Abstract
BACKGROUND
Epidermal growth factor receptor overexpression is associated with poor outcomes in urothelial carcinoma (UC). Cetuximab (CTX) exhibited an antitumor effect in in vivo UC models. The efficacy of gemcitabine/cisplatin (GC) with or without CTX in patients with advanced UC was evaluated.
METHODS
Patients with advanced UC, measurable disease, and adequate organ function were randomized 1:2 to cisplatin (70 mg/m2) on day 1 plus gemcitabine (1000 mg/m2) on days 1, 8, and 15 (arm A) or GC plus CTX (500 mg/m2) on days 1 and 15 (arm B). The primary endpoint was the overall response rate. The secondary endpoints were the response duration, safety, progression-free survival, overall survival, determination of whether or not CTX sensitized nonresponders to GC, and exploratory biomarker analysis. The accrual targets were 27 and 54 patients for the 2 arms, respectively. The overall response rate was reported by arm with binomial confidence intervals (CIs). Kaplan-Meier methods were used for time-to-event endpoints.
RESULTS
Eighty-eight eligible patients were randomized; 87 were toxicity-evaluable, and 85 were response-evaluable. The overall response rates were 57.1% for arm A (95% CI = 37%–76%) and 61.4% for arm B (95% CI = 48%–74%). The median progression-free survival times were 8.5 months for arm A (95% CI = 5.7–10.4 months) and 7.6 months for arm B (95% CI = 6.1–8.7 months). The median overall survival times were 17.4 months for arm A (95% CI = 12.8 months to unreached) and 14.3 months for arm B (95% CI = 11.6–22.2 months). The most common grade 3/grade 4 adverse events in both arms were myelosuppression and nausea. Thromboembolism, acneiform rash, fatigue, pain, hypersensitivity reactions, elevated transaminases, hyponatremia, and hypomagnesemia were more common in arm B; 3 grade 5 adverse events occurred in arm B. The presence of primary disease significantly correlated with thromboembolism. An increased soluble E-cadherin level after cycle 2 correlated with a higher risk of death.
CONCLUSIONS
GC plus CTX was feasible but was associated with more adverse events and no improvements in outcomes.
Keywords: urothelial carcinoma, chemotherapy, gemcitabine, cetuximab, cisplatin
INTRODUCTION
Gemcitabine/cisplatin (GC) is standard first-line chemotherapy for advanced urothelial carcinoma (UC) patients with an approximately 50% objective response rate, a time to progression of 7 to 8 months, a median survival time of 14 to 15 months, and a 5-year overall survival (OS) rate of 13% to 15%.1
Epidermal growth factor receptor (EGFR) overexpression has been described in solid tumors, including UC.2–4 EGFR signaling functions as an oncogene promoting cell proliferation, differentiation, survival, invasion, angiogenesis, and metastasis.5,6 In UC, EGFR overexpression in the primary tumor has been associated with higher tumor grade, stage, disease progression, and shorter survival.7–14 UC metastases frequently overexpress EGFR protein: a study reported 85% EGFR membranous expression (65% overexpression) in metastatic sites with a relatively high concordance expression pattern between primary and metastatic specimens.11 In contrast to colorectal cancer, KRAS mutations are rare in UC (0%–5%).15–19 Thus, the KRAS mutation status is not expected to modulate the response to EGFR targeting. In vitro data indicate that EGFR is activated in response to chemotherapy attenuating apoptosis.20 An EGFR tyrosine kinase inhibitor (TKI), gefitinib, inhibited chemotherapy-induced EGFR activation in bladder cancer cell lines; a combined treatment with gefitinib/etoposide significantly increased apoptosis in comparison with etoposide alone.20 Synergy has been shown between GC and lapatinib, an anti-EGFR/human epidermal growth factor receptor 2 (HER2) TKI.21 These results suggest that a combination of EGFR blockade with chemotherapy may overcome drug resistance, and this is further supported by clinical data for other solid tumors.22,23
Cetuximab (CTX) is a recombinant, human/murine–chimeric monoclonal antibody that specifically binds the EGFR extracellular domain, and this results in EGFR inhibition and downregulation of downstream signaling pathways. It is Food and Drug Administration–approved for other cancers.22,23 CTX exerts dose-dependent cytostatic effects on bladder cancer cell lines and is associated with dose-dependent downregulation of angiogenic factors and significant regression of established UC xenografts in athymic mice; this provides supportive evidence for its clinical investigation in patients with UC.24
On the basis of these data, we hypothesized that targeting EGFR with CTX would result in higher response rates and longer times to progression when it was added to GC.
MATERIALS AND METHODS
Key Eligibility Criteria
Eligible patients had a pathological diagnosis of UC (pure or mixed histology) from primary, metastatic, locally recurrent, or locally advanced/unresectable (T4bN0 or TanyN2–3) and measurable bladder or nonbladder disease on imaging; an Eastern Cooperative Oncology Group performance status ≤ 2; a life expectancy ≥ 12 weeks; and adequate organ function (absolute neutrophil count ≥ 1500 µL, platelet count ≥ 1500 µL, serum creatinine level ≤ 1.5 mg/dL or creatinine clearance ≥ 50 mL/min, and total bilirubin level ≤ 1.5 mg/dL). Prior neoadjuvant or adjuvant chemotherapy was allowed if 6 months or more had passed since a non–cisplatin-based regimen or 1 year or more had passed since a cisplatin-based regimen. Patients with an asymptomatic pulmonary embolus or deep vein thrombosis (DVT) were eligible if they were on anticoagulation and at the physician’s discretion. Because of the lack of conclusive data regarding its predictive value in UC and other carcinomas for which CTX has Food and Drug Administration approval, patients were not preselected on the basis of the status of the EGFR expression level. The study protocol was approved by the institutional review board of each participating center, and all patients provided written informed consent before study entry. The study was registered with ClinicalTrials.gov (identifier NCT00645593).
