Abstract
This study examined the association of progression-free survival at 6 months with overall survival in the context of second-line therapy of advanced urothelial carcinoma in pooled patient-level data from 10 phase II trials and then externally validated in a large phase III trial. Progression-free survival at 6 months was significantly correlated with overall survival and is an innovative primary endpoint to evaluate new agents in this setting.
Objective
Second-line systemic therapy for advanced urothelial carcinoma (UC) has substantial unmet needs, and current agents show dismal activity. Second-line trials of metastatic UC have used response rate (RR) and median progression-free survival (PFS) as primary endpoints, which may not reflect durable benefits. A more robust endpoint to identify signals of durable benefits when investigating new agents in second-line trials may expedite drug development. PFS at 6 months (PFS6) is a candidate endpoint, which may correlate with overall survival (OS) at 12 months (OS12) and may be applicable across cytostatic and cytotoxic agents.
Methods
Ten second-line phase II trials with individual patient outcomes data evaluating chemotherapy or biologics were combined for discovery, followed by external validation in a phase III trial. The relationship between PFS6/RR and OS12 was assessed at the trial level using Pearson correlation and weighted linear regression, and at the individual level using Pearson chi-square test with Yates continuity correction.
Results
In the discovery dataset, a significant correlation was observed between PFS6 and OS12 at the trial (R2 = 0.55, Pearson correlation = 0.66) and individual levels (82%, Қ = 0.45). Response correlated with OS12 at the individual level less robustly (78%, Қ = 0.36), and the trial level association was not statistically significant (R2 = 0.16, Pearson correlation = 0.37). The correlation of PFS6 (81%, Қ = 0.44) appeared
Keywords: Advanced urothelial carcinoma, Intermediate endpoint, Overall survival, Progression-free survival at 6 months, Second-line treatment
Introduction
Advanced urothelial carcinoma (UC) has not had major improvements in outcomes for more than 2 decades. Despite initial high response rates (RRs) of 40% to 70% with cisplatin-based frontline combination chemotherapy, these regimens are generally not curative and yield a 5-year overall survival (OS) of 4% to 20%.1–3 Multiple agents have demonstrated limited activity in the second-line setting, with RRs of 5% to 20%, median progression-free survival (PFS) of 2 to 4 months, and median OS of 6 to 9 months.4–14 Thus, there are significant unmet medical needs, particularly in the second-line setting.
Trials of metastatic UC in the second-line setting have commonly used RR or median PFS as the primary endpoint to evaluate activity and as a surrogate for OS. However, response may not capture the activity of cytostatic agents, and both of these endpoints do not lend confidence with regard to the durability of benefits. PFS at a fixed time point beyond the usual median PFS at 6 months (PFS6) may warrant further study as an intermediate endpoint for OS at 12 months (OS12). Indeed, a strong association between PFS6 and OS has been found in similar aggressive malignancies, glioblastoma multiforme, and small-cell lung cancer.15–17 We hypothesized that PFS6 correlates with OS12 in the context of second-line therapy for advanced UC and may be a robust endpoint to identify signals of durable benefits when investigating new agents.
Patients and Methods
Eligible Trials and Patients
Individual patient-level data were pooled from 10 phase II trials (8 single arm and 2 randomized) evaluating second-line chemotherapy or biologics (except the trial by Choueiri et al,5 which allowed ≤ 3 prior lines of therapy after enrolling 65 of 149 patients) (Table 1). Prior therapy may have been administered in the metastatic or perioperative setting. The study by Wong et al14 was a noncomparative randomized trial that randomized patients to 2 arms (cetuximab and cetuximab-paclitaxel) but discontinued enrollment on cetuximab after accruing 11 patients because of futility. Patients with available progression data by 6 months and survival data by 12 months were eligible for analysis, and others were censored.4–10,12–14 Progression was defined as objective tumor progression (by Response Evaluation Criteria in Solid Tumors [RECIST] 1.0 in 9 trials and World Health Organization criteria in 1 trial by Sternberg et al9), or death from any cause.
Table 1.
