Abstract
Objective
CXCL12 encodes stromal cell-derived factor 1 alpha (SDF-1), which binds to the receptor encoded by CXCR4. Variation at the CXCL12 locus is associated with coronary artery disease (CAD) and endothelial progenitor cell (EPC) numbers, while variation at the CXCR4 locus is associated with leukocyte telomere length (LTL), which has been shown to be associated with CAD. We therefore examined the relations of plasma SDF-1 levels to cardiovascular disease (CVD)-related outcomes, risk factors, LTL, and EPCs.
Approach and Results
SDF-1 was measured in 3359 Framingham Heart Study participants. We used Cox regression to examine relations of SDF-1 to new-onset CVD, myocardial infarction (MI), heart failure (HF), and all-cause mortality; we used linear regression to evaluate associations of SDF-1 with risk factors, LTL, and CD34+ cell phenotypes. In multivariable models, higher SDF-1 levels were associated with older age, lower levels of HDL cholesterol, and cigarette smoking. Higher SDF-1 levels were associated with lower CD34+ cell frequency (p=0.02), but not with LTL. During follow-up (median 9.3 years), there were 263 new-onset CVD events, 160 MIs, 200 HF events, and 385 deaths. After adjusting for clinical risk factors, SDF-1 levels were associated with HF (p=0.04) and all-cause mortality (p=0.003), but not with CVD (p=0.39) or MI (p=0.10). The association of SDF-1 levels with MI was attenuated after adjustment for HDL cholesterol.
Conclusions
After adjusting for traditional CVD risk factors, SDF-1 is associated with HF and all-cause mortality risk. Further studies are needed to determine whether measurement of SDF-1 levels has clinical utility.
Keywords: Cardiovascular disease, epidemiology, myocardial infarction, heart failure, mortality, stromal cell-derived factor 1, progenitor cells
Introduction
Genome-wide association studies (GWAS) of coronary artery disease (CAD) and myocardial infarction (MI) have identified 10q11.21 as a risk locus.[1–4] 10q11.21 harbors CXCL12, which encodes stromal cell-derived factor 1 alpha (SDF-1). In addition to the association of CXCL12 with CAD and MI, we reported that its receptor, CXCR4, is associated in GWAS with leukocyte telomere length (LTL).[5] We and others have shown that LTL, in turn, is associated with CAD and MI.[5, 6] The dual GWAS links of the CXCR4-CXCL12 axis with CAD/MI and with LTL makes SDF-1 an attractive protein biomarker for cardiovascular disease (CVD) and related phenotypes.
The CXCR4-CXCL12 axis has been explored in experimental models and in population studies. SDF-1 governs the homing of endothelial progenitor cells (EPCs) from bone marrow to areas of vascular injury for angiogenesis and repair.[7] The Bruneck study reported that plasma SDF-1 levels are inversely related to circulating EPC numbers.[8] Additionally, in the same study, there was an association between CXCL12 genetic variation, circulating SDF-1 levels, and circulating EPCs.[9] CD34+ cell count is an indicator of progenitor cell activity [10], is associated with CVD [11], and promotes neovascularization in the context of vascular disease.[12,13] Thus, alterations in CXCL12 expression – and by inference, increased SDF-1 levels – may affect CD34+ abundance and recruitment in the context of CVD.
Collectively, these findings are consistent with the hypothesis that plasma SDF-1 levels and genetic variations at the CXCL12 and CXCR4 loci are linked to CVD risk and to CVD-related phenotypes through effects on progenitor cell recruitment and LTL dynamics. We thus sought to study the association of plasma SDF-1 levels with CVD-related outcomes and to investigate possible mechanistic connections. We hypothesized that higher plasma SDF-1 levels would be associated with increased risk for CVD-related outcomes, with a greater burden of CVD risk factors, and with shorter LTL and lower CD34+ cell numbers. To that end, we examined these relations in 3359 participants from the Framingham Heart Study (FHS).
Methods and Materials
Methods and Materials are available in the online-only Data Supplement.
Results
Baseline characteristics
The mean age of the study sample was 59 years and 53% were women. The mean SDF-1 level was 1894 pg/mL (range 742 pg/mL to 17,633 pg/mL). Two individuals with SDF-1 levels >5SD were excluded from analyses, leaving 3357 with SDF-1 data; 3216 participants had no missing covariates. Baseline clinical characteristics of the 3357 participants with measured SDF-1 levels are summarized in Table 1.