Treatment Plan
Patients were stratified by disease status (unresectable versus recurrent/metastatic) and randomized 1:2 to receive GC (arm A) or GC plus CTX (arm B). The starting therapy for both arms was as follows: gemcitabine (1000 mg/m2) on days 1, 8, and 15 and cisplatin (70 mg/m2) on day 1, every 28 days. Because of a higher than expected thromboembolic event (TEE) rate in arm B, the gemcitabine dose for arm B was decreased to 800 mg/m2 after 42 patients had enrolled in arm B (and 19 patients in arm A). Patients were also required to start baby aspirin (once daily). Arm B patients received CTX (500 mg/m2) on days 1 and 15. Arm A patients who had progressed after 2 cycles (at the first efficacy evaluation) could have CTX added to GC. After 6 treatment cycles, chemotherapy was stopped in both arms; however, arm B patients without disease progression continued on CTX until progression or unacceptable toxicity if it had been deemed clinically beneficial. Arm B patients who had completed ≥4 GC cycles, had achieved stable disease or better, were tolerating CTX, but were deemed not able to tolerate additional GC could proceed to maintenance CTX until disease progression or unacceptable toxicity. Patients in either arm with locally advanced disease who responded to treatment were offered local therapy as deemed medically appropriate. Arm B patients who received local therapy discontinued CTX. Patients in either arm who elected not to receive or were not candidates for local therapy could continue the protocol therapy.
Patient Evaluation
The baseline evaluation included a complete history/physical examination, a comprehensive metabolic profile, magnesium, and a complete blood count. C-reactive protein (CRP) and D-dimer baseline levels were added to the study after 37 patients had been enrolled to assess any correlation with TEEs. Baseline imaging included abdominal/ pelvic computed tomography or magnetic resonance imaging, chest X-ray or computed tomography, and a radionucleotide bone scan. Imaging was repeated every 8 weeks while patients were on the study therapy. After the completion of the study therapy, imaging was required every 12 ± 2 weeks for the first 24 weeks and then every 16 weeks for a year. Thereafter, imaging was required every 6 months for years 3 to 5 and otherwise as clinically indicated until progression was documented. The Common Terminology Criteria for Adverse Events (version 3.0) of the National Cancer Institute were used for adverse event reporting.
Correlative Studies
Serum soluble E-cadherin (sE-cad) was evaluated as a potential predictor of outcome; this was based on data suggesting that a sensitivity to EGFR inhibition requires E-cadherin expression in UC cells.25 E-cadherin is expressed in epithelial cells, and data suggest that EGFR could mediate E-cadherin proteolysis and the production of sE-cad.26,27 We hypothesized that patients with advanced UC would have decreased sE-cad levels in response to anti-EGFR therapy and that this would correlate with a higher response rate. Serum was collected at the baseline, at the end of cycle 2, at the completion of chemotherapy, and at disease progression so that the sE-cad level could be measured. Blood was drawn in a standard serum separator tube and was spun at 1300g for 10 minutes. Serum was harvested immediately in labeled cryovials (Fisher Scientific) and stored at −80°C. sE-cad was measured with an enzyme-linked immunosorbent assay (R&D Systems Quantikine kit) according to the manufacturer’s instructions. In addition, baseline CRP and D-dimer levels were measured to evaluate any correlation with TEEs.
Statistical Considerations
The primary endpoint was the overall response rate (ORR; ie, Complete Response (CR) + Partial Response (PR), which was defined as the best confirmed response at any time point during the trial in accordance with the Response Evaluation Criteria in Solid Tumors (version 1.0).28 Secondary endpoints included the response duration, safety, progression-free survival (PFS), OS, and ORR after crossover to CTX in patients progressing on chemotherapy alone.
This was a randomized phase 2 trial with patients randomized 1:2 to GC and GC plus CTX. Historical response rates with GC are variable and depend on the extent of disease and sites; hence, this design ensured a relevant comparison group by including a control arm and allowed more experience to be gained with the experimental agent (CTX). It was hypothesized that adding CTX to chemotherapy would increase ORR by 15%. The randomized selection design was used to compare treatment regimens.29 Under the assumption that chemotherapy (control) would result in a 50% ORR and that a difference in ORR of 15% (an experimental arm with an ORR ≥ 65%) would be clinically meaningful and with the use of a 1:2 randomization schema, it was estimated that 27 patients would need to be randomized to the control arm and 54 would need to be randomized to the experimental arm to result in a 90% probability that the arm with the higher ORR would be found. The primary endpoint of best ORR is reported for each arm with associated 95% binomial confidence intervals (CIs). Descriptive proportions with frequencies and mean ages with age ranges are reported. Median PFS values, OS values, and response durations are reported with product-limit estimates from Kaplan-Meier methods with corresponding 95% CIs and log-rank tests. Tested toxicity comparisons are reported with mid P values.
Exploratory correlative analyses of sE-cad levels were completed. Enzyme-linked immunosorbent assay triplicates were averaged for each sample. Differences from the baseline (before treatment began) were calculated for each sample taken after treatment had started (after cycle 2, at the end of chemotherapy, and at disease progression). PFS and OS associations with the baseline sE-cad level and the sE-cad level change after cycle 2, at the end of chemotherapy, and at progression in comparison with the baseline were individually tested with Coxmodels. When a statistically significant association was found, further exploration with multivariate models was performed. To determine whether sE-cad was predictive or prognostic, interactions of the sE-cad level and the treatment were tested in the models.
RESULTS
Baseline Characteristics and Treatment Summary (Tables 1 and 2)
TABLE 1.
Baseline Characteristics of Eligible Patients (n = 88)
| Arm A: Gemcitabine/ Cisplatin (n = 28) |
Arm B: Gemcitabine/ Cisplatin + Cetuximab (n = 60) |
|
|---|---|---|
| Sex, n (%) | ||
| Male | 23 (82) | 46 (77) |
| Female | 5 (18) | 14 (23) |
| Age, ya | 65.8 (41–79.9) | 60.9 (32.8–79.4) |
| ECOG performance status, n (%) | ||
| 0 | 18 (64) | 33 (55) |
| 1 | 8 (29) | 26 (43) |
| 2 | 2 (7) | 1 (2) |
| Bladder primary, n (%) | 20 (71) | 45 (75) |
| Renal pelvis, n (%) | 5 (18) | 12 (20) |
| Ureter, n (%) | 2 (7) | 2 (3) |
| Urethral, n (%) | 1 (4) | 1 (2) |
| Distant metastasis, n (%) | 26 (93) | 54 (90) |
| Liver only ± lymph nodes | 3 (12) | 9 (17) |
| Lung only ± lymph nodes | 3 (12) | 11 (20) |
| Bone only ± lymph nodes | 1 (4) | 4 (7) |
| Lymph nodes only | 7 (27) | 16 (30) |
| Multiple metastatic sites | 12 (46) | 14 (26) |
| Local recurrence, n (%) | 1 (3.5) | 2 (3) |
| Unresectable disease, n (%) | 1 (3.5) | 4 (7) |
| Prior cystectomy or nephroureterectomy, n (%) | 10 (36) | 13 (22) |
| Primary in place, n (%) | 18 (64) | 47 (78) |
| Prior neoadjuvant or adjuvant chemotherapy, n (%) | 6 (21.4) | 8 (13.3) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
The data are presented as medians and ranges.
