Abstract
The ability to induce and study neuronal plasticity in single dendritic spines has greatly advanced our understanding of the signaling mechanisms that mediate long-term potentiation. It is now clear that in addition to compartmentalization by the individual spine, subcompartmentalization of biochemical signals occurs at specialized microdomains within the spine. The spatiotemporal coordination of these complex cascades allows for the concomitant remodeling of the postsynaptic density actin spinoskeleton and for the regulation of membrane traffic to express functional and structural plasticity. Here, we highlight recent findings in the signaling cascades at spine microdomains as well as the challenges and approaches to studying plasticity at the spine level.
Keywords: long-term potentiation, LTP, hippocampus, neuron, memory
INTRODUCTION
Dendritic spines are tiny, bulbous structures that protrude from the dendrites of most neurons that receive fast excitatory synaptic input in the brain. These beautiful structures, first documented by Ramón y Cajal more than 100 years ago, compartmentalize the postsynaptic machinery and biochemical signaling molecules needed to respond to input from single presynaptic terminals. It is now widely accepted that this compartmentalization serves a major function of spine structure: input specificity of synaptic plasticity.
The plastic nature of individual synapses is thought to underlie the brain's ability to encode and store information. The long-lasting increase and decrease in synaptic strength, known as long-term potentiation (LTP) and long-term depression (LTD), respectively, are generally accepted as cellular correlates to learning and memory and are essential for normal cognitive function. Dysfunction of synaptic plasticity is a feature of affective disorders, neurodegenerative disease, and the cognitive decline associated with normal aging (1). Experimentally, LTP can be induced in an animal by a behavioral learning task or by patterned stimulation of neurons (2). Throughout the brain, various forms of LTP occur, but the most robust and widely studied is the NMDA receptor (NMDAR)-dependent LTP of Schaffer collateral–CA1 synapses in the hippocampus. Understanding the biochemical signals in dendritic spines that induce and express this form of LTP has been a major focus of neuroscience research over the past several decades.
Despite this extended period of intense study, only in the past decade have technological advances enabled the study of neuronal plasticity at the single-spine level. Previously, LTP studies relied on stimulation and recordings of thousands of synapses, limiting temporal and spatial resolution and the understanding of the signaling cascades that mediate these changes. However, this limitation was overcome by the development of two-photon glutamate uncaging (3–5). Two-photon uncaging uses a two-photon excitation process to break a chemical bond linking glutamate and a caging compound, thus releasing glutamate only at the focus of laser beam with a spatial resolution of <1 μm. By using this technique, LTP could be induced in a single spine of known location (6). These experiments revealed that LTP is expressed as an increase in postsynaptic glutamate sensitivity and is accompanied by physical growth of spines, termed structural plasticity (6). Moreover, LTP could be induced and expressed in single spines independently of the presynaptic neuron (6). Ca2+ imaging in single spines has revealed that presynaptic mechanisms also contribute to the expression of LTP (7), although this topic is outside of the scope of this review. The ability to study LTP with high spatiotemporal resolution has enabled scientists to begin to elucidate the complex web of signaling molecules that transduce transient input signals into long-lasting structural and functional changes in the spine.
In a simplified view of LTP signaling, LTP is induced by NMDAR-dependent Ca2+ influx, followed by a transient activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) (8, 9). These events trigger signaling cascades that lead to long-lasting changes in synaptic strength that are mediated by an increase in functional AMPA receptors (AMPARs) at the synapse. The signaling cascades that transduce transient CaMKII signals into long-lasting receptor incorporation and the regulation of LTP are not fully understood but are the focus of much research. One emerging theme is that the signaling networks in dendritic spines depend on highly regulated coordination in space and time of compartmentalized protein activity. Whereas research efforts previously centered on the compartmentalization of signaling within individual spines (10), it is now clear that within the tiny femtoliter volumes of spines, microdomains are present to subcompartmentalize and coordinate the complex signaling cascades that mediate spine plasticity. The minute size of these microdomains poses great technological challenges that are only beginning to be overcome with advances such as superresolution fluorescence imaging and targeted observation and manipulation of protein dynamics (see sidebar, Technical Advances for Spine Plasticity Studies)
In this review, we focus on early (~10-min) protein signals at spine microdomains that enable transient synaptic input to be transduced into long-lasting synaptic changes in hippocampal CA1 NMDAR-dependent LTP. We review recent findings in (a) the remodeling of the postsynaptic density (PSD), (b) the remodeling of the actin cytoskeleton, and (c) the regulation of membrane trafficking and discuss the coordination of these mechanisms. Finally, we emphasize the challenges of disentangling these signaling networks and of understanding their interdependence at spine microdomains as well as potential approaches with which to address these challenges.
REMODELING THE POSTSYNAPTIC DENSITY
The PSD is a proteinaceous specialization that is adherent to the postsynaptic membrane and that scaffolds the receptors, enzymes, and signaling molecules required for synaptic transduction. Not surprisingly, the long-lasting increase in synaptic strength of LTP is concomitant with remodeling of the PSD protein content. Studies have revealed that LTP-inducing stimuli induce the recruitment or loss from the PSD of various proteins, including CaMKII, Shank, PSD-95, and RNA-binding proteins. One estimate suggests that ~2% of the PSD proteome is altered upon a chemical LTP-inducing stimulus (11), although this is likely an underestimate. This PSD remodeling, in turn, leads to the stable incorporation of additional AMPARs at the synapse to express LTP (12, 13) (Figure 1).
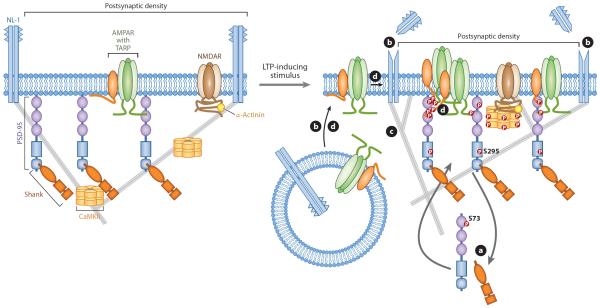
Figure 1.
Remodeling of the postsynaptic density (PSD) during early long-term potentiation (LTP) expression. Upon NMDA receptor (NMDAR)-dependent Ca2+ influx, the PSD undergoes rapid changes, including the recruitment of Ca2+/calmodulin-dependent protein kinase II (CaMKII) via its interaction with NMDAR and subsequent downstream phosphorylation events. These events lead to PSD remodeling by (a) the transient dissociation and subsequent stabilization of scaffold proteins PSD-95 and Shank, (b) extracellular cleavage and surface recruitment of neuroligin-1 (NL-1), (c) the remodeling of actin filaments, and (d) the trapping of surface and newly exocytosed AMPA receptors (AMPAR) in the PSD through both direct (phosphorylation-dependent) and indirect (molecular crowding) mechanisms. TARP denotes transmembrane AMPAR-regulatory protein.
PSD Structure and Organization
Ultrastructure studies, and more recently superresolution microscopy, have revealed that the PSD is a highly organized structure: Proteins are localized in an ordered fashion, with specific orientations and locations in both the longitudinal and transverse axes (14–16). This organization appears to define a functional microstructure with relevance for transmission. For example, NMDARs tend to be localized centrally in the PSD, whereas AMPARs appear to be more lateral (17). Fluorescence imaging suggests that AMPARs are localized in microclusters that may directly oppose hot spots of presynaptic glutamate release (18, 19). Much of this organization is defined by interactions between proteins, the cytoskeleton, and perhaps specialized lipid domains. Although the mechanisms are not clear, the micro-organization of the PSD must be important in mediating its reorganization during LTP. For example, as described below, specific localization of Ca2+-dependent signaling proteins, such as CaMKII, defines downstream signaling events (9). Furthermore, local changes in the density of PSD proteins during LTP may mediate AMPAR trapping by molecular crowding or protein-protein interactions (19, 20).
CaMKII Recruitment to the PSD
Activation of CaMKII is both required and sufficient for LTP induction (9, 21–24). The CaMKII holoenzyme consists of 12 subunits, primarily CaMKIIα and -β subunits in mature hippocampal neurons, that are assembled in a sixfold symmetric ring structure (25, 26). Upon Ca2+ influx, Ca2+/CaM binds to CaMKII subunits, inducing a conformational change that activates the kinase domain. Activation of two adjacent subunits causes transautophosphorylation, which induces an alternate state of kinase activation that is independent of Ca2+/CaM binding (25, 26). Initially touted as a molecular mediator of memory due to its ability to maintain activity despite the dissociation of Ca2+/CaM, CaMKII was recently demonstrated to be active for only a few minutes during LTP induction by using 2P FLIM–FRET (two-photon fluorescence lifetime imaging microscopy–Förster resonance energy transfer) (27) (see sidebar, Technical Advances for Spine Plasticity Studies). Thus, LTP expression must rely on signaling cascades downstream of CaMKII activation.
