Abstract
Background
Elderly patients with metastatic renal cell carcinoma (mRCC) may require special treatment considerations, particularly when comorbidities are present. An understanding of the efficacy and safety of targeted agents in elderly patients with mRCC is essential to provide individualized therapy.
Objective
To evaluate the efficacy and safety of everolimus in elderly patients (those ≥65 and ≥70 yr of age) enrolled in RECORD-1.
Design, setting, and participants
The multicenter randomized RECORD-1 phase 3 trial (Clinicaltrials.gov identifier, NCT00410124; http://www.clinicaltrials.gov) enrolled patients with mRCC who progressed during or within 6 mo of stopping sunitinib and/or sorafenib treatment (n = 416).
Intervention
Everolimus 10 mg once daily (n = 277) or placebo (n = 139) plus best supportive care. Treatment was continued until disease progression or unacceptable toxicity.
Measurements
Median progression-free survival (PFS), median overall survival (OS), and time to deterioration in Karnofsky performance status (TTD-KPS) were assessed using the Kaplan-Meier method; the log-rank test was used to compare treatment arms. Other outcomes evaluated included reduction in tumor burden, overall response rate (ORR), and safety.
Results and limitations
In RECORD-1, 36.8% of patients were ≥65 yr and 17.5% were ≥70 yr of age. PFS, OS, TTD-KPS, reduction in tumor burden, and ORR were similar in the elderly and the overall RECORD-1 population. Everolimus was generally well tolerated in elderly patients, and most adverse events were grade 1 or 2 in severity. The toxicity profile of everolimus was generally similar in older patients and the overall population; however, peripheral edema, cough, rash, and diarrhea were reported more frequently in the elderly regardless of treatment. The retrospective nature of the analyses was the major limitation.
Conclusions
Everolimus is effective and tolerable in elderly patients with mRCC. When selecting targeted therapies in these patients, the specific toxicity profile of each agent and any patient comorbidities should be considered.
Keywords: Adverse events, Kidney cancer, mTOR inhibitor, Pneumonitis
1. Introduction
Over the last 5 yr, the treatment options available for the management of metastatic renal cell carcinoma (mRCC) have increased, with the approval of several agents targeting specific angiogenic or growth and proliferation pathways. Although these agents (ie, sunitinib, sorafenib, pazopanib, bevacizumab, temsirolimus, and everolimus) are now widely used in patients with mRCC, safety data continue to emerge from long-term follow-up and expanded access programs. Improved understanding of the efficacy and safety profiles of targeted agents in specific populations may enhance the ability of clinicians to provide individualized therapy and improve outcomes in mRCC.
Elderly patients constitute a large component of the mRCC population because the incidence of mRCC increases with age, with a median age of 62 yr at diagnosis [1]. Comorbid conditions are generally more prevalent in elderly patients compared with their younger counterparts. In a population-based study, serious concomitant diseases were present in 9%, 25%, 49%, and 60% of patients with newly diagnosed cancer <45, 45–59, 60–74, and ≥75 yr of age, respectively [2]. Another study reported the most prevalent comorbidities observed in patients (n = 363) to be arthrosis-arthritis (31%), hypertension (29%), digestive diseases (23%), cardiac disease (21%), and vascular disease (19%) [3]. In addition, elderly patients with cancer are more likely to have a compromised performance status: In one study of 593 patients, a baseline Eastern Cooperative Oncology Group performance status ≥1 was observed in 30% of patients ≥70 yr of age versus 9% of patients <70 yr [4]. The presence of comorbidities and decreased performance status in an older patient may result in a decreased ability to tolerate cancer therapy and therefore to receive the intended dose intensity. An additional concern is that medications taken to manage comorbidities may interact with cancer treatments. Although clinical trials have not been performed directly comparing the safety and efficacy of targeted agents in the elderly population, retrospective analyses of outcomes in elderly subsets enrolled in large clinical trials may provide useful information about how age affects the efficacy and tolerability of individual targeted agents.
Everolimus is a mammalian target of rapamycin (mTOR) inhibitor approved in 65 countries for use in patients with mRCC who have failed prior vascular endothelial growth factor receptor-tyrosine kinase inhibitor (VEGFr-TKI) therapy. The phase 3 RECORD-1 trial demonstrated a significant improvement in progression-free survival (PFS) with everolimus. Median PFS by independent central review was 4.9 mo with everolimus versus 1.9 mo with placebo (p < 0.001) [5,6]. Stomatitis, infection, asthenia, and fatigue, the most commonly reported adverse events (AEs) with everolimus, were manageable and mainly grade 1 or 2 in severity.
In RECORD-1, age (<65 vs ≥65 yr) was not reported to have significant prognostic value for either PFS or overall survival (OS) [6]; however, a detailed subgroup analysis in elderly patients was not performed. Here we compare the outcomes and toxicities in patients ≥65 and ≥70 yr of age enrolled in RECORD-1 with those of the overall study population to further explore the tolerability and efficacy of everolimus in elderly patients.