Table 1.
Unadjusted baseline characteristics according to quartiles of SDF-1 levels
| SDF-1 Quartiles | |||||||
|---|---|---|---|---|---|---|---|
| Quartile 1 (n=810) | Quartile 2 (n=889) | Quartile 3 (n=815) | Quartile 4 (n=843) | p-value per quartile increment in ln(SDF-1)† | p-value per 1 SD increment in ln(SDF-1) | ||
| Mean SDF-1 [range] (pg/mL)* | 1330 [742–1518] | 1676 [1539–1818] | 1973 [1839–2135] | 2588 [2156–5980] | |||
| Age (years) | mean (SD) | 56 (9) | 58 (9) | 60 (10) | 63 (10) | <0.0001 | < 0.0001 |
| Women | n (%) | 457 (56) | 436 (49) | 441 (54) | 441 (52) | 0.36 | 0.075 |
| BMI (kg/m2) | mean(SD) | 28 (5) | 28 (5) | 28 (5) | 28 (5) | 0.28 | 0.71 |
| Waist circumference (cm) | mean (SD) | 38 (5) | 38 (5) | 39 (5) | 38 (5) | 0.97 | 0.37 |
| Total cholesterol (mg/dl) | mean (SD) | 205 (38) | 205 (36) | 206 (38) | 207 (49) | 0.31 | 0.70 |
| HDL cholesterol (mg/dl) | mean (SD) | 54 (16) | 52 (16.3) | 50 (16) | 48 (16) | <0.0001 | < 0.0001 |
| LDL cholesterol (mg/dl) | mean (SD) | 124 (35) | 127 (32) | 127 (34) | 129 (39) | 0.012 | 0.008 |
| Triglycerides (mg/dl) | mean (SD) | 135 (93) | 135 (88) | 144 (111) | 152 (210) | 0.0035 | 0.008 |
| Systolic blood pressure (mmHg) | mean (SD) | 127 (19) | 127 (18) | 130 (19) | 130 (19) | 0.0001 | < 0.0001 |
| Diastolic blood pressure (mmHg) | mean (SD) | 76 (9) | 75 (9) | 76 (10) | 74 (10) | 0.0001 | < 0.0001 |
| Glucose (mg/dl) | mean (SD) | 103 (26) | 103 (25) | 103 (25) | 107 (31) | 0.006 | 0.0002 |
| BNP | mean (SD) | 11 (18) | 14 (17) | 16 (20) | 26 (36) | <0.0001 | < 0.0001 |
| Smoking status (current) | n (%) | 111 (14) | 141 (16) | 122 (15) | 134 (16) | 0.32 | 0.63 |
| CVD | n (%) | 22 (3) | 35 (4) | 42 (5) | 69 (8) | <0.0001 | < 0.0001 |
| Diabetes (yes) | n (%) | 69 (9) | 72 (8) | 74 (9) | 116 (14) | 0.0003 | < 0.0001 |
| Hypertension treatment (yes) | n (%) | 301 (37) | 334 (38) | 377 (46) | 379(45) | <0.0001 | < 0.0001 |
| Statin treatment | n (%) | 81 (0.1) | 89 (0.1) | 82 (0.1) | 84 (0.1) | 0.17 | 0.10 |
Ln(SDF-1) range: Q1 [6.61–7.33]; Q2 [7.33–7.52]; Q3[7.52–7.67]; Q4 [7.67–8.69]
Values are unadjusted (single variable analysis)
1 standard deviation of ln(SDF-1): 0.26
In unadjusted analyses, higher SDF-1 levels were associated with older age (p<0.0001), and waist circumference, higher prevalence of diabetes, hypertension treatment, and CVD (p<0.0001). Additionally, increasing quartiles of SDF-1 were associated with higher levels of low-density lipoprotein (LDL) cholesterol, triglycerides, systolic blood pressure, glucose, and BNP (p<0.01). In contrast, HDL cholesterol decreased across SDF-1 quartiles (p<0.0001). In multivariable analyses (Supplemental Table I), higher SDF-1 levels were associated with older age; lower SDF-1 levels were associated with lower BMI, HDL cholesterol, and systolic blood pressure.