Ca2+ elevation in spines can trigger the clustering of CaMKII at the postsynaptic site (28–30). This clustering is dependent on the binding of the CaMKIIα subunit to the GluN2B subunit of NMDAR (31–33). The NMDAR-CaMKII interaction positions the CaMKII enzyme well to respond to nanodomains of high Ca2+ concentration through NMDARs, as was demonstrated by imaging of CaMKII activity (27). This localization may ensure full activation of the enzyme and signaling to downstream protein partners in the PSD (34). Additionally, CaMKII translocation to the PSD recruits the proteasome into the spine and is important for activity-dependent degradation of polyubiquitinated proteins (35). Disruption of the CaMKII-NMDAR interaction impairs LTP as well as phosphorylation of downstream targets thought to be involved in LTP, including the phosphorylation of AMPARs and AMPAR auxiliary proteins (31, 36). In a similar mechanism, CaMKII forms signaling complexes with voltage-gated Ca2+ channels, where it can be efficiently activated by high-Ca2+ nanodomains and can phosphorylate downstream targets (37, 38). These studies indicate that precise localization, even in the small volume of spines, is essential for coordinating complex signaling cascades.
Indeed, as a primary mediator of Ca2+ signaling in the spine, CaMKII recruitment to the PSD allows CaMKII to regulate PSD remodeling by phosphorylating PSD proteins, including AMPARs, NMDARs, PSD-95, and stargazin (39). Moreover, the CaMKIIβ subunit, in its inactive form, binds to and bundles actin, serving a structural role in maintaining PSD stability (40). However, upon activation, CaMKII dissociates from actin, allowing for actin destabilization and subsequent PSD remodeling. CaMKII then moves into the PSD due to the CaMKIIα association with NR2B. With its subsequent deactivation, CaMKII may dissociate from PSD and reassociate with actin, stabilizing LTP-induced changes. It is still elusive whether CaMKII localization to the PSD serves as long-lasting memory storage (41) or whether such localization is required only transiently to transduce NMDAR activation into downstream signaling events that mediate LTP expression and maintenance (9).
Remodeling of PSD Structural Proteins
PSD scaffolding proteins contain multiple protein interaction domains and bind either directly or indirectly to synaptic receptors, actin-binding proteins (ABPs), small GTPase regulators, and other signaling proteins (42). In addition to helping define synaptic structure, scaffolding proteins, through their numerous interactions, likely coordinate many of the changes underlying LTP expression. The most abundant scaffold protein in the PSD is PSD-95 (43). PSD-95 has been implicated in playing an essential role in LTP and in mediating AMPAR numbers at the synapse (44–46). During LTP induction, a significant fraction of PSD-95 transiently dissociates from the synapse, indicative of PSD remodeling (47). This trafficking is dependent on CaMKII-mediated phosphorylation of PSD-95 at the serine-73 residue and directs the dissociation of an interacting scaffold protein, Shank. In addition to being phosphorylated at serine-73, PSD-95 is phosphorylated at a different residue, serine-295, in a Rac-JNK-dependent manner that enhances the stability of PSD-95 at the synapse (48). Phosphorylation states at these residues, therefore, provide bidirectional control of PSD-95 stability at the synapse.
Neuroligin is a transmembrane cell adhesion molecule that is anchored in the PSD of excitatory synapses and makes contact with the presynaptic neuron through binding to neurexin. One function of the neuroligin/neurexin interaction is to stabilize the connection between the pre- and the postsynaptic neuron. Recent work has demonstrated that this interaction may be modulated during LTP and that neuroligin may play a more direct role in signals underlying LTP expression. Neuroligin-1 (NL-1) is important for maintaining NMDAR-mediated currents and Ca2+ influx in spines in cortical layer 2/3 pyramidal neurons and plays an important role in spinogenesis that is dependent on transneuronal competition (49). Also, upon chemical LTP induction, NL-1 appears to be trafficked to the plasma membrane, increasing the surface pool of NL-1 at spines (50). Interestingly, increased levels of NL-1, through the interaction of NL-1 with PSD-95, reduce AMPAR surface diffusion (51), potentially playing a role in activity-dependent trapping of AMPARs through molecular crowding (see below). Knockdown of NL-1, but not that of NL-3, by shRNA led to a loss of synapses and LTP deficits in young animals (52). Recently, NL-1 was also implicated in a mechanism of transsynaptic signaling. Activation of single spines with two-photon glutamate uncaging led to rapid cleavage of the extracellular domain of NL-1. This cleavage, mediated by matrix metalloprotease 9, was NMDAR and CaMK dependent. Cleavage of NL-1, in turn, disrupted neurexin binding and reduced presynaptic neurotransmitter release probability (53). Thus, NL-1 cleavage and recruitment may play sequential roles mediating the transient dissociation of synaptic structure, allowing for spine growth and remodeling, followed by the subsequent stabilization of the potentiated synapse.
AMPAR Regulation in the PSD
The incorporation of additional AMPARs into the PSD is believed to be the central mechanism of LTP expression (12, 13, 54). AMPARs are the primary mediators of glutamatergic transmission in the brain. Recently, a proteomic characterization of the multimeric AMPAR complex identified a total of 34 different protein components, including the four AMPAR subunits (GLUA1–4) and known associated proteins, such as transmembrane AMPAR-regulatory proteins (TARPs). More than 60% of the identified proteins, however, had not been previously characterized as interacting with AMPARs (55). This work suggests that our understanding of AMPAR regulation is still very incomplete. The complexity of AMPARs indicates significant modulation of both AMPAR gating properties and trafficking, which likely play an important role in plasticity (55).
Tagging AMPAR subunits with pH-sensitive fluorophores allows for the detection of a surface pool of AMPARs (56, 57). Combined with fluorescence recovery after photobleaching (FRAP), this technique revealed that the enrichment of AMPARs following LTP induction depends primarily on the activity-induced synaptic trapping of a pool of extrasynaptic surface AMPARs (58, 59). Single-particle tracking of endogenous surface AMPARs revealed that this pool of receptors is highly mobile and traverses the spine membrane, entering and exiting the synapse (60). Upon local increases in Ca2+, however, extrasynaptic AMPARs become immobilized at the PSD (60). The activity-dependent mechanism of this trapping has received intense study, although a comprehensive understanding remains elusive.
One appealing mechanism of AMPAR trapping is an activity-dependent modification of AMPARs that may increase binding of AMPARs to the PSD during LTP. Indeed, CaMKII, protein kinase A, and protein kinase C can phosphorylate the C terminus of the AMPAR GluA1 subunit, a subunit that has been implicated in LTP-induced AMPAR enrichment (61–63). Moreover, GluA1 phosphorylation can regulate AMPAR trafficking to the PSD. Interestingly, both CaMKII and protein kinase A are recruited to synapses by NMDAR activity, a potential activity-dependent regulation of their function (28, 29, 64). An additional CaMKII phosphorylation site on the intracellular loop of the GluA1 AMPAR subunit may regulate PSD trapping without affecting exocytosis (65). A similar mechanism mediated by TARPs, including stargazin (TARP γ2), has also been proposed. Stargazin is phosphorylated by CaMKII, disrupting an interaction between a C-tail-containing PDZ-binding domain and the plasma membrane, releasing the PDZ-binding domain and thus allowing for its interaction with the PSD (66). Such CaMKII-dependent phosphorylation immobilizes AMPARs in the PSD (67). Furthermore, phosphomimetic mutations of the C-tail of stargazin potentiate synaptic transmission (66), and disruption of the stargazin-PSD complex leads to the dissociation of AMPARs from the synapse (45).
Although an activity-dependent association between the AMPAR complex and PSD proteins is likely involved in the normal expression of LTP, a recent study demonstrated that such an association is surprisingly not required. By overexpression of mutant AMPAR subunits following the genetic knockout of all major AMPAR subunits (GluA1–3), Granger et al. (68) demonstrated that the minimum requirement for LTP expression is the presence of an extrasynaptic pool of glutamate receptors, independent of subtype or C terminus–mediated interactions. In fact, in the absence of AMPARs, kainate receptors can support LTP expression (68). Although this study did not directly address whether stargazin may have been involved in the trafficking of mutant AMPARs or kainate receptors, stargazin is not thought to regulate kainate trafficking, despite the high degree of homology between the receptors (69).