2. Patients and methods
2.1. Eligibility and treatment
The study design of the randomized double-blind multicenter phase 3 RECORD-1 trial was previously reported [5,6]. Adult patients with metastatic clear cell RCC who experienced disease progression on or within 6 mo of stopping treatment with sunitinib, sorafenib, or both, were enrolled. Prior therapy with bevacizumab, interleukin-2, or interferon-α was allowed. Patients were assigned to receive everolimus 10 mg/d plus best supportive care (BSC) or placebo plus BSC. Randomization was stratified by Memorial Sloan-Kettering Cancer Center risk and number of prior VEGFr-TKI therapies (one vs two). Treatment continued until disease progression or unacceptable toxicity. Patients receiving placebo were allowed to cross over to the everolimus arm upon disease progression (during the blinded period of study) or at the end of the blinded study period.
2.2. Study design and outcome variables
Retrospective subgroup analyses compared efficacy and safety outcomes, including PFS, OS, reduction in tumor burden, time to deterioration of Karnofsky performance status (KPS), and the frequency and severity of AEs, in patients ≥65 and ≥70 yr of age versus the overall RECORD-1 population. Tumor measurements were performed by calculating the sum of the longest diameter of all target lesions as assessed by computed tomography or magnetic resonance imaging at baseline and every 8 wk thereafter until study discontinuation. Disease progression was assessed by a blinded independent central review committee. AEs were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, v.3.0.
2.3. Statistical analysis
Analyses were performed on the final RECORD-1 data set [6]. Subgroup analyses of efficacy were performed on the intent-to-treat population (n = 416). Subgroup analyses of safety were performed on the safety population (n = 411), which included patients who received one or more dose of the study drug with one or more valid postbaseline safety assessment. The Kaplan-Meier method was used to estimate PFS and median time to definitive worsening of KPS; the log-rank test was used to test the difference between the treatment arms. Descriptive statistics were used to compare safety outcomes. Definitive worsening was defined as a decrease in performance status by one or more Karnofsky category (ie, at least 10 points less) compared with baseline.
3. Results
3.1. Patients
Among the 416 patients enrolled in the RECORD-1 study, 36.8% were ≥65 yr and 17.5% were ≥70 yr of age. Of those ≥65 yr, 112 patients and 41 patients received everolimus or placebo, respectively. Of those ≥70 yr, 53 patients and 20 patients received everolimus or placebo, respectively. Table 1 summarizes the baseline characteristics.
Table 1.
Demographics and baseline characteristics of elderly patients and the total study population
| Age ≥65 yr | Age ≥70 yr | All patients | ||||
|---|---|---|---|---|---|---|
| Everolimus plus BSC (n = 112) |
Placebo plus BSC (n = 41) |
Everolimus plus BSC (n = 53) |
Placebo plus BSC (n = 20) |
Everolimus plus BSC (n = 277) |
Placebo plus BSC (n = 139) |
|
| Age, yr, median (range) | 69.0 (65–85) | 69.0 (65–79) | 74.0 (70–85) | 72.5 (70–79) | 61.0 (27–85) | 60.0 (29–79) |
| MSKCC risk, n (%) | ||||||
| Favorable | 25 (22) | 10 (24) | 13 (25) | 3 (15) | 81 (29) | 39 (28) |
| Intermediate | 70 (63) | 24 (59) | 33 (62) | 14 (70) | 156 (56) | 79 (57) |
| Poor | 17 (15) | 7 (17) | 7 (13) | 3 (15) | 40 (14) | 21 (15) |
| Prior VEGFr-TKI, n (%) | ||||||
| Sorafenib only | 42 (38) | 17 (42) | 18 (34) | 11 (55) | 81 (29) | 43 (31) |
| Sunitinib only | 40 (36) | 14 (34) | 17 (32) | 4 (20) | 124 (45) | 60 (43) |
| Sorafenib and sunitinib | 30 (27) | 10 (24) | 18 (34) | 5 (25) | 72 (26) | 36 (26) |
| Other prior systemic therapy, n (%) | ||||||
| Immunotherapy | 71 (63) | 29 (71) | 33 (62) | 15 (75) | 179 (65) | 93 (67) |
| Chemotherapy | 13 (12) | 7 (17) | 5 (9) | 4 (20) | 37 (13) | 22 (16) |
| Hormonal therapy | 3 (3) | 2 (5) | 2 (4) | 2 (10) | 5 (2) | 5 (4) |
| Prior surgery (nephrectomy), n (%) | 107 (96) | 40 (98) | 51 (96) | 20 (100) | 269 (97) | 133 (96) |
BSC = best supportive care; MSKCC = Memorial-Sloan Kettering Cancer Center; VEGFr-TKI = vascular endothelial growth factor receptor-tyrosine kinase inhibitor.
