Abstract
Background and Purpose
Stroke treatment is constrained by limited treatment windows, and the clinical inefficacy of agents that showed preclinical promise. Yet animal and clinical data suggest considerable post-stroke plasticity, which could allow for treatment with recovery-modulating agents. Memantine (MEM) is a well-tolerated N-methyl-D-aspartate (NMDA) glutamate receptor antagonist in common use for Alzheimer's disease.
Methods
MEM, 30mg/kg/day, or vehicle, was delivered chronically in drinking water beginning >2 hours after photothrombotic stroke.
Results
Though there was no difference in infarct size, behavior, or optical intrinsic signal (OIS) maps in the first seven days after stroke, mice treated chronically with MEM showed significant improvements in motor control, measured by cylinder test and grid walking performance, compared to vehicle treated animals. OIS revealed an increased area of forepaw sensory maps at 28 days after stroke. There was decreased reactive astrogliosis and increased vascular density around the infarcted cortex. Peri-infarct Western blots revealed increased brain-derived neurotrophic factor (BDNF) and phosphorylated-Tropomyosin-related-kinase-B receptor (p-TrkB) expression.
Conclusions
Our results suggest that MEM improves stroke outcome in an apparently non-neuroprotective manner involving increased BDNF signaling, reduced reactive astrogliosis and improved vascularization, associated with improved recovery of sensory and motor cortical function. The clinical availability and tolerability of MEM make it an attractive candidate for clinical translation.
Keywords: memantine, photothrombosis, stroke recovery, BDNF
Introduction
Stroke is the fourth leading cause of death, and a major cause of morbidity worldwide1. Despite an enormous amount of research, treatment options are limited. Tissue plasminogen activator is restricted to the few hours after stroke; beyond this window treatment is limited to supportive care, secondary prevention, and rehabilitation2,3. Neuroprotection, the prevention of cell death beyond the boundaries of the infarct core, has been disappointing in human studies. Yet despite the loss of neuronal tissue, considerable plasticity is retained after stroke. Manipulation of the recovery process has emerged as an alternative and potentially more tractable target in stroke research4,5.
Memantine (MEM) is a non-competitive, use-dependent NMDA antagonist, which is used to treat Alzheimer's disease6,7. MEM is neuroprotective, reducing infarct size when given acutely8–14. However, MEM has mechanisms that may be relevant beyond the acute setting15,16. We treated mice chronically with MEM, using doses designed to mimic usual serum concentrations in humans17, delivered to avoid neuroprotection and isolate recovery effects.
Materials and Methods
Protocols were approved by the Animal Research Committee of UCLA.
Focal Ischemia
Male C57BL/6J mice (n=82;28-32g) underwent photothrombosis (2-mm diameter irradiation, positioned 1.5mm lateral from Bregma, 5min after 200μl of a 10mg/ml Rose Bengal solution was injected intraperitoneally) or sham treatment as described4,18. Animals were then allocated to one type of follow-up experiment (behavior, sensory mapping, histology, or Western blot; see below) following treatment with MEM or vehicle.
Memantine Treatment
Mice were randomly assigned to treatment for 28 days with MEM (Sigma; 30 mg/kg/day in 2% sucrose solution17) or 2% sucrose vehicle delivered continuously in drinking water, beginning 2 hours after photothrombosis. This dosing regimen results in serum concentrations of ~1μM in C57Bl/6J mice, comparable to therapeutic concentration in humans17,19.
Behavioral testing
Cylinder test and grid-walking test were performed 7 days before, and 7,14,21, and 28 days after stroke (8 animals/group; Figure 1A). Video analysis was by blinded raters. Cylinder test. Animals were video-recorded in a clear acrylic cylinder for 10min in order to determine forelimb preference. Limb contacts were counted and the index for preference was obtained: Index=(Left–Right)/(Left+Right+simultaneous)20–22. Grid-walking test was performed as described4,5. Mice walked on an elevated wire grid for 5min while being video-recorded. Total normal steps and foot-faults were counted for each limb, and ratio between foot-faults and total-steps calculated.
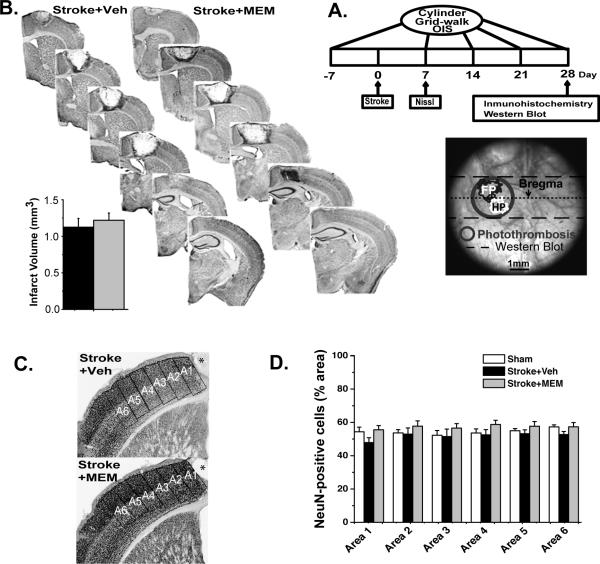
Figure 1.