The above result suggests the presence of a mechanism that relies on activity-dependent remodeling of the PSD to trap diffusing AMPARs independent of the requirement for specific modifications of the AMPAR complex, including stargazin. One proposed mechanism that fits these characteristics is the diffusional trapping of AMPARs via molecular crowding in the PSD (70, 71). Indeed, lipids that are not enriched within the synapse, and that thus are thought not to be directly anchored to the PSD, also show reduced mobility at the synapse. This trapping is mediated by nonspecific interactions with proteins in the dense protein mesh of the PSD. Interestingly, although the PSD is highly organized and ordered, the overall structure is flexible in nature (72). Imaging of PSD-95 as well as of other PSD scaffolding proteins suggests that, although the protein composition of the PSD is stable, the shape and size of the PSD are highly dynamic, rapidly (within minutes) altering the local protein density without changing overall protein composition within the PSD. This flexibility is mediated by actin dynamics, which, when inhibited, immobilize the PSD (19, 72). Importantly, the flexible nature of the PSD structure is also activity dependent, with an increase in dynamics upon synaptic activity (19, 72). Thus, by altering local protein density, this flexibility may aid in the molecular trapping of proteins that are regulated during LTP, such as AMPARs. Indeed, two-color superresolution imaging of PSD-95 and the AMPAR subunit GluA2 demonstrated that AMPARs are enriched in microclusters of high scaffold density (19). Modeling further demonstrated that this clustering can shape postsynaptic responses to glutamate (19). Therefore, an activity-dependent increase in PSD flexibility may be a potential mechanism for protein trapping. In addition, it may also be a more general mechanism for PSD remodeling including the activity-dependent release of proteins, consistent with the increased turnover of PSD scaffolding proteins during LTP (47).
REMODELING THE ACTIN CYTOSKELETON
LTP expression and the associated structural plasticity require rapid actin dynamics and a long-term increase in polymerized actin in spines (73, 74). Recent work has indicated the presence of a complex network of actin in the spine termed the spinoskeleton. Upon LTP-inducing stimuli, this network is uniquely regulated within microdomains to modulate spine morphology, PSD structure, and membrane trafficking (75–77).
Actin Spinoskeleton
Within the spine, actin is the primary cytoskeletal element. Each actin monomer dynamically cycles between monomeric G-actin and filamentous F-actin through a process known as tread-milling. In this cycle, ATP-bound G-actin binds to one end of an actin filament (the barbed end), whereas ADP-bound actin is depolymerized from the other end (the pointed end). Monomers in the actin filament (F-actin) slowly hydrolyze ATP into ADP. Together with a large family of ABPs, actin filaments (F-actin) can further assemble into highly branched networks connected to the plasma membrane, the PSD, and other subspine structures, as well as to microtubules that run along the parent dendritic shaft, as beautifully shown in platinum replica electron microscopy (78). The spine neck, which had previously been considered to contain bundled actin filaments, was also found to contain highly branched actin filaments (78). The specific ABPs associated with actin determine whether it is stabilized to maintain structure or whether force is generated to induce membrane deformation and remodeling. For example, ABPs that promote cross-linking of actin filaments, such as α-actinin, increase stability, whereas ABPs such as the Arp2/3 complex, which nucleates new actin filament branches, increase network expansion. In spines, actin is thought to be organized into at least two pools: a dynamic pool, in which the majority of filaments undergo complete turnover within a minute, and a stable core, turning over in tens of minutes (77, 79).
By using single-particle tracking with nanometer resolution, the dynamics of individual actin monomers in actin filaments were recently visualized (80–82). This approach revealed that tread-milling occurs in both retrograde (spine-tip-to-neck) and anterograde (spine-neck-to-tip) directions, with the net flow in the retrograde direction. The approach also identified multiple, discrete sites of high actin velocity that were discriminated at spine subdomains, including the PSD, extrasynaptic sites, and even the spine neck (80). This suggests a mechanism for rapid actin-mediated changes that can be regulated uniquely at microdomains. Indeed, spine stimulation induces rapid actin polymerization, which is required for LTP expression (74). However, actin stability at the spine core is also required, playing a role in stabilization of spine structure. Consistently, the size of the stable pool of actin correlates with spine size (79).
Actin Regulation of Structural Plasticity
The regulation of actin in the spine is mediated through complex signaling cascades, including Rho GTPases, that transduce membrane signals to ABPs (83). Growing evidence suggests that ABPs are segregated into distinct subspine microdomains and that their regulated localization may be a mechanism for subcompartmentalization of Rho signaling (84). For example, ABPs responsible for stabilization, including drebrin, are enriched within the core of the spine (84). Recent work suggests that drebrin may alter the properties of actin filaments, rendering them resistant to depolymerization (85). Moreover, larger spines with increased PSD size are enriched in drebrin A (86), suggesting that it may play a role in stabilizing spine enlargement induced by LTP (87).
In contrast, ABPs that are involved in mediating rapid actin dynamics are concentrated in more peripheral regions of the spine (84). For example, cofilin, which depolymerizes actin and is required for actin turnover, is enriched adjacent to the spine membrane and is sparse in the spine core (88). Cofilin is regulated through phosphorylation by the upstream LIM kinase in an activity-dependent manner. Upon LTP induction, cofilin activity is first increased and is subsequently decreased, allowing first for actin remodeling and then for an increase in F-actin and associated spine enlargement (73, 89–91).
Arp2/3, a multimeric complex responsible for initiating actin branching (92), is also localized to the peripheral region of the spine surrounding the spine core but is not directly adjacent to the membrane (84). Activation of the Arp2/3 complex induces spine head enlargement (93). Recently, the Arp2/3 complex was shown to be essential for LTP-associated structural plasticity. Knockout of a critical subunit of the Arp2/3 complex reduces transient spine enlargement by ~50% and eliminates sustained spine enlargement (94). Furthermore, Arp2/3 knockout mice are severely impaired in episodic memory (94). Overall, the coordinated regulation of distinct ABPs in discrete subdomains (e.g., activation of drebrin and Arp2/3 and rapid activation followed by inhibition of cofilin) is likely required for efficient remodeling of the actin spinoskeleton to support structural plasticity.
Upstream Regulation of Actin
The complex regulation of actin has curtailed a clear delineation of signaling pathways that mediate LTP-induced actin remodeling. Ca2+ influx through the NMDAR reorganizes actin dynamics through several layers of signal regulation that is mediated by many proteins, including the Rho family of small GTPases and ABPs. Each Rho GTPase is regulated by multiple activators [guanine nucleotide exchange factors (GEFs)] and inhibitors [GTPase-activating proteins (GAPs)] with overlapping specificity (95) and signals to multiple downstream molecules that regulate ABPs (96). Recent work has measured, with high spatiotemporal resolution, the activity of two members of the Rho GTPase family, Cdc42 and RhoA, during LTP at single spines. In response to an LTP-inducing stimulus and CaMKII activity, both proteins are rapidly activated. Their activity precedes spine enlargement and decays within minutes to a lower level of activity that is sustained for more than 30 min (97). Both GTPases show similar time courses of activity but unique spatial profiles of activation: RhoA is active in the stimulated spine and spreads into the parent dendrite over ~5 μm, whereas Cdc42 activation is restricted to the stimulated spine. Furthermore, RhoA and Cdc42 demonstrate differential control of spine morphology. The RhoA GTPase pathway and its downstream signaling through Rho-associated kinase are essential for both initial and sustained spine growth. In contrast, the Cdc42 GTPase and its downstream target, p21-activated kinase (PAK), are required for sustained structural plasticity, but not for initial spine enlargement, demonstrating a functional segregation at this level of signaling (97).
Evidence points to the presence of signaling complexes as a means of regulating complex actin dynamics. These complexes physically localize upstream activators or inhibitors, small GTPases, and downstream effectors as a means of subcompartmentalizing functional outputs. For example, βPix, a GEF for Cdc42 and Rac, binds to PAK, a downstream effector, to promote the interaction of PAK with these GTPase proteins. Moreover, βPix also complexes with GIT, a GAP for the ADP ribosylation factor small GTPase (98), potentially serving as a means of cross talk between signaling pathways. The βPix signaling complex is localized to the PSD by its interaction with the Shank family of PSD scaffolding proteins (99). This localization may position βPix well to undergo Ca2+-dependent phosphorylation via CaMKI to enhance its GAP activity (100).
Another example of an actin-regulating signal complex is the WAVE/WRP/ARP2/3 complex. WAVE-1, a Rac effector, is a modular protein that contains several protein interaction domains. These domains scaffold both upstream Rac regulators and downstream ABPs. Specifically, WAVE-1 binds to WRP, a Rac GAP, and to the Arp2/3 complex, which nucleates actin branching (101). Disruption of WAVE-WRP binding in knock-in animals alters spine morphology, LTP expression, and memory retention, indicating the importance of the complex in the regulation of the actin spinoskeleton (102). The presence of protein complexes to regulate the signaling pathways involved in the remodeling of the spinoskeleton may be a general mechanism by which to define subcompartmentalization of signaling events within spine microdomains.