3.2. Efficacy
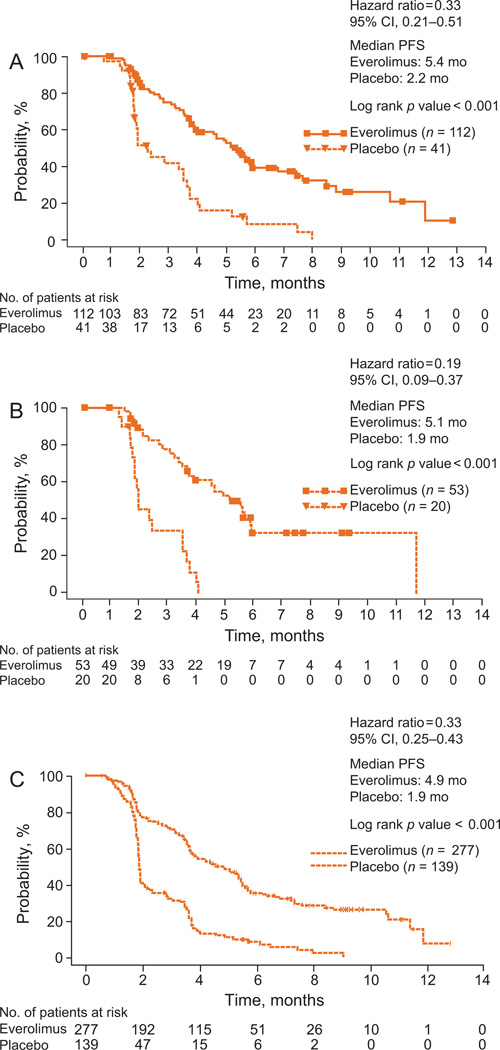
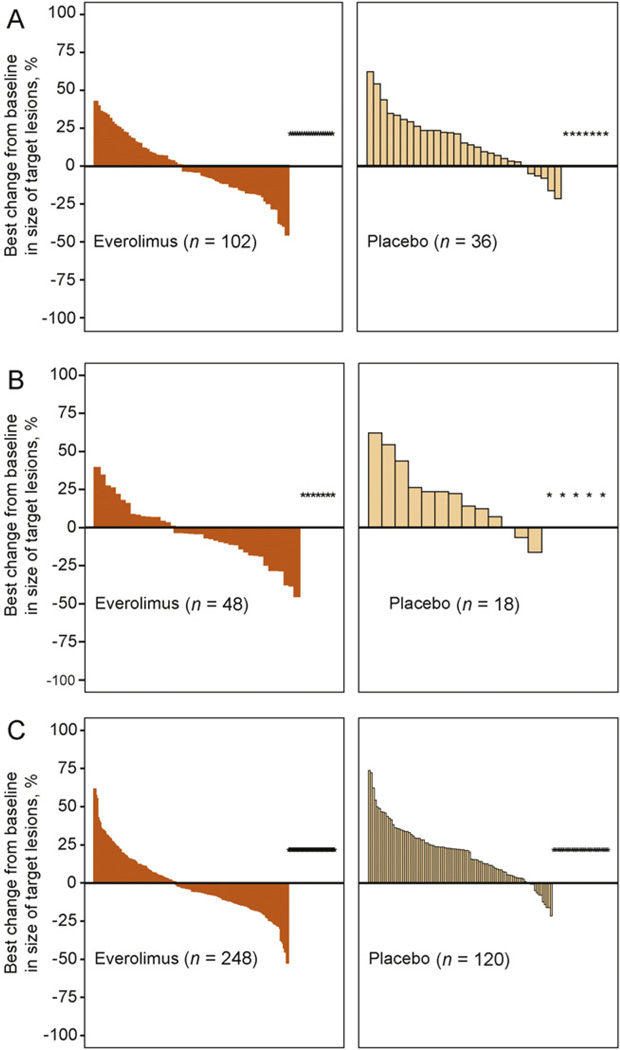
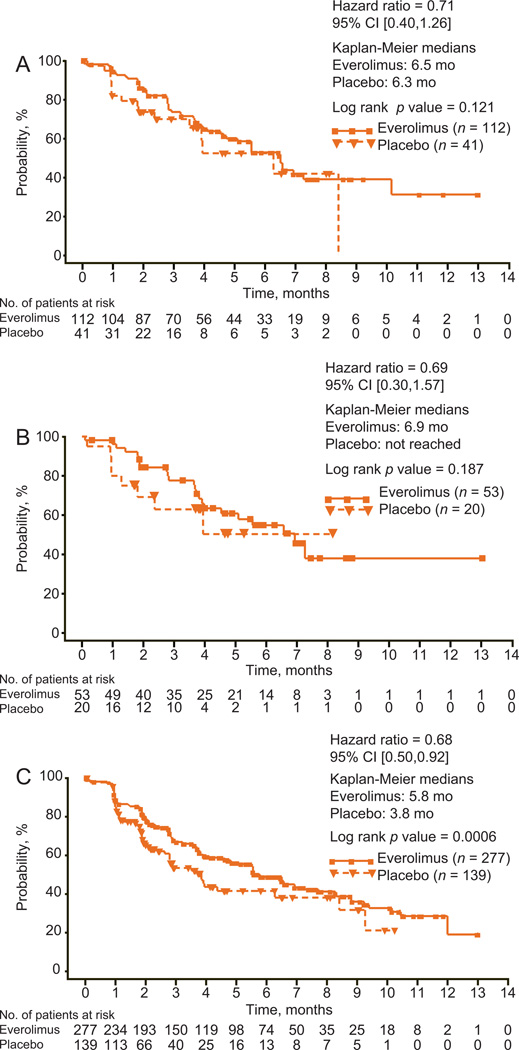
Everolimus significantly improved PFS over placebo in elderly patients, to an extent consistent with that seen in the overall study population (Fig. 1). By central review, median PFS with everolimus and placebo, respectively, was 5.4 mo versus 2.2 mo in patients ≥65 yr of age (hazard ratio [HR]: 0.33; 95% confidence interval [CI], 0.21–0.51; p < 0.001), 5.1 mo versus 1.9 mo in patients ≥70 yr of age (HR: 0.19; 95% CI, 0.09–0.37; p < 0.001), and 4.9 mo versus 1.9 mo in the overall study population (HR: 0.33; 95% CI, 0.25–0.43; p < 0.001). Measurements of best percentage change in target lesion size from baseline were comparable in elderly patients and the overall study population (Fig. 2). In patients ≥65 yr, ≥70 yr, and in all patients, 44.1%, 52.1%, and 46.9% of everolimus-treated patients and 13.9%, 11.1%, and 10% of patients receiving placebo, respectively, had a decrease in tumor burden. Although statistically significant in the overall study population, in elderly patients there was no difference in the time to definitive deterioration of KPS with everolimus versus with placebo (Fig. 3; 6.5 mo vs 6.3 mo, >65 yr; 6.9 mo vs not reached, ≥70 yr).
Fig. 1.
Kaplan-Meier estimates of progression-free survival (PFS) associated with everolimus versus placebo in (A) patients ≥65 yr, (B) patients ≥70 yr, and (C) the total study population as determined by central review. CI = confidence interval.
Fig. 2.
Best percentage change from baseline in sum of the longest tumor diameters (ΔSLD) based on central radiology review associated with the everolimus and placebo groups in (A) patients ≥65 yr of age, (B) patients ≥70 yr of age, and (C) the total study population. Patients for whom the best ΔSLD was not available, or for whom the best ΔSLD was contradicted by an unknown overall lesion response, were excluded from the analysis (everolimus vs placebo, respectively, in patients ≥70 yr [5 and 2 patients], patients ≥65 yr [10 and 5 patients], and the total study population [34 and 19 patients]. CI = confidence interval.