A.Timeline. Separate groups of animals were used for each kind of experiment, following identical stroke, recovery, and treatment procedures. Schematic: OIS functional mapping preparation, showing photothrombosis and boundaries of cortex taken for Western blot. B.Post-stroke MEM treatment shows no structural or cellular evidence of neuroprotection. Representative 40μm Nissl-stained sections collected 7 days after infarct for vehicle- and MEM-treated animals. Inset: no significant difference in mean (±SEM) infarct area in mm3; 4 animals, >/=6 sections per group. C.Representative NeuN-stained sections from vehicle and MEM-treated animals. A1-A6: regions-of-interest (ROI); *infarct core. D.Percent-area occupied by NeuN-positive cells in 350μm-wide ROIs, increasing distances from infarct core. There was no significant difference in area between groups (4 animals, >/=6 sections per group).
Sensory mapping
OIS imaging (5 animals/group) was performed 7 days before and 7,14,21 and 28 days after focal ischemia, as described18. Briefly, electrical stimulation (50Hz, 0.001s pulse width, 0.14-0.22mA) was delivered to the forepaw and hindpaw (FP, HP) through pairs of subdermal needle electrodes, while 617nm reflectance was recorded through the exposed skull. Images were normalized to averaged prestimulus frames. Functional maps were quantified as the area whose pixels showed a stimulus response > 50% of maximum response. Image analysis was performed using either plugins or custom routines written for ImageJ23.
Histology
Full histological methods appear in the Data Supplement. Briefly, mice were perfused transcardially with 0.9% NaCl followed by 4% paraformaldehyde in phosphate-buffered saline, 7 or 28 days after photothrombosis. Forty-μm frozen sections were prepared as described4,24. Every third section was collected to quantify infarct volumes using Nissl stain (4 animals, >/=6 sections each per group). Brightfield immunohistochemistry (4 animals, >/=6 sections each per group) was performed using biotinylated secondary antibodies, biotin-avidin-peroxidase complex and diaminobenzidine as the developing agent25. Primary antibodies were: rabbit anti-GFAP, rat anti-PECAM-1, bio-NeuN. Stained sections were examined and photographed using brightfield microscopy. Immunohistochemical images from animals with stroke were analyzed from the border of the glial scar to a lateral distance of 2100μm, divided into 6 regions for analysis. Sham treated animals were analyzed over identical cortical regions. Because post-stroke scarring causes changes in cortical thickness, all measures were normalized to the area of the region analyzed
Western blot
Brains were placed in a mouse matrix in which a 2mm coronal section was cut, -1mm to +1mm from bregma. This section included the whole ischemic area and cognate regions from the contralateral hemisphere (4-6 animals/group; Figure 1A,B). Hemispheres were separately frozen in dry ice. Protein extracts were prepared using standard methods26. Primary antibodies used for Western blots were anti-VEGF, anti-GDNF, anti-BDNF, anti-Trk-B, anti-phospho-Trk-B. Quantitative densitometric analyses were performed using the Gel Analyzer Plugin (ImageJ).
Statistical Analysis
Experiments were performed in accordance with ARRIVE and NINDS guidelines27,28. Group sizes were determined from a combination of power analysis (see Data Supplement) and our previous work in similar models4,5,18. Animals were randomized to treatment vs. sham groups, and experimenters and raters were blinded to group identity. Comparisons were made with one- or two-way-ANOVA with post-hoc Tukey test for pairwise comparisons. A p<0.05 was considered statistically significant. Data are expressed as mean± standard error of the mean (SEM).
Results
Identical infarct size and neuronal density in MEM- and vehicle-treated animals
Consistent with previous work4,18 an infarct centered 1.5mm lateral to Bregma affected primary motor cortex as well as FP and HP sensory cortex29, with HP sensory cortex being more severely affected. There was no difference in infarct size between MEM- and vehicle-treated animals (Figure 1B), consistent with other work in which MEM was given more than 2 hours after ischemia10,12. NeuN staining revealed no difference in neuronal density between treated and untreated animals (Figure 1C,D). Though histological measures alone do not rule out all types of neuroprotection30, we inferred that MEM was not exerting significant neuroprotective effects using our treatment regimen.