SDF-1 and clinical outcomes
For analyses of new-onset atherosclerotic CVD, participants with prevalent atherosclerotic CVD (n = 168) and missing covariates (n=23) were excluded, and among the 3168 remaining participants, there were 263 incident atherosclerotic CVD events during follow-up (median [minimum, maximum] = 9.3 years [0.03, 15.7]). 160 of these 263 individuals developed MI. For analyses of HF, participants with prevalent HF (n=36) and missing covariates (n=27) were excluded, and the available sample size was 3296. There were 200 incident HF events during follow-up (9.3 years [0.005, 15.5]). For analyses of death from all causes, participants with missing covariates (n=26) were excluded and the available sample size was 3,333. There were 385 deaths during follow-up (9.3 years [0.3, 14.1]).
Table 2 displays the relations of SDF-1 levels (quartiles and continuous measures) to clinical outcomes. In age- and sex- adjusted analyses (Model 1), a one standard deviation increment in SDF-1 was associated with incident atherosclerotic CVD (hazards ratio [HR] 1.2, 95% confidence interval [CI] 1.1–1.4; p=0.009), MI (HR 1.3, 95% CI 1.1–1.6; p=0.009), HF (HR 1.4, 95% CI 1.1–1.6; p=0.0012), and death from all causes (HR 1.2, 95% CI 1.1–1.4; p=0.0001).
Table 2.
Risk for new-onset atherosclerotic cardiovascular disease, myocardial infarction, heart failure, or all-cause mortality according to levels of SDF-1
| Ln (SDF-1) quartiles | ||||||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Per quartile increment in ln(SDF-1) | Per 1 SD increment in ln(SDF-1) | |
| HR [95% CI] | HR [95% CI] | HR [95% CI] | HR [95% CI] | HR [95% CI] (p-value) | HR [95% CI] (p-value§) | |
| Cardiovascular disease (CVD), n=263 | ||||||
| Model 1 | Referent | 1.0 [0.69–1.6] | 1.2 [0.77–1.8] | 1.3 [0.89–2.1] | 1.1 [1.0–1.3] (0.10) | 1.2 [1.1–1.4] (0.009) |
| Model 2* | Referent | 1.0 [0.68–1.6] | 1.1 [0.74–1.7] | 1.1 [0.68–1.7] | 1.0 [0.90–1.2] (0.70) | 1.1 [0.9–1.2] (0.39) |
| Model 3 | Referent | 1.0 [0.68–1.6] | 1.1 [0.73–1.7] | 1.1 [0.64–1.6] | 1.0 [0.90–1.20] (0.90) | 1.1 [0.9–1.3] (0.31) |
| Myocardial Infarction (MI), n=160 | ||||||
| Model 1 | Referent | 1.1 [0.61–1.9] | 1.1 [0.63–1.9] | 1.7 [1.0–2.9] | 1.2 [1.0–1.4] (0.03) | 1.3 [1.1–1.6] (0.009) |
| Model 2* | Referent | 1.0 [0.57–1.7] | 1.0 [0.59–1.8] | 1.4 [0.80–2.5] | 1.1 [0.9–1.4] (0.20) | 1.1 [0.9–1.2] (0.10) |