TABLE 2.
Treatment Summary (n = 88)
| Gemcitabine/ Cisplatin (n = 28) |
Gemcitabine/ Cisplatin + Cetuximab (n = 60) |
|
|---|---|---|
| Eligible, n | 28 | 60 |
| Toxicity evaluable, n | 28 | 59a |
| Efficacy evaluable, n | 28 | 57b |
| Number of chemotherapy cycles, n (%)c | ||
| <4 | 4 (14) | 15 (25) |
| 4 | 3 (11) | 13 (22) |
| 5 | 1 (4) | 3 (5) |
| 6 | 20 (71) | 28 (47) |
| Dose intensity per cycle, mg/m2d | ||
| Gemcitabine | 2267 ± 459.8 | 2066 ± 602.5 |
| Cisplatin | 65 ± 8.1 | 65 ± 10.8 |
| Patients on cetuximab maintenance, n (%) | 26 (44) | |
| Cycles, ne | NA | 3 (1–36) |
| Patients crossed over to cetuximab, n | 1f | — |
| Patients off therapy because of toxicity/change in condition at any time during study, n (%) | 3 (10.7) | 10 (16.9) |
Abbreviation: NA, not applicable.
One patient withdrew consent before treatment was started.
Two patients withdrew consent before they had completed 4 weeks of therapy.
There were 87 evaluable patients.
The data are presented as means and standard deviations.
The data are presented as medians and ranges.
Thirty-two cycles of cetuximab.
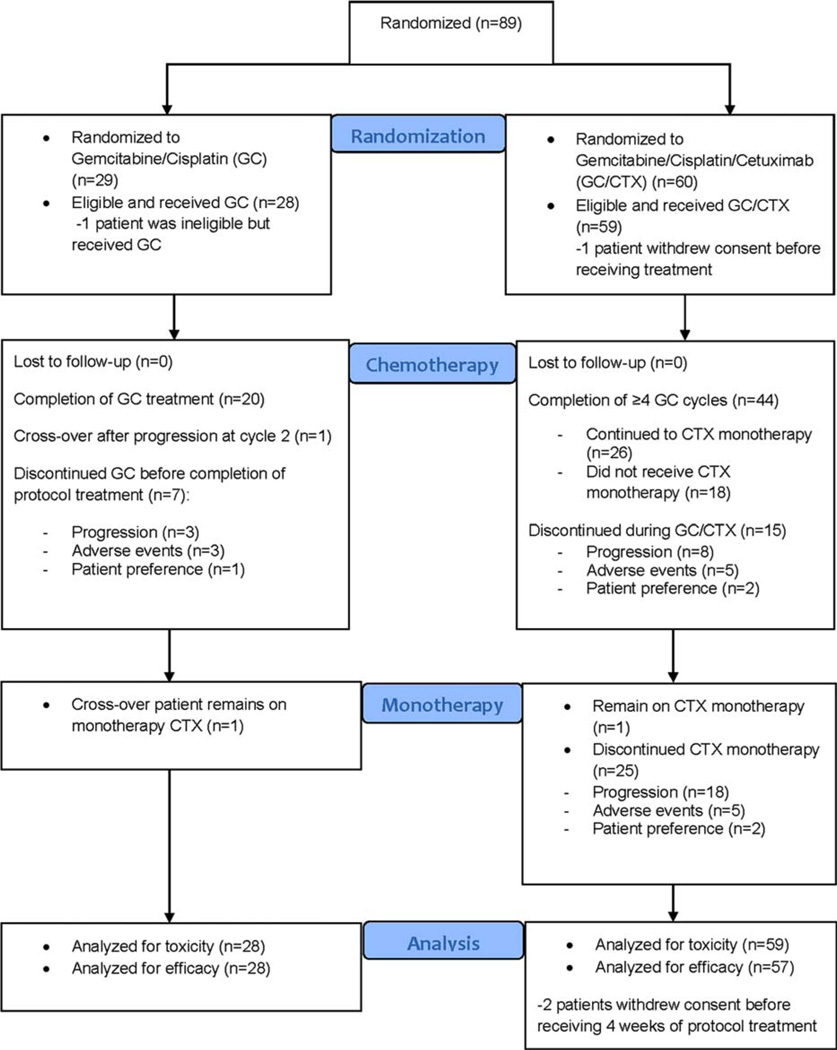
Between June 2008 and January 2011, 89 patients were accrued from 12 institutions; 88 of the 89 eligible patients were randomized (1 patient was randomized to arm A, treated, and developed grade 4 [G4] neutropenia but was found to be ineligible upon histology review and was removed from the study). Follow-up continued until data cutoff in May 2013. Patient characteristics were well balanced between the arms. More than 70% of the patients had bladder primary, and approximately 90% had systemic metastasis. Before receiving treatment, 1 patient randomized to arm B withdrew consent; thus, 87 patients were evaluable for toxicity (28 in arm A and 59 in arm B). After receiving <4 weeks of treatment, 2 patients in arm B withdrew consent (patients’ wishes) and thus were not evaluable with respect to their response (Fig. 1 and Table 2). The median number of treatment cycles was 6 in arm A (71% of the patients received 6 cycles) and 5 in arm B (47% of the patients received 6 cycles); the median duration of treatment (including maintenance CTX) was approximately 5 months for both arms (Table 2). The mean dose intensities of GC per cycle were similar in the 2 arms (Table 2). In arm B, 26 patients (44%) continued on CTX monotherapy with a median of 3 cycles (1–36); 8 of them received >6 cycles of CTX maintenance. Only 1 patient in arm A (bladder primary and metastases in the lymph nodes, liver, and lung) had disease progression after 2 chemotherapy cycles and was eligible to receive CTX; she received 32 cycles of CTX and was still on treatment at the time of the last follow-up.
Figure 1.
Consolidated Standards of Reporting Trials flow diagram. GC indicates gemcitabine/cisplatin; CTX, cetuximab.