Improved forepaw behavioral recovery in MEM-treated animals
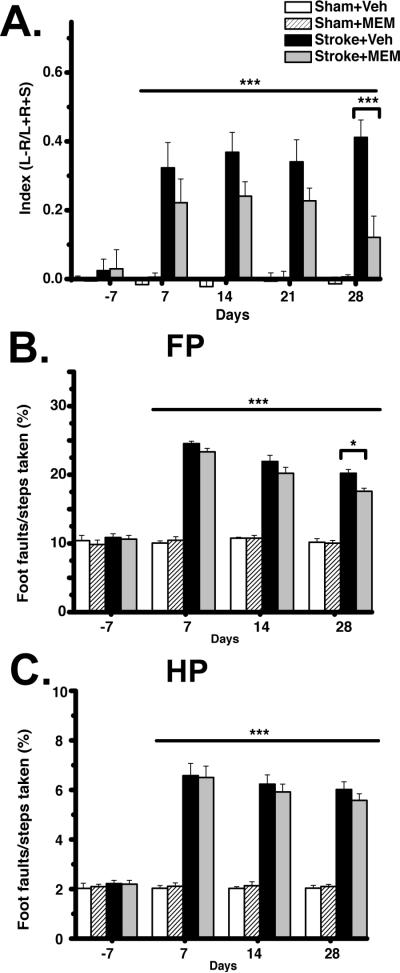
Despite identical infarct volumes, there was significant improvement in behavioral measures of recovery in MEM-treated compared to vehicle-treated animals. Though both MEM- and vehicle-treated animals showed a significant increase in forelimb use asymmetry after stroke, MEM-treated animals showed a progressive recovery of the impaired limb on cylinder test, which was significant at 28 days post-stroke (Figure 2A). For grid-walking test, there was a significant increase in FP and HP foot faults after stroke and a subsequent slow recovery of function. MEM-treated animals showed a greater reduction in FP (but not HP) foot-faults, which became significant 28 days after stroke (Figure 2B,C). Taken together, these data show that MEM treatment was associated with improved recovery of FP, but not HP, function after stroke.
Figure 2. Improved behavioral recovery from stroke with MEM treatment.
A.Cylinder test. Both MEM- and vehicle-treated animals showed significant forepaw use asymmetry after stroke. There was significant improvement in forepaw asymmetry at 28 days of MEM vs. vehicle treatment. B,C.Grid-Walking test. Both MEM- and vehicle-treated animals showed a significant increase in foot-faults after stroke. There was a significant reduction in forelimb but not hindlimb foot-faults at 28 days of MEM compared to vehicle treatment (***p<0.001; *p<0.05, Repeated-measures two-way ANOVA; post-hoc Tukey Test; 8 animals per group).
Improved recovery of forepaw sensory maps with MEM treatment
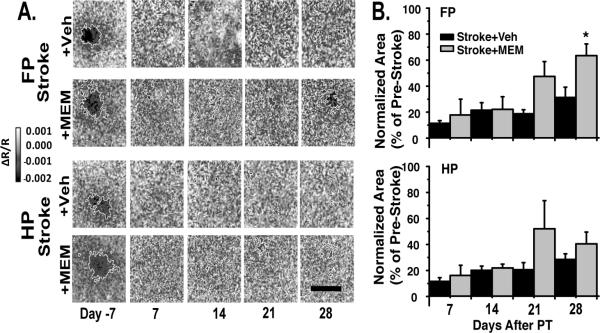
We used OIS to measure the sensory physiology of the peri-infarct cortex during recovery. FP and HP stimulation produced distinct regions of activation in primary somatosensory cortex (Figures 1A,3A), which were essentially abolished after stroke. Supporting a lack of meaningful neuroprotection, there was no significant difference in area of FP or HP activation between MEM- and vehicle treated animals seven days after stroke. Activation area for both FP and HP sensory maps slowly increased during stroke recovery, but remained substantially below pre-stroke conditions for all animals (Figure 3A,B). FP maps showed a significant increase in activation area in MEM-treated compared to vehicle-treated animals at 28 days after stroke. There was no significant difference in HP maps with MEM treatment. Our functional activation data thus showed a similar pattern to behavioral data, with improved recovery in FP but not HP with MEM treatment.
Figure 3. Significant recovery of forepaw sensory maps with MEM treatment.
A.FP and HP sensory maps in representative vehicle and MEM-treated animals. B.Area of activation for FP and HP. There was a significant increase in FP, but not HP, activation area at 28 days of MEM treatment compared to vehicle-treated animals (*p<0.05, twoway repeated-measures ANOVA; post-hoc Tukey Test; 5 animals per group).
Though the behavioral and functional activation data were collected in separate animals, we examined whether there was a correlation between the two measures. There was a significant negative correlation between OIS response area and forepaw use asymmetry (Pearson correlation coefficient (r)=-0.9413; r2=0.8860; p=0.0169 (two-tailed), 95% confidence intervals -0.9962 to -0.3476), supporting an association between functional hemodynamic recovery and behavioral improvement.