| Model 3 | Referent | 1.0 [0.57–1.7] | 1.0 [0.59–1.8] | 1.4 [0.78–2.4] | 1.1 [0.9–1.3] (0.40) | 1.1 [0.9–1.3] (0.31) |
| Heart failure (HF), n=200 | ||||||
| Model 1 | Referent | 1.1 [0.64–1.9] | 1.4[0.84–2.4] | 1.9[1.1–3.1] | 1.2 [1.0–1.50] (0.006) | 1.4 [1.1–1.6] (0.0012) |
| Model 2† | Referent | 1.1 [0.65–1.9] | 1.4[0.83–2.4] | 1.6 [0.92–2.6] | 1.2 [1.0–1.40] (0.06) | 1.2 [1.0–1.5] (0.04) |
| Model 3 | Referent | 1.0 [0.62–1.7] | 1.2[0.72–2.1] | 1.1 [0.66–1.9] | 1.0 [0.90–1.20] (0.60) | 1.1 [0.9–1.3] (0.27) |
| Death from all causes, n=385 | ||||||
| Model 1 | Referent | 1.3 [0.96–1.8] | 1.4 [1.0–2.0] | 1.6 [1.1–2.2] | 1.1 [1.0–1.2] (0.007) | 1.2 [1.1–1.4] (0.0001) |
| Model 2‡ | Referent | 1.3 [0.91–1.7] | 1.4 [1.0–1.9] | 1.4 [1.0–1.9] | 1.1 [1.0–1.2] (0.04) | 1.2 [1.1–1.3] (0.003) |
| Model 3 | Referent | 1.2 [0.91–1.7] | 1.4 [1.0–1.9] | 1.3 [0.9–1.8] | 1.1 [1.0–1.2] (0.10) | 1.2 [1.0–1.3] (0.02) |
Model 1: Adjusted for age and sex
Model 2: Adjusted for age, sex, systolic blood pressure, hypertension treatment, total cholesterol, HDL, diabetes, smoking status, statin use. After excluding HDL from Model 2, the HR [95% CI] per 1 SD increment in ln(SDF-1) did not change for CVD, and the p-value was insignificant (p=0.11). For MI, exclusion of HDL from Model 2 had a HR= 1.2 [1.0–1.5] and p-value = 0.020.
Model 2: Adjusted for age, sex, systolic blood pressure, hypertension treatment, HDL, BMI, diabetes, smoking status, atrial fibrillation, coronary heart disease, valvular heart disease
Model 2: Adjusted for age, sex, systolic blood pressure, hypertension treatment, total cholesterol, HDL, diabetes, smoking status, angina, atherosclerotic CVD, and HF
Model 3: Adjusted for respective Model 2 outcome+ BNP
1 standard deviation of ln(SDF-1): 0.26
P-value for the association of ln(SDF-1) as a continuous variable with clinical outcomes
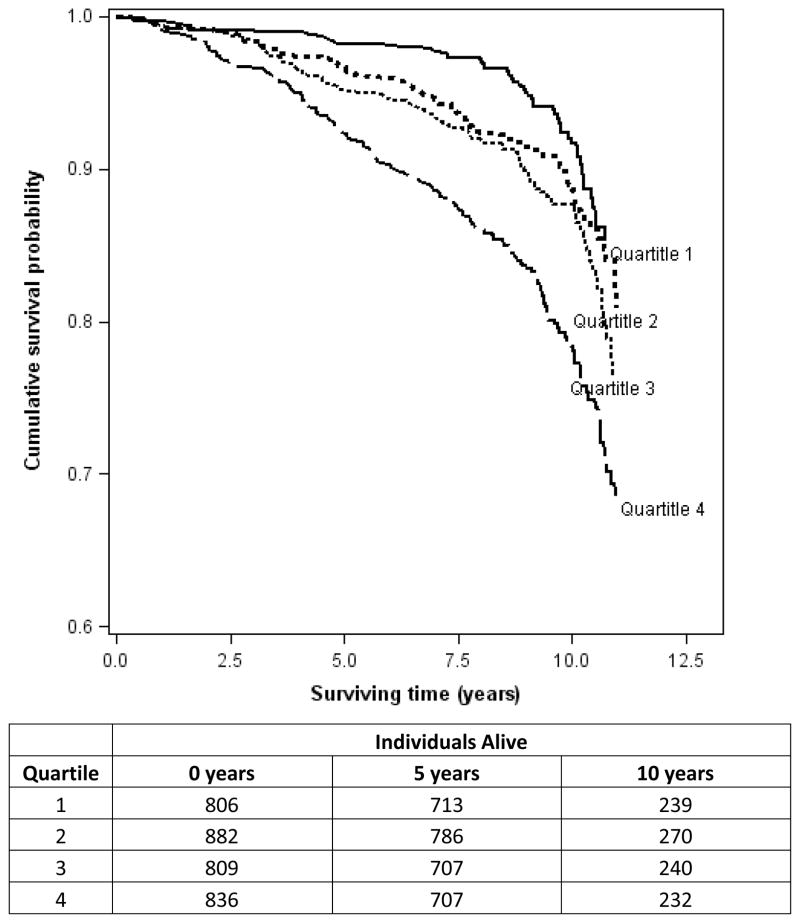
After additionally adjusting for clinical covariates (Model 2), SDF-1 remained associated with HF and death from all causes (p<0.05), but not with atherosclerotic CVD or MI. However, when HDL cholesterol was excluded from the multivariable model, SDF-1 was associated with MI (HR = 1.3, 95% CI 1.0–1.5; p=0.02). When additionally adjusting for BNP level (Model 3), SDF-1 remained associated with death from all causes. A Kaplan-Meier plot of survival as a function of baseline SDF-1 quartile is displayed in Figure 1.