Safety and Tolerability
Table 3 summarizes the most frequent (>5% of eligible patients) G3 to G5 adverse events. The most common G3/G4 adverse events in both arms were myelosuppression and nausea. In arm B, 17 patients (29%) experienced G3/G4 TEEs, whereas 3 patients (11%) in arm A did (P = .08). Nineteen patients with G3/G4 TEEs had an unresected primary tumor; only 1 patient with prior primary tumor resection had a TEE (P = .01). In arm A, 17% of the patients with an unresected primary tumor had G3/G4 TEEs, whereas 0% with a resected primary tumor did; 35% and 8%, respectively, had G3/G4 TEEs in arm B (P = .01). No association was found between the metastatic site and G3/G4 TEEs (P = .44). Four of 17 G3/G4 TEEs in arm B occurred after the amendment that decreased the gemcitabine dose. The G3/G4 TEE proportions in arm B were 30% before the amendment and 24% after the amendment. In all, there were 20 G3/G4 TEEs in the study: 7 were lower extremity DVT, 10 were pulmonary embolisms (with or without DVT), 1 was port-related right jugular vein thrombosis, 1 was non–port/catheter-related left upper extremity vein thrombosis, and 1 was left lower limb ischemia.
TABLE 3.
Treatment-Related Grade 3 to 5 Adverse Events (>5% of Toxicity-Evaluable Patients)a
| Adverse Event | Arm A: Gemcitabine/ Cisplatin (n = 28) Grade 3/Grade 4 |
Arm B: Gemcitabine/ Cisplatin + Cetuximab (n = 59) Grade 3/Grade 4/Grade 5 |
|---|---|---|
| All grade 3/4+ toxicities | 75 | 83 |
| Neutropeniab | 32.1/10.7 | 27.1/13.6/0 |
| Thrombocytopenia | 28.6/10.7 | 15.3/10.2/0 |
| Leukopenia | 17.9/3.6 | 27.1/3.4/0 |
| Anemia | 10.7/0 | 8.5/0/0 |
| Lymphopenia | 3.6/0 | 8.5/0/0 |
| Nausea | 7.1/0 | 8.5/0/0 |
| Thromboembolism | 7.1/3.6 | 13.6/15.3/0 |
| Acneiform rash | 0/0 | 25.4/0/0 |
| Fatigue | 7.1/0 | 13.6/0/0 |
| Hyponatremia | 3.6/0 | 10.2/0/0 |
| Hypomagnesemia | 0/0 | 8.5/3.4/0 |
| Elevated transaminases | 0/0 | 5.1/0/0 |
| Infection | 7.2/0 | 6.8/1.7/0 |
| Neuropathy | 3.6/0 | 5.1/0/0 |
| Hypersensitivity reaction | 0/0 | 5.1/0/0 |
| Pain | 0/0 | 8.5/0/0 |
| Pneumonitis (lung infiltrates) | 3.6/0 | 1.7/0/1.7c |
| Sudden (unexpected) death | 0/0 | 0/0/1.7d |
| Myocardial infarction | 0/0 | 0/0/1.7 |
The data are presented as percentages.
Two patients in arm A and 3 patients in arm B had febrile neutropenia.
One patient died of respiratory failure 5 months after an unresolved episode of pneumonitis.
One patient died suddenly and unexpectedly 1 week after cycle 2 (day 1) of therapy.
G3/G4 acneiform rash, fatigue, hypersensitivity reactions, pain, elevated transaminases, hyponatremia, and hypomagnesemia were more common in arm B (Table 3). Five patients discontinued CTX monotherapy because of adverse events: 3 patients had skin toxicity, another had G4 left lower limb ischemia due to severe peripheral vascular atherosclerosis (an adverse event was also deemed possibly related to CTX), and another patient with no documented baseline pulmonary pathology developed G4 interstitial pneumonitis that was deemed possibly related to CTX because no other etiology was identified. There were 3 treatment-related deaths, all in arm B (5%, 95% CI = 0.01–0.14). The patient with pneumonitis improved but ultimately died of ongoing/worsening respiratory failure 5 months later with no evidence of disease recurrence. Another patient died suddenly 1 week after cycle 2 of therapy (day 1); this was unexpected without an identified cause and was deemed possibly related to therapy. A third patient died of acute myocardial infraction 4 days after cycle 3 (day 1) of therapy; he had hypertension and coronary artery disease (undiagnosed at the time), and this event was deemed to be probably related to therapy.
Efficacy Analysis
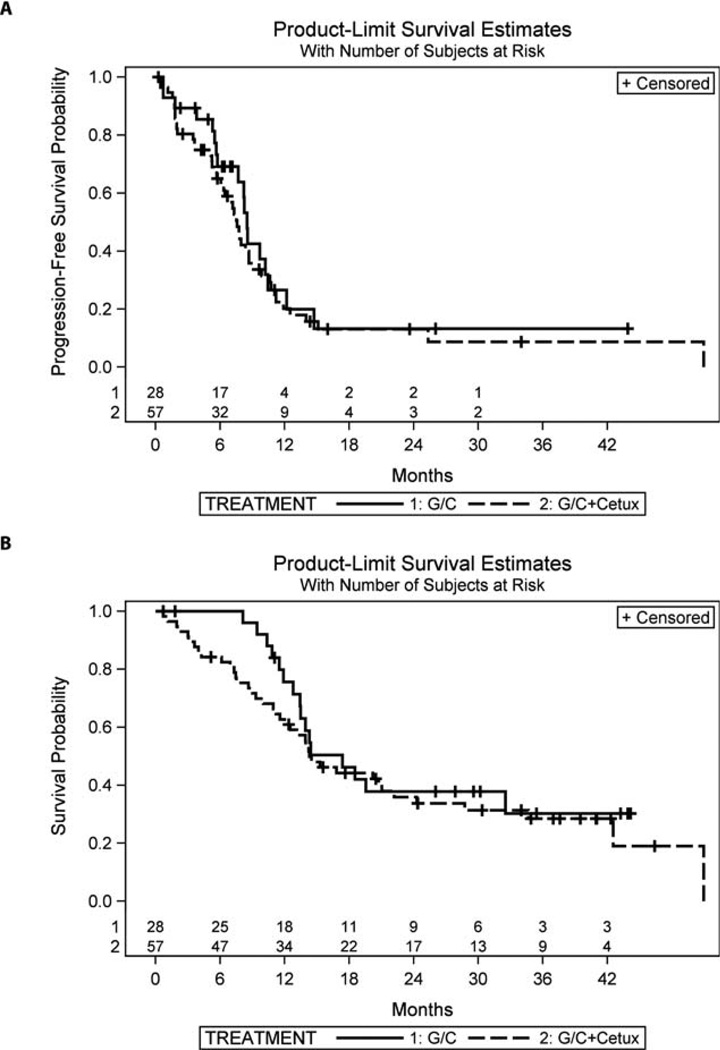
ORR was 57% in arm A and 61% in arm B with overlapping 95% CIs (Table 4). ORR in arm B did not significantly change after gemcitabine dose adjustments (26/41 [63.4%] before and 9/16 [56.3%] after, P = .76). In addition, 32% (arm A) and 19% (arm B) had the best response of stable disease. The median duration of stable disease was similar for the 2 arms: 8.5 months for arm A and 8.7 months for arm B. The median PFS was 8.5 months (95% CI = 5.7–10.4 months) in arm A and 7.6 months (95% CI = 6.1–8.7 months) in arm B (P = .47; Fig. 2A and Table 4). The median survival time was 17.4 months (95% CI = 12.8 months to not reached) in arm A and 14.3 months (95% CI = 11.6–22.2 months) in arm B (P = .43; Fig. 2B and Table 4). One patient in arm A and 1 (despite lymph node progression) of 4 patients in arm B with an unresectable tumor underwent surgical resection after chemotherapy. One patient (arm B) was receiving CTX monotherapy without progression at the time of the last follow-up. The patient who progressed on chemotherapy in arm A and had CTX added achieved long-term disease control and was continuing on CTX monotherapy at the time of the last follow-up.
