±0.47 | 0.19±0.10 | 0.13±0.10 | 0.13±0.07 | 0.10±0.10 | 0.18±0.13 |
| EMCL/H2O*100 | 2.33±1.42 | 1.32±1.0 | 2.52±1.41 | 2.0±0.93 | 1.24±1.08 | 0.68±0.56 | 4.03±1.98 | 2.33±1.93 |
| IMCL/EMCL | 0.7±0.6 | 0.7±0.6 | 0.2±0.2 | 0.1±0.1 | 0.2±0.2 | 0.4±0.3 | 0.03±0.04 | 0.3±0.5 |
| TMA/H2O*100 | 1.3±0.5 | 1.4±0.2 | 0.8±0.3* | 1.2±0.2 | 1.0±0.6 | 1.0±0.3 | 0.7±0.3 | 1.0±0.3 |
| tCr/H2O*100 | 1.0±0.3 | 0.9±0.1 | 0.8±0.6* | 1.0±0.3 | 0.4±0.3 | 0.6±0.2 | 1.4±2.0 | 1.0±0.3 |
| TMA/tCr | 1.5±0.4 | 1.8±0.4 | 1.4±0.6 | 1.4±0.3 | 2.2±1.1 | 1.5±0.4 | 1.4±0.5 | 1.0±0.4 |
Values are mean ± SD. DMD, Duchenne muscular dystrophy group; CON, control group; IMCL, intramyocellular lipid; EMCL, extramyocellular lipid; H2O, water; Sol, soleus; MG, medial gastroenemius; TA, tibialis anterior; Per, peroneal muscles; TMA, trimethyl ammonium; tCr, total creatine.
p< 0.05.
Discussion
This is the first study to evaluate intramuscular lipid and metabolites from four different muscles of the lower leg in children with DMD. Examining how the disease process affects different muscles needed for functional mobility and walking is a key step to understand the disease progression of DMD and how novel interventions may affect its course. We used 1H-MRS as a means of objectively measuring the replacement of muscle by intramuscular lipid. Overall, the results of this study show considerable variations among lower leg muscle responses in DMD with age. We also found significant relationships between intramuscular lipid fraction with both function and strength.
In this study we examined the Sol, TA, MG, and Per of the lower leg. We included assessment of the Per musculature as this muscle group has been reported to have a high degree of lipid infiltration when assessed qualitatively via MRI [16]. Our results confirm this observation as we found the Per to have the highest degree of fatty tissue infiltration of the lower leg muscles studied. The Per muscle was also the only muscle assessed that demonstrated a significant difference in lipid fraction across all three age groups for the boys with DMD (5–7 years, 8–10 years, and 11+ years), suggesting it is affected early in the course of the disease. It is possible that the Per muscle provides more stabilization about the ankle joint during gait in children with DMD due to altered kinetics/kinematics from the disease [25,26]. This potential increase in muscular contractions at the Per may contribute to a more rapid intramuscular infiltration of lipid. We observed less fatty infiltration in the TA relative to the other lower leg muscles. Torriani et al [18] also reported that the TA had approximately one third of the lipid fraction of the Sol muscle when assessed via 1H-MRS (TA = 9.2% vs. Sol = 28.4%), and the qualitative rating of lipid infiltration via MRI was lower than the Per, Sol, GM, and lateral gastrocnemius muscles.
Furthermore, we examined how these lipid levels were related to functional ability in boys with DMD. We found moderate to strong relationships between lipid fractions from all four lower leg muscles and function. Others have also noted strong relationships between measures of intramuscular fat in the legs of boys with DMD and their functional abilities [16, 17, 19]. The global functional scale of the Modified Brooke Lower Extremity score was highly correlated with the lipid fraction from the Sol and Per muscles and moderately correlated with the MG and TA muscles. The lipid fraction from the TA muscle consistently demonstrated the lowest relationship for all functional scores/tests. Our findings do differ somewhat from those by Torriani [16] in that they found a strong correlation between the lipid fraction of the TA and a global functional score. However, they did not find any significant relationship between the lipid fraction from the TA and any of the specific functional measures used in that study (the 6 minute walk test or the 10 m walk). The weaker correlations we found with the lipid fraction of the TA compared to those of the other 3 muscles examined may be in part due to the lesser degree of involvement of this muscle, resulting in lower fatty infiltration. This difference between our results and those of Torriani may also be due in part to our subjects being approximately 2 years younger on average than those used in the other study.
