Abstract
BACKGROUND
A profound difference between cancer and normal tissues is the preferential utilization of glycolysis by cancer cells. To translate this paradigm in the clinic, we completed a phase I study of 2-deoxyglucose (2DG), and assessed 2DG uptake with fluorodeoxyglucose (FDG) positron emission tomography (PET) and the autophagy substrate p62 as a marker of 2DG resistance.
METHODS
Patients received 2DG orally on days 1–14 of a 21-day cycle in cohorts of three in a dose-escalating manner. Correlative assessments included PET scans at baseline and day 2 and p62 protein in peripheral blood mononuclear cells as a potential marker of 2DG resistance.
RESULTS
The dose of 45 mg/kg was defined as the recommended phase II dose, secondary to dose-limiting toxicity of grade 3 asymptomatic QTc prolongation at a dose of 60 mg/kg. PK evaluation of 2DG revealed linear pharmacokinetics with Cmax 45 μg/ml (277 μM), 73.7 μg/ml (449 μM), and 122 μg/ml (744 μM) in dose levels 30, 45, and 60 mg/kg, respectively. Five of eight patients assessed with FDG-PET scanning demonstrated decreased FDG uptake by day 2 of therapy, suggesting competition of 2DG with FDG. Five of six patients assessed for p62 had a decrease in p62 at 24 hr.
CONCLUSIONS
These data support the safety of 2DG, defined 2DG PK, demonstrated the effect of 2DG on FDG-PET imaging, and demonstrated the feasibility of assessment of p62 as an autophagic resistance marker. These data support future studies of 2DG alone or in combination with approaches to abrogate autophagy.
Keywords: deoxyglucose, metabolism, prostate cancer, autophagy, p62
INTRODUCTION
The investigation of cancer cell metabolism as a therapeutic target is a rapidly developing research paradigm. The likelihood of therapeutic success by targeting metabolic pathways was predicted in the 1920s when Otto Warburg discovered a major exploitable difference between the metabolism of glucose in normal and cancer cells [1]. Warburg established that cancer cells preferentially utilize the inefficient process of aerobic glycolysis, which can convert each molecule of glucose to two molecules of ATP, rather than oxidative phosphorylation, which can generate up to 36 molecules of ATP. The fact that cancer cells prefer glycolysis to convert glucose to ATP forms the basis of tumor imaging with fluorodeoxyglucose (FDG) positron emission tomography (PET) that demonstrates increased glucose uptake in tumor compared to normal tissue. These initial clues to exploitable differences in metabolism highlight the importance of translational drug development focused on targeting metabolic pathways.
Oncogenic events may additionally increase the metabolic fragility of cancer cells. Recent data demonstrate that certain oncogenes exert some of their oncogenic effects through the modulation of the glycolytic pathway, potentially making tumor cells more sensitive to inhibition of glycolysis [2]. These effects have been shown to occur either at the level of glucose uptake through the modulation of glucose transporters or through the direct up-regulation of glycolytic enzymes [3]. Events that promote glycolysis include activation of growth factor receptors, PI-3 kinase, or disruption of PTEN with increased activity of Akt, which also functions as an anti-apoptotic survival factor [4–8]. Growth factors and activated Akt increase surface expression of glucose transporters, stimulate mitochondrial association of hexokinase, and phosphorylate phosphofructokinase, which increase glycolysis [9].
Prostate cancer may be particularly sensitive to modulation of metabolic pathways. Most prostate cancers display altered or deleted activity of PTEN and increased activation of PI3K/Akt signaling [10]. Our prior studies also demonstrated induction of multiple glycolytic enzymes resulting from autocrine stimulation in prostate cancer cells, suggesting that inhibition of glycolysis could exploit the altered metabolism of prostate cancer cells to induce cytotoxicity with an acceptable therapeutic index [11]. Additional studies demonstrated that the glucose analog, 2-deoxyglucose (2DG), an inhibitor of the glycolytic pathway, is cytotoxic to prostate cancer cells in preclinical studies and that the process of autophagy was a significant mechanism of 2DG resistance [12,13].