Figure 1.
10-year survival according to quartile of plasma SDF-1. Kaplan-Meier survival plots are unadjusted for covariates. A log-rank test of survival across quartiles of SDF-1 is highly significant (p<0.001).
In secondary analyses we explored the association of SDF-1 with cause-specific mortality (CVD deaths [n=110], cancer deaths [n=199], and deaths from other or unknown causes [n=176]; Supplemental Table II). In risk factor adjusted models, SDF-1 was associated with CVD death (p=0.02) and other or unknown causes of death (p=0.003), but not with cancer death (p=0.22).
SDF-1 levels and LTL
Of the 1185 individuals who had LTL data, the mean telomere length was 6.9 kilobase pairs (kbp) in men and 7.0 kbp in women. There was no statistically significant association between SDF-1 and LTL (Table 3).
Table 3.
Leukocyte telomere length according to SDF-1 quartiles
| Ln (SDF-1) quartiles | Quartile 1* (n=270) | Quartile 2* (n=325) | Quartile 3* (n=295) | Quartile 4* (n=295) | p-value per quartile increment in ln(SDF-1) | p-value† per 1 SD‡ increment in ln(SDF-1) |
|---|---|---|---|---|---|---|
| Leukocyte telomere length, LTL (kbps) | ||||||
| Model 1 | 7.0 (0.03) | 7.0 (0.03) | 7.0 (0.03) | 6.9 (0.03) | 0.20 | 0.18 |
| Model 2 | 7.0 (0.03) | 7.0 (0.03) | 7.0 (0.03) | 7.0 (0.03) | 0.40 | 0.37 |
Values reflect least square means (standard error)
P-value for the association of ln(SDF-1) as a continuous variable with LTL
1 standard deviation of ln(SDF-1): 0.26
Model 1: Adjusted for age and sex
Model 2: Adjusted for age, sex, BMI, total cholesterol/HDL cholesterol ratio, smoking status, diabetes, systolic blood pressure, hypertension treatment
SDF-1 and CD34+ cell phenotypes
We examined natural log-transformed CD34+ cell numbers (n=1579) in relation to SDF-1 levels. CD34+ frequency was associated with higher SDF-1 levels in both age- and sex- adjusted and multivariable adjusted models (p=0.02 for one standard deviation increment in SDF-1, Table 4). The association of SDF-1 with CD34+ cells remained statistically significant after adjustment for clinical covariates (p=0.02)
Table 4.
CD34+ frequency according to SDF-1 quartiles
| Ln(SDF-1) quartiles | Quartile 1* (n=424) | Quartile 2* (n=438) | Quartile 3* (n=383) | Quartile 4* (n=358) | p-value per quartile increment in ln(SDF-1) | p-value† per 1 SD‡ increment in ln(SDF-1) |
|---|---|---|---|---|---|---|
|
CD 34+ (% of CD34+ cells in peripheral blood excluding red blood cells, platelets and cell debris) [95% CI] | ||||||
| Model 1 | 0.081 (0.077–0.085) | 0.076 (0.073–0.080) | 0.075 (0.070–0.079) | 0.073 (0.069–0.077) | 0.01 | 0.02 |
| Model 2 | 0.080 (0.076–0.084) | 0.076 (0.073–0.080) | 0.074 (0.071–0.078) | 0.073 (0.069–0.077) | 0.01 | 0.02 |
Values reflect least square means (standard error)
P-value for the association of ln(SDF-1) as a continuous variable with CD34+
1 standard deviation of ln(SDF-1): 0.26
Model 1: Adjusted for age and sex
Model 2: Adjusted for age, sex, BMI, total cholesterol/HDL cholesterol ratio, smoking status, diabetes, systolic blood pressure, hypertension treatment
Discussion
Based on GWAS results linking the CXCL12-CXCR4 axis to CAD [2–4] and LTL [5], we explored the relations of plasma SDF-1, the protein coded by CXCL12, to CVD outcomes and CVD risk factors. Additionally, we examined LTL and CD34+ cell frequency as intermediate phenotypes.[9] Our findings are three-fold. First, SDF-1 levels were associated with several CVD risk factors. Second, higher SDF-1 levels were associated with HF (continuous model adjusted for clinical HF risk factors, p= 0.03) and all-cause mortality (continuous model adjusted for risk factors, p=0.003), but not with atherosclerotic CVD. Finally, SDF-1 levels were associated with CD34+ cell frequency (continuous model adjusted for CVD risk factors, p=0.02), but not with LTL.
Extensive phenotypic data from the FHS allowed us to investigate the relations of SDF-1 to several CVD risk factors in a large sample size of 3359 individuals. In multivariable adjusted models, we found that SDF-1 levels were associated with age, smoking status, and HDL cholesterol (Supplemental Table I). Prior studies have reported similar associations between plasma SDF-1 and clinical CVD risk factors.[14, 15]
To further explore the association of SDF-1 with CVD, we analyzed the association of SDF-1 with MI and found that SDF-1 was associated with MI when HDL cholesterol was not included as a covariate in the multivariable model, (p=0.02), however, that association was attenuated when HDL cholesterol was included as a covariate (Supplemental Table II). Thus, we conclude that the (inverse) association of SDF-1 with HDL cholesterol is partly responsible for the attenuation of the association of SDF-1 with MI. Analyses of cause-specific mortality revealed that SDF-1 levels were associated to deaths due to CVD (p=0.02) and deaths from other or unknown causes (p=0.003), but not with cancer deaths (Supplemental Table II).
Single nucleotide polymorphisms (SNPs) rs501120 and rs1746048 at the CXCL12 locus that were previously reported to be associated with MI/CAD [2, 4, 16] were found to be associated with increased SDF-1 levels in our Framingham participants (p-value ≤0.0005 in age- and sex-adjusted models; Supplemental Table III). These findings are consistent with the PennCath Study that found a similar effect size and directionality between rs1746048 and CAD/MI,[17] and the Bruneck Study that found an association between rs1746048 and carotid intimal-medial thickness.[18] While the literature and our preliminary data suggest that the rs1746048-C allele is associated with higher SDF-1 levels, inferences linking the SDF-1 risk allele to atherogenesis should be made with caution.
We found that SDF-1 levels were associated with risk of HF and death. Higher SDF-1 levels were associated with a 20% increase in risk of new-onset HF (HR per one standard deviation increment in ln(SDF-1)=1.2, 95% CI [1.0–1.5]; p=0.03]) after adjusting for HF clinical risk factors; this association was attenuated after adjusting for plasma BNP levels. We also found an association of SDF-1 levels with all-cause mortality (HR per one SD increment = 1.2, 95% CI [1.1–1.3], p=0.003 after adjusting for clinical covariates). The association between SDF-1 and all-cause mortality in the general population is novel.
A recent prospective study by Mehta et al reported an association of SDF-1 with incident MI, and death in a kidney disease cohort. Our results for MI and HF complement their findings. Of note, as was the case in our analyses, Metha et al found an association between SDF-1 and MI in a model that did not adjust for HDL.[15] Additionally, an investigation from the Diabetes Heart Study found an association of rs1746048 at the CXCL12 locus with all-cause mortality in people with type 2 diabetes mellitus.[19]
We found that plasma SDF-1 was associated with reduced frequency of CD34+ cell phenotypes, an association that persisted after adjusting for CVD risk factors. This inverse relationship contrasts with mouse models that suggest a direct association between SDF-1 and CD34+, a circulating cell marker associated with progenitor cell activity. Previous studies have found that SDF-1 binds to the CXCR4 receptor found on CD34+ cells and directs CD34+ cell release from the bone marrow.[20] The release of CD34+ cells aids in neovascularization and angiogenesis, processes that may mediate tissue healing in the context of CVD.[13] Our findings are consistent with population-based studies that have shown that CD34+ frequency is inversely related to CVD-risk factors (age and smoking status) and a higher Framingham Risk Score.[21]
Several lines of evidence implicate an association of SDF-1 with HDL cholesterol, and we therefore hypothesize that HDL cholesterol modulates the CXCL12-CXCR4 axis. HDL cholesterol was significantly (p<0.0001) associated with SDF-1 levels in our multivariable model. Additionally, HDL cholesterol was the major covariate that attenuated the association of SDF-1 with MI. Of note, a GWAS of HDL cholesterol identified a suggestive signal for rs768676 (p=4.3×10−6) located near CXCL12.[22] Although these merging lines of evidence are highly speculative, the relationship of SDF-1 to HDL cholesterol warrants further exploration.
Our study has several limitations. We measured SDF-1 levels at one time point. Evidence suggests that there is acute modulation of SDF-1 levels in humans.[23] The number of incident atherosclerotic CVD and HF events in our analyses was modest, thereby limiting our power. Our study was restricted to a predominantly white, middle-aged sample; thus, our ability to generalize our findings to other groups is limited. Last, the association of SDF-1 with mortality and HF warrants replication in other studies.
Supplementary Material
Significance.
We report an association of higher plasma SDF-1 levels with risk of HF and mortality. We found no association of SDF-1 levels with risk of atherosclerotic CVD after adjusting for CVD risk factors. SDF-1 was associated with several CVD risk factors, most notably HDL cholesterol. We explored several intermediate phenotypes and found that higher SDF-1 levels were associated with reduced CD34+ cell frequency. We speculate that HDL cholesterol may mediate the association between SDF-1 and MI. Molecular studies may be helpful to evaluate the role of HDL cholesterol in these associations. Additional population-based studies are needed to validate the associations of SDF-1 with HF and mortality.
Acknowledgments
Sources of funding: The Framingham Heart Study of the National Heart, Lung, and Blood Institute is funded by NIH contract N01-HC-25195; the Division of Intramural Research of the National Heart, Lung, and Blood Institute also supported this research. Dr. Abraham Aviv’s research was supported by NIH grants R01AG030678 and R01HD071180.
Abbreviations
- BMI
Body mass index
- CAD
Coronary artery disease
- CVD
Cardiovascular disease
- EPC
Endothelial progenitor cell
- GWAS
Genome-wide association study
- HDL
High-density lipoprotein
- HF
Heart failure
- LTL
Leukocyte telomere length
- MI
Myocardial infarction
- SDF-1
Stromal cell-derived factor 1 alpha
Footnotes
Disclosures: Pieter Muntendam contributed to this project while affiliated with BG Medicine, Inc., a biomarker discovery company
References
- 1.Angelakopoulou A, Shah T, Sofat R, et al. Comparative analysis of genome-wide association studies signals for lipids, diabetes, and coronary heart disease: Cardiovascular Biomarker Genetics Collaboration. Eur Heart J. 2012;33:393–407. doi: 10.1093/eurheartj/ehr225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kathiresan S, Voight BF, et al. Myocardial Infarction Genetics Consortium. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preuss M, König IR, Thompson JR, et al. Design of the Coronary ARtery DIsease Genome-Wide Replication And Meta-Analysis (CARDIoGRAM) Study: A Genome-wide association meta-analysis involving more than 22 000 cases and 60 000 controls. Circ Cardiovasc Genet. 2010;3:475–483. doi: 10.1161/CIRCGENETICS.109.899443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samani NJ, Deloukas P, Erdmann J, Hengstenberg C, Kuulasmaa K, McGinnis R, Schunkert H, Soranzo N, Thompson J, Tiret L, Ziegler A Coronary Artery Disease Consortium. Large scale association analysis of novel genetic loci for coronary artery disease. Arterioscler Thromb Vasc Biol. 2009;29:774–780. doi: 10.1161/ATVBAHA.108.181388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Donnell CJ, Demissie S, Kimura M, Levy D, Gardner JP, White C, D’Agostino RB, Wolf PA, Polak J, Cupples LA, Aviv A. Leukocyte telomere length and carotid artery intimal medial thickness: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2008;28:1165–1171. doi: 10.1161/ATVBAHA.107.154849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Codd V, Nelson CP, Albrecht E, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45:422–427. doi: 10.1038/ng.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunner S, Winogradow J, Huber BC, Zaruba MM, Fischer R, David R, Assmann G, Herbach N, Wanke R, Mueller-Hoecker J, Franz WM. Erythropoietin administration after myocardial infarction in mice attenuates ischemic cardiomyopathy associated with enhanced homing of bone marrow-derived progenitor cells via the CXCR-4/SDF-1 axis. FASEB J. 2009;23:351–361. doi: 10.1096/fj.08-109462. [DOI] [PubMed] [Google Scholar]