Fig. 3.
Kaplan-Meier estimates of time to deterioration of KPS with everolimus versus placebo in (A) patients ≥65 yr of age, (B) patients ≥70 yr of age, and (C) the total study population as determined by central review.
Objective response rates were 2.7%, 3.8%, and 1.8% in patients ≥65 yr, ≥70 yr, and in all patients, respectively, with no responses observed in any group receiving placebo (Table 2). Consistent with the overall study population, no significant difference in median OS was observed in everolimus-treated patients compared with those receiving placebo age ≥65 yr and ≥70 yr (Table 2).
Table 2.
Best overall tumor response and median overall survival in elderly patients and the total study population
| Patient population | ||||||
|---|---|---|---|---|---|---|
| Age ≥65 yr | Age ≥70 yr | All patients | ||||
| Everolimus plus BSC (n = 112) |
Placebo plus BSC (n = 41) |
Everolimus plus BSC (n = 53) |
Placebo plus BSC (n = 20) |
Everolimus plus BSC (n = 277) |
Placebo plus BSC (n = 139) |
|
| Best overall tumor response, n (%) | ||||||
| CR | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PR | 3 (2.7) | 0 (0.0) | 2 (3.8) | 0 (0.0) | 5 (1.8) | 0 (0.0) |
| SD | 77 (68.8) | 18 (43.9) | 38 (71.7) | 7 (35.0) | 185 (66.8) | 45 (32.4) |
| PD | 21 (18.8) | 18 (43.9) | 8 (15.1) | 11 (55.0) | 57 (20.6) | 74 (53.2) |
| Unknown | 11 (9.8) | 5 (12.2) | 5 (9.4) | 2 (10.0) | 30 (10.8) | 20 (14.4) |
| ORR | 3 (2.7) | 0 (0.0) | 2 (3.8) | 0 (0.0) | 5 (1.8) | 0 (0.0) |
| Overall survival, mo, median (95% CI) | 14.78 (11.96–20.27) | 16.13 (8.48–22.93) | 13.57 (9.82–21.82) | 13.63 (5.09–22.93) | 13.57 (11.96–17.87) | 13.01 (10.09–16.66) |
| HR (95% CI) | 1.07 (0.69–1.67) | 0.85 (0.47–1.55) | 0.9 (0.71–1.14) | |||
| p value | 0.381 | 0.301 | 0.183 | |||
BSC = best supportive care; CR = complete response; PR = partial response; SD = stable disease; PD = progressive disease; ORR = overall response rate; ORR = CR plus PR; CI = confidence interval; HR = hazard ratio.