Taken together, these data support assessment of 2DG in clinical trials and assessment of markers of autophagy as potential clinical markers of drug resistance. Recent studies agree that one mechanism of resistance to therapeutic starvation is the process of autophagy, a response to starvation in which cellular organelles and bulk cytoplasm are targeted to lysosomes for degradation to supply an alternate energy source during periods of nutrient limitation [14]. Our group also demonstrated that the process of autophagy degrades sequestered proteins such as p62, which may serve as a reliable marker of autophagy induction in patients [15].
These prior studies support the translational development of therapeutic starvation in cancer. We hypothesized that a phase I study with the agent 2DG, as a prototypical glycolytic inhibitor, will provide data on which additional studies can be designed. Furthermore, we hypothesized that we can develop FDG-PET imaging as a marker of drug uptake, define 2DG pharmacokinetics, and establish markers of auto-phagy as biomarkers of glycolytic inhibitor resistance. We also hypothesized that the development of such markers of autophagy in patients will have high impact for future studies, as research and the importance of autophagy has rapidly grown in recent years [14].
PATIENTS AND METHODS
Patient Eligibility
This study was approved by the local institutional review board. Patients age ≥18 years with a histologically proven prostate cancer or other solid tumor malignancy without a standard option of therapy were eligible for this trial. Patients with prostate cancer were maintained on androgen suppression therapy and had disease progression after bicalutamide or flutamide withdrawal of 6 and 4 weeks, respectively. Patients were required to have an ECOG performance status of ≥2 and adequate bone marrow, hepatic, and renal function (as defined by granulocytes of ≥1,500/μml, hemoglobin of ≥10.0 g/dl, platelet count of ≥100,000/L, normal total bilirubin, aspartate aminotransferase, and alanine aminotransferase of ≤2.5 × upper limit of normal, and serum creatinine of ≤1.5 mg/dl or creatinine clearance >50 ml/min). All patients had fasting blood glucose below the institutional upper limit of normal. There was no limit on the number of other prior systemic therapy regimens. Patients with the following conditions were also excluded: patients with a history of glucose intolerance; diabetes or hypoglycemia; seizure disorder; autonomic dysfunction; uncontrolled gastrointestinal disorder; G6PD deficiency; coagulopathies or anticoagulation; second primary malignancies except most in situ carcinomas; known brain metastasis; allergy to methyparaben or propylparaben; active clinically significant infection requiring antibiotics; history of clinically significant unexplained episodes of hypotension, fainting, dizziness, or lightheadedness; history or symptoms of cardiovascular disease (NYHA Class 2, 3, or 4; New York Heart Association Criteria) within the last 6 months, particularly coronary artery disease, arrhythmias, or conduction defects with risk of cardiovascular instability, uncontrolled hypertension, clinically signifi-cant pericardial effusion, or congestive heart failure; history of transient ischemic attack, stroke, or seizure disorder or any other CNS disease considered to be significant by the investigator; major surgery within 4 weeks of the start of study treatment, without complete recovery; anti-tumor therapy within 28 days of the start of study treatment; inability to discontinue prohibited medications (including proton pump inhibitors, H-2 antagonists, antacids, drugs for diarrhea or constipation, anti-diabetics; mannitol or sucralfate) for 24 hr before and after dosing on cycle 1, day 1 of weeks 1 and 2; patients who are unable (as per investigator discretion) or unwilling to give written informed consent.
Study Design
This trial was an open-label multiple dose level phase I study. The primary objective was to determine the safety and optimal dose of 2DG administered as a single agent on a daily schedule to patients with advanced solid tumors. Secondary objectives include the pharmacokinetic analysis of 2DG administered on a daily schedule, the effects of 2DG on PET imaging, and the assessment of peripheral blood mononuclear cells (PBMCs) for protein markers of autophagy, as a potential mechanism of resistance of cellular starvation to 2DG.