Decreased astrogliosis and increased vascular density in peri-infarct cortex of MEM-treated animals
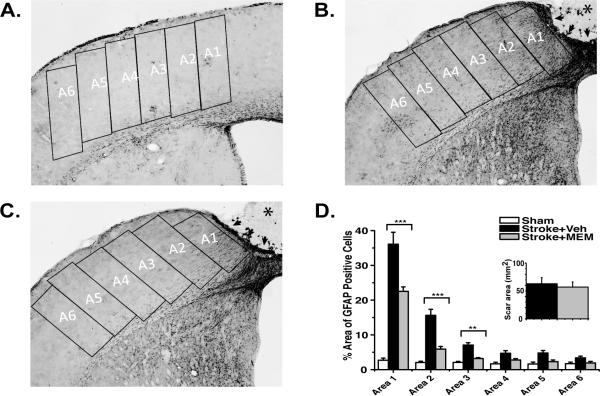
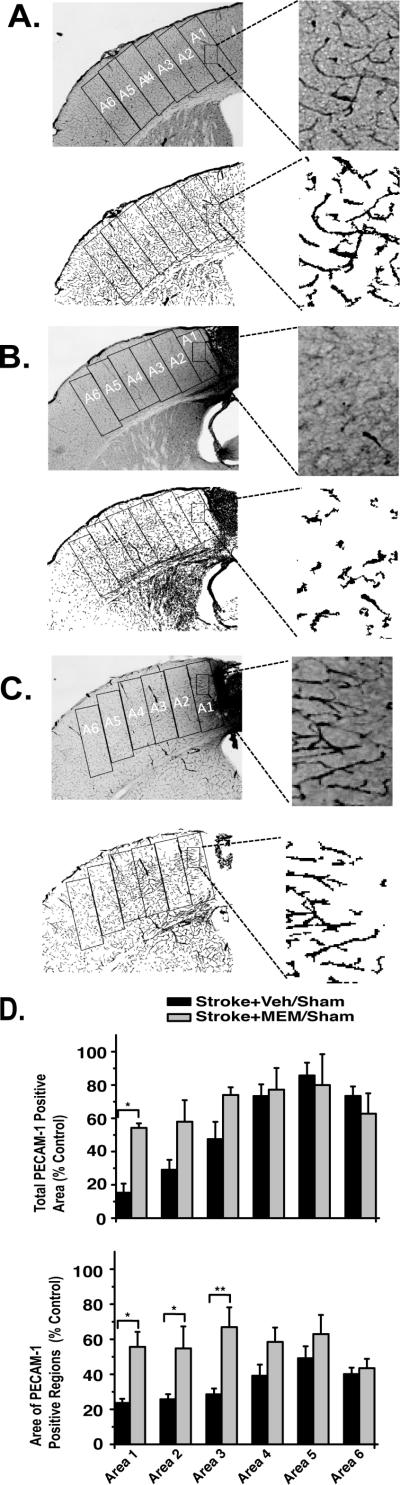
Stroke centered over forepaw motor cortex resulted in a full-cortical-thickness lesion, which at 28 days consisted of a core region of necrotic tissue (stroke+vehicle:1.13±0.12mm3; stroke+MEM:1.22±0.10mm3), encircled by an border of compact glial scar and a surrounding zone of reactive astrogliosis31 that exhibited a gradient of elevated GFAP expression (highest near the lesion; indistinguishable from control hemisphere >1770μm from the lesion border; Figure 4B,C). Vascular density, measured by PECAM1 staining of the endothelium, was maximally decreased in the immediate vicinity of the infarct lesion and became indistinguishable from control hemisphere at distances >2mm from the lesion border (Figure 5B,C). GFAP and PECAM1 immunoreactivity were significantly altered in MEM-treated animals. Total GFAP-expressing-cell area was significantly reduced in each region of interest, becoming indistinguishable from control hemisphere at 1050μm from the lesion border (Figure 4D). PECAM1 expression was significantly increased in MEM-treated vs. vehicle treated animals within the first three zones of tissue (0-1050μm) adjacent to the lesion border (Figure 5D).
Figure 4. Decreased reactive astrocytosis in MEM-treated animals.
A,B,C.Representative GFAP inmuhistochemistry from sham-, vehicle- and MEM-treated groups 28 days after photothrombosis. A1-6: regions of interest; *infarct core; arrows: glial scar. D.There was a significant reduction in percent area occupied by GFAP-positive cells in MEM- compared to vehicle-treated animals (significant differences indicated by asterisks). Inset: no significant difference in the area of the glial scar between MEM- and vehicle-treated animals (One-way ANOVA with post-hoc Tukey Test *p<0.05, **p<0.01, ***p<0.001; 4 animals; >/=6 sections each per group).