3.3. Safety
Toxicities in elderly patients were similar to those in the overall study population. Stomatitis was the most common AE of any grade in everolimus-treated patients, including the elderly populations (Table 3). Some AEs, including peripheral edema, cough, rash, and diarrhea, occurred at higher rates in the elderly subgroups for both everolimus and placebo-treated patients compared with the overall study population. Rates of grade 3/4 AEs were low in the elderly and generally consistent with rates reported in all patients (Table 4). The most common grade 3/4 toxicities in patients ≥65 yr and ≥70 yr were anemia (14% and 12%, respectively), infection (11% and 12%, respectively), lymphopenia (9% and 10%, respectively), and hyperglycemia (8% in both subgroups).
Table 3.
Incidence in elderly patients and the total study population of adverse events and laboratory abnormalities (all grades)*
| Age ≥65 yr | Age ≥70 yr | All patients | ||||
|---|---|---|---|---|---|---|
| Everolimus plus BSC (n = 111) |
Placebo plus BSC (n = 39) |
Everolimus plus BSC (n = 52) |
Placebo plus BSC (n = 20) |
Everolimus plus BSC (n = 274) |
Placebo plus BSC (n = 137) |
|
| Adverse event, % | ||||||
| Stomatitis | 42 | 5 | 46 | 0 | 44 | 8 |
| Cough | 36 | 21 | 40 | 20 | 30 | 16 |
| Infection | 36 | 15 | 33 | 10 | 37 | 18 |
| Asthenia | 33 | 26 | 37 | 25 | 33 | 23 |
| Rash | 32 | 13 | 33 | 0 | 29 | 7 |
| Peripheral edema | 32 | 10 | 37 | 20 | 25 | 8 |
| Diarrhea | 32 | 8 | 38 | 10 | 30 | 7 |
| Fatigue | 29 | 26 | 37 | 20 | 31 | 27 |
| Anorexia | 28 | 10 | 27 | 10 | 25 | 14 |
| Nausea | 25 | 13 | 27 | 15 | 26 | 19 |
| Dyspnea | 23 | 8 | 23 | 5 | 24 | 15 |
| Pyrexia | 22 | 5 | 27 | 0 | 20 | 9 |
| Vomiting | 21 | 10 | 21 | 15 | 20 | 12 |
| Mucosal inflammation | 21 | 0 | 25 | 0 | 19 | 1 |
| Headache | 16 | 5 | 15 | 0 | 19 | 9 |
| Epistaxis | 16 | 0 | 15 | 0 | 18 | 0 |
| Dysgeusia | 14 | 3 | 17 | 5 | 10 | 2 |
| Pneumonitis | 11 | 0 | 10 | 0 | 14 | 0 |
| Pain in extremity | 10 | 13 | 14 | 15 | 10 | 7 |
| Pruritus | 10 | 10 | 10 | 15 | 14 | 7 |
| Dry skin | 9 | 3 | 8 | 5 | 13 | 5 |
| Laboratory abnormality, % | ||||||
| Hemoglobin decreased | 88 | 56 | 92 | 55 | 92 | 79 |
| Cholesterol increased | 71 | 39 | 71 | 30 | 77 | 35 |
| Triglycerides increased | 69 | 26 | 73 | 20 | 73 | 34 |
| Creatinine increased | 65 | 36 | 71 | 30 | 50 | 34 |
| Lymphocytes decreased | 63 | 26 | 62 | 20 | 51 | 28 |
| Glucose increased | 60 | 26 | 54 | 40 | 57 | 25 |
| Phosphate decreased | 33 | 10 | 31 | 5 | 37 | 8 |
| Platelets decreased | 31 | 0 | 27 | 0 | 23 | 2 |
| Alanine transaminase increased | 20 | 3 | 17 | 0 | 21 | 4 |
| Aspartate transaminase increased | 18 | 5 | 17 | 5 | 25 | 7 |
| Neutrophils decreased | 17 | 3 | 12 | 5 | 14 | 4 |
BSC = best supportive care.
Regardless of relation to treatment, occurring in >10% of all patients in the everolimus arm.
Table 4.