Actin Regulation of the PSD
Direct contact between actin and PSD proteins occurs through interactions with ABPs. One example is the ABP α-actinin, which is involved in the cross-linking of F-actin to stabilize its structure. α-Actinin binds the NMDAR and has an activity-dependent association with CaMKII, allowing CaMKII to regulate LTP-induced remodeling of the PSD (103). Acute inhibition of actin polymerization with latrunculin leads to the rapid dissociation of PSD-localized synaptic proteins, such as GKAP, Shank, and Homer, but not to the dissociation of PSD-95 (104, 105). This indicates that ongoing actin dynamics play a role in regulating the protein composition of the PSD. Furthermore, imaging of PSD proteins suggests that actin mediates ongoing changes in the microdensity of proteins within the PSD, increasing this modulation when Ca2+ is elevated (72).
Actin Regulation of Membrane Traffic
The regulation of membrane traffic is a critical component of LTP expression. Membrane trafficking relies on actin as tracks for myosin-driven vesicle movement and for generating force during vesicle fission and fusion (106). Thus, although the mechanisms are not well described, the regulation of actin and membrane trafficking must be coordinated during LTP. The unidirectional movement of vesicles by specific myosin motors along actin filaments, of which polarity seems to be relatively random, also plays an important role in directing vesicles to the right locations. This may be particularly crucial at the spine neck, which may be a filter for vesicularly transported cargo. The spine neck, although narrow (~0.2 μm), consists of complex, branched networks of actin filaments (78). This likely serves as a means by which to regulate endosomal vesicle traffic, as transit through this network may require vesicles to contain multiple motors to traverse actin filaments of different polarity (77).
Not only does actin regulate vesicle traffic through spine necks, but actin regulation plays a role in LTP-induced exocytosis and endocytosis. Following LTP induction, a transient enhancement of cofilin activity (a depolymerizing ABP) is required to induce AMPAR exocytosis and LTP (83). Subsequently, cofilin is inactivated by phosphorylation to aid in spine growth and stabilization (91). Protein interacting with C kinase 1 (PICK-1), which is implicated in the NMDAR-dependent endocytosis of AMPARs (107), binds to and inhibits the Arp2/3 complex. Moreover, disruption of this interaction blocks AMPAR internalization (108). The complex regulation of vesicle trafficking, as discussed below, requires actin dynamics, stable actin filaments, and the regulation of motor and signaling proteins.
REGULATION OF MEMBRANE TRAFFIC
LTP expression relies on the rapid insertion of membrane and membrane proteins to support the enlargement of spines and protein remodeling at the synapse (Figure 2). Dysregulation of membrane traffic, by disruption of endosomal trafficking or exocytosis, blocks LTP expression (109, 110). Within the femtoliter volume of the spine, the many membranous structures include endosomes; exocytotic vesicles; multivesicular bodies; and, in ~80% of large mushroom spines (111), the spine apparatus (SA), whose function is largely unknown. Additionally, smooth endoplasmic reticulum, Golgi outposts, and recycling endosomes termed recycling outposts (ROs) (112) are present in the dendrites at the spine base. Endocytic zones (EZs), which are specialized for endocytosis, are also defined in the spine, are localized adjacent to the synapse, and are tethered to the PSD through protein interactions (113, 114). Exocytotic domains, in contrast, do not seem to be as clearly structurally defined, existing either within the spine or in the underlying dendrite (59, 109, 115–117).
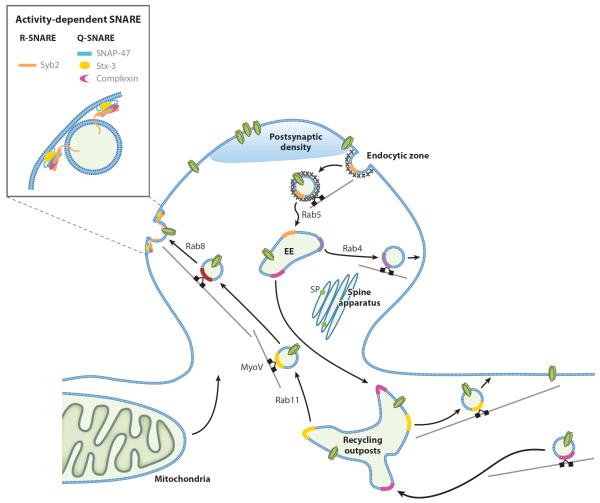
Figure 2.
Membrane traffic regulation in the spine. During LTP induction, transmembrane proteins and lipids are rapidly delivered to the spine from dendritically localized recycling outposts through activity-dependent vesicle transport and exocytosis mediated by specific Rab proteins, motor proteins, and SNARE machinery. The constitutive endocytosis and recycling of membrane proteins through the endocytic zone, early endosome (EE), and recycling outposts are also highly regulated by the Rab GTPases and are essential for maintaining synaptic transmission. In addition, potentiated spines often contain spine apparatus, which may provide local stores of Ca2+ and membrane. Other abbreviations: MyoV, class V myosin; SP, synaptopodin; Stx-3, syntaxin-3; Syb2, synaptobrevin 2.
Membrane traffic through the endosomal pathway in other cell types and its regulation by the Rab family of small GTPases (which is part of the Ras superfamily) are well described (118, 119). Rab GTPases are targeted to specific membrane components (e.g., early endosomes, recycling endosomes) and are involved in regulating the budding, motility, and fusion of vesicles. Exocytosis of membrane proteins to the plasma membrane is regulated by Rab8, whereas proteins that are internalized into early endosomes are regulated in a Rab5-dependent manner. From the early endosome, internalized proteins and lipids can be either recycled directly back to the surface through a Rab4 mediated pathway or recycled by an indirect pathway through the recycling endosome (or ROs near spines) mediated by Rab11. The regulation of Rab proteins, like that of other GTPases, is through their localization to specific membranes as well as through interaction with diverse Rab family GEFs and GAPs.
LTP-Induced Changes in Endocytosis and Exocytosis
Although little is known about the LTP-induced activity of Rab proteins in spines, dominant negative overexpression studies have suggested that the Rab4 pathway is important for constitutive recycling of postsynaptic compartments, whereas the Rab8 and Rab11 pathways seem to be required for LTP-induced exocytosis of vesicles (120). LTP induces a rapid, approximately fivefold increase in the rate of exocytosis; this increased rate persists throughout the stimulation, and the rate decreases within 1 min after stimulation (59). The time course of exocytosis (as measured by exocytosis of AMPARs) parallels that of spine volume increase, suggesting that membrane insertion from the fusion of vesicles contributes to spine volume changes (59). Moreover, although most LTP-induced AMPAR recruitment to the PSD is due to the trapping of extrasynaptic membrane-resident AMPARs (58), approximately 10–30% of additional AMPARs are derived from activity-induced exocytosis of AMPAR-containing vesicles (59). LTP induces the movement of RO-derived vesicles to the stimulated spine (109, 121). This LTP-induced movement of endosomal vesicles depends on class V myosin (MyoV) motors and their interaction with the actin cytoskeleton (121, 122). Specifically, a Ca2+-dependent complex of Rab11 and MyoV motor proteins is believed to tether RO-derived vesicles to the actin cytoskeleton, mediating their transport into dendritic spines (121, 122).
In addition to an activity-dependent transport of exocytotic vesicles, SNARE machinery that mediates regulated LTP-induced exocytosis of AMPARs, but not constitutive AMPAR exocytosis, was recently identified. Specifically, the plasma membrane–associated Q-SNAREs SNAP-47 and syntaxin-3 (Stx-3) are required for LTP-induced exocytosis and LTP expression. The binding of Stx-3 to complexin appears to be essential for this regulation (123, 124). Although syntaxin-4 had previously been implicated in mediating Ca2+-dependent exocytosis (115), it was not found to be required for LTP-induced exocytosis of AMPARs or for LTP expression (124). Constitutive AMPAR exocytosis is mediated by a different Q-SNARE, SNAP25. The discrimination between Ca2+-dependent exocytosis and constitutive exocytosis appears to be regulated by Q-SNAREs, as the vesicularly localized R-SNARE synaptobrevin 2 is involved in both pathways (124). The activity-dependent step associated with this machinery is still unknown, but the description of a specific LTP-dependent SNARE complex should aid in its identification.
In addition to the importance of Rab signaling, pharmacological studies indicate that the Ras-ERK signaling pathway plays an important role in regulating exocytosis (59). Consistently, the spatial profile of exocytosis mimics the spread of LTP-induced Ras activity, which increases in the stimulated spine and ~10 μm along the parent dendrite (59). This activity profile may correlate with the location of ROs, which are present in dendrites in a distributed manner [~1 for every 10–20 spines (125)]. Interestingly, the stimulation-induced increase in the exocytosis rate appears to be independent of CaMKII activation, although later incorporation of AMPARs at the PSD requires CaMKII (59). The precise role of exocytosis in LTP is not fully understood, as evidenced by the requirement for exocytosis in LTP despite the relatively low contribution of exocytosis to the incorporation of additional AMPARs at the synapse (58, 59). These seemingly contradictory findings may suggest a role of exocytosis for introducing other proteins or lipids that are essential for LTP into the spine membrane.