Dose Escalation and Toxicity Rules
The starting dose for this phase I dose escalation study was 30 mg/kg of 2DG administered orally on a daily schedule for 2 weeks (days 1–14) of a 3-week (21 days) cycle. Dose level 2 was 45 mg/kg. Dose escalation after dose level 2 was determined based on a 33% increase over the previous dose level. Three patients were treated at each dose level until one DLT was observed, at which time the cohort size was planned to be expanded to six patients. If two or more DLTs occur in a cohort of 3–6 subjects, the dose level below is considered the MTD. DLT was defined as any grade ≥2 renal; grade 3 or 4 non-hematologic toxicity (including neurotoxicity and asthenia and excluding alopecia, anemia, lymphopenia, or untreated nausea and vomiting) regardless of relationship to 2DG; grade 4 hematologic toxicity for >5 days. DLTs were only considered in the first cycle of therapy (one cycle was 3 weeks) to assess acute toxicity. Patients were assessed for toxicity in other cycles and dose adjusted and reported. Any seizure was considered a DLT. Formal assessment for seizure was at the discretion of the investigator. Hyperglycemia was not considered a DLT unless blood glucose >300 mg/dl for ≥6 hr or blood glucose >250 mg/dl for any duration associated with other persistent and significant signs or symptoms such as acidosis. If a persistent (≥2 weeks) AE that compromised the patient's ability to participate in the study occurred or if an AE did not resolve to baseline or ≤grade 1, 2DG was discontinued and the patient was discontinued from the study. If >2 dose reductions were needed 2DG was discontinued and the patient was discontinued from the study. Patients experiencing any grade 3 or 4 non-hematologic adverse event regardless of relationship to 2DG had the dose to be held until the event recovered to baseline or grade ≤1. If the adverse event did not resolve within 2 weeks, the patient has to be removed from the study. For grade 3 and 4 toxicities, treatment should was withheld until the toxicity resolved to grade ≤1, then reinstituted (if medically appropriate) at a 25% dose reduction.
Drug Formulation and Administration
2DG was supplied by Threshold Pharmaceuticals in aqueous solution for oral administration. 2DG was stored in a secure area with limited access under controlled conditions including refrigeration (2–8°C). 2DG was supplied in a 40-ml clear glass screw cap vial containing 20 ml nominal (23 ml target fill) of a solution of 2DG formulated at a concentration of 100 mg/ml in water. Each oral dose of 2DG was administered 1 hr before breakfast. Each dose of 2DG was followed by administration of one rinse of the dosing container of approximately 50 ml of water.
Pharmacokinetic,Imaging, and Laboratory Correlative Studies
To determine the ability of imaging to detect 2DG uptake, PET scans were obtained at baseline, C1 day 2, and C2 day 15. To determine 2DG pharmacokinetics, blood samples (5 ml/interval) were collected predose and hourly up to 6 hr following 2DG administration on day 1 and at 24 and 48 hr after day 1 of cycle 1 administration and again on day 1, cycle 2 of the study. For the measurement of 2DG in plasma, the sample extracts were derivatized to 2-aminobenzioc acid derivatives, and determined using highly sensitive high-performance liquid chromatography fluorometric method (HPLC-FL). To assess protein markers of autophagy, PBMCs were collected and assessed for protein expression by Western blot. Assessment for p62 was done to assess the feasibility of obtaining this marker of autophagy, as previously demonstrated in our prior studies [15,16].
For assessment of p62, PBMC cell extracts were made in Laemmli buffer and separated by SDS–PAGE (30 mg protein) and transferred to polyvinylidene fluoride (PDVF) membranes (Millipore, Billerica, MA). Blots were incubated with primary antibodies p62 (Biomol, Plymouth Meeting, PA), α-tubulin (Sigma, St. Louis, MO) and developed with HRP-conjugated secondary antibody using the ECL system (GE Healthcare, Piscataway, NJ). The protein expression images were scanned on HP Scanjet 4570c scanner with the following optical density analyses using ImageJ software (NIH, Bethesda, MA). Results for p62 were normalized by α-tubulin.
RESULTS
Patient's Characteristics
Twelve patients (Table I) received treatment of 2DG within one of three dose levels (30, 45, and 60 mg/kg). The median age of patients was 65.5 years. Nine patients were diagnosed with advanced castrate-resistant prostate cancer (CRPC), one with cervical cancer, one with nasopharyngeal cancer, and one with non-small cell lung cancer. Six patients had received and progressed after prior chemotherapy. Of the nine patients with prostate cancer, all had progression on prior androgen ablation therapy and three had progression after at least one prior chemotherapy regimen.