Figure 5. Increased vascular density in MEM-treated animals.
A,B,C.Micrographs show representative PECAM-1 inmuhistochemistry for sham-, vehicle- and MEM-treated animals, 28 days after photothrombosis. Skeletonized figures in each panel are the same figures after image processing. D.PECAM-1 quantification. Percent area occupied by PECAM-1 inmunoreactivity in 350μm wide regions of interest beginning at the glial scar margin is a proxy for vascular density; average area of each PECAM-1-positive image region is a proxy for vascular length and diameter. There was a significant difference in both measures between MEM-and vehicle-treated animals (indicated by asterisks). (ANOVA with post-hoc Tukey Test; *p<0.05, 4 animals, >/=6 sections each per group). .
Increased BDNF pathway signaling in peri-infarct cortex of MEM-treated animals
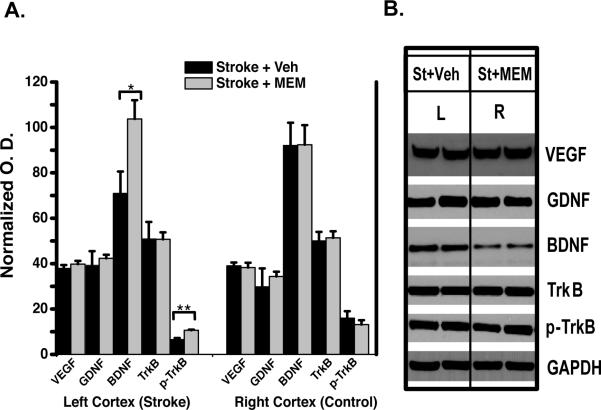
BDNF expression is increased during stroke recovery5,32,33, and our in glial reactivity and vascular morphology results motivated an examination of GDNF and VEGF. We found no difference in GDNF or VEGF expression by Western blot. However, there was a significant increase in peri-infarct BDNF and p-TrkB expression in MEM-treated compared to vehicle-treated animals (Figure 6).
Figure 6. Increased BNDF pathway signaling in MEM-treated animals.
A.Quantification (mean optical density, normalized to GADPH) of Western Blots (representative blots in B.). There was a significant increase in BDNF and phospho-TrkB expression, consistent with activation of the BDNF pathway. (*p<0.05, Student's t-test; 4-6 animals per group).
Discussion
We have shown that chronic treatment with a clinically tolerated medication, dosed to deliver concentrations comparable to human use, improves stroke outcome. The improvements occurred despite the fact that MEM was delivered orally after the stroke, and the lack of any significant difference in infarct size, OIS maps or behavioral testing in the first 7 days after photothrombosis suggests that neuroprotection did not play a significant role in this improvement. The translational significance of our findings is two-fold. Firstly, it suggests a treatment for stroke recovery that is clinically feasible. Secondly, it suggests that stroke recovery can be improved without the stringent time-dependency of neuroprotective strategies.
Recovery-promoting vs. neuroprotective effects of orally-dosed MEM
MEM is neuroprotective when given before12, and sometimes within the first two hours after stroke8–10,12,14. Though we cannot completely rule out all aspects of neuroprotection (e.g. changes in synaptic physiology or dendritic structure/function)30, we are fairly confident that neuroprotection did not play a significant role in our results, as we observed no significant difference between MEM- and vehicle-treated animals in either cell number, behavior, or OIS maps during the first seven days after stroke. This is important as it allows us to specifically assess MEM effects on post-stroke recovery.
Improved sensorimotor recovery in MEM-treated animals
Cylinder and grid walking tests document stroke recovery4,5 and OIS imaging has been used to demonstrate sensory map plasticity after stroke18,34. We observed improvements in both behavior and OIS following MEM compared to vehicle treatment, with a significant correlation between the two measures (albeit collected from separate groups of animals). Interestingly, both tests showed improvement in FP, but not HP, behavior and sensory maps. This is likely due to greater destruction of HP cortex by our stroke technique (Figures 1,3). Alternatively, MEM is an activity-dependent blocker of NMDA receptors35: differential use of the FP compared to HP (e.g. for exploratory activity) might account for a greater effect on FP sensory maps and behavioral function than HP. There are also intrinsic differences in FP and HP excitability and plasticity which might explain a difference between the two cortices36.
Increased vascular density
OIS maps are generated primarily by increases in blood volume or oxygenation specific to the activated region of cortex37, and functional stroke recovery is correlated with recovery of vascular density in peri-infarct regions38. We found that PECAM1 staining, which outlines vascular endothelium, was increased adjacent to the lesion border in MEM-treated compared to vehicle-treated animals. This area corresponds to regions activated on OIS mapping (Figures 1,3). Vascular integrity is a prerequisite for survival of peri-infarct tissue and subsequent functional recovery24,38. Given the use-dependent nature of both post-stroke angiogenesis39 and the neurovascular coupling relationship, it is likely that OIS map plasticity, increased vascular density, and behavioral recovery were mutually dependent processes in MEM-treated animals. Regarding mechanism, BDNF signaling is involved in angiogenesis40,41, and BDNF polymorphisms, associated with poor stroke outcome in humans, show reduced angiogenesis in animal models42. Our peri-infarct BDNF increases may be relevant in this regard.