In contrast to the case for exocytosis, the importance of the acute regulation of endocytosis in LTP is not yet known, although such regulation is essential in mediating AMPAR internalization during LTD (126). A subdomain for endocytosis, the EZ, is defined in the perisynaptic membrane adjacent to the PSD (113). The EZ is tethered to the PSD through interactions between dynamin 2/3 and the PSD scaffolding proteins Homer and Shank (114). It has been hypothesized that the EZ is essential for maintaining a highly mobile pool of available AMPARs for LTP by trapping AMPARs that laterally diffuse out of the synapse, internalizing and recycling them back to the surface (127, 128). The disruption of the EZ-PSD interaction, or disruption of an interaction between the AMPAR subunit GluA1 and the internalization adaptor protein AP2, leads to a decrease in AMPARs at the synapse and to an alteration in surface AMPAR mobility (114, 127). Interestingly, the immediate early gene Arc, whose transcription and local translation are upregulated following LTP-inducing stimulation and during learning tasks, is thought to interact with dynamin and endophilin to enhance endocytosis of AMPAR (129–131). However, in a study in which endocytosis was disrupted through the overexpression of a dominant negative Rab5, no effect on either basal transmission or LTP was seen (120). These seemingly contradictory results suggest that endocytosis is differentially regulated constitutively and during LTP induction, expression, and consolidation. Further study of the role of endocytosis with greater temporal precision is warranted.
LTP-Induced Organelle Traffic
During LTP, in addition to regulation of acute trafficking of small vesicles, there is longer-term remodeling of membrane structures, including the recruitment of mitochondria into the stimulated spine (132) and the appearance of the SA (133). Although the appearance of SA has not been directly associated with synaptic potentiation, the SA is present primarily in large, mushroom-shaped spines (a morphological characteristic of potentiated spines) (111, 134). Moreover, spines that contain synaptopodin (SP), a marker protein for the SA, have twice as large of a response to glutamatergic input as those without SP (135) and tend to be more sensitive to mGluR-dependent LTD (136). Finally, SP knockout mice, which lack SA, show a decrease in LTP (137). The function of this membrane structure is largely unknown, although the structure has been proposed to be analogous to smooth endoplasmic reticulum and to be both a Ca2+ reservoir and a membrane reserve (138, 139). The SA is also closely tied to the actin cytoskeleton by its interaction with ABPs such as SP and α-actinin (84), providing a potential point of coordination between membrane traffic and the actin spinoskeleton.
CHALLENGES, TOOLS, AND PERSPECTIVES
Over the past decade, the study of LTP at the single-spine level has gradually shifted focus from compartmentalization of signaling molecules in the spine toward subcompartmentalization of signaling at microdomains within the spine. Recent work has highlighted activity-induced changes in the PSD, spinoskeleton, and membrane that underlie LTP expression. The signaling mechanisms that mediate and coordinate these changes are only beginning to be described.
Disentangling the complex signaling network at spine microdomains is technically challenging. Perhaps the greatest impediment is the small size of the spine. Because of an average spine size with submicrometer diameter and subfemtoliter volume (140), discriminating microdomains within the spine requires resolution near or beyond the diffraction limit of light. Moreover, studies have estimated that some proteins (even exogenously expressed) may have only several copies present in a single spine, thus requiring techniques with high sensitivity. [Even for a relatively highly expressing protein (~1 μM), a typical spine with 0.1-fl volume contains only 60 copies.] Finally, hundreds of species of proteins have been implicated in mediating or modulating LTP. To begin to understand their integration and coordination at microdomains, the activity of many proteins needs to be monitored during LTP with high spatiotemporal resolution.
Undoubtedly, the task is daunting. However, as the data reviewed here demonstrate, advances in technology (see sidebar, Technical Advances for Spine Plasticity Studies), including super-resolution imaging and the targeted monitoring and manipulation of protein activity with high spatiotemporal resolution, are beginning to address the challenges associated with studying LTP in a single spine. The continued development of these techniques and the development of as-yet-unforeseen tools that improve spatial and temporal resolution for detecting and probing of signaling dynamics in the spine will enhance our understanding of the networks underlying LTP. Such developments will hopefully lead to a greater understanding of synaptic plasticity at the level of the spine, the encoding of memory in neurons, and the disease processes associated with disruptions of plasticity.
TECHNICAL ADVANCES FOR SPINE PLASTICITY STUDIES.
Superresolution imaging: Superresolution microscopy holds great promise for advancing the LTP field by offering a 2–10-fold improvement in the resolution of light-based techniques. The major approaches—structured illumination microscopy (SIM), stimulated emission depletion (STED), RESOLFT, and single-molecule localization microscopy (PALM/STORM)—achieve resolutions of 20–100 nm (141–145). Although these techniques require stringent sample preparation and high levels of light, thus limiting their application, recent improvements allow for live-cell imaging with lower light intensity and faster imaging speeds (146). These advances suggest that live-cell discrimination of signaling at spine microdomains is feasible.
Optical monitoring and manipulation of protein activity: The use of Förster resonance energy transfer (FRET)-based sensors allows protein interactions and enzyme activity to be monitored (147). Combined with two-photon fluorescence lifetime microscopy (2P FLIM), this approach can measure spatial and temporal patterns of signaling activity during single-spine LTP (27, 97, 148, 149). Furthermore, through target-specific sensor design, subcompartmentalization of signaling pathways can be discriminated. In addition, methods to optically turn on or off proteins were recently developed by taking advantage of light-sensitive conformational changes (150). Modulation of individual signaling nodes in the LTP network with high spatiotemporal control while changes in the activity of other nodes are monitored may enable the integration of signaling proteins into an LTP model (148).
SUMMARY POINTS.
The functional and structural plasticity of spines is mediated by complex signaling cascades that are compartmentalized at spine microdomains.
The highly ordered structure of the PSD forms a flexible matrix that is regulated by the actin cytoskeleton and that undergoes rapid restructuring following LTP-inducing stimuli.
The enrichment of AMPARs at the PSD is likely mediated by a combination of specific protein interactions and nonspecific trapping due to molecular crowding.
The spinoskeleton consists of complex networks of actin filaments that interconnect microdomains within the spine and, thus, likely coordinates PSD remodeling with membrane traffic and spine growth. Subcompartmentalization of signaling to regulate actin dynamics is mediated by discrete localization of ABPs and by the presence of multiprotein signaling complexes.
Activity-induced exocytosis is mediated by specific SNARE machinery and is required for LTP expression and structural plasticity.
The study of plasticity at the spine level is technically challenging due to the small size of spines and the complexity of the signaling cascades involved. The continued development of experimental tools—including superresolution microscopy, targeted signal manipulation, and monitoring of protein activity and interactions—provides great potential for unraveling the complex signals that mediate spine plasticity.
ACKNOWLEDGMENTS
We thank Dr. Scott Soderling for critical reading and the members of the Yasuda lab for discussion.