TABLE I.
Patient Characteristics
| Patient characteristics | No. of patients | |
|---|---|---|
| Total | 12 | |
| Male | 11 | |
| Female | 1 | |
| Age (years) | ||
| Median | 65 | |
| Range | 36–83 | |
| ECOG performance status | ||
| 0 | 8 | |
| 1 | 4 | |
| Primary site of disease | ||
| Prostate | 9 | |
| Nasopharynx | 1 | |
| Lung | 1 | |
| Cervix | 1 | |
| Prior chemotherapy regimens | ||
| 0 | 6 | |
| 1 | 1 | |
| 2 | 3 | |
| 3 | 1 | |
| Greater or equal to 4 | 1 |
Adverse Events and Clinical Outcomes
Therapy was well tolerated with few grade 3 or 4 toxicities, as shown in Table II. The most common grade 1 and 2 toxicities were fatigue and dizziness (grade 2 fatigue in three patients and grade 1 dizziness in two patients). Dose-limiting toxicity of grade 3 asymptomatic QTc prolongation was seen in two patients treated at a dose of 60 mg/kg. Therefore, two additional patients were safely enrolled at the dose of 45 mg/kg. None of the five patients dosed at 45 mg/kg had a DLT and 45 mg/kg was selected as the recommended phase II dose. In seven patients with prostate cancer who have completed study therapy, no declines in PSA were seen. Three patients with CRPC remained on study beyond three cycles with radiographically stable disease (8, 12, and 21 cycles).
TABLE II.
Toxicity
| Number of patients with toxicity |
||||
|---|---|---|---|---|
| Toxicity category | Grade | 30 mg/kg (n = 3) | 45 mg/kg (n = 5) | 60 mg/kg (n = 4) |
| Constitutional—fatigue | 1/2 | 1 | 2 | |
| 3 | 1 | |||
| Cardiac—QTc prolongation | 1/2 | 1 | 1 | |
| 3 | 2 | |||
| Cardiac—AV block | 1/2 | 1 | ||
| Cardiac—bradycardia | 1/2 | 1 | ||
| Gastrointestinal—taste alteration | 1/2 | 1 | ||
| Neurological—dizziness | 1/2 | 1 | 1 | 1 |
| Pain—gastric pain | 1/2 | 1 | ||
Pharmacokinetics
Pharmacokinetics of 2DG for cycles 1 and 2 are shown in Table III. Mean AUC in the first cycle was 821.2±236.2, 1107.7±213.3, and 2086.2±743.2 (μmol/ L hr±SD) at doses of 30, 45, and 60 mg/kg, respectively. The Cmax in the first cycle also increased with increasing dose levels, which were 276.7±18.6, 449.2±168.5, and 744.0±289.1 (μM±SD) in dose levels 30, 45, and 60 mg/kg, respectively. The half-life at the recommended phase II dose level of 45 mg/kg was 7.3±1.6 in cycle 1 and 8.2±1.9 (hr±SD) in cycle 2.
TABLE III.
Pharmacokinetics
| Dose levels (mg/kg/day) | Cmax (μM ± SD) | AUC (μmol/Lhr ± SD) | Clearance (ml/hr ± SD) | t1/2 (hr ± SD) |
|---|---|---|---|---|
| Cycle 1 | ||||
| 30 (n = 3) | 276.7 ± 18.6 | 821.2 ± 236.2 | 232.0 ± 63.8 | 4.5 ± 0.6 |
| 45 (n = 4) | 449.2 ± 168.5 | 1107.7 ± 213.3 | 243.3 ± 49.4 | 7.3 ± 1.6 |
| 60 (n = 4) | 744.0 ± 289.1 | 2086.2 ± 743.2 | 185.0 ± 52.6 | 5.2 ± 1.6 |
| Cycle 2 | ||||
| 30 (n = 3) | 224.0 ± 12.6 | 751.3 ± 181.7 | 246.3 ± 56.6 | 5.3 ± 1.1 |
| 45 (n = 4) | 425.8 ± 144.9 | 1087.1 ± 441.8 | 270.4 ± 126.4 | 8.2 ± 1.9 |
| 60 (n = 4) | 605.6 ± 277.3 | 1226.9 ± 571.2 | 314.9 ± 108.7 | 6.1 ± 3.9 |
n, number of patients/dose level.
Imaging and Molecular Markers
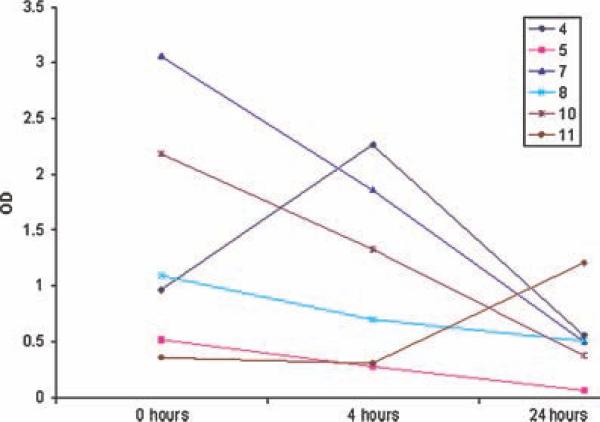
Nine patients underwent assessment with FDG-PET scanning before treatment and on day 2 to determine the effect of 2DG on FDG uptake (Table IV). Using the ratio of SUV at selected tumor sites over the liver, as a control, decreased FDG uptake occurred in five patients in either dose level 2 (patients numbered 6 and 11) or 3 (patients numbered 7, 8, and 9). Based on prior studies demonstrating the effect of autophagy on p62 protein levels, assessment of p62 was assessed in PBMCs of patients before and during treatment with 2DG. Of six patients with PBMCs available for assessment of p62 by immunoblot, five of six patients had a decrease in p62 level at 24 hr (Fig. 1).
TABLE IV.
FDG-PET Imaging Marker Assessment
| Patient number | Tumor | Dose level | Tumor site assessed | Baseline SUV (ratio site/liver) | Day 2 SUV (ratio site/liver) |
|---|---|---|---|---|---|
| 4 | Prostate | 2 | Mediastinum | 4.6 | 4.7 |
| 5 | Lung | 2 | Chest | 6.3 | 11.6 |
| 6 | Cervical | 2 | Mediastinum | 4.3 | 2 |
| 7 | Prostate | 3 | L1 vertebral body | 1.2 | 1 |
| 8 | Prostate | 3 | Ischium | 2.8 | 2.0 |
| 9 | Prostate | 3 | Mediast | 2.1 | 1.7 |
| 10 | Prostate | 3 | Pelvic LN | 3.8 | 4.8 |
| 11 | Prostate | 2 | Ilium | 3.8 | 3 |
Fig. 1.
Assessment of p62 protein levels from peripheral blood mononuclear cells obtained at baseline,4 hr,and 24 hr in six patients. Optical density of p62 was normalized to α-tubulin.
DISCUSSION
This is the first study to define the recommended phase II dose, pharmacokinetics, and molecular marker data for 2DG, a prototypical inhibitor of glycolysis, as a single agent. The ability to use 2DG in the clinic lays an important foundation in the effort to target metabolism in cancer. While the abnormal metabolism of cancer cells has long been recognized, the underlying genetic abnormalities driving aberrant metabolism are starting to come into focus necessitating the development of clinical agents that can exploit metabolic differences between cancer cells and the normal host environment. 2DG has long been recognized as an effective inhibitor of glucose metabolism. When 2DG is taken into the cell, 2DG is phosphorylated to 2DG-P, which is trapped in the cell and cannot be utilized for energy, leading to inhibition of glycolysis and depletion of cellular ATP [17–21].
Multiple preclinical studies have evaluated the efficacy of 2DG as a single agent and have identified agents that may effectively be combined with 2DG to enhance efficacy. In vitro studies with 2DG have demonstrated activity in osteosarcoma cells that were defective in oxidative phosphorylation implying that cells relying on glycolysis are more susceptible to 2DG [12]. Other studies have demonstrated that 2DG increased the efficacy of death receptor mediated apoptosis with TNF and TRAIL [22]. In vivo, 2DG has enhanced the cytotoxicity of adriamycin and paclitaxel in osteosarcoma and lung cancer xenograft models [23], and increased cytotoxicity when combined with the microtubule inhibitor 2-methoxyestradiol [24]. 2DG has also been combined with radiation preclinically and in clinical trials where 2DG was administered prior to treatment with high-dose radiation fractions administered on a weekly basis [25]. More recently, our group demonstrated the cytotoxic effect of 2DG in prostate cancer cell lines and demonstrated that the process of autophagy was a significant mechanism of resistance to 2DG [16].
In our clinical study, 2DG was well tolerated at the recommended phase II dose of 45 mg/kg. At the higher dose of 60 mg/kg, the QT interval was prolonged. This finding is in agreement with prior data demonstrating prolonged QT interval in studies of 2DG stimulation of gastric acid production. In this prior study, when used as a vagal stimulator to test for completeness of surgical vagotomies, 2DG induced non-specific T wave flattening and QT prolongation, without inducing serious arrythmias [26]. In our study, we utilized a schedule of daily dosing for 2 weeks with 1 week off to allow recovery from induction of glucopenia. The PK was linear with dose level, demonstrated a Cmax median of 744 μM at the highest dose level, and had relatively little inter-patient variability. Although this study supports the safety of 2DG as a single agent at blood concentrations that reach 0.7 mM concentrations, our prior laboratory studies demonstrated that cytotoxic effects below 1 mM were enhanced by the abrogation of autophagy as a mechanism of resistance of 2DG [16]. These data, therefore, suggest that future studies of 2DG may be best approached in combination with an autophagy inhibitor.
In fact, the process of autophagy is now known to be a cell survival mechanism to multiple agents capable of tumor cell starvation including 2DG, mTOR inhibitors, VEGF, and tyrosine kinase inhibitors [14]. More recently, our group demonstrated that autophagy regulates the expression of p62, suggesting that this may be a potential surrogate clinical marker of autophagy [15]. In an effort to begin to test this hypothesis that autophagy decreases the expression of p62, we assessed the feasibility of measuring p62 in patients PBMCs. As shown in Figure 1, p62 decreased in 5/6 patients assessed by 24 hr. Although only a small cohort of patients, these data are important and form the basis to test p62 as a marker of the modulation of autophagy in larger studies. In our study, FDG-PET scans also demonstrated possible competitive inhibition of glucose uptake in the majority of patients, which may be tested further as a useful pharmacodynamic marker.
In summary, this phase I and correlative study defined the recommended phase II dose of 2DG, defined 2DG pharmacokinetics, assessed the effect of 2DG on FDG-PET, and determined the feasibility of assessment of p62 as a potential marker of autophagy. Given parallel emerging laboratory data on autophagy as a mechanism of resistance to many agents capable of tumor cell starvation, the feasibility of measuring p62 as a marker of autophagy is additionally important for the development of future studies. Future studies with 2DG in combination with agents that abrogate autophagy are also warranted.
Acknowledgments
Grant sponsor: Department of Defense, Threshold Pharmaceuticals; Grant number: W81XWH-05-1-0036.
Footnotes
Views and opinions of, and endorsements by, the authors do not reflect those of the US Army or the Department of Defense.