Decreased GFAP expression
GFAP expression is a hallmark of reactive astrogliosis31. Our observation of a decrease in GFAP immunoreactivity in peri-infarct cortex after MEM-treatment is consistent with other reports associating improved stroke recovery with reduced astrocytic reactivity43,44. Moreover, in a similar animal model, BDNF treatment was associated with a significant decrease in astrogliosis, which is consistent with our results45. It remains unclear whether decreased astrogliosis is a cause or consequence of the recovery process.
Increased BDNF signaling
Increases in BDNF expression have been reported in peri-infarct cortex after stroke; attenuation of BDNF activity worsens outcome46–48. In human studies, BDNF polymorphisms affect stroke outcome49. We observed an increase in BDNF and p-TrkB expression in MEM- compared to vehicle-treated animals after stroke. This increase was specific to the infarcted hemisphere, suggesting that signaling related to peri-infarct recovery and plasticity is important.
MEM may supplement an endogenous tendency toward BDNF increase after stroke: treatment at levels comparable to ours has been shown to increase BDNF mRNA expression across the brain15,50. The mechanism of MEM-induced BDNF increase is not clear: BDNF increase has been associated with both activation51,52 and suppression15,53 of NMDA receptor activity. Moreover, such effects might be independent of MEM's NMDA antagonism54.
Conclusions and translational relevance
We have shown that chronic MEM treatment improves stroke outcome in an apparently non-neuroprotective manner, concomitant with sensory map recovery, decreased reactive astrocytosis, increased vascular density, and increased BDNF/TrkB expression. MEM has been used for many years in treatment of Alzheimer's and other neurological diseases, and has proven well tolerated in an elderly, medically complex population. Recently, MEM has been used to successfully treat aphasia55, showing its promise in post-stroke populations. Further evaluation of MEM in the clinical setting may be warranted.
Supplementary Material
Acknowledgements
Experimental design: HLV,ANC,MVS,STC,KCB; OIS, behavior, Western blot: HLV; surgeries, histology, behavior: ANC; immunohistochemistry: HLV,YA; imaging tools: AC; data analysis: HLV,ANC,YA,MVS,STC,KCB; wrote manuscript: HLV,ANC,MVS, STC,KCB.
Sources of funding
Larry L. Hillblom Foundation(HLV,ACC,STC); American Heart Association Postdoctoral Fellowship; Repatriation Fellowship, New Zealand Neurological Foundation; Sir Charles Hercus Fellowship(ANC); National Institutes of Health NS053957(STC), NS057624(MVS); NS059072,NS070084(KCB).
Footnotes
Disclosures:None.
References
- 1.Kinlay S. Changes in stroke epidemiology, prevention, and treatment. Circulation. 2011;124:e494–496. doi: 10.1161/CIRCULATIONAHA.111.069633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benowitz LI, Carmichael ST. Promoting axonal rewiring to improve outcome after stroke. Neurobiol. Dis. 2010;37:259–266. doi: 10.1016/j.nbd.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmichael ST. Targets for neural repair therapies after stroke. Stroke. 2010;41:S124–126. doi: 10.1161/STROKEAHA.110.597146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarkson AN, Overman JJ, Zhong S, Mueller R, Lynch G, Carmichael ST. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J. Neurosci. 2011;31:3766–3775. doi: 10.1523/JNEUROSCI.5780-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rammes G, Danysz W, Parsons CG. Pharmacodynamics of memantine: an update. Curr Neuropharmacol. 2008;6:55–78. doi: 10.2174/157015908783769671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas SJ, Grossberg GT. Memantine: a review of studies into its safety and efficacy in treating Alzheimer's disease and other dementias. Clin Interv Aging. 2009;4:367–377. doi: 10.2147/cia.s6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Wang YF, Rayudu PV, Edgecomb P, Neill JC, Segal MM, et al. Neuroprotective concentrations of the N-methyl-D-aspartate open-channel blocker memantine are effective without cytoplasmic vacuolation following post-ischemic administration and do not block maze learning or long-term potentiation. Neuroscience. 1998;86:1121–1132. doi: 10.1016/s0306-4522(98)00163-8. [DOI] [PubMed] [Google Scholar]