Incidence of grades 3 and 4 adverse events in elderly patients and the total study population*
| Age ≥65 yr | Age ≥70 yr | All patients | ||||
|---|---|---|---|---|---|---|
| Everolimus plus BSC (n = 111) |
Placebo plus BSC (n = 39) |
Everolimus plus BSC (n = 52) |
Placebo plus BSC (n = 20) |
Everolimus plus BSC (n = 274) |
Placebo plus BSC (n = 137) |
|
| Adverse event, % | ||||||
| Anemia | 14 | 3 | 12 | 0 | 10 | 5 |
| Infection | 11 | 3 | 12 | 0 | 10 | 1 |
| Lymphopenia | 9 | 0 | 10 | 0 | 4 | 0 |
| Hyperglycemia | 8 | 3 | 8 | 5 | 6 | 2 |
| Stomatitis | 6 | 0 | 4 | 0 | 4 | 0 |
| Fatigue | 3 | 3 | 6 | 0 | 5 | 3 |
BSC = best supportive care.
Adverse events with combined grades 3 and 4 incidence of >5% in the everolimus arm of either elderly subpopulation are shown.
Elderly patients did not experience a higher rate of noninfectious pneumonitis with everolimus: Pneumonitis of any grade was reported in 11% of patients age ≥65 yr and 10% of patients ≥70 yr, compared with 14% among all RECORD-1 patients. Grade 3 pneumonitis was reported in 2% of elderly patients (two patients ≥65 yr and one patient ≥70 yr) compared with 3.6% (n = 10) in the overall population. No grade 4 pneumonitis was observed in either elderly subpopulation. Regardless of study drug relationship, age did not affect the incidence of serious AEs (everolimus vs placebo, respectively; ≥65 yr: 37.8% vs 41.0%; ≥70 yr: 38.5% vs 35.0%; overall: 40.9% vs 47.4%). However, patients who were ≥70 yr of age had a greater occurrence of more than one dose reduction and/or interruption, as well as increased frequency of AEs resulting in dose reduction and/or interruption compared with patients ≥65 yr of age and the overall study population (Table 5). Consistent with this, the mean dose intensity was lower in everolimus-treated patients ≥70 yr compared with patients ≥65 yr and all patients. Nevertheless, median everolimus treatment duration appeared slightly longer in elderly patients. Elderly patients receiving everolimus were administered a slightly higher mean number of concomitant medications than the overall population.
Table 5.
Dose reductions, dose interruptions, and concomitant medications in elderly patients and the total study population
| Age ≥65 yr | Age ≥70 yr | All patients | ||||
|---|---|---|---|---|---|---|
| Everolimus plus BSC (n = 111) |
Placebo plus BSC (n = 39) |
Everolimus plus BSC (n = 52) |
Placebo plus BSC (n = 20) |
Everolimus plus BSC (n = 274) |
Placebo plus BSC (n = 137) |
|
| No. of dose reductions/interruptions, % | ||||||
| 0 | 56 (50.5) | 34 (87.2) | 23 (44.2) | 18 (90.0) | 147 (53.6) | 116 (84.7) |
| 1 | 30 (27.0) | 5 (12.8) | 13 (25.0) | 2 (10.0) | 75 (27.4) | 15 (10.9) |
| >1 | 25 (22.5) | 0 | 16 (30.8) | 0 | 52 (19.0) | 6 (4.4) |
| Reasons for dose reduction/interruption, % | ||||||
| Adverse event | 44 (39.6) | 3 (7.7) | 27 (51.9) | 2 (10.0) | 107 (39.1) | 12 (8.8) |
| Dosing error | 13 (11.7) | 1 (2.6) | 4 (7.7) | 0 | 27 (9.9) | 7 (5.1) |
| Laboratory test abnormality | 6 (5.4) | 1 (2.6) | 3 (5.8) | 0 | 7 (2.6) | 3 (2.2) |
| Scheduling conflict | 2 (1.8) | 0 | 2 (3.8) | 0 | 7 (2.6) | 1 (0.7) |
| Treatment duration, d | ||||||
| Median (min, max) | 157.0 (20, 451) | 84.0 (21, 284) | 150.0 (28, 402) | 111.0 (25, 237) | 141.0 (19, 451) | 60.00 (21, 295) |
| Dose intensity, mg/d | ||||||
| Mean (min, max) | 9.03 (2.7, 10.0) | 9.96 (9.3, 10.0) | 8.69 (2.7, 10.0) | 9.97 (9.4, 10.0) | 9.18 (2.7, 10.0) | 10.01 (5.0, 20.0) |
| No. of concomitant medications* | ||||||
| Mean | 15.1 | 13.3 | 15.2 | 12.0 | 13.8 | 14.2 |
| Median (min, max) | 14 (2, 41) | 11 (2, 34) | 14 (4, 39) | 11.5 (2, 27) | 12 (1, 46) | 12 (1, 55) |
Used by each patient after the start of the study drug.
4. Discussion
This retrospective study represents the first detailed report of efficacy and safety results with everolimus in elderly patients with mRCC. In RECORD-1, everolimus provided significant clinical benefit in patients ≥65 yr and ≥75 yr of age. Tumor burden reduction in elderly patients was improved with everolimus compared with placebo, consistent with the overall study population. Median PFS was prolonged and higher rates of disease stabilization were observed in patients treated with everolimus compared with placebo, regardless of age.