This is also the first study we are aware of to examine the relationship between strength measures (peak torque) and 1H-MRS measures of lipid in children with DMD. Strength decline occurs progressively in patients with DMD, and this developing weakness has devastating consequences with the eventual loss of the ability to walk. We found moderate inverse relationships between lipid fraction and PF strength (r values ranging from −0.49 to −0.56), however, these were not as strong as those found between lipid fraction and timed functional tests (r values ranging from 0.70 to 0.52). Our findings using 1H-MRS revealed similar results to Wren and colleagues [17] who used Dixon MRI to examine the relationship between lipid and functional ability in boys with DMD. These findings suggest that future studies examining the effects of muscle pathophysiology and replacement by noncontractile tissue in boys with DMD may consider placing greater importance on including functional testing than on assessments of strength.
The results of this study also provide important information concerning both the methods and the results of quantifying metabolites, IMCL, and EMCL concentrations in the lower leg muscle of boys with DMD. While others have reported on TMA and tCr values in the Sol muscle of boys with DMD previously [28,29], this is the first study to provide values for these metabolites in other lower leg muscles as well as for the IMCL and EMCL levels in this patient population. A key finding findings from our study is the difficulty found in obtaining spectra with adequate resolution to accurately determine IMCL and EMCL levels in dystrophic muscle. In the healthy control subjects, these values were able to be determined in the majority of the spectra; however, this was not the case for the data from the DMD group as many of the spectra were unable to be confidently resolved (most noted for quantifying the EMCL and IMCL values) even when TMA and tCr were clearly spectrally resolved. We implemented the criterion recommended by Boesch et al [40] of requiring a visual “step” or “split” between IMCL and EMCL to determine if a spectrum could be analyzed for determining IMCL and EMCL. With the increased lipid infiltration from the disease process the spectra were less frequently able to be analyzed, and overall nearly half of the spectra did not meet the above criteria to be reliably analyzed. Other algorithms may be more appropriate to use in better distinguishing spectra to be resolved in this patient population.
We could not confirm the findings of Hsieh and colleagues [29] as our results differed somewhat in the TMA and tCr ratios. While the values we reported were on average less in boys with DMD compared to controls, this difference did not reach significance in the Sol muscle (as it did for Hsieh) and only reached significance for the MG. This difference may be partially explained by the different age ranges of the samples used (6–9 years for Hsieh [29], and 5–15 years in our study) as well as voxel size and positioning. We can also not rule out that our results are an underestimation of the metabolites in boys with DMD who had more disease progression since the spectra acquired in the older subjects were typically not able to be analyzed as often as the younger subjects. Another potential contributor to this difference in results may be from the use of corticosteroids. While all of our subjects with DMD were currently taking steroids, Hsieh et al did not indicate whether the subjects were taking steroids at the time of their study. It is possible that our subjects had more intracellular water content from this steroid use that lowered the TMA/H2O and tCr/H2O levels.
Limitations
A limitation to our investigation is that we did not examine boys who were not receiving corticosteroids as a comparison. While the mechanism of action for corticosteroids is not fully understood, the use of corticosteroids in patients with DMD slows disease progression. The results of several studies have demonstrated improved muscle strength/function as well as prolonging of ambulation in boys with DMD [10, 11, 51–56]. Another limitation to this study is that we did not assess muscles beyond the lower leg. We decided to concentrate on the muscles of the lower leg because these muscles may be more advantageous than the thigh/hip muscles due to the disease progression affecting the lower leg muscles over a longer period of time. While others have also focused on examining lower leg muscles in DMD [16, 29, 49], it is important to recognize more proximal muscles (ie muscles of the thigh, hip, and back) also impact functional ability. Therefore, we can not exclude the potential effect the changes in these more proximal muscles may have had on the correlations reported. Finally, we acknowledge a limitation in using two different MR scanner vendors and magnetic fields for data collection. These differences between the two scanners could potentially have had an effect on the results, though we minimized this possibility by using ratios to quantify lipid/metabolite concentrations.