TABLE 4.
Overall Response Rate, Progression-Free Survival, and Overall Survival of the Evaluable Patients (n = 85)
| Endpoint | Gemcitabine/Cisplatin (n = 28) | Gemcitabine/Cisplatin + Cetuximab (n = 57) | P |
|---|---|---|---|
| Overall response rate, %a | 57.1 (37–76) | 61.4 (48–74) | .81 |
| Best confirmed response, n (%) | .71 | ||
| Complete Response (CR) | 3 (10.7) | 2 (3.5) | |
| Partial Response (PR) | 13 (46.4) | 33 (57.9) | |
| Stable Disease (SD) | 9 (32.1) | 11 (19.3) | |
| Median progression-free survival, moa | 8.5 (5.7–10.4) | 7.6 (6.1–8.7) | .47 |
| Median survival, moa | 17.4 (12.8 to not reached) | 14.3 (11.6–22.2) | .43 |
The values in parentheses are 95% confidence intervals.
Figure 2.
Kaplan-Meier curves: (A) progression-free survival and (B) overall survival. G/C indicates gemcitabine/cisplatin; Cetux, cetuximab.
Serum sE-cad
The baseline sE-cad levels did not differ between the arms (arm A, median = 43.0 ng/mL [range = 27–511 ng/mL]; arm B, median = 43.6 ng/mL [range = 20–147 ng/mL]; P = .57) and did not correlate with ORR, PFS, or OS. The sE-cad level change versus the baseline differed with the treatment arm after cycle 2 (arm A, median = 43 [range = 26–91]; arm B, median = 40 [range = 16–228]; paired-difference P = .009), at the completion of chemotherapy (arm A, median = 48 [range = 26–80]; arm B, median = 40 [range = 25–71]; paired-difference P = .036), and at progression (arm A, median = 54 [range = 34–73]; arm B, median = 37 [range = 25–80]; paired-difference P = .01); the sE-cad level increased with treatment in arm A but decreased in arm B (Fig. 3). In the single patient who crossed over, the sE-cad level increased after 2 cycles of chemotherapy but decreased after 4 treatment cycles in arm B. In an exploratory analysis of sE-cad as a prognostic or predictive biomarker, the sE-cad level change at the end of cycle 2 in comparison with the baseline did not correlate with ORR or PFS, but it was significantly associated with the risk of death. An increase in the sE-cad level of 5 ng/mL after cycle 2 resulted in a 13% higher risk of death (hazard ratio = 1.13, 95% CI = 1.03–1.25). This association remained significant in a multivariate analysis (hazard ratio = 1.14, 95% CI = 1.03–1.26) that accounted for the treatment arm, age, gender, primary tumor site, history of prior chemotherapy, and disease status (unresectable vs metastatic). However, the sE-cad level change from the baseline to the end of treatment or time of disease progression was not significantly associated with the risk of death. The baseline sE-cad level or the sE-cad level change after 2 cycles did not correlate significantly with TEEs.
Figure 3.
Soluble E-cadherin level changes after cycle 2 versus the baseline per treatment arm. G/C indicates gemcitabine/ cisplatin; Cetux, cetuximab.
CRP and D-Dimers
There were 41 patients (15 in arm A and 26 in arm B) with available baseline samples; 53% and 46% patients had abnormal CRP levels, whereas 67% and 81% had abnormal D-dimer levels in arms A and B, respectively. There was no significant association between the baseline CRP or D-dimer levels and TEEs in either arm. The baseline CRP and D-dimer levels were not significantly associated with ORR, PFS, or OS.
DISCUSSION
Adding CTX to standard first-line GC was feasible, but it did not improve outcomes and was associated with a higher rate of G3/G4 adverse events, including TEEs. Bladder cancer and chemotherapy are associated with TEEs30; however, the rate observed in arm B was unexpected, particularly because as a single agent CTX was not previously known to be associated with an increased TEE risk.31 A recent meta-analysis of prospective randomized trials concluded that anti-EGFR agents, especially CTX, are associated with a significantly higher TEE risk.32 However, this was not known when the study was amended to reduce the gemcitabine dose. The latter was based on the reduced TEE rate noted after a gemcitabine dose reduction in a previous trial of GC with bevacizumab in UC patients in which a high TEE rate was encountered.33 Interestingly, in our study, no patient with a previously resected primary tumor had TEEs, which were mostly lower extremity DVT and/or pulmonary embolisms. It is not clear whether this observation is a function of a locoregional effect of the tumor or systemic effects related to the presence of the primary tumor.