Retrospective analyses of other targeted therapies have reported an increase in the frequency of AEs in elderly patients with mRCC [7–9]; however, the specific AEs associated with each agent varied. The individual safety profile of an agent is an important consideration when making treatment decisions in elderly patients, especially when comorbidities are present. The toxicity profiles of VEGF-targeted therapies differ significantly from those of mTOR inhibitors. The most common grade 3/4 AEs associated with the VEGFr-TKI sunitinib in a pivotal study were hypertension (8%), fatigue (7%), diarrhea (5%), and hand-foot syndrome (5%) [10]. In an expanded-access study, severe toxicity requiring dose reduction or discontinuation of sunitinib significantly correlated with increased age (p = 0.006) [7]. In a pivotal trial of sorafenib, the most common grade 3/4 AEs were hand-foot skin reaction (6%), hypertension (4%), diarrhea (3%), and fatigue (3%) [11]. A subgroup analysis of elderly patients in this study demonstrated higher rates of grade 3 AEs with sorafenib in patients ≥70 yr of age (40% vs 29% in patients <70 yr), although the incidence of grade 4 events was similar (6% vs 7%, respectively) [8]. However, in an expanded-access study, the incidence of grade 3/4 AEs associated with sorafenib was similar in patients ≥65 and <65 yr, although fatigue and rash/desquamation occurred more frequently in patients ≥65 yr (8% vs 4% and 6% vs 4% in patients <65 yr, respectively) [12]. In a trial of bevacizumab plus interferon-α, bevacizumab-associated grade 3/4 toxicity included hypertension (10%), anorexia (17%), fatigue (37%), and proteinuria (15%) [13], with serious AEs occurring more frequently in patients ≥65 yr (40%) versus <65 yr (23%) [9]. Not surprisingly, the safety profile of the mTOR inhibitor temsirolimus is similar to everolimus. The most common grade 3/4 AEs in temsirolimus-treated patients were anemia (20%), asthenia (11%), hyperglycemia (11%), and dyspnea (9%) [14]. Age had little effect on the incidence of grade 3/4 toxicities [15].
In RECORD-1, everolimus was well tolerated in the elderly, with a toxicity profile similar to that observed in younger patients. The most common grade 3/4 AEs were anemia (12% and 14% in patients ≥65 and ≥70 yr) and infection (11% and 12% in patients ≥65 and ≥70 yr). The frequency of several AEs including peripheral edema, cough, rash, and diarrhea were higher in the elderly; however, these AEs were more frequent both in patients receiving everolimus and placebo and were generally manageable. No increases in grade 3/4 AEs were observed in elderly patients compared with the overall population, and notably, elderly patients also did not appear to have an increased risk of developing noninfectious pneumonitis compared with younger patients.
This study has several limitations. Analyses were retrospective, and they were not designed to allow statistical comparison across the different elderly subpopulations. Patients were not stratified by age; thus an imbalance in subgroups is possible. Finally, selection of previous therapy was at the physician’s discretion and may have been biased by previous experience with sorafenib and sunitinib in elderly patients.
5. Conclusions
In RECORD-1, everolimus provided clinical benefit over placebo in elderly patients and the overall study population. Age had no apparent detrimental effect on PFS or reduction in tumor burden observed with everolimus. Everolimus was well tolerated in elderly patients and the overall study population, with low rates of grade 3/4 AEs. Some toxicities including peripheral edema, cough, rash, and diarrhea were reported more frequently in elderly patients, irrespective of treatment; however, no increase in everolimus-related pneumonitis was observed compared with younger patients. Elderly patients have a higher obvious incidence of comorbidities, and many are unable to tolerate therapeutic regimens appropriate for the general mRCC population. With a favorable safety and efficacy profile, everolimus may be considered a suitable targeted agent in the treatment of relatively healthy elderly patients with mRCC.
Acknowledgment statement
The authors acknowledge editorial assistance provided by ApotheCom (Yardley, PA, USA).
I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Camillo Porta has received honoraria from Novartis, Bayer-Schering, Pfizer, Hoffman La Roche, and GlaxoSmithKline and research funding from Novartis and Bayer-Schering. Emiliano Calvo has served as a consultant to and received honoraria from Novartis and Pfizer and investigational grants from Pfizer, Novartis, Roche, GlaxoSmithKline, and Bayer. Ulka Vaishampayan has received honoraria and research funding from Novartis. Alain Ravaud is a member of global, European, and/or French boards on urologic tumors for Pfizer, Novartis, GlaxoSmithKline, Bayer-Schering, and Dendreon, and he has received institutional grant support from Pfizer, Novartis, and Roche. Sergio Bracarda has served as an advisory board member to Pfizer, GlaxoSmithKline, Novartis, and Bayer, and he has received honoraria from Pfizer and Novartis. Thomas E. Hutson has served as a consultant to and received honoraria and research funding from Pfizer, Bayer, Novartis, GlaxoSmithKline, and Genentech. Bernard Escudier has served as a consultant to and received honoraria from Bayer, Pfizer, Roche, Novartis, and GlaxoSmithKline. Viktor Grunwald has served as a consultant to Roche, Bayer, Novartis, Pfizer, GlaxoSmithKline, and Aveo/Astellas, has received honoraria from GlaxoSmithKline, Novartis, and Pfizer, and has received research funding from Pfizer and GlaxoSmithKline. Dennis Kim is an employee and owns stock in Novartis. Ashok Panneerselvam and Oezlem Anak are employees of Novartis. Robert J. Motzer has served as a consultant to and received honoraria from Pfizer, and he has received research funding from Novartis, Pfizer, GlaxoSmithKline, Aveo, and Bristol-Myers Squibb.