Trial and Patient Characteristics
| Therapy and Study, Author | Total No. of Subjects |
No. of Evaluable Subjects |
Median Age in Years (Range) |
Male Gender N (%) |
Visceral Metastasis (%) |
ECOG-PS (%) | Imaging Interval |
|---|---|---|---|---|---|---|---|
| GP (6 cycles vs. to PD)a Albers et al12 |
102 | 98 | 65 (43–81) | 73 (74) | 40% | 0: 49% 1: 21% 2: 14% NA: 15% |
Every 60 d |
| Irinotecan Beer et al4 |
45 | 38 | 63 (46–80) | 28 (74) | 87% | 0: 24% 1: 61% 2: 11% NA: 5% |
Every 63 d |
| Docetaxel + vandetanib/placeboa Choueiri et al5 |
152 | 144 | 66 (37–88) | 99 (69) | 70% | 0: 51% 1: 71 (49) |
At 42 d then every 63 d |
| Vinflunine Culine et al6 |
57 | 57 | 63 (41–81) | 46 (81) | 49% | 0: 53% 1: 44% 2: 4% |
Every 42 d |
| Nab-paclitaxel Ko et al7 |
48 | 48 | 68 (39–88) | 40 (83) | 58% | 0: 33% 1: 50% 2: 17% |
Every 42 d |
| Gefitinib Petrylak et al8 |
31 | 31 | 68 (37–82) | 25 (81) | 74% | 0: 55% 1: 32% 2: 3% 3: 3% NA: 6% |
Every 60 d |
| GP Sternberg et al9 |
41 | 36 | 64 (33–78) | 28 (78) | 53% | 0: 36% 1: 11% 2: 47% 3: 2 (6) |
Every 42 d |
| Pazopanib Pili et al13 |
23 | 7 | 66 (40–76) | 3 (43) | 57% | 0: 43% 1: 57% |
Every 30 d |
| Vinflunine Vaughn et al10 |
151 | 148 | 66 (31–83) | 119 (80) | 49% | 0: 68% 1: 32% |
Every 42 d |
| Cetuximab or cetuximab-paclitaxel Wong et al14,b |
39 | 39 | 69 (46–83) | 34 (87) | 87% | 0: 41% 1: 51% 2: 8% |
Every 60 d |
| All studies | 689 | 646 | 65 (31–88) | 495 (77) | 59% | 0: 50% 1: 39% 2: 8% 3: < 1% NA: 3% |
Patients with available progression and survival information.
Abbreviations: ECOG-PS = Eastern Cooperative Oncology Group Performance Status; GP = gemcitabine plus paclitaxel; NA = not available; OS12 = overall survival at 12 months; PD = progressive disease; PFS6 = progression-free survival at 6 months.
No differences were seen for primary efficacy endpoint between arms of the trial (overall outcomes are shown for these studies and not outcomes by arm).
Noncomparative randomized trial that randomized patients to 2 arms (cetuximab and cetuximab-paclitaxel) but discontinued enrollment on cetuximab arm after 11 patients because of futility (overall outcomes are shown for this study and outcomes by arm were published separately). All studies used RECIST 1.0 to evaluate response except the trial by Sternberg et al, which used World Health Organization criteria.
A second-line phase III trial of patients with advanced UC was used for external validation.11 In this trial, 370 patients who had received 1 prior regimen for metastatic disease were treated with vinflunine plus best supportive care (BSC) (n = 253) or BSC alone (n = 117). This trial used central radiology review and RECIST 1.0 for objective tumor assessment.
Statistical Analysis
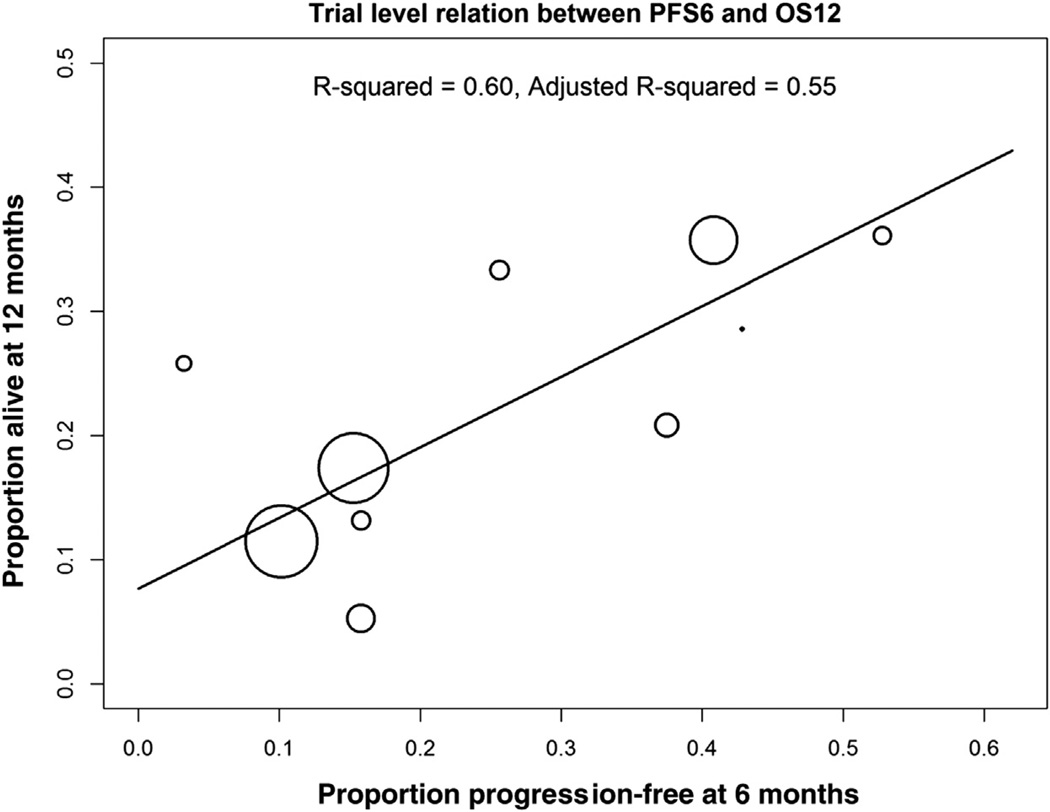
Unadjusted and adjusted binomial confidence intervals (CIs) for PFS6, OS12, and response were reported, with adjustment for variability between trials using random effects models. To get an estimate of PFS6 with an appropriate estimate of standard error, we fit generalized linear mixed models with normal random effects for trial, using a penalized quasi-likelihood estimation approach as implemented in the glmmPQL function of the MASS package in “R”.18,19 The relationship between PFS6/RR and OS12 was assessed at the trial level using weighted linear regression, with larger studies having more influence and Pearson correlation. For the weighted linear regression correlation, the fitted line is from a weighted least-squares regression model with weights proportional to the study size. The circles are proportional to the study size. The equation for the regression model is y = 0.07694 + 0.5685*x. If there was perfect agreement between OS12 and PFS6, the slope would be 1.00. In contrast, the Pearson correlation treats all trials as equal regardless of size. For the Pearson correlation, values from 0.3 to 0.5 generally indicate a large positive association.