Although the study was not powered to detect significant differences, the observed lack of improvement in the antitumor effect with the addition of CTX to GC could be related to several factors. The gemcitabine dose reduction did not appear to be a significant factor because at the time of the dose-reduction implementation, two-thirds of the patients were randomized, and there was no significant ORR difference based on the gemcitabine dose. The lower median number of chemotherapy cycles in arm B may have played some role. The unselected design may also be a contributor because the study may have been underpowered to detect outcome differences in patients with EGFR overexpression. However, to date, there is no validated predictive biomarker for a response to anti-EGFR therapies in UC, and the tissue EGFR expression level has not been established as a predictor of a response to EGFR-targeted therapy. However, the strong association between EGFR and poor outcomes and the recent genomic data indicating a high gene copy number of EGFR in a number of UC tumors suggest that a patient subset might benefit from EGFR inhibition.34,35
Another consideration is whether CTX is the appropriate anti-EGFR agent in UC. As a single agent, CTX had no activity in patients with previously treated UC.36 In a trial using lapatinib as a single agent in 34 patients with platinum-resistant advanced UC, 1 objective response was identified, and 18 patients had stable disease; the median time to progression was 8.6 weeks, and the median OS was 17.9 weeks.37 However, in a subset analysis based on EGFR/HER2 expression levels, 17 of 19 patients with a clinical benefit from lapatinib had tumors overexpressing EGFR and/or HER2, and this suggested that biomarker-based enrichment may be important. As a single agent, gefitinib, another anti-EGFR TKI, was ineffective as a second-line therapy for metastatic UC.38
In combination with chemotherapy, CTX appeared to modestly augment the activity of paclitaxel in 39 patients with previously treated UC,36 whereas gefitinib did not appear to improve outcomes in combination with GC in comparison with historical controls using GC alone.39 Collectively, reported studies, including ours, have failed to show a significant benefit from anti-EGFR agents in patients with advanced UC. However, there appear to be occasional patients, including some in our study, who seem to derive a benefit from anti-EGFR therapy. Other ongoing trials will help to shed light on the potential role of targeting EGFR in UC; lapatinib with GC is being tested as a first-line treatment (ClinicalTrials.gov identifier NCT00623064), whereas docetaxel/lapatinib is being evaluated as a second-line therapy (NCT01382706) for UC. An ongoing phase 2 trial is evaluating docetaxel/gefitinib as maintenance after first-line chemotherapy (NCT00479089).
Targeting only EGFR may not be sufficient for a complex disease such as UC. Moreover, heterodimerization of EGFR with the other HERs might circumvent EGFR inhibition and drive parallel mitogen-activating signaling.40,41 Therefore, more potent inhibitors with an irreversible mechanism and broader anti-HER activity may provide more comprehensive signaling inhibition and greater antitumor effects. Dacomitinib, a novel, potent, and irreversible pan-HER inhibitor, has shown promising antitumor activity in human bladder cancer models.42 A better understanding of UC biology can elucidate potential predictors of response and resistance, help to select patients, and guide the successful development of targeted therapies in UC.34,35,43–46
In agreement with our hypothesis, sE-cad decreased with CTX. This may represent a loss of the epithelial tumor component, an inhibition of EGFR-mediated E-cadherin proteolysis, or a cell epithelial-to-mesenchymal transition. This sE-cad level reduction did not correlate with a response to either therapy or PFS; however, an increased sE-cad level was independently associated with a higher risk of death, regardless of treatment. Most studies have associated tissue E-cadherin expression loss with UC recurrence/progression.47–60 A higher plasma sE-cad level has been associated with bladder cancer in comparison with healthy controls; a higher preoperative plasma sE-cad level has been associated with nodal metastases and shorter PFS in patients treated with radical cystectomy.61 Our findings are exploratory, and the role of sE-cad in UC merits further investigation.
In conclusion, the combination of CTX and GC did not improve outcomes. This multicenter trial demonstrated the feasibility of accruing patients and conducting large randomized phase 2 trials investigating targeted agents in UC in a reasonable time period. The lack of benefit from an agent that targets a seemingly important pathway and has an established role in other solid tumors clearly highlights the importance of the biological context to the use of targeted therapies and the need to understand the molecular biology of UC to better inform drug development and affect outcomes.
Acknowledgments
FUNDING SUPPORT
The drug supply was provided by Lilly/ImClone. The National Comprehensive Cancer Network was the direct sponsor of this trial, and funding was provided by Lilly/ImClone.
Dr. Hussain received research funding for this study from the National Comprehensive Cancer Network and Lilly/ImClone (contract with the University of Michigan). She also received research funding from Pfizer, Genentech, Millennium, Medivation, and EMD Serono (contracts with the University of Michigan). Ms. Daignault received research support from Lilly/ImClone. Dr. Grivas received grant support from Genmab and personal fees from Myriad. Dr. Yu received research funding from ImClone/Lilly. Dr. Mark L. Day received grant support from Eli Lilly, Genmab, and Pharco and personal fees from Pharco. Dr. Siefker-Radtke received personal fees and other nonfinancial support for clinical trial support and advisory board work for Dendreon and Bristol-Myers Squibb. Dr. Siefker-Radtke also received clinical trial support from Millennium, AstraZeneca, and Genentech. Dr. Agarwal received research support for the University of Utah from Amgen, Bristol-Myers Squibb, GlaxoSmithKline, ImClone Systems, Medivation, Merrimack, Millennium, Novartis, Pfizer, and Spectrum Pharmaceuticals.
Footnotes
Presented in part at the 48th Annual Meeting of the American Society of Clinical Oncology; June 1–5, 2012; Chicago, IL.