Conclusions
In this study we quantified lipid levels of four lower leg muscles with 1H-MRS and found moderate to strong correlations between these levels and functional and strength measurements in children with DMD. Lipid levels differed across muscles and increased with older age groups. These differences between DMD and controls were the smallest for the TA muscle and greatest for the Per muscle, which may be linked to altered gait mechanics from the disease. Further investigation should continue to examine what factors contribute to the variability of these lipid levels across muscles in boys with DMD and how future clinical interventions affect the fatty infiltration of skeletal muscle and their functional deficits. Due to the dire need for nonambulatory measures of disease progression in DMD, future research should examine lipid and metabolite levels in the upper extremities with disease progression in older boys with DMD.
Acknowledgements
This study was supported by the Muscular Dystrophy Association (MDA 4170), Parent Project Muscular Dystrophy (8509), and the National Institutes of Health (U54AR052646, R01AR056973, and RC1AR058189). In addition, Dr. Lott was supported by the Rehabilitation Research Career Development Program (K12-HD055929).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Emery AE. Population frequencies of inherited neuromuscular diseases – a world survey. Neuromuscul Disord. 1991;1(1):19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- 2.Mendell JR, Shilling C, Leslie ND, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71(3):304–313. doi: 10.1002/ana.23528. [DOI] [PubMed] [Google Scholar]
- 3.Hsu JD, Furumasu J. Gait and posture changes in the Duchenne muscular dystrophy child. Clin Orthop Relat Res. 1993;(288):122–125. [PubMed] [Google Scholar]
- 4.Bakker JP, De Groot IJ, Beelen A, et al. Predictive factors of cessation of ambulation in patients with Duchenne muscular dystrophy. Am J Phys Med. 2002;81(12):906–912. doi: 10.1097/00002060-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Bushby K, Finkel R, Birnkrant DJ, et al. DMD Care Considerations Working Group. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9(1):77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 6.Doglio L, Pavan E, Pernigotti I, et al. Earyl signs of gait deviation in Duchenne muscular dystrophy. Eur J Phys Rehabil Med. 2011 Dec;47(4):587–594. [PubMed] [Google Scholar]
- 7.Pardo AC, Do T, Ryder T, et al. Combination of steroids and ischial weight-bearing knee ankle foot orthoses in Duchenne’s muscular dystrophy prolongs ambulation past 20 years of age—a case report. Neuromuscul Disord. 2011;21(11):800–802. doi: 10.1016/j.nmd.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Fenichel GM, Florence JM, Pestronk A, et al. Long-term benefit from prednisone therapy in Duchenne muscular dystrophy. Neurology. 1991;41(12):1874–1877. doi: 10.1212/wnl.41.12.1874. [DOI] [PubMed] [Google Scholar]
- 9.Bonifati MD, Ruzza G, Bonometto P, et al. A multicenter, double-blind, randomized trial of deflazacort versus prednisone in Duchenne muscular dystrophy. Muscle Nerve. 2000;23(9):1344–1347. doi: 10.1002/1097-4598(200009)23:9<1344::aid-mus4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Balaban B, Matthews DJ, Clayton GH, et al. Corticosteroid treatment and functional improvement in Duchenne muscular dystrophy: long-term effect. Am J Phys Med Rehabil. 2005;84(11):843–850. doi: 10.1097/01.phm.0000184156.98671.d0. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi F, Yonemoto N, Nakamura H, et al. Prednisolone improves walking in Japanese Duchenne muscular dystrophy patients. J Neurol. 2013;260(12):3023–3029. doi: 10.1007/s00415-013-7104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yilmaz O, Karaduman A, Topaloglu H. Prednisolone therapy in Duchenne muscular dystrophy prolongs ambulation and prevents scoliosis. Eur J Neurol. 2004;11(8):541–544. doi: 10.1111/j.1468-1331.2004.00866.x. [DOI] [PubMed] [Google Scholar]