Glossary
- Long-term potentiation (LTP)
a long-lasting enhancement of synaptic transmission
- Long-term depression (LTD)
a long-lasting decrease in synaptic transmission
- NMDAR (N-methyl-d-aspartate receptor)
a glutamate receptor channel that is important for the induction of plasticity
- Two-photon glutamate uncaging
the photolysis of chemically caged glutamate in a restricted volume by the absorption of two photons of light to release glutamate
- Structural plasticity
the sustained enlargement of spines that accompanies the expression of LTP
- Ca2+/calmodulin-dependent protein kinase II (CaMKII)
a Ca2+/calmodulin-activated holoenzyme that is critical for LTP induction
- AMPAR (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor)
a glutamate receptor channel that mediates most fast synaptic transmission in the central nervous system
- Superresolution imaging
light microscope techniques that achieve higher resolution than the diffraction limit of light
- Postsynaptic density (PSD)
a protein-dense specialization that is adjacent to the postsynaptic membrane and that compartmentalizes receptors and signaling molecules involved in receiving synaptic input
- Two-photon fluorescence lifetime imaging microscopy (2P FLIM)
a technique that measures the fluorescence decay time of a fluorophore by using two-photon excitation, allowing for more quantitative measurements of FRET
- Förster resonance energy transfer (FRET)
the transfer of nonradiative energy between a donor chromophore and an acceptor chromophore in close proximity to one another
- Actin-binding proteins (ABPs)
a large family of proteins that bind and regulate actin dynamics
- Transmembrane AMPAR-regulatory proteins (TARPs)
a family of proteins that complex with the AMPAR and regulate its trafficking and gating
- Fluorescence recovery after photobleaching (FRAP)
an optical technique measuring fluorescence recovery into a photobleached region; used to measure the mobility of fluorescently tagged proteins
- PDZ domain (postsynaptic density protein, Drosophila disc large tumor suppressor, and zonula occludens-1 protein domain)
a protein interaction domain that is found in numerous proteins, including PSD-95, and that binds to short amino acid sequences of interacting proteins
- Recycling outpost (RO)
a specialized recycling endosome present at the spine base
- Endocytic zone (EZ)
a stable subdomain that is enriched in clathrin and that is the putative site of endocytosis in neuronal spines
Footnotes
DISCLOSURE STATEMENT The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–29. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–97. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 3.Pettit DL, Wang SS, Gee KR, Augustine GJ. Chemical two-photon uncaging: a novel approach to mapping glutamate receptors. Neuron. 1997;19:465–71. doi: 10.1016/s0896-6273(00)80361-x. [DOI] [PubMed] [Google Scholar]
- 4.Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 2001;4:1086–92. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 6.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–66. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enoki R, Hu YL, Hamilton D, Fine A. Expression of long-term plasticity at individual synapses in hippocampus is graded, bidirectional, and mainly presynaptic: optical quantal analysis. Neuron. 2009;62:242–53. doi: 10.1016/j.neuron.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Lüscher C, Malenka RC. NMDA receptor–dependent long-term potentiation and long-term depression (LTP/LTD) Cold Spring Harb. Perspect. Biol. 2012;4:a005710. doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 2012;13:169–82. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu. Rev. Physiol. 2002;64:313–53. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- 11.Zhang G, Neubert TA, Jordan BA. RNA binding proteins accumulate at the postsynaptic density with synaptic activity. J. Neurosci. 2012;32:599–609. doi: 10.1523/JNEUROSCI.2463-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann. NY Acad. Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- 13.Nicoll RA, Roche KW. Long-term potentiation: peeling the onion. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.02.010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Winters C, Azzam R, Li X, Galbraith JA, et al. Organization of the core structure of the postsynaptic density. Proc. Natl. Acad. Sci. USA. 2008;105:4453–58. doi: 10.1073/pnas.0800897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dani A, Huang B, Bergan J, Dulac C, Zhuang X. Superresolution imaging of chemical synapses in the brain. Neuron. 2010;68:843–56. doi: 10.1016/j.neuron.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi MK, Tang C, Verpelli C, Narayanan R, Stearns MH, et al. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell. 2009;137:159–71. doi: 10.1016/j.cell.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kharazia VN, Weinberg RJ. Tangential synaptic distribution of NMDA and AMPA receptors in rat neocortex. Neurosci. Lett. 1997;238:41–44. doi: 10.1016/s0304-3940(97)00846-x. [DOI] [PubMed] [Google Scholar]
- 18.Kerr JM, Blanpied TA. Subsynaptic AMPA receptor distribution is acutely regulated by actin-driven reorganization of the postsynaptic density. J. Neurosci. 2012;32:658–73. doi: 10.1523/JNEUROSCI.2927-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacGillavry HD, Song Y, Raghavachari S, Blanpied TA. Nanoscale scaffolding domains within the postsynaptic density concentrate synaptic AMPA receptors. Neuron. 2013;78:615–22. doi: 10.1016/j.neuron.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opazo P, Sainlos M, Choquet D. Regulation of AMPA receptor surface diffusion by PSD-95 slots. Curr. Opin. Neurobiol. 2012;22:453–60. doi: 10.1016/j.conb.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Lledo PM, Hjelmstad GO, Mukherji S, Soderling TR, Malenka RC, Nicoll RA. Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proc. Natl. Acad. Sci. USA. 1995;92:11175–79. doi: 10.1073/pnas.92.24.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pettit DL, Perlman S, Malinow R. Potentiated transmission and prevention of further LTP by increased CaMKII activity in postsynaptic hippocampal slice neurons. Science. 1994;266:1881–85. doi: 10.1126/science.7997883. [DOI] [PubMed] [Google Scholar]
- 23.Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the αcalcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–73. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 24.Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in α-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–6. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- 25.Stratton MM, Chao LH, Schulman H, Kuriyan J. Structural studies on the regulation of Ca2+/calmodulin dependent protein kinase II. Curr. Opin. Struct. Biol. 2013;23:292–301. doi: 10.1016/j.sbi.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chao LH, Pellicena P, Deindl S, Barclay LA, Schulman H, Kuriyan J. Intersubunit capture of regulatory segments is a component of cooperative CaMKII activation. Nat. Struct. Mol. Biol. 2010;17:264–72. doi: 10.1038/nsmb.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–66. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- 29.Strack S, Choi S, Lovinger DM, Colbran RJ. Translocation of autophosphorylated calcium/calmodulin-dependent protein kinase II to the postsynaptic density. J. Biol. Chem. 1997;272:13467–70. doi: 10.1074/jbc.272.21.13467. [DOI] [PubMed] [Google Scholar]
- 30.Otmakhov N, Tao-Cheng JH, Carpenter S, Asrican B, Dosemeci A, et al. Persistent accumulation of calcium/calmodulin-dependent protein kinase II in dendritic spines after induction of NMDA receptor–dependent chemical long-term potentiation. J. Neurosci. 2004;24:9324–31. doi: 10.1523/JNEUROSCI.2350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 32.Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW. Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-d-aspartate receptor. Proc. Natl. Acad. Sci. USA. 1999;96:3239–44. doi: 10.1073/pnas.96.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strack S, McNeill RB, Colbran RJ. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-d-aspartate receptor. J. Biol. Chem. 2000;275:23798–806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- 34.Merrill MA, Chen Y, Strack S, Hell JW. Activity-driven postsynaptic translocation of CaMKII. Trends Pharmacol. Sci. 2005;26:645–53. doi: 10.1016/j.tips.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Bingol B, Wang CF, Arnott D, Cheng D, Peng J, Sheng M. Autophosphorylated CaMKIIα acts as a scaffold to recruit proteasomes to dendritic spines. Cell. 2010;140:567–78. doi: 10.1016/j.cell.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Takahashi E, Li W, Halt A, Wiltgen B, et al. Interactions between the NR2B receptor and CaMKII modulate synaptic plasticity and spatial learning. J. Neurosci. 2007;27:13843–53. doi: 10.1523/JNEUROSCI.4486-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J. Cell Biol. 2005;171:537–47. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wheeler DG, Groth RD, Ma H, Barrett CF, Owen SF, et al. CaV1 and CaV2 channels engage distinct modes of Ca2+ signaling to control CREB-dependent gene expression. Cell. 2012;149:1112–24. doi: 10.1016/j.cell.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimura Y, Shinkawa T, Taoka M, Kobayashi K, Isobe T, Yamauchi T. Identification of protein substrates of Ca2+/calmodulin-dependent protein kinase II in the postsynaptic density by protein sequencing and mass spectrometry. Biochem. Biophys. Res. Commun. 2002;290:948–54. doi: 10.1006/bbrc.2001.6320. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc. Natl. Acad. Sci. USA. 2007;104:6418–23. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanhueza M, Fernandez-Villalobos G, Stein IS, Kasumova G, Zhang P, et al. Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J. Neurosci. 2011;31:9170–78. doi: 10.1523/JNEUROSCI.1250-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng W, Zhang M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nat. Rev. Neurosci. 2009;10:87–99. doi: 10.1038/nrn2540. [DOI] [PubMed] [Google Scholar]
- 43.Cheng D, Hoogenraad CC, Rush J, Ramm E, Schlager MA, et al. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol. Cell. Proteomics. 2006;5:1158–70. doi: 10.1074/mcp.D500009-MCP200. [DOI] [PubMed] [Google Scholar]