The relationship between PFS6/RR and OS12 at the individual level was assessed using Pearson chi-square test with Yates continuity correction. Statistical analyses used “R” statistical computing software, version 2.8.0. A secondary analysis was conducted to examine the trial and individual-level associations of PFS6 and OS12 based on prior chemotherapy in the perioperative disease or metastatic settings. The second-line phase III trial comparing BSC with vinflunine plus BSC was used for external validation.11
Results
Trial and Patient Characteristics
Of 689 patients enrolled in 10 phase II trials used as the discovery dataset, 646 were evaluable for PFS analysis (with available trial-defined progression and survival data). Patients were censored because of loss to follow-up and removal from trial for reasons other than progression (eg, toxicities or patient decision). A total of 560 were evaluable for response (with available baseline measurable disease and survival data) (Table 1). The agents evaluated in these trials included chemotherapy (gemcitabine-paclitaxel, nanoparticle-albumin-bound (nab)- paclitaxel, irinotecan, docetaxel, vinflunine), biologic agents (gefitinib, pazopanib, cetuximab), and the combination of chemotherapeutic and biologic agents (docetaxel-vandetanib, paclitaxel-cetuximab). The overall median age of patients was 65 years, the majority were male (77%), visceral metastases were present in 59%, and the majority (89%) had Eastern Cooperative Oncology Group Performance Status (ECOG-PS) 0–1 (Table 1). Of the 646 patients with progression events, 535 had objective tumor progression and 111 had died. The overall PFS6 was 22% (95% CI, 17–24), and adjusted PFS6 was 23% (95% CI, 15–34). The overall OS12 was 20% (95% CI, 17–24), and adjusted OS12 was 21% (95% CI, 15–29) (Table 2). Among patients evaluable for response and OS, the RR was 22% (95% CI, 18–25) and adjusted RR was 21% (95% CI, 13–32) (Table 2).
Table 2.
Overall and Trial-Level Outcomes
| Trial | No. of Subjects |
PFS6 % (95% Exact Binomial CI) |
Adjusted PFS6a % (95% CI) |
OS12 % (95% Exact Binomial CI) |
Adjusted OS12a % (95% CI) |
RR % (95% Exact Binomial CI) |
Adjusted RRa % (95% CI) |
|---|---|---|---|---|---|---|---|
| Albers et al12 | 98 | 41 (31–51) | 40 (27–53) | 36 (26–46) | 34 (25–44) | 40 (30–52) | 39 (26–54) |
| Beer et al4 | 38 | 16 (6–31) | 17 (11–26) | 13 (4–28) | 16 (11–22) | 12 (3–27) | 14 (8–22) |
| Choueiri et al5 | 144 | 15 (10–22) | 16 (10–24) | 17 (12–25) | 18 (12–25) | 9 (5–16) | 10 (6–17) |
| Culine et al6 | 57 | 16 (7–28) | 17 (10–26) | 5 (1–15) | 10 (7–14) | 35 (16–57) | 32 (20–46) |
| Ko et al7 | 48 | 38 (24–53) | 36 (24–49) | 21 (10–35) | 21 (15–29) | 25 (14–40) | 24 (15–37) |
| Petrylak et al8 | 31 | 3 (0–17) | 9 (5–14) | 26 (12–45) | 24 (17–33) | 4 (0–18) | 9 (5–14) |
| Sternberg et al9 | 36 | 53 (35–70) | 48 (35–61) | 36 (21–54) | 32 (23–41) | 61 (43–77) | 55 (41–69) |
| Pili et al13 | 7 | 43 (10–82) | 32 (22–45) | 29 (4–71) | 23 (16–31) | 14 (0–58) | 18 (11–28) |
| Vaughn et al10 | 148 | 10 (6–16) | 11 (7–18) | 11 (7–18) | 13 (9–18) | 16 (10–23) | 16 (10–26) |
| Wong et al14 | 39 | 26 (13–42) | 25 (16–37) | 33 (19–50) | 30 (22–39) | 18 (8–34) | 19 (11–29) |
| All studies combined | 646 | 22 (17–24)b | 23 (15–34) | 20 (17–24)b | 21 (15–29) | 22 (18–25)b | 21 (13–33) |
Abbreviations: CI = confidence interval; OS12 = overall survival at 12 months; PFS6 = progression-free survival at 6 months.
The adjusted models take into account heterogeneity between studies with a random study effect. See Statistical Methods.
This CI does not take into account heterogeneity between studies. That is, the basic (probably false) assumption for this CI is that the true PFS6 or OS12 is the same for all the trials, and the variability between trials is entirely due to chance.
Patient characteristics and outcomes in the phase III trial used for external validation have been described by Bellmunt et al.11 To summarize, the median age of patients was approximately 65 years, visceral metastasis was present in approximately 74% of patients, and all patients had an ECOG-PS 0–1. In this trial, OS was not statistically better by intention-to-treat analysis, but an extension of median OS was observed in the eligible population (n = 357, 6.9 vs. 4.9 months, P = .04) and after adjusting for prognostic factors (hazard ratio, 0.77; P = .036). Overall median PFS was statistically better for vinflunine plus BSC compared with BSC alone (3.0 vs. 1.5 months, P = .001). Of the 357 eligible patients, 17 with PFS censored before 6 months or OS censored before 12 months were excluded, leaving 340 evaluable patients for external validation of PFS6.11 Of the progression events, 231 were objective tumor progression and 104 were deaths, and 5 patients were alive with PFS > 6 months and OS > 12 months. This trial accrued 270 patients with baseline measurable disease who were evaluable for response. The overall RR was better for vinflunine plus BSC compared with BSC (8.6% vs. 0%, P = .0063).
Overall Association of PFS6 With OS12
For overall trial-level association, the P value for significance of the regression line was .0086 (Fig. 1). The adjusted squared correlation coefficient R2 equaled 0.55, which indicates that PFS6 explains 55% of the variability in OS12. The Pearson correlation between trial-level PFS6 and OS12 was 0.66 (P = .037).
Figure 1. Trial-Level Relation Between PFS6 and OS12. The fitted Line is From a Weighted Least-Squares Regression Model With Weights Proportional to the Study Size (ie, the Circles are Proportional to the Study Size). R2 = 0.55 (P = .0086).
Abbreviations: OS12 = overall survival at 12 months; PFS6 = progression-free survival at 12 months.
The individual-level agreement between PFS6 and OS12 was 82% (Қ = 0.45, Pearson chi-square with Yates continuity correction = 130.69, df = 1, P < .0001). As shown in Table 3, the Pearson chi-square for the 2 × 2 table shows that the additional agreement over chance is highly statistically significant. The Pearson chi-square constructs a statistic from the “observed” minus the “expected” counts.
Table 3.
Individual-Level Association of PFS6 and OS12
| OS12 | ||
|---|---|---|
| Alive at 12 Mo Count (Expected Count) | Dead at 12 Mo Count (Expected Count) | |
| PFS6 (Evaluable = 646) | ||
| No progression at 6 mo | 78 (29.0) | 65 (114.0) |
| Progression at 6 mo | 53 (102.0) | 450 (401.0) |
| Response (Evaluable = 560) | ||
| No response | 64 (99.1) | 374 (338.9) |
| Response | 62 (26.9) | 57 (92.1) |
The table has observed counts with expected counts in parentheses. Expected counts computed assuming no relationship between (unadjusted) PFS6 and OS12. Pearson chi-square with Yates continuity correction = 130.69, df = 1, P = 2.9e-30. The Pearson chi-square for the 2 × 2 table shows that the additional agreement over chance is highly statistically significant. The Pearson chisquare constructs a statistic from the “observed” minus the “expected” counts. For the PFS6 dataset, 78 + 450 = 528/646 = 82% of the subjects show agreement between OS12 and PFS6. One would expect 29 + 401 = 430/646 = 67% to show agreement by chance (numbers in parentheses). Similar calculations for the response dataset yield 78% agreement between response and OS12.
Abbreviations: OS12 = overall survival at 12 months; PFS6 = progression-free survival at 6 months.
Overall Association of Response and OS12
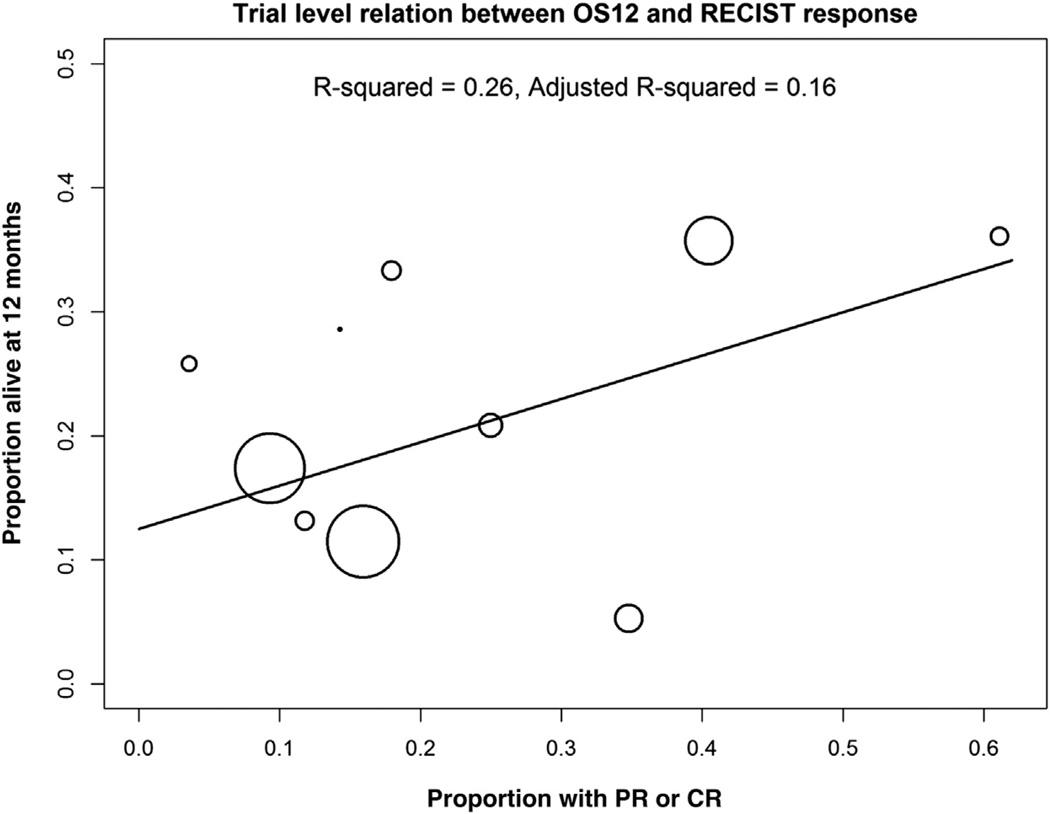
For the trial-level correlation of response and OS12, by a weighted least-squares regression model, the P value for significance of the regression line was .1359, and the adjusted R2 equaled 0.16 (Fig. 2). The Pearson correlation between trial-level response and OS12 was 0.37 (P = .30). The individual-level agreement between response, and OS12 was 78% (Қ = 0.36, P < .0001, Pearson chisquare (Yates corrected) = 90.4, df = 2, P < .001) (Table 3).
Figure 2. Trial-Level Relation Between OS12 and RECIST Response. The Fitted Line is From a Weighted Least-Squares Regression Model With Weights Proportional to the Study Size (ie, the Circles are Proportional to the Study Size). R2 = 0.16 (P =.1359).
Abbreviations: CR = complete response; OS12 = overall survival at 12 months; PR = partial response.
Association of PFS6 and Response With OS12 Based on Prior Chemotherapy in Perioperative or Metastatic Settings
Data regarding the setting of prior chemotherapy were available for 605 patients. Of these patients, 214 had received prior perioperative chemotherapy and 391 had received prior chemotherapy for metastatic disease. Trial- and individual-level associations for PFS6 with OS12 appeared stronger than the associations for response and OS12 in those receiving prior therapy in both the perioperative and metastatic disease settings (Table 4).
Table 4.
Trial- and Individual-Level Associations for PFS6 and Response With OS12 Based on Setting of Prior Therapy
| Setting of Prior Chemotherapy |
Association of PFS6 With OS12 | Association of Response With OS12 | ||
|---|---|---|---|---|
| Trial-level | Individual-level | Trial-level | Individual-level | |
| Perioperative | Adjusted R2 = 0.82 Pearson = 0.92 |
Kappa = 0.51 | Adjusted R2 = 0.54 Pearson = 0.78 |
Kappa = 0.40 |
| Metastatic | Adjusted R2 = 0.22 Pearson = 0.38 |
Kappa = 0.43 | Adjusted R2 = −0.13 Pearson = −0.018 |
Kappa = 0.34 |
Abbreviations: OS12 = overall survival at 12 months; PFS6 = progression-free survival at 6 months.
External Validation of Association of PFS6 and Response With OS12
The individual-level agreement between PFS6 and OS12 was 81% (Қ = 0.44, Pearson chi-square with Yates continuity correction = 63.73, P < .0001). The correlation in individual risk groups based on prognostic factors, hemoglobin < 10 g/dL versus > 10 g/dL, ECOG-PS (1 vs. 0), and liver metastasis was examined.20 PFS6 and OS12 were associated when stratified by risk group and individual prognostic factors (P < .0001 by Cochran–Mantel–Haenszel statistics), except the group with hemoglobin < 10 g/dL (P = .8787).
The individual-level agreement between response and OS12 in 270 evaluable patients with measurable disease at baseline was 76% (Қ = 0.17, Pearson chi-square with Yates continuity correction = 13.57, P = .0002). Excluding the 108 patients in the BSC arm (who exhibited no responses) showed a stronger individual-level agreement between response and OS12 of 82% (Қ = 0.53, Pearson chi-square with Yates continuity correction = 62.98, P < .0001).
Discussion
This study shows a significant correlation between PFS6 and OS12 at the trial and individual levels in the context of second-line therapy using cytotoxic and/or biologic agents for advanced UC. Although response correlated with OS12 at the individual level (albeit less robustly), the trial-level association was not statistically significant. Thus, PFS6 captures durable benefits more robustly than RR. This study was conducted in one of the largest assembled datasets of second-line therapy for advanced UC (646 evaluable of 689 patients) and shows a significant correlation between PFS6 and OS12 at the trial (R2 = 0.55, Pearson correlation = 0.66) and individual levels (82%, Қ = 0.45). The second-line therapy included both cytotoxic and/or biologic agents, and included patients who had received prior perioperative therapy only or therapy for metastatic disease. Although response correlated with OS12 at the individual level albeit less robustly (78%, Қ = 0.36), the trial-level association was not statistically significant (R2 = 0.16, Pearson correlation = 0.37). In addition, we externally validated the significant correlation of PFS6 with OS12 at the individual patient level (81%, Қ = 0.44) in a large phase III trial (n = 340 evaluable of 370 patients). Thus, PFS6 may be useful and appears robust as an intermediate endpoint to facilitate the screening of a broad spectrum of agents in phase II trials.
Secondary analyses based on prognostic risk groups and setting of prior therapy also demonstrated stronger associations for PFS6 with OS12 compared with response with OS12. Of note, both PFS6 and response appeared to be more robustly associated with OS12 in the context of prior chemotherapy in the perioperative setting as opposed to the metastatic setting. However, this finding may be a result of the smaller number of patients who had received prior perioperative chemotherapy or greater variability in association with those who had received prior therapy for metastatic disease. The RR correlated with OS12 at the individual patient level in the external validation dataset more strongly (82%, Қ = 0.53) in the vinflunine plus BSC arm compared with the overall correlation when combining both arms (76%, Қ = 0.17), although both associations were statistically significant. These data suggest that in the absence of a cytotoxic agent that induces tumor regressions, response is unlikely to be a useful intermediate endpoint. The caveat is that in some settings, certain biologic cytostatic agents may extend PFS without necessarily extending OS by promoting certain resistance mechanisms. Moreover, the number of patients in subsets receiving chemotherapy alone, biologic agent alone, or both chemotherapy and biologic agents was considered suboptimal for such secondary analyses examining each of these subsets. However, the majority of evaluable patients in the discovery and validation datasets had received chemotherapeutic agents. Thus, our data require further validation in large datasets of patients receiving different classes of biologic agents and in larger numbers of patients receiving combination chemotherapy and biologic agents.
Study Limitations
Our analysis suffers from limitations typical to retrospective studies. The phase II trials objectively assessed disease at varying intervals (6–12 weeks). However, PFS6 essentially represents a landmark analysis beyond the median PFS and partly overcomes the confounding impact of imaging intervals on median PFS when determining progression (because most patients would have undergone multiple radiographic assessments by 6 months). Although most deaths in the context of second-line therapy are attributable to tumor progression, a proportion of deaths may be attributable to other events, which may inflate the association of progression and death. Nevertheless, the majority of patients in both the discovery dataset (535/646, ie, 82.8%) and the validation dataset (231/340, ie, 68%) exhibited objective tumor progression as the progression event rather than death. Therefore, we do not expect an analysis excluding death as a progression event to alter our findings. Moreover, we used the conventional definition of PFS, which accounts for both objective progression and death, whichever comes first. A positive attribute of our study is that the discovery dataset is one of the largest such second-line UC datasets assembled. The dataset included only well-conducted prospective phase II trials by respected institutions and investigators, which are likely to adequately capture progression events compared with a retrospectively assembled nontrial dataset. Censoring for the primary analysis examining the association of PFS6 and OS12 occurred in only 43 of 689 patients (6.2%) in the discovery dataset and in only 17 of 357 patients (4.8%) in the validation dataset. Moreover, the external validation was conducted in the largest phase III trial reported in the second-line advanced UC context. However, this phase III trial was considered to be inadequate in size for a formal surrogacy analysis. In the future, a larger second-line dataset may be able to conduct such a stringent analysis.
One pitfall is that capturing PFS6 does require a longer follow-up than the time required to capture response. However, given that the transition of drug development from phase II to phase III trials has yielded a poor rate of subsequent successful phase III trials (ie, showing benefit for the experimental agent), a more thorough evaluation at the phase II stage of drug development is in order. One measure to avoid a misleading positive signal at the phase II stage of drug development is to better capture durable activity. Indeed, PFS6 was studied as a candidate endpoint for our study, and PFS at shorter time-points (eg, 3 or 4 months) was not studied because it does not reflect a durable benefit. Another caveat is that similar to RR, PFS6 is likely to vary as a function of baseline prognosis of patients and definition of second-line therapy. Thus, PFS6 needs to be defined in different prognostic groups to enable its optimal use in nonrandomized trials. This problem is illustrated by the variable PFS6 (3%–53%) in the individual phase II trials combined in the discovery dataset (Table 2). Furthermore, the definition of second-line therapy requires standardization and consensus. Therefore, randomized phase II trials with PFS6 as the primary endpoint should be preferred to identify signals of benefit with novel regimens compared with conventional regimens. Moreover, the magnitude of trial-level improvement in PFS6 that may translate to trial-level extension of OS needs further study but may be informed by the finding that an improvement in PFS6 from 13.25% with BSC to 26.48% with vinflunine plus BSC translated to a 23% reduction in hazard of death in the phase III trial (personal communication in September 2012, R. Fougeray).11
Conclusions
This study was conducted in one of the largest assembled datasets of second-line therapy for advanced UC and shows a significant correlation between PFS6 and OS12 at the trial and individual levels. The second-line therapy included both cytotoxic and biologic agents, and included patients who had received prior perioperative therapy only or therapy for metastatic disease. Although response correlated with OS12 at the individual level albeit less robustly, the trial-level association was not statistically significant. In addition, we externally validated the significant correlation of PFS6 with OS12 at the individual patient level in a large phase III trial. Thus, we conclude that PFS6 may be useful and seems robust as an endpoint to facilitate the screening of a broad spectrum of novel agents in future phase II trials in the setting of second-line therapy of advanced UC. Our data require further validation in large datasets of patients receiving different classes of biologic agents and in larger numbers of patients receiving combination chemotherapy and biologic agents.
Clinical Practice Points.
The goal of this retrospective study was to examine the association of PFS at 6 months with OS in the context of second-line therapy of advanced UC.
Individual patient data were pooled from 10 available phase II trials enrolling a total of 646 evaluable patients that investigated second-line chemotherapy or biologics for advanced UC.
External validation was conducted using individual patient data from the largest phase III trial conducted in this setting enrolling 340 evaluable patients for this analysis.
This retrospective analysis of individual patient data from 10 pooled prospective phase II trials followed by external validation in a phase III trial suggests that PFS at 6 months is a more optimal and innovative primary endpoint than RR to evaluate new agents for the second-line therapy of advanced UC.
Acknowledgments
G. Sonpavde, N. J. Vogelzang: research support to institution from Boehringer-Ingelheim. R. Fougeray: employee of Pierre-Fabre. T. K. Choueiri, A. Q. Qu, J. E. Rosenberg, G. Sonpavde: research support to institution from AstraZeneca. J. Bellmunt: research support to institution from Pierre-Fabre. Y.-N. Wong: research support to institution from BMS. Y.-J. Ko, S. S. Sridhar, G. Sonpavde: research support to institution from Celgene. U. N. Vaishampayan: research support to institution from GSK. M. D. Galsky: research support to institution from Eli-Lilly. G. Niegisch and P. Albers: research support to institution from Eli Lilly and BMS.
Footnotes
Disclosure
N. Agarwal, K. M. Boucher, B. L. Maughan, A. M. Mehta, D. P. Petrylak, T. M. Beer, C. N. Sternberg: no relevant conflicts.
Presented in part at: the poster discussion session, American Society of Clinical Oncology conference, June 1–5, 2012, Chicago, Illinois, and as an oral presentation at: the European Society for Medical Oncology Congress, September 28 to October 2, 2012, Vienna, Austria
References
- 1.Saxman SB, Propert KJ, Einhorn LH, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1997;15:2564–2569. doi: 10.1200/JCO.1997.15.7.2564. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. 2006;42:50–54. doi: 10.1016/j.ejca.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 3.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 4.Beer TM, Goldman B, Nichols CR, et al. Southwest Oncology Group phase II study of irinotecan in patients with advanced transitional cell carcinoma of the urothelium that progressed after platinum-based chemotherapy. Clin Genitourin Cancer. 2008;6:36–39. doi: 10.3816/cgc.2008.n.006. [DOI] [PubMed] [Google Scholar]
- 5.Choueiri TK, Ross RW, Jacobus S, et al. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol. 2012;30:507–512. doi: 10.1200/JCO.2011.37.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Culine S, Theodore C, De Santis M, et al. A phase II study of vinflunine in bladder cancer patients progressing after first-line platinum-containing regimen. Br J Cancer. 2006;94:1395–1401. doi: 10.1038/sj.bjc.6603118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko YJ, Canil CM, Mukherjee SD, et al. Nanoparticle albumin-bound paclitaxel for second-line treatment of metastatic urothelial carcinoma: a single group, multicentre, phase 2 study. Lancet oncol. 2013;14:769–776. doi: 10.1016/S1470-2045(13)70162-1. [DOI] [PubMed] [Google Scholar]
- 8.Petrylak DP, Tangen CM, Van Veldhuizen PJ, Jr, et al. Results of the Southwest Oncology Group phase II evaluation (study S0031) of ZD1839 for advanced transitional cell carcinoma of the urothelium. BJU Int. 2010;105:317–321. doi: 10.1111/j.1464-410X.2009.08799.x. [DOI] [PubMed] [Google Scholar]
- 9.Sternberg CN, Calabro F, Pizzocaro G, et al. Chemotherapy with an every-2-week regimen of gemcitabine and paclitaxel in patients with transitional cell carcinoma who have received prior cisplatin-based therapy. Cancer. 2001;92:2993–2998. doi: 10.1002/1097-0142(20011215)92:12<2993::aid-cncr10108>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Vaughn DJ, Srinivas S, Stadler WM, et al. Vinflunine in platinum-pretreated patients with locally advanced or metastatic urothelial carcinoma: results of a large phase 2 study. Cancer. 2009;115:4110–4117. doi: 10.1002/cncr.24460. [DOI] [PubMed] [Google Scholar]
- 11.Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27:4454–4461. doi: 10.1200/JCO.2008.20.5534. [DOI] [PubMed] [Google Scholar]
- 12.Albers P, Park SI, Niegisch G, et al. Randomized phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: short-term versus prolonged treatment [German Association of Urological Oncology (AUO) trial AB 20/99] Ann Oncol. 2011;22:288–294. doi: 10.1093/annonc/mdq398. [DOI] [PubMed] [Google Scholar]
- 13.Pili R, Qin R, Flynn PJ, et al. A Phase II Safety and Efficacy Study of the Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitor Pazopanib in Patients With Metastatic Urothelial Cancer. Clin Genitourin Cancer. 2013 Jul 25; doi: 10.1016/j.clgc.2013.05.005. [e-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong YN, Litwin S, Vaughn D, et al. Phase II trial of cetuximab with or without paclitaxel in patients with advanced urothelial tract carcinoma. J Clin Oncol. 2012;30:3545–3551. doi: 10.1200/JCO.2012.41.9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polley MY, Lamborn KR, Chang SM, et al. Six-month progression-free survival as an alternative primary efficacy endpoint to overall survival in newly diagnosed glioblastoma patients receiving temozolomide. Neuro Oncol. 2010;12:274–282. doi: 10.1093/neuonc/nop034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballman KV, Buckner JC, Brown PD, et al. The relationship between sixmonth progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007;9:29–38. doi: 10.1215/15228517-2006-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster NR, Qi Y, Shi Q, et al. Tumor response and progression-free survival as potential surrogate endpoints for overall survival in extensive stage small-cell lung cancer. Cancer. 2011;117:1262–1271. doi: 10.1002/cncr.25526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg JE, von der Maase H, Seigne JD, et al. A phase II trial of R115777, an oral farnesyl transferase inhibitor, in patients with advanced urothelial tract transitional cell carcinoma. Cancer. 2005;103:2035–2041. doi: 10.1002/cncr.21023. [DOI] [PubMed] [Google Scholar]
- 19.Venables WN, Ripley BD. Modern Applied Statistics with S. 4th ed. New York NY: Springer; 2002. [Google Scholar]
- 20.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28:1850–1855. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]