- 13.Eagle M, Baudouin SV, Chandler C, et al. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002;12(10):926–929. doi: 10.1016/s0960-8966(02)00140-2. [DOI] [PubMed] [Google Scholar]
- 14.Kieny P, Chollet S, Delalande P, et al. Evolution of life expectancy of patients with Duchenne muscular dystrophy at AFM Yolaine de Kepper centre between 1981 and 2011. Ann Phys Rehabil Med. 2013 Sep;56(6):443–454. doi: 10.1016/j.rehab.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Jones DA, Round JM, Edwards RH, et al. Size and composition of the calf and quadriceps muscles in Duchenne muscular dystrophy. J Neurol Sci. 1983;60:307–322. doi: 10.1016/0022-510x(83)90071-0. [DOI] [PubMed] [Google Scholar]
- 16.Torriani M, Townshead E, Thomas BJ, et al. Lowe leg muscle involvement in Duchenne muscular dystrophy: an MR imaging and spectroscopy study. Skeletal Radiol. 2012;41(4):437–445. doi: 10.1007/s00256-011-1240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wren TA, Bluml S, Tseng-Ong L, et al. Three-point technique of fat quantification of muscle tissue as a marker of disease progression in Duchenne muscular dystrophy: preliminary study. AJR Am J Roentgenol. 2008;190(1):W8–W12. doi: 10.2214/AJR.07.2732. [DOI] [PubMed] [Google Scholar]
- 18.Schreiber A, Smith WL, Ionasescu V, et al. Magnetic resonance imaging of children with Duchenne muscular dystrophy. Pediatr Radiol. 1987;17(6):495–497. doi: 10.1007/BF02388288. [DOI] [PubMed] [Google Scholar]
- 19.Liu GC, Jong YJ, Chiang CH, et al. Duchenne muscular dystrophy: MR grading system with functional correlation. Radiology. 1993;186(2):475–480. doi: 10.1148/radiology.186.2.8421754. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, Majumdar S, Genant HK, et al. Quantitative MR relaxometry study of muscle composition and function in Duchenne muscular dystrophy. J Magn Reson Imaging. 1994;4(1):59–64. doi: 10.1002/jmri.1880040113. [DOI] [PubMed] [Google Scholar]
- 21.Pichiecchio A, Uggetti C, Egitto MG, et al. Quantitative MR evaluation of body composition in patients with Duchenne muscular dystrophy. Eur Radiol. 2002;12(11):2704–2709. doi: 10.1007/s00330-002-1392-4. [DOI] [PubMed] [Google Scholar]
- 22.Marden FA, Connolly AM, Siegel MJ, et al. Compositional analysis of muscle in boys with Duchenne muscular dystrophy using MR imaging. Skeletal Radiol. 2005;34(3):140–148. doi: 10.1007/s00256-004-0825-3. [DOI] [PubMed] [Google Scholar]
- 23.Gaeta M, Messina S, Mileto A, et al. Muscle fat-fraction and mapping in Duchenne muscular dystrophy: evaluation of disease distribution and correlation with clinical assessments. Preliminary experience. Skeletal Radiol. 2012;41(8):955–961. doi: 10.1007/s00256-011-1301-5. [DOI] [PubMed] [Google Scholar]
- 24.Hollingsworth KG, Garrood P, Eagle M, et al. Magnetic resonance imaging in Duchenne muscular dystrophy: longitudinal assessment of natural history over 18 months. Muscle Nerve. 2013;48(4):586–588. doi: 10.1002/mus.23879. [DOI] [PubMed] [Google Scholar]
- 25.Kim HK, Merrow AC, Shiraj S, et al. Analysis of fatty infiltration and inflammantion of the pelvic and thigh muscles in boys with Duchenne muscular dystrophy (DMD): grading of disease involvement on MR imaging and correlation with clinical assessments. Pediatr Radiol. 2013;43(10):1327–1335. doi: 10.1007/s00247-013-2696-z. [DOI] [PubMed] [Google Scholar]
- 26.Wokke BH, Bos C, Reijnierse M, et al. Comparison of Dixon and T1-weighted MR methods to assess the degree of fat infiltration in duchenne muscular dystrophy patients. J Magn Reson Imaging. 2013 Sep;38(3):619–624. doi: 10.1002/jmri.23998. [DOI] [PubMed] [Google Scholar]