- 44.Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–43. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 45.Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–34. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 46.Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–20. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Steiner P, Higley MJ, Xu W, Czervionke BL, Malenka RC, Sabatini BL. Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron. 2008;60:788–802. doi: 10.1016/j.neuron.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim MJ, Futai K, Jo J, Hayashi Y, Cho K, Sheng M. Synaptic accumulation of PSD-95 and synaptic function regulated by phosphorylation of serine-295 of PSD-95. Neuron. 2007;56:488–502. doi: 10.1016/j.neuron.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Kwon HB, Kozorovitskiy Y, Oh WJ, Peixoto RT, Akhtar N, et al. Neuroligin-1-dependent competition regulates cortical synaptogenesis and synapse number. Nat. Neurosci. 2012;15:1667–74. doi: 10.1038/nn.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim J, Jung SY, Lee YK, Park S, Choi JS, et al. Neuroligin-1 is required for normal expression of LTP and associative fear memory in the amygdala of adult animals. Proc. Natl. Acad. Sci. USA. 2008;105:9087–92. doi: 10.1073/pnas.0803448105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mondin M, Labrousse V, Hosy E, Heine M, Tessier B, et al. Neurexin-neuroligin adhesions capture surface-diffusing AMPA receptors through PSD-95 scaffolds. J. Neurosci. 2011;31:13500–15. doi: 10.1523/JNEUROSCI.6439-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shipman SL, Nicoll RA. A subtype-specific function for the extracellular domain of neuroligin 1 in hippocampal LTP. Neuron. 2012;76:309–16. doi: 10.1016/j.neuron.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peixoto RT, Kunz PA, Kwon H, Mabb AM, Sabatini BL, et al. Transsynaptic signaling by activity-dependent cleavage of neuroligin-1. Neuron. 2012;76:396–409. doi: 10.1016/j.neuron.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 2012;22:461–69. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwenk J, Harmel N, Brechet A, Zolles G, Berkefeld H, et al. High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron. 2012;74:621–33. doi: 10.1016/j.neuron.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 56.Ashby MC, De La Rue SA, Ralph GS, Uney J, Collingridge GL, Henley JM. Removal of AMPA receptors (AMPARs) from synapses is preceded by transient endocytosis of extrasynaptic AMPARs. J. Neurosci. 2004;24:5172–76. doi: 10.1523/JNEUROSCI.1042-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J. Neurosci. 2006;26:2000–9. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron. 2009;64:381–90. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patterson MA, Szatmari EM, Yasuda R. AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proc. Natl. Acad. Sci. USA. 2010;107:15951–56. doi: 10.1073/pnas.0913875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borgdorff AJ, Choquet D. Regulation of AMPA receptor lateral movements. Nature. 2002;417:649–53. doi: 10.1038/nature00780. [DOI] [PubMed] [Google Scholar]
- 61.Lee HK, Takamiya K, Han JS, Man H, Kim CH, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–43. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 62.Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–88. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- 63.Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J. Biol. Chem. 1997;272:32727–30. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- 64.Zhong H, Sia GM, Sato TR, Gray NW, Mao T, et al. Subcellular dynamics of type II PKA in neurons. Neuron. 2009;62:363–74. doi: 10.1016/j.neuron.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu W, Isozaki K, Roche KW, Nicoll RA. Synaptic targeting of AMPA receptors is regulated by a CaMKII site in the first intracellular loop of GluA1. Proc. Natl. Acad. Sci. USA. 2010;107:22266–71. doi: 10.1073/pnas.1016289107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sumioka A, Yan D, Tomita S. TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron. 2010;66:755–67. doi: 10.1016/j.neuron.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, et al. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67:239–52. doi: 10.1016/j.neuron.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 68.Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA. LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature. 2013;493:495–500. doi: 10.1038/nature11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen L, El-Husseini A, Tomita S, Bredt DS, Nicoll RA. Stargazin differentially controls the trafficking of α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate and kainate receptors. Mol. Pharmacol. 2003;64:703–6. doi: 10.1124/mol.64.3.703. [DOI] [PubMed] [Google Scholar]
- 70.Renner ML, Cognet L, Lounis B, Triller A, Choquet D. The excitatory postsynaptic density is a size exclusion diffusion environment. Neuropharmacology. 2009;56:30–36. doi: 10.1016/j.neuropharm.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 71.Santamaria F, Gonzalez J, Augustine GJ, Raghavachari S. Quantifying the effects of elastic collisions and non-covalent binding on glutamate receptor trafficking in the post-synaptic density. PLoS Comput. Biol. 2010;6:e1000780. doi: 10.1371/journal.pcbi.1000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blanpied TA, Kerr JM, Ehlers MD. Structural plasticity with preserved topology in the postsynaptic protein network. Proc. Natl. Acad. Sci. USA. 2008;105:12587–92. doi: 10.1073/pnas.0711669105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–60. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- 74.Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat. Neurosci. 2004;7:1104–12. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 75.Bosch M, Hayashi Y. Structural plasticity of dendritic spines. Curr. Opin. Neurobiol. 2012;22:383–88. doi: 10.1016/j.conb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dent EW, Merriam EB, Hu X. The dynamic cytoskeleton: backbone of dendritic spine plasticity. Curr. Opin. Neurobiol. 2011;21:175–81. doi: 10.1016/j.conb.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frost NA, Kerr JM, Lu HE, Blanpied TA. A network of networks: cytoskeletal control of compartmentalized function within dendritic spines. Curr. Opin. Neurobiol. 2010;20:578–87. doi: 10.1016/j.conb.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Korobova F, Svitkina T. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol. Biol. Cell. 2010;21:165–76. doi: 10.1091/mbc.E09-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Honkura N, Matsuzaki M, Noguchi J, Ellis-Davies GC, Kasai H. The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron. 2008;57:719–29. doi: 10.1016/j.neuron.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 80.Frost NA, Shroff H, Kong H, Betzig E, Blanpied TA. Single-molecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron. 2010;67:86–99. doi: 10.1016/j.neuron.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Izeddin I, Specht CG, Lelek M, Darzacq X, Triller A, et al. Super-resolution dynamic imaging of dendritic spines using a low-affinity photoconvertible actin probe. PLoS ONE. 2011;6:e15611. doi: 10.1371/journal.pone.0015611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tatavarty V, Kim EJ, Rodionov V, Yu J. Investigating sub-spine actin dynamics in rat hippocampal neurons with super-resolution optical imaging. PLoS ONE. 2009;4:e7724. doi: 10.1371/journal.pone.0007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saneyoshi T, Fortin DA, Soderling TR. Regulation of spine and synapse formation by activity-dependent intracellular signaling pathways. Curr. Opin. Neurobiol. 2010;20:108–15. doi: 10.1016/j.conb.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Racz B, Weinberg RJ. Microdomains in forebrain spines: an ultrastructural perspective. Mol. Neurobiol. 2013;47:77–89. doi: 10.1007/s12035-012-8345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma S, Grintsevich EE, Phillips ML, Reisler E, Gimzewski JK. Atomic force microscopy reveals drebrin induced remodeling of F-actin with subnanometer resolution. Nano Lett. 2011;11:825–27. doi: 10.1021/nl104159v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kobayashi C, Aoki C, Kojima N, Yamazaki H, Shirao T. Drebrin A content correlates with spine head size in the adult mouse cerebral cortex. J. Comp. Neurol. 2007;503:618–26. doi: 10.1002/cne.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Biou V, Brinkhaus H, Malenka RC, Matus A. Interactions between drebrin and Ras regulate dendritic spine plasticity. Eur. J. Neurosci. 2008;27:2847–59. doi: 10.1111/j.1460-9568.2008.06269.x. [DOI] [PubMed] [Google Scholar]
- 88.Racz B, Weinberg RJ. Spatial organization of cofilin in dendritic spines. Neuroscience. 2006;138:447–56. doi: 10.1016/j.neuroscience.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 89.Chen LY, Rex CS, Casale MS, Gall CM, Lynch G. Changes in synaptic morphology accompany actin signaling during LTP. J. Neurosci. 2007;27:5363–72. doi: 10.1523/JNEUROSCI.0164-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fedulov V, Rex CS, Simmons DA, Palmer L, Gall CM, Lynch G. Evidence that long-term potentiation occurs within individual hippocampal synapses during learning. J. Neurosci. 2007;27:8031–39. doi: 10.1523/JNEUROSCI.2003-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gu J, Lee CW, Fan Y, Komlos D, Tang X, et al. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat. Neurosci. 2010;13:1208–15. doi: 10.1038/nn.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rotty JD, Wu C, Bear JE. New insights into the regulation and cellular functions of the ARP2/3 complex. Nat. Rev. Mol. Cell Biol. 2013;14:7–12. doi: 10.1038/nrm3492. [DOI] [PubMed] [Google Scholar]
- 93.Kim Y, Sung JY, Ceglia I, Lee KW, Ahn JH, et al. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature. 2006;442:814–17. doi: 10.1038/nature04976. [DOI] [PubMed] [Google Scholar]