REFERENCES
- 1.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, Thompson CB. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 3.Dipaola RS, Thompson IM. National cooperative group trials. Urology;2005;65:23–29. doi: 10.1016/j.urology.2005.03.042. discussion 9. [DOI] [PubMed] [Google Scholar]
- 4.Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353–361. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT—A major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Morgensztern D, McLeod HL. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs. 2005;16:797–803. doi: 10.1097/01.cad.0000173476.67239.3b. [DOI] [PubMed] [Google Scholar]
- 8.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16:525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 9.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24:7465–7474. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- 11.Dvorzhinski D, Thalasila A, Thomas PE, Nelson D, Li H, White E, Dipaola RS. A novel proteomic coculture model of prostate cancer cell growth. Proteomics. 2004;4:3268–3275. doi: 10.1002/pmic.200400847. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Hu YP, Savaraj N, Priebe W, Lampidis TJ. Hyper-sensitization of tumor cells to glycolytic inhibitors. Biochemistry. 2001;40:5542–5547. doi: 10.1021/bi002426w. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Savaraj N, Priebe W, Lampidis TJ. Hypoxia increases tumor cell sensitivity to glycolytic inhibitors: A strategy for solid tumor therapy (model C). Biochem Pharmacol. 2002;64:1745–1751. doi: 10.1016/s0006-2952(02)01456-9. [DOI] [PubMed] [Google Scholar]
- 14.White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15:5308–5316. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, Dipaola RS, Karantza-Wadsworth V, White E. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiPaola RS, Dvorzhinski D, Thalasila A, Garikapaty V, Doram D, May M, Bray K, Mathew R, Beaudoin B, Karp C, Stein M, Foran DJ, White E. Therapeutic starvation and autophagy in prostate cancer: A new paradigm for targeting metabolism in cancer therapy. Prostate. 2008;68:1743–1752. doi: 10.1002/pros.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 18.Maher JC, Krishan A, Lampidis TJ. Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer Chemother Pharmacol. 2004;53:116–122. doi: 10.1007/s00280-003-0724-7. [DOI] [PubMed] [Google Scholar]
- 19.McComb RB, Yushok WD. Metabolism of ascites tumor cells. IV. Enzymatic reactions involved in adenosinetriphosphate degradation induced by 2-deoxyglucose. Cancer Res. 1964;24:198–205. [PubMed] [Google Scholar]
- 20.Karczmar GS, Arbeit JM, Toy BJ, Speder A, Weiner MW. Selective depletion of tumor ATP by 2-deoxyglucose and insulin, detected by 31P magnetic resonance spectroscopy. Cancer Res. 1992;52:71–76. [PubMed] [Google Scholar]
- 21.Wick AN, Drury DR, Nakada HI, Wolfe JB. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem. 1957;224:963–969. [PubMed] [Google Scholar]
- 22.Munoz-Pinedo C, Ruiz-Ruiz C, Ruiz de Almodovar C, Palacios C, Lopez-Rivas A. Inhibition of glucose metabolism sensitizes tumor cells to death receptor-triggered apoptosis through enhancement of death-inducing signaling complex formation and apical procaspase-8 processing. J Biol Chem. 2003;278:12759–12768. doi: 10.1074/jbc.M212392200. [DOI] [PubMed] [Google Scholar]
- 23.Maschek G, Savaraj N, Priebe W, Braunschweiger P, Hamilton K, Tidmarsh GF, De Young LR, Lampidis TJ. 2-Deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64:31–34. doi: 10.1158/0008-5472.can-03-3294. [DOI] [PubMed] [Google Scholar]
- 24.Tagg SL, Foster PA, Leese MP, Potter BV, Reed MJ, Purohit A, Newman SP. 2-Methoxyoestradiol-3,17-O,O-bis-sulphamate and 2-deoxy-D-glucose in combination: A potential treatment for breast and prostate cancer. Br J Cancer. 2008;99:1842–1848. doi: 10.1038/sj.bjc.6604752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohanti BK, Rath GK, Anantha N, Kannan V, Das BS, Chandramouli BA, Banerjee AK, Das S, Jena A, Ravichandran R, Sahi UP, Kumar R, Kapoor N, Kalia VK, Dwarakanath BS, Jain V. Improving cancer radiotherapy with 2-deoxy-D-glucose: Phase I/II clinical trials on human cerebral gliomas. Int J Radiat Oncol Biol Phys. 1996;35:103–111. doi: 10.1016/s0360-3016(96)85017-6. [DOI] [PubMed] [Google Scholar]
- 26.Burckhardt D, Stalder GA. Cardiac changes during 2-deoxy-dglucose test. A study in patients with selective vagotomy and pyloroplasty. Digestion. 1975;12:1–8. doi: 10.1159/000197647. [DOI] [PubMed] [Google Scholar]