- 27.Sharma U, Atri S, Sharma MC, et al. Skeletal muscle metabolism in Duchenne muscular dystrophy: an in-vitro proton NMR spectroscopy study. Magn Reson Imaging. 2003;21(2):145–153. doi: 10.1016/s0730-725x(02)00646-x. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh TJ, Wang CK, Chuang HY, et al. In vivo proton magnetic resonance spectroscopy assessment for muscle metabolism in neuromuscular diseases. J Pediatr. 2007;151(3):319–321. doi: 10.1016/j.jpeds.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh TJ, Law TS, Chuang HY, et al. Muscle metabolism in Duchenne muscular dystrophy assessed by in vivo proton magnetic resonance spectroscopy. J Comput Asssit Tomogr. 2009;33(1):150–154. doi: 10.1097/RCT.0b013e318168f735. [DOI] [PubMed] [Google Scholar]
- 30.Tarnopolsky MA, Mahoney DJ, Vajsar J, et al. Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy. Neurology. 2004;62(10):1771–1777. doi: 10.1212/01.wnl.0000125178.18862.9d. [DOI] [PubMed] [Google Scholar]
- 31.Banerjee B, Sharma U, Balasubramanian K, et al. Effect of creatine monohydrate in improving cellular energetics and muscle strength in ambulatory Duchenne muscular dystrophy patients: a randomized, placebo-controlled 31P MRS study. Magn Reson Imaging. 2010;28(5):698–707. doi: 10.1016/j.mri.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Shah PK, Gregory CM, Stevens JE, et al. Non-invasive assessment of lower extremity muscle composition after incomplete spinal cord inury. Spinal Cord. 2008;46(8):565–670. doi: 10.1038/sc.2008.10. [DOI] [PubMed] [Google Scholar]
- 33.Bredella MA, Lin E, Brick DJ, et al. Effects of GH in women with abdominal adiposity: a 6-month randomized, double-blind, placebo-controlled trial. Eur J Endocrinol. 2012;166(4):601–611. doi: 10.1530/EJE-11-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wijsman CA, van Opstal AM, Kan HE, et al. Proton magnetic resonance spectroscopy shows lower intramyocellular lipid accumulation in middle-aged subjects predisposed to familial longevity. Am J Physiol Endocrinol Metab. 2012;302(3):E344–E348. doi: 10.1152/ajpendo.00455.2011. [DOI] [PubMed] [Google Scholar]
- 35.Yokota T, Kinugawa S, Yamato M, et al. Systemic oxidative stress is associated with lower aerobic capacity and impaired skeletal muscle energy metabolism in patients with metabolic syndrome. Diabetes Care. 2013 May;36(5):1341–1346. doi: 10.2337/dc12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perseghin G, Lattuada G, Danna M, et al. Insulin resistance, intramyocellular lipid content, and plasma adiponectin in patients with type 1 diabetes. Am J Physiol Endocrinol Metab. 2003;285(6):E1174–E1181. doi: 10.1152/ajpendo.00279.2003. [DOI] [PubMed] [Google Scholar]
- 37.Thamer C, Machann J, Bachmann O, et al. Intramyocellular lipids: anthropometric determinants and relationships with maximal aerobic capacity and insulin sensitivity. J Clin Endocrinol Metab. 2003;88(4):1785–1791. doi: 10.1210/jc.2002-021674. [DOI] [PubMed] [Google Scholar]
- 38.Ingram KH, Lara-Castro C, Gower BA, et al. Intramyocellular lipid and insulin resistance: differential relationships in European and African Americans. Obesity (Silver Spring) 2011;19(7):1469–1475. doi: 10.1038/oby.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akima H, Lott D, Senesac C, et al. Relationships of thigh muscle contractile and non-contractile tissue with function, strength, and age in boys with Duchenne muscular dystrophy. Neuromuscul Disor. 2012;22(1):16–25. doi: 10.1016/j.nmd.2011.06.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boesch C, Machann J, Vermathen P, et al. Role of proton MR for the study of muscle lipid metabolism. NMR Biomed. 2006;19(7):968–988. doi: 10.1002/nbm.1096. [DOI] [PubMed] [Google Scholar]
- 41.Krssak M, Mlynarik V, Meyerspeer M, et al. 1H NMR relaxation times of skeletal muscle metabolites at 3 T. MAGMA. 2004;16:155–159. doi: 10.1007/s10334-003-0029-1. [DOI] [PubMed] [Google Scholar]