- 9.Görgülü A, Kinş T, Cobanoglu S, Unal F, Izgi NI, Yanik B, et al. Reduction of edema and infarction by Memantine and MK-801 after focal cerebral ischaemia and reperfusion in rat. Acta Neurochir (Wien) 2000;142:1287–1292. doi: 10.1007/s007010070027. [DOI] [PubMed] [Google Scholar]
- 10.Lapchak PA. Memantine, an uncompetitive low affinity NMDA open-channel antagonist improves clinical rating scores in a multiple infarct embolic stroke model in rabbits. Brain Res. 2006;1088:141–147. doi: 10.1016/j.brainres.2006.02.093. [DOI] [PubMed] [Google Scholar]
- 11.Hao J, Mdzinarishvili A, Abbruscato TJ, Klein J, Geldenhuys WJ, Van der Schyf CJ, et al. Neuroprotection in mice by NGP1-01 after transient focal brain ischemia. Brain Res. 2008;1196:113–120. doi: 10.1016/j.brainres.2007.11.075. [DOI] [PubMed] [Google Scholar]
- 12.Babu CS, Ramanathan M. Pre-ischemic treatment with memantine reversed the neurochemical and behavioural parameters but not energy metabolites in middle cerebral artery occluded rats. Pharmacol. Biochem. Behav. 2009;92:424–432. doi: 10.1016/j.pbb.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Liu C, Lin N, Wu B, Qiu Y. Neuroprotective effect of memantine combined with topiramate in hypoxic-ischemic brain injury. Brain Res. 2009;1282:173–182. doi: 10.1016/j.brainres.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 14.Shih AY, Blinder P, Tsai PS, Friedman B, Stanley G, Lyden PD, et al. The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nat. Neurosci. 2013;16:55–63. doi: 10.1038/nn.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marvanová M, Lakso M, Pirhonen J, Nawa H, Wong G, Castrén E. The neuroprotective agent memantine induces brain-derived neurotrophic factor and trkB receptor expression in rat brain. Mol. Cell. Neurosci. 2001;18:247–258. doi: 10.1006/mcne.2001.1027. [DOI] [PubMed] [Google Scholar]
- 16.Meisner F, Scheller C, Kneitz S, Sopper S, Neuen-Jacob E, Riederer P, et al. Memantine upregulates BDNF and prevents dopamine deficits in SIV-infected macaques: a novel pharmacological action of memantine. Neuropsychopharmacology. 2008;33:2228–2236. doi: 10.1038/sj.npp.1301615. [DOI] [PubMed] [Google Scholar]
- 17.Minkeviciene R, Banerjee P, Tanila H. Memantine improves spatial learning in a transgenic mouse model of Alzheimer's disease. J. Pharmacol. Exp. Ther. 2004;311:677–682. doi: 10.1124/jpet.104.071027. [DOI] [PubMed] [Google Scholar]
- 18.Clarkson A, Lopez-Valdes H, Overman J, Charles A, Brennan K, Carmichael S. Multimodal examination of structural and functional remapping in the mouse photothrombotic stroke model. J Cereb Blood Flow Metab. 2013;33:716–23. doi: 10.1038/jcbfm.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kornhuber J, Quack G. Cerebrospinal fluid and serum concentrations of the N-methyl-D-aspartate (NMDA) receptor antagonist memantine in man. Neurosci. Lett. 1995;195:137–139. doi: 10.1016/0304-3940(95)11785-u. [DOI] [PubMed] [Google Scholar]
- 20.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp. Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Kleim JA, Boychuk JA, Adkins DL. Rat models of upper extremity impairment in stroke. ILAR J. 2007;48:374–384. doi: 10.1093/ilar.48.4.374. [DOI] [PubMed] [Google Scholar]
- 23.Rasband WS. [5/1/13];Image J. 1997 http://imagej.nih.gov/ij/.