- 94.Kim IH, Racz B, Wang H, Burianek L, Weinberg R, et al. Disruption of Arp2/3 results in asymmetric structural plasticity of dendritic spines and progressive synaptic and behavioral abnormalities. J. Neurosci. 2013;33:6081–92. doi: 10.1523/JNEUROSCI.0035-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–77. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 96.Penzes P, Cahill ME. Deconstructing signal transduction pathways that regulate the actin cytoskeleton in dendritic spines. Cytoskeleton. 2012;69:426–41. doi: 10.1002/cm.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–4. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang H, Webb DJ, Asmussen H, Niu S, Horwitz AF. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J. Neurosci. 2005;25:3379–88. doi: 10.1523/JNEUROSCI.3553-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park E, Na M, Choi J, Kim S, Lee JR, et al. The Shank family of postsynaptic density proteins interacts with and promotes synaptic accumulation of the βPIX guanine nucleotide exchange factor for Rac1 and Cdc42. J. Biol. Chem. 2003;278:19220–29. doi: 10.1074/jbc.M301052200. [DOI] [PubMed] [Google Scholar]
- 100.Saneyoshi T, Wayman G, Fortin D, Davare M, Hoshi N, et al. Activity-dependent synaptogenesis: regulation by a CaM-kinase kinase/CaM-kinase I/βPIX signaling complex. Neuron. 2008;57:94–107. doi: 10.1016/j.neuron.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Soderling SH, Binns KL, Wayman GA, Davee SM, Ong SH, et al. The WRP component of the WAVE-1 complex attenuates Rac-mediated signalling. Nat. Cell Biol. 2002;4:970–75. doi: 10.1038/ncb886. [DOI] [PubMed] [Google Scholar]
- 102.Soderling SH, Guire ES, Kaech S, White J, Zhang F, et al. A WAVE-1 and WRP signaling complex regulates spine density, synaptic plasticity, and memory. J. Neurosci. 2007;27:355–65. doi: 10.1523/JNEUROSCI.3209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Merrill MA, Malik Z, Akyol Z, Bartos JA, Leonard AS, et al. Displacement of α-actinin from the NMDA receptor NR1 C0 domain by Ca2+/calmodulin promotes CaMKII binding. Biochemistry. 2007;46:8485–97. doi: 10.1021/bi0623025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuriu T, Inoue A, Bito H, Sobue K, Okabe S. Differential control of postsynaptic density scaffolds via actin-dependent and -independent mechanisms. J. Neurosci. 2006;26:7693–706. doi: 10.1523/JNEUROSCI.0522-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J. Neurosci. 1998;18:2423–36. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lanzetti L. Actin in membrane trafficking. Curr. Opin. Cell Biol. 2007;19:453–58. doi: 10.1016/j.ceb.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 107.Hanley JG, Henley JM. PICK1 is a calcium-sensor for NMDA-induced AMPA receptor trafficking. EMBO J. 2005;24:3266–78. doi: 10.1038/sj.emboj.7600801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rocca DL, Martin S, Jenkins EL, Hanley JG. Inhibition of Arp2/3-mediated actin polymerization by PICK1 regulates neuronal morphology and AMPA receptor endocytosis. Nat. Cell Biol. 2008;10:259–71. doi: 10.1038/ncb1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, et al. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–30. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–75. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- 111.Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J. Neurosci. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kelly EE, Horgan CP, McCaffrey MW, Young P. The role of endosomal-recycling in long-term potentiation. Cell. Mol. Life Sci. 2011;68:185–94. doi: 10.1007/s00018-010-0516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–49. doi: 10.1016/s0896-6273(02)00979-0. [DOI] [PubMed] [Google Scholar]
- 114.Lu J, Helton TD, Blanpied TA, Racz B, Newpher TM, et al. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron. 2007;55:874–89. doi: 10.1016/j.neuron.2007.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kennedy MJ, Davison IG, Robinson CG, Ehlers MD. Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell. 2010;141:524–35. doi: 10.1016/j.cell.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yudowski GA, Puthenveedu MA, Leonoudakis D, Panicker S, Thorn KS, et al. Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J. Neurosci. 2007;27:11112–21. doi: 10.1523/JNEUROSCI.2465-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, et al. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat. Neurosci. 2009;12:879–87. doi: 10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu. Rev. Biochem. 2012;81:637–59. doi: 10.1146/annurev-biochem-052810-093700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stenmark H. The Rabs: a family at the root of metazoan evolution. BMC Biol. 2012;10:68. doi: 10.1186/1741-7007-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brown TC, Correia SS, Petrok CN, Esteban JA. Functional compartmentalization of endosomal trafficking for the synaptic delivery of AMPA receptors during long-term potentiation. J. Neurosci. 2007;27:13311–15. doi: 10.1523/JNEUROSCI.4258-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Correia SS, Bassani S, Brown TC, Lise MF, Backos DS, et al. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat. Neurosci. 2008;11:457–66. doi: 10.1038/nn2063. [DOI] [PubMed] [Google Scholar]
- 122.Wang Z, Edwards JG, Riley N, Provance DW, Jr, Karcher R, et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–48. doi: 10.1016/j.cell.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ahmad M, Polepalli JS, Goswami D, Yang X, Kaeser-Woo YJ, et al. Postsynaptic complexin controls AMPA receptor exocytosis during LTP. Neuron. 2012;73:260–67. doi: 10.1016/j.neuron.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jurado S, Goswami D, Zhang Y, Molina AJ, Südhof TC, Malenka RC. LTP requires a unique postsynaptic SNARE fusion machinery. Neuron. 2013;77:542–58. doi: 10.1016/j.neuron.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cooney JR, Hurlburt JL, Selig DK, Harris KM, Fiala JC. Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. J. Neurosci. 2002;22:2215–24. doi: 10.1523/JNEUROSCI.22-06-02215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carroll RC, Beattie EC, von Zastrow M, Malenka RC. Role of AMPA receptor endocytosis in synaptic plasticity. Nat. Rev. Neurosci. 2001;2:315–24. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- 127.Petrini EM, Lu J, Cognet L, Lounis B, Ehlers MD, Choquet D. Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron. 2009;63:92–105. doi: 10.1016/j.neuron.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lüscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, et al. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–58. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- 129.Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–59. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, et al. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–84. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–74. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–87. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 133.Segal M, Vlachos A, Korkotian E. The spine apparatus, synaptopodin, and dendritic spine plasticity. Neuroscientist. 2010;16:125–31. doi: 10.1177/1073858409355829. [DOI] [PubMed] [Google Scholar]
- 134.Bas Orth C, Vlachos A, Del Turco D, Burbach GJ, Haas CA, et al. Lamina-specific distribution of Synaptopodin, an actin-associated molecule essential for the spine apparatus, in identified principal cell dendrites of the mouse hippocampus. J. Comp. Neurol. 2005;487:227–39. doi: 10.1002/cne.20539. [DOI] [PubMed] [Google Scholar]
- 135.Vlachos A, Korkotian E, Schonfeld E, Copanaki E, Deller T, Segal M. Synaptopodin regulates plasticity of dendritic spines in hippocampal neurons. J. Neurosci. 2009;29:1017–33. doi: 10.1523/JNEUROSCI.5528-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Holbro N, Grunditz A, Oertner TG. Differential distribution of endoplasmic reticulum controls metabotropic signaling and plasticity at hippocampal synapses. Proc. Natl. Acad. Sci. USA. 2009;106:15055–60. doi: 10.1073/pnas.0905110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Deller T, Korte M, Chabanis S, Drakew A, Schwegler H, et al. Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity. Proc. Natl. Acad. Sci. USA. 2003;100:10494–99. doi: 10.1073/pnas.1832384100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jedlicka P, Vlachos A, Schwarzacher SW, Deller T. A role for the spine apparatus in LTP and spatial learning. Behav. Brain Res. 2008;192:12–19. doi: 10.1016/j.bbr.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 139.Vlachos A. Synaptopodin and the spine apparatus organelle—regulators of different forms of synaptic plasticity? Ann. Anat. 2012;194:317–20. doi: 10.1016/j.aanat.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 140.Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J. Neurosci. 1992;12:2685–705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sigrist SJ, Sabatini BL. Optical super-resolution microscopy in neurobiology. Curr. Opin. Neurobiol. 2012;22:86–93. doi: 10.1016/j.conb.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 142.Hell SW, Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994;19:780–82. doi: 10.1364/ol.19.000780. [DOI] [PubMed] [Google Scholar]
- 143.Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–45. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 144.Rust MJ, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nat. Methods. 2006;3:793–95. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gustafsson MG. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 2000;198:82–87. doi: 10.1046/j.1365-2818.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- 146.Testa I, Urban NT, Jakobs S, Eggeling C, Willig KI, Hell SW. Nanoscopy of living brain slices with low light levels. Neuron. 2012;75:992–1000. doi: 10.1016/j.neuron.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 147.Yasuda R. Imaging spatiotemporal dynamics of neuronal signaling using fluorescence resonance energy transfer and fluorescence lifetime imaging microscopy. Curr. Opin. Neurobiol. 2006;16:551–61. doi: 10.1016/j.conb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 148.Yasuda R. Studying signal transduction in single dendritic spines. Cold Spring Harb. Perspect. Biol. 2012;4:a005611. doi: 10.1101/cshperspect.a005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–40. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhou XX, Chung HK, Lam AJ, Lin MZ. Optical control of protein activity by fluorescent protein domains. Science. 2012;338:810–14. doi: 10.1126/science.1226854. [DOI] [PMC free article] [PubMed] [Google Scholar]