- 42.Mathur S, Lott DJ, Senesac C, et al. Age-related differences in lower-limb muscle cross-sectional area and torque production in boys with Duchenne muscular dystrophy. Arch Phys Med Rehabil. 2010;91(7):1051–1058. doi: 10.1016/j.apmr.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDonald CM, Abresch RT, Carter GT, et al. Profiles of neuromuscular diseases. Duchenne muscular dystrophy. Am J Phys Med Rehabil. 1995;74(5 Suppl):S70–S92. doi: 10.1097/00002060-199509001-00003. [DOI] [PubMed] [Google Scholar]
- 44.Pradhan S, Ghosh D, Srivastava NK, et al. Prednisolone in Duchenne muscular dystrophy with imminent loss of ambulation. J Neurol. 2006;253(10):1309–1316. doi: 10.1007/s00415-006-0212-1. [DOI] [PubMed] [Google Scholar]
- 45.Skura CL, Fowler EG, Wetzel GT, et al. Albuterol increases lean body mass in ambulatory boys with Duchenne or Becker muscular dystrophy. Neurology. 2008;70(2):137–143. doi: 10.1212/01.WNL.0000287070.00149.a9. [DOI] [PubMed] [Google Scholar]
- 46.Brooke MH, Griggs RC, Mendell JR, et al. Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve. 1981;4(3):186–197. doi: 10.1002/mus.880040304. [DOI] [PubMed] [Google Scholar]
- 47.Brooke MH, Fenichel GM, Griggs RC, et al. Clinical investigation in Duchenne dystrophy: 2. Determination of the “power” of therapeutic trials based on the natural history. Muscle Nerve. 1983;6(2):91–103. doi: 10.1002/mus.880060204. [DOI] [PubMed] [Google Scholar]
- 48.Brooke MH, Fenichel GM, Griggs RC, et al. Duchenne muscular dystrophy: patterns of clinical progression and effects of supportive therapy. Neurology. 1989;39(4):475–481. doi: 10.1212/wnl.39.4.475. [DOI] [PubMed] [Google Scholar]
- 49.Arpan I, Forbes SC, Lott DJ, et al. T2 mapping provides multiple approaches for the characterization of muscle involvement in neuromuscular diseases: a cross-sectional study of lower leg muscles in 5–15-year-old boys with Duchenne muscular dystrophy. NMR Biomed. 2013;26(3):320–328. doi: 10.1002/nbm.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaudreault N, Gravel D, Nadeau, et al. Gait patterns comparison of children with Duchenne muscular dystrophy to those of control subjects considering the effect of gait velocity. Gait Posture. 2010;32(3):342–347. doi: 10.1016/j.gaitpost.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Mendell JR, Province MA, Moxley RT, 3rd, et al. Clinical investigation of Duchenne muscular dystrophy. A methodology for therapeutic trials based on natural history controls. Arch Neurol. 1987 Aug;44(8):808–811. doi: 10.1001/archneur.1987.00520200012009. [DOI] [PubMed] [Google Scholar]
- 52.Angelini C, Pegoraro E, Turella E, et al. Deflazacort in Duchenne dystrophy: study of long-term effect. Muscle Nerve. 1994;17(4):386–391. doi: 10.1002/mus.880170405. [DOI] [PubMed] [Google Scholar]
- 53.Rahman MM, Hannan MA, Mondol BA, et al. Prednisolone in Duchenne muscular dystrophy. Bangladesh Med Res Counc Bull. 2001;27(1):38–42. [PubMed] [Google Scholar]
- 54.Biggar WD, Harris VA, Eliasoph L, et al. Long-term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscul Disord. 2006 Apr;16(4):249–255. doi: 10.1016/j.nmd.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 55.Manzur AY, Kuntzer T, Pike M, et al. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008 Jan;23(1):CD003725. doi: 10.1002/14651858.CD003725.pub3. [DOI] [PubMed] [Google Scholar]
- 56.Henricson EK, Abresch RT, Cnaan A, et al. CINRG Investigators. The cooperative international neuromuscular research group Duchenne natural history study: glucocorticoid treatment preserves clinically meaningful functional milestones and reduces rate of disease progression as measured by manual muscle testing and other commonly used clinical trial outcome measures. Muscle Nerve. 2013;48(1):55–67. doi: 10.1002/mus.23808. [DOI] [PMC free article] [PubMed] [Google Scholar]