- 24.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J. Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Coria H, Green KN, Billings LM, Kitazawa M, Albrecht M, Rammes G, et al. Memantine improves cognition and reduces Alzheimer's-like neuropathology in transgenic mice. Am. J. Pathol. 2010;176:870–880. doi: 10.2353/ajpath.2010.090452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tennant KA, Adkins DL, Donlan NA, Asay AL, Thomas N, Kleim JA, et al. The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cereb. Cortex. 2011;21:865–876. doi: 10.1093/cercor/bhq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corbett D, Nurse S. The problem of assessing effective neuroprotection in experimental cerebral ischemia. Prog. Neurobiol. 1998;54:531–548. doi: 10.1016/s0301-0082(97)00078-6. [DOI] [PubMed] [Google Scholar]
- 31.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ploughman M, Windle V, MacLellan CL, White N, Doré JJ, Corbett D. Brainderived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke. 2009;40:1490–1495. doi: 10.1161/STROKEAHA.108.531806. [DOI] [PubMed] [Google Scholar]
- 33.Ke Z, Yip SP, Li L, Zheng X-X, Tong K-Y. The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: a rat brain ischemia model. PLoS ONE. 2011;6:e16643. doi: 10.1371/journal.pone.0016643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown CE, Li P, Boyd JD, Delaney KR, Murphy TH. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J. Neurosci. 2007;27:4101–4109. doi: 10.1523/JNEUROSCI.4295-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H-SV, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J. Neurochem. 2006;97:1611–1626. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- 36.David-Jürgens M, Churs L, Berkefeld T, Zepka RF, Dinse HR. Differential effects of aging on fore- and hindpaw maps of rat somatosensory cortex. PLoS ONE. 2008;3:e3399. doi: 10.1371/journal.pone.0003399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frostig RD, Lieke EE, Ts'o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gertz K, Priller J, Kronenberg G, Fink KB, Winter B, Schröck H, et al. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ. Res. 2006;99:1132–1140. doi: 10.1161/01.RES.0000250175.14861.77. [DOI] [PubMed] [Google Scholar]
- 40.Kermani P, Hempstead B. Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends Cardiovasc. Med. 2007;17:140–143. doi: 10.1016/j.tcm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santhanam AVR, Smith LA, Katusic ZS. Brain-derived neurotrophic factor stimulates production of prostacyclin in cerebral arteries. Stroke. 2010;41:350–356. doi: 10.1161/STROKEAHA.109.564492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin L, Kim E, Ratan R, Lee FS, Cho S. Genetic variant of BDNF (Val66Met) polymorphism attenuates stroke-induced angiogenic responses by enhancing anti-angiogenic mediator CD36 expression. J. Neurosci. 2011;31:775–783. doi: 10.1523/JNEUROSCI.4547-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 44.Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Jametti LP, Kilic E, Kilic U, et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–2251. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- 45.Schäbitz W-R, Berger C, Kollmar R, Seitz M, Tanay E, Kiessling M, et al. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004;35:992–997. doi: 10.1161/01.STR.0000119754.85848.0D. [DOI] [PubMed] [Google Scholar]
- 46.Sulejczak D, Ziemlińska E, Czarkowska-Bauch J, Nosecka E, Strzalkowski R, Skup M. Focal photothrombotic lesion of the rat motor cortex increases BDNF levels in motor-sensory cortical areas not accompanied by recovery of forelimb motor skills. J. Neurotrauma. 2007;24:1362–1377. doi: 10.1089/neu.2006.0261. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, et al. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J. Neurosci. 2005;25:2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacLellan CL, Keough MB, Granter-Button S, Chernenko GA, Butt S, Corbett D. A critical threshold of rehabilitation involving brain-derived neurotrophic factor is required for poststroke recovery. Neurorehabil Neural Repair. 2011;25:740–748. doi: 10.1177/1545968311407517. [DOI] [PubMed] [Google Scholar]
- 49.Kim J-M, Stewart R, Park M-S, Kang H-J, Kim S-W, Shin I-S, et al. Associations of BDNF genotype and promoter methylation with acute and long-term stroke outcomes in an East Asian cohort. PLoS ONE. 2012;7:e51280. doi: 10.1371/journal.pone.0051280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Molinaro G, Battaglia G, Riozzi B, Di Menna L, Rampello L, Bruno V, et al. Memantine treatment reduces the expression of the K(+)/Cl(−) cotransporter KCC2 in the hippocampus and cerebral cortex, and attenuates behavioural responses mediated by GABA(A) receptor activation in mice. Brain Res. 2009;1265:75–79. doi: 10.1016/j.brainres.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 51.Dietrich WD, Truettner J, Prado R, Stagliano NE, Zhao W, Busto R, et al. Thromboembolic events lead to cortical spreading depression and expression of c-fos, brain-derived neurotrophic factor, glial fibrillary acidic protein, and heat shock protein 70 mRNA in rats. J. Cereb. Blood Flow Metab. 2000;20:103–111. doi: 10.1097/00004647-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 52.Chen LY, Rex CS, Pham DT, Lynch G, Gall CM. BDNF signaling during learning is regionally differentiated within hippocampus. J. Neurosci. 2010;30:15097–15101. doi: 10.1523/JNEUROSCI.3549-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia LSB, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 54.Jantas D, Szymanska M, Budziszewska B, Lason W. An involvement of BDNF and PI3-K/Akt in the anti-apoptotic effect of memantine on staurosporine-evoked cell death in primary cortical neurons. Apoptosis. 2009;14:900–912. doi: 10.1007/s10495-009-0370-6. [DOI] [PubMed] [Google Scholar]
- 55.Berthier ML, Green C, Lara JP, Higueras C, Barbancho MA, Dávila G, et al. Memantine and constraint-induced aphasia therapy in chronic poststroke aphasia. Ann. Neurol. 2009;65:577–585. doi: 10.1002/ana.21597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.