CONFLICT OF INTEREST DISCLOSURES
REFERENCES
- 1.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson RI, Gee JMW, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37:S9–S15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 3.Chow NH, Liu HS, Lee EI, et al. Significance of urinary epidermal growth factor and its receptor expression in human bladder cancer. Anticancer Res. 1997;17:1293–1296. [PubMed] [Google Scholar]
- 4.Salomon DS, Brandt R, Ciardiello F, et al. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 5.Wells A. Tumor invasion: role of growth factor-induced cell motility. Adv Cancer Res. 2000;78:31–101. doi: 10.1016/s0065-230x(08)61023-4. [DOI] [PubMed] [Google Scholar]
- 6.Eccles SA. Cell biology of lymphatics metastasis. The potential role of c-erbB oncogene signaling. Recent Results Cancer Res. 2000;157:41–54. doi: 10.1007/978-3-642-57151-0_5. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen PL, Swanson PE, Jaszcz W, et al. Expression of epidermal growth factor receptor in invasive transitional cell carcinoma of the urinary bladder. A multivariate survival analysis. Am J Clin Pathol. 1994;101:166–176. doi: 10.1093/ajcp/101.2.166. [DOI] [PubMed] [Google Scholar]
- 8.Neal DE, Marsh C, Bennett MK, et al. Epidermal-growth-factor receptors in human bladder cancer: comparison of invasive and superficial tumours. Lancet. 1985;1:366–368. doi: 10.1016/s0140-6736(85)91386-8. [DOI] [PubMed] [Google Scholar]
- 9.Neal DE, Sharples L, Smith K, et al. The epidermal growth factor receptor and the prognosis of bladder cancer. Cancer. 1990;65:1619–1625. doi: 10.1002/1097-0142(19900401)65:7<1619::aid-cncr2820650728>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Messing EM. Clinical implications of the expression of epidermal growth factor receptors in human transitional cell carcinoma. Cancer Res. 1990;50:2530–2537. [PubMed] [Google Scholar]
- 11.Bue P, Wester K, Sjostrom A, et al. Expression of epidermal growth factor receptor in urinary bladder cancer metastases. Int J Cancer. 1998;76:189–193. doi: 10.1002/(sici)1097-0215(19980413)76:2<189::aid-ijc4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 12.Lipponen P, Eskelinen M. Expression of epidermal growth factor receptor in bladder cancer as related to established prognostic factors, oncoprotein (c-erbB-2, p53) expression and long-term prognosis. Br J Cancer. 1994;69:1120–1125. doi: 10.1038/bjc.1994.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer C, Klasmeyer K, Bojar H, et al. Heparin-binding epidermal growth factor-like growth factor isoforms and epidermal growth factor receptor/ErbB1 expression in bladder cancer and their relation to clinical outcome. Cancer. 2007;109:2016–2024. doi: 10.1002/cncr.22627. [DOI] [PubMed] [Google Scholar]
- 14.Mellon K, Wright C, Kelly P, et al. Long-term outcome related to epidermal growth factor receptor status in bladder cancer. J Urol. 1995;153:919–925. [PubMed] [Google Scholar]
- 15.Kroft SH, Oyasu R. Urinary bladder cancer: mechanisms of development and progression. Lab Invest. 1994;71:158–174. [PubMed] [Google Scholar]
- 16.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 17.Kompier LC, Lurkin I, van der Aa MN, et al. FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS One. 2010;5:e13821. doi: 10.1371/journal.pone.0013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visvanathan KV, Pocock RD, Summerhayes IC. Preferential and novel activation of H-ras in human bladder carcinomas. Oncogene Res. 1988;3:77–86. [PubMed] [Google Scholar]
- 19.Karimianpour N, Mousavi-Shafaei P, Ziaee AA, et al. Mutations of RAS gene family in specimens of bladder cancer. Urol J. 2008;5:237–242. [PubMed] [Google Scholar]
- 20.Munk M, Memon AA, Nexo E, et al. Inhibition of the epidermal growth factor receptor in bladder cancer cells treated with the DNA-damaging drug etoposide markedly increases apoptosis. BJU Int. 2007;99:196–201. doi: 10.1111/j.1464-410X.2006.06510.x. [DOI] [PubMed] [Google Scholar]
- 21.McHugh LA, Kriajevska M, Mellon JK, et al. Combined treatment of bladder cancer cell lines with lapatinib and varying chemotherapy regimens—evidence of schedule-dependent synergy. Urology. 2007;69:390–394. doi: 10.1016/j.urology.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 24.Perrotte P, Matsumoto T, Inoue K, et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 1999;5:257–265. [PubMed] [Google Scholar]
- 25.Black PC, Brown GA, Inamoto T, et al. Sensitivity to epidermal growth factor receptor inhibitor requires E-cadherin expression in urothelial carcinoma cells. Clin Cancer Res. 2008;14:1478–1486. doi: 10.1158/1078-0432.CCR-07-1593. [DOI] [PubMed] [Google Scholar]
- 26.Grabowska MM, Sandhu B, Day ML. EGF promotes the shedding of soluble E-cadherin in an ADAM10-dependent manner in prostate epithelial cells. Cell Signal. 2012;24:532–538. doi: 10.1016/j.cellsig.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuo JH, Zhu W, Li MY, et al. Activation of EGFR promotes squamous carcinoma SCC10A cell migration and invasion via inducing EMT-like phenotype change and MMP-9-mediated degradation of E-cadherin. J Cell Biochem. 2011;112:2508–2517. doi: 10.1002/jcb.23175. [DOI] [PubMed] [Google Scholar]
- 28.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 29.Simon R, Wittes RE, Ellenberg SS. Randomized phase II clinical trials. Cancer Treat Rep. 1985;69:1375–1381. [PubMed] [Google Scholar]
- 30.Botten J, Sephton M, Tillett T, et al. Thromboembolic events with cisplatin-based neoadjuvant chemotherapy for transitional cell carcinoma of urinary bladder. J Clin Oncol. 2013;31(suppl 6):277. [Google Scholar]
- 31.Pessino A, Artale S, Sciallero S, et al. First-line single-agent cetuximab in patients with advanced colorectal cancer. Ann Oncol. 2008;19:711–716. doi: 10.1093/annonc/mdm516. [DOI] [PubMed] [Google Scholar]
- 32.Petrelli F, Cabiddu M, Borgonovo K, et al. Risk of venous and arterial thromboembolic events associated with anti-EGFR agents: a meta-analysis of randomized clinical trials. Ann Oncol. 2012;23:1672–1679. doi: 10.1093/annonc/mdr592. [DOI] [PubMed] [Google Scholar]
- 33.Hahn NM, Stadler WM, Zon RT, et al. Phase II trial of cisplatin, gemcitabine, and bevacizumab as first-line therapy for metastatic urothelial carcinoma: Hoosier Oncology Group GU 04-75. J Clin Oncol. 2011;29:1525–1530. doi: 10.1200/JCO.2010.31.6067. [DOI] [PubMed] [Google Scholar]
- 34.Ross J, Wang K, Al-Rohil RN, et al. Advanced urothelial carcinoma: next-generation sequencing reveals diverse genomic alterations and targets of therapy. Mod Pathol. 2014;27:271–280. doi: 10.1038/modpathol.2013.135. [DOI] [PubMed] [Google Scholar]