- 8.Xiao Q, Kiechl S, Patel S, Oberhollenzer F, Weger S, Mayr A, Metzler B, Reindl M, Hu Y, Willeit J, Xu Q. Endothelial progenitor cells, cardiovascular risk factors, cytokine levels and atherosclerosis--results from a large population-based study. PloS one. 2007;2:e975. doi: 10.1371/journal.pone.0000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao Q, Ye S, Oberhollenzer F, Mayr A, Jahangiri M, Willeit J, Kiechl S, Xu Q. SDF1 gene variation is associated with circulating SDF1alpha level and endothelial progenitor cell number: the Bruneck Study. PloS one. 2008;3:e4061. doi: 10.1371/journal.pone.0004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duda DG, Cohen KS, Scadden DT, Jain RK. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc. 2007;2:805–810. doi: 10.1038/nprot.2007.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49:741–752. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 12.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 13.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 14.Stellos K, Ruf M, Sopova K, Kilias A, Rahmann A, Stamatelopoulos K, Jorbenadze R, Geisler T, Gawaz M, Bigalke B. Plasma levels of stromal cell-derived factor-1 in patients with coronary artery disease: effect of clinical presentation and cardiovascular risk factors. Atherosclerosis. 2011;219:913–916. doi: 10.1016/j.atherosclerosis.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Mehta NN, Matthews GJ, Krishnamoorthy P, et al. Higher plasma CXCL12 levels predict incident myocardial infarction and death in chronic kidney disease: findings from the Chronic Renal Insufficiency Cohort study. Eur Heart J. 2013 doi: 10.1093/eurheartj/eht481. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schunkert H, König IR, Kathiresan S, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta NN, Li M, William D, et al. The novel atherosclerosis locus at 10q11 regulates plasma CXCL12 levels. Eur Heart J. 2011;32:963–71. doi: 10.1093/eurheartj/ehr091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiechl S, Laxton RC, Xiao Q, et al. Coronary artery disease-related genetic variant on chromosome 10q11 is associated with carotid intima-media thickness and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2678–2683. doi: 10.1161/ATVBAHA.110.213785. [DOI] [PubMed] [Google Scholar]
- 19.Adams JN, Raffield LM, Langefeld CD, Ng MCY, Carr JJ, Cox AJ, Bowden DW. Analysis of common and coding variants with cardiovascular disease in the diabetes heart study. Cardiovascular Diabetology. 2014;13:77. doi: 10.1186/1475-2840-13-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen KS, Cheng S, Larson MG, Cupples LA, McCabe EL, Wang YA, Ngwa JS, Martin RP, Klein RJ, Hashmi B, Ge Y, O’Donnell CJ, Vasan RS, Shaw SY, Wang TJ. Circulating CD34(+) progenitor cell frequency is associated with clinical and genetic factors. Blood. 2013;121:e50–e56. doi: 10.1182/blood-2012-05-424846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kathiresan S, Manning AK, Demissie S, D’Agostino RB, Surti A, Guiducci C, Gianniny L, Burtt NP, Melander O, Orho-Melander M, Arnett DK, Peloso GM, Ordovas JM, Cupples LA. A genome-wide association study for blood lipid phenotypes in the Framingham Heart Study. BMC Med Genet. 2007;8 (Suppl 1):S17. doi: 10.1186/1471-2350-8-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stellos K, Bigalke B, Langer H, Geisler T, Schad A, Kögel A, Pfaff F, Stakos D, Seizer P, Müller I, Htun P, Lindemann S, Gawaz M. Expression of stromal-cell-derived factor-1 on circulating platelets is increased in patients with acute coronary syndrome and correlates with the number of CD34+ progenitor cells. Eur Heart J. 2009;30:584–593. doi: 10.1093/eurheartj/ehn566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.