Funding/Support and role of the sponsor: Novartis Pharmaceuticals Corporation helped design and conduct the study, manage, analyze, and interpret the data, and prepare, review, and approve the manuscript.
Footnotes
Author contributions: Camillo Porta had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Porta, Hutson, Motzer, Kim.
Acquisition of data: Porta, Calvo, Climent, Vaishampayan, Osanto, Ravaud, Bracarda, Hutson, Escudier, Grunwald, Motzer.
Analysis and interpretation of data: Porta, Calvo, Climent, Vaishampayan, Osanto, Ravaud, Bracarda, Hutson, Escudier, Grunwald, Kim, Panneerselvam, Anak, Motzer.
Drafting of the manuscript: Porta, Calvo, Climent, Vaishampayan, Osanto, Ravaud, Bracarda, Hutson, Escudier, Grunwald, Kim, Panneerselvam, Anak, Motzer.
Critical revision of the manuscript for important intellectual content: Porta, Calvo, Climent, Vaishampayan, Osanto, Ravaud, Bracarda, Hutson, Escudier, Grunwald, Kim, Panneerselvam, Anak, Motzer.
Statistical analysis: Panneerselvam.
Obtaining funding: Kim, Anak.
Administrative, technical, or material support: None.
Supervision: None.
Other (specify): None.
Financial disclosures: Miguel A. Climent has nothing to disclose. Susanne Osanto has nothing to disclose.
References
- 1.Aron M, Nguyen MM, Stein RJ, Gill IS. Impact of gender in renal cell carcinoma: an analysis of the SEER database. Eur Urol. 2008;54:133–142. doi: 10.1016/j.eururo.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Coebergh JW, Janssen-Heijnen ML, Razenberg PP. Prevalence of comorbidity in newly diagnosed patients with cancer: a population-based study. Crit Rev Oncol Hematol. 1998;27:97–100. doi: 10.1016/s1040-8428(97)10011-7. [DOI] [PubMed] [Google Scholar]
- 3.Repetto L, Comandini D. Cancer in the elderly: assessing patients for fitness. Crit Rev Oncol Hematol. 2000;35:155–160. doi: 10.1016/s1040-8428(00)00091-3. [DOI] [PubMed] [Google Scholar]
- 4.Repetto L, Venturino A, Vercelli M, et al. Performance status and comorbidity in elderly cancer patients compared with young patients with neoplasia and elderly patients without neoplastic conditions. Cancer. 1998;82:760–765. [PubMed] [Google Scholar]
- 5.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116:4256–4265. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 7.van der Veldt AA, Boven E, Helgason HH, et al. Predictive factors for severe toxicity of sunitinib in unselected patients with advanced renal cell cancer. Br J Cancer. 2008;99:259–265. doi: 10.1038/sj.bjc.6604456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisen T, Oudard S, Szczylik C, et al. Sorafenib for older patients with renal cell carcinoma: subset analysis from a randomized trial. J Natl Cancer Inst. 2008;100:1454–1463. doi: 10.1093/jnci/djn319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escudier BJ, Ravaud A, Bracarda S, et al. Efficacy and safety of first-line bevacizumab (BEV) plus interferon-a2a (IFN) in subgroups of patients (pts) with metastatic renal cell carcinoma (mRCC); Paper presented at: American Society of Clinical Oncology Genitourinary Cancers Symposium; February 14–16, 2008; San Francisco, CA, USA. [Google Scholar]
- 10.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 12.Bukowski RM, Stadler WM, Figlin RA, et al. Safety and efficacy of sorafenib in elderly patients >65 years: a subset analysis from the Advanced Renal Carcinoma Sorafenib (ARCCS) expanded access program in North America [abstract 5045] J Clin Oncol. 2008;26(Suppl) [Google Scholar]
- 13.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol. 2010;28:2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 15.Dutcher JP, Szczylik N, Tannir N, et al. Correlation of survival with tumor histology, age and prognostic risk group for previously untreated patients with advanced renal cell carcinoma receiving temsirolimus or interferon-alpha [abstract 5033] J Clin Oncol. 2007;25:18S. [Google Scholar]