- 35.Iyer G, Al-Ahmadie H, Schultz N, et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. J Clin Oncol. 2013;31:3133–3140. doi: 10.1200/JCO.2012.46.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong YN, Litwin S, Vaughn D, et al. Phase II trial of cetuximab with or without paclitaxel in patients with advanced urothelial tract carcinoma. J Clin Oncol. 2012;30:3545–3551. doi: 10.1200/JCO.2012.41.9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wulfing C, Machiels JP, Richel DJ, et al. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer. 2009;115:2881–2890. doi: 10.1002/cncr.24337. [DOI] [PubMed] [Google Scholar]
- 38.Petrylak DP, Tangen CM, Van Veldhuizen PJ, Jr, et al. Results of the Southwest Oncology Group phase II evaluation (study S0031) of ZD1839 for advanced transitional cell carcinoma of the urothelium. BJU Int. 2010;105:317–321. doi: 10.1111/j.1464-410X.2009.08799.x. [DOI] [PubMed] [Google Scholar]
- 39.Philips GK, Halabi S, Sanford BL, et al. A phase II trial of cisplatin (C), gemcitabine (G) and gefitinib for advanced urothelial tract carcinoma: results of Cancer and Leukemia Group B (CALGB) 90102. Ann Oncol. 2009;20:1074–1079. doi: 10.1093/annonc/mdn749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wheeler DL, Huang S, Kruser TJ, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–3956. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai YS, Cheng HL, Tzai TS, et al. Clinical significance of ErbB receptor family in urothelial carcinoma of the bladder: a systematic review and meta-analysis. Adv Urol. 2012;2012:181964. doi: 10.1155/2012/181964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grivas PD, Day KC, Karatsinides A, et al. Evaluation of the antitumor activity of dacomitinib in models of human bladder cancer. Mol Med. 2013;19:367–376. doi: 10.2119/molmed.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Figueroa JD, Ye Y, Siddiq A, et al. Genome-wide association study identifies multiple loci associated with bladder cancer risk. Hum Mol Genet. 2014;23:1387–1398. doi: 10.1093/hmg/ddt519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solomon DA, Kim JS, Bondaruk J, et al. Frequent truncating mutations of STAG2 in bladder cancer. Nat Genet. 2013;45:1428–1423. doi: 10.1038/ng.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo G, Sun X, Chen C, et al. Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation. Nat Genet. 2013;45:1459–1463. doi: 10.1038/ng.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross JS, Wang K, Gay L, et al. A high frequency of activating extracellular domain ERBB2 (HER2) mutation in micropapillary urothelial carcinoma. Clin Cancer Res. 2014;20:68–75. doi: 10.1158/1078-0432.CCR-13-1992. [DOI] [PubMed] [Google Scholar]
- 47.Omran OM. CD10 and E-cad expression in urinary bladder urothelial and squamous cell carcinoma. J Environ Pathol Toxicol Oncol. 2012;31:203–212. doi: 10.1615/jenvironpatholtoxicoloncol.v31.i3.20. [DOI] [PubMed] [Google Scholar]
- 48.Reis ST, Leite KR, Mosconi Neto A, et al. Immune expression of E-cadherin and α, β and γ-catenin adhesion molecules and prognosis for upper urinary tract urothelial carcinomas. Int Braz J Urol. 2012;38:466–473. doi: 10.1590/s1677-55382012000400005. [DOI] [PubMed] [Google Scholar]
- 49.Khorrami MH, Hadi M, Gharaati MR, et al. E-cadherin expression as a prognostic factor in transitional cell carcinoma of the bladder after transurethral resection. Urol J. 2012;9:581–585. [PubMed] [Google Scholar]
- 50.Cui D, Han BM, Jing YF, et al. Analysis of N-E cadherin switch as an independent predictive parameter of bladder cancer survival outcomes [in Chinese] Zhonghua Yi Xue Za Zhi. 2012;92:380–383. [PubMed] [Google Scholar]
- 51.Muramaki M, Miyake H, Terakawa T, et al. Expression profile of E-cadherin and N-cadherin in non-muscle-invasive bladder cancer as a novel predictor of intravesical recurrence following transurethral resection. Urol Oncol. 2012;30:161–166. doi: 10.1016/j.urolonc.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Erdemir F, Ozcan F, Kilicaslan I, et al. The relationship between the expression of E-cadherin and tumor recurrence and progression in high-grade stage T1 bladder urothelial carcinoma. Int Urol Nephrol. 2007;39:1031–1037. doi: 10.1007/s11255-006-9159-5. [DOI] [PubMed] [Google Scholar]
- 53.Byrne RR, Shariat SF, Brown R, et al. E-cadherin immunostaining of bladder transitional cell carcinoma, carcinoma in situ and lymph node metastases with long-term followup. J Urol. 2001;165:1473–1479. [PubMed] [Google Scholar]
- 54.Hu X, Ruan Y, Cheng F, et al. p130Cas, E-cadherin and β-catenin in human transitional cell carcinoma of the bladder: expression and clinicopathological significance. Int J Urol. 2011;18:630–637. doi: 10.1111/j.1442-2042.2011.02793.x. [DOI] [PubMed] [Google Scholar]
- 55.Shariat SF, Pahlavan S, Baseman AG, et al. E-cadherin expression predicts clinical outcome in carcinoma in situ of the urinary bladder. Urology. 2001;57:60–65. doi: 10.1016/s0090-4295(00)00892-x. [DOI] [PubMed] [Google Scholar]
- 56.Serdar A, Turhan C, Soner G, et al. The prognostic importance of e-cadherin and p53 gene expression in transitional bladder carcinoma patients. Int Urol Nephrol. 2005;37:485–492. doi: 10.1007/s11255-005-0919-4. [DOI] [PubMed] [Google Scholar]
- 57.Mahnken A, Kausch I, Feller AC, et al. E-cadherin immunoreactivity correlates with recurrence and progression of minimally invasive transitional cell carcinomas of the urinary bladder. Oncol Rep. 2005;14:1065–1070. [PubMed] [Google Scholar]
- 58.Fromont G, Roupret M, Amira N, et al. Tissue microarray analysis of the prognostic value of E-cadherin, Ki67, p53, p27, survivin and MSH2 expression in upper urinary tract transitional cell carcinoma. Eur Urol. 2005;48:764–770. doi: 10.1016/j.eururo.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 59.Kashibuchi K, Tomita K, Schalken JA, et al. The prognostic value of E-cadherin, alpha-, beta-, and gamma-catenin in urothelial cancer of the upper urinary tract. Eur Urol. 2006;49:839–845. doi: 10.1016/j.eururo.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 60.Mialhe A, Louis J, Montlevier S, et al. Expression of E-cadherin and alpha-, beta- and gamma-catenins in human bladder carcinomas: are they good prognostic factors? Invasion Metastasis. 1997;17:124–137. [PubMed] [Google Scholar]
- 61.Matsumoto K, Shariat SF, Casella R, et al. Preoperative plasma soluble E-cadherin predicts metastases to lymph nodes and prognosis in patients undergoing radical cystectomy. J Urol. 2003;170(pt 1):2248–2252. doi: 10.1097/01.ju.0000094189.93805.17. [DOI] [PubMed] [Google Scholar]