Abstract
Objective
There is increasing evidence that altered glutamate (Glu) homeostasis is involved in the pathophysiology of multiple sclerosis (MS). The aim of this study was to evaluate the in vivo effects of excess brain Glu on neuroaxonal integrity measured by N-acetylaspartate (NAA), brain volume, and clinical outcomes in a large, prospectively followed cohort of MS subjects.
Methods
We used multivoxel spectroscopy at 3T to longitudinally estimate Glu and NAA concentrations from large areas of normal-appearing white and gray matter (NAWM and GM) in MS patients (n = 343) with a mean follow-up time of 5 years. Using linear mixed-effects models, Glu was examined as a predictor of NAA decline, annualized percentage brain volume change, and evolution of clinical outcomes (Multiple Sclerosis Functional Composite [MSFC], Paced Auditory Serial Addition Test-3 [PASAT], and Expanded Disability Status Scale). Glu/NAA ratio was tested as a predictor of brain volume loss and clinical outcomes.
Results
Baseline Glu[NAWM] was predictive of accelerated longitudinal decline in NAA[GM] (–0.06mM change in NAA[GM]/yr for each unit increase in Glu; p = 0.004). The sustained elevation of Glu[NAWM] was predictive of a loss of 0.28mM/yr in NAA[NAWM] (p < 0.001) and 0.15mM/yr in NAA[GM] (p = 0.056). Each 10% increase in Glu/NAA[NAWM] was associated with a loss of 0.33% brain volume/yr (p = 0.001), 0.009 standard deviations/yr in MSFC z-score (p < 0.001), and 0.17 points/yr on the PASAT (p < 0.001).
Interpretation
These results indicate that higher Glu concentrations increase the rate of NAA decline, and higher Glu/NAA[NAWM] ratio increases the rate of decline of brain volume, MSFC, and PASAT. This provides evidence of a relationship between brain Glu and markers of disease progression in MS.
Multiple sclerosis (MS) is an immune-mediated disorder in which inflammatory cells attack the myelin of the central nervous system (CNS), leading to varying extents of neuroaxonal injury. There is increasing evidence that glutamate (Glu) is involved in the patho-physiology of MS and its animal model, experimental autoimmune encephalomyelitis (EAE). Increased extracellular Glu concentrations result in neuronal and glial cell death via excitotoxic mechanisms.1 Evidence that Glu is involved in MS pathophysiology includes: (1) the abnormal elevation of levels of phosphate-activated glutaminase, the principal enzyme for the production of Glu, in macrophages and microglial cells over lesional white matter (WM) and dystrophic axons2; (2) the deficient expression of Glu transporters on the surface of oligodendrocytes responsible for Glu reuptake in MS WM3; (3) the overexpression of metabotropic Glu receptors on injured axons in MS4; (4) the elevation of Glu concentrations in the cerebrospinal fluid of MS patients experiencing relapses and progressive disability worsening5,6; and (5) the association of Glu receptor genes with MS susceptibility.7 In EAE, treatment with different Glu receptor antagonists has been shown to ameliorate clinical outcome, reduce spinal cord neurodegeneration and dephosphorylation of axonal neurofilament, and increase oligodendrocyte survival independently of lymphocyte infiltration and lesion size.8–11
Proton magnetic resonance spectroscopic imaging (1H-MRSI) at 3T offers a unique method to noninvasively measure and resolve Glu and glutamine resonances in vivo, which remains challenging with lower field strength spectroscopy. It has been demonstrated12 that the zeroth component of a 2-dimensional (2D) J-resolved spectrum results in an unobstructed detection of Glu at 2.35ppm that is distinct from glutamine and N-acetylaspartate (NAA), a specific marker of axonal integrity and mitochondrial dysfunction.13 This technique, called TE-Averaging, shows elevated Glu concentrations in contrast-enhancing WM lesions and normal-appearing WM (NAWM) but not chronic lesions of MS patients.14 To better assess Glu levels in large areas of NAWM and gray matter (GM), we combined TE-Averaging with a fast multivoxel spectroscopic imaging scheme (TE-Averaged 1H-MRSI) acquired within a clinically reasonable time.15 We recently used TE-Averaged 1H-MRSI to measure Glu concentration as an endophenotypic trait and assessed the extent to which Glu concentration is under genomic control (whole genome DNA variants); using pathway-based analysis, we identified a module of 70 genes with high relevance to Glu biology.16
This work is the first longitudinal study of the association between in vivo brain Glu concentration and markers of brain injury in MS. Whereas other investigators have recently used single-echo, single-voxel magnetic resonance spectroscopy,17 we use TE-Averaged 1H-MRSI to estimate the levels of Glu and NAA from large areas of NAWM and GM centered around the corpus callosum18 of MS patients. Our hypothesized model was that in vivo brain Glu would be associated with loss of neuroaxonal integrity, determined by an acceleration of NAA decline over time, which would in turn lead to decreased brain volume and, ultimately, a worse clinical outcome. Finding relationships in the data that are supportive of this model of brain injury would provide in vivo evidence for the hypothesis of Glu excitotoxicity in MS.
Subjects and Methods
Research Participants
These patients were part of a large, prospective, phenotype– genotype biomarker study conducted at the University of California, San Francisco (UCSF) Multiple Sclerosis Center between January 2005 and December 2010. White patients aged 18 to 65 years who fulfilled 2005 McDonald criteria for MS19 were offered the opportunity to participate. A cohort of >500 MS patients of all stages and clinical subtypes of the disease20 was assembled and followed longitudinally with annual clinical visits and brain magnetic resonance imaging (MRI) scans for an average of 5 years. TE-Averaged 1H-MRSI baseline data were collected on 402 of 583 patients as part of their annual study brain MRI. Subjects were excluded from the MRI and spectroscopy acquisition if they had experienced a clinical relapse or received treatment with corticosteroids within the month prior to their scan, or if time did not allow for spectroscopy acquisition. The concomitant use of disease-modifying therapies for MS was permitted. In addition, 42 adult healthy control subjects were recruited and scanned at 1 time point during the study period. The protocol was approved by the Committee on Human Research at UCSF, and informed consent was obtained from all participants.
Study Design
The first step in our hypothesized pathway of MS brain tissue injury was that increased Glu would predict NAA decline. As such, the main entry criterion for a subject to be included in this analysis was that they must have at least 1 valid baseline Glu and at least 2 valid NAA measurements (after quality checking the spectroscopy data). Of the 402 subjects who had baseline spectroscopy measurements, 343 (N1) fit the entry criterion and comprise the main study population included herein. In addition, we considered a definition of sustained elevation of Glu as a predictor of NAA decline. To be included in this sub-analysis, patients needed at least 2 valid consecutive Glu and at least 2 valid NAA measurements. There were 211 (N2) such subjects.
Image Acquisition
Structural MRI scans were performed on all subjects at each annual study visit. Images were acquired using an 8-channel phased array coil in reception and a body coil in transmission on a 3T GE Excite scanner that did not undergo hardware upgrades throughout the duration of the study period (January 2005–December 2010). A 3D inversion recovery spoiled gradient-echo (IRSPGR) T1-weighted isotropic volumetric sequence (1 × 1 × 1mm3, 180 slices) was acquired for brain volume measurements (echo time [TE]/repetition time [TR]/ inversion time = 2/7/400 milliseconds, flip angle = 15°, 256 × 256 × 180 matrix, 240 × 240 × 180mm3 field of view, number of excitations = 1).
Spectral Acquisition, Repositioning, and Quantification
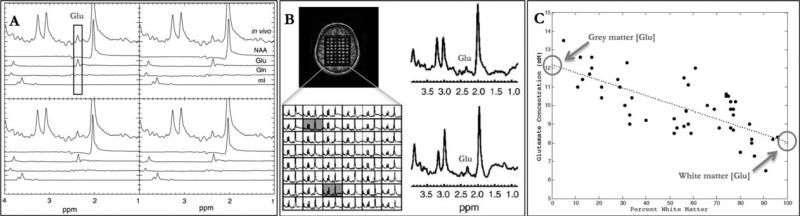
TE-Averaged 1H-MRSI (TR 5 1 second, 64 echo time steps starting at TE = 35 milliseconds with an echo time increment of 2.5 milliseconds, total acquisition time = 21 minutes)15 spectra were acquired with an in-plane resolution of 1.2 × 1.0cm over a single slice of thickness 1.5cm in the supratentorial brain just above the corpus callosum.18 Briefly, we have previously demonstrated using single-voxel spectroscopy that a multiecho technique, TE-Averaging, can obtain an unobstructed Glu measurement (ie, separate from glutamine and NAA) compared to single-echo techniques.12 Subsequently, to measure Glu from larger areas of the brain than a single-voxel technique would allow, we extended the TE-Averaged point resolved spectroscopy (PRESS) technique to a 2D multivoxel imaging scheme (TE-Averaged 1H-MRSI). We were again able to obtain an unobstructed measure of Glu (Fig, 1A).15 To keep the acquisition time clinically reasonable, TE-Averaged 1H-MRSI acquires data in a flyback echo planar (ie, spiral) gradient, which are then transformed back into a 2D grid (see Fig, 1B).
FIGURE 1.
(A) Spectra from a subset of proton magnetic resonance spectroscopic imaging (1H-MRSI) voxels obtained from phantoms containing only glutamate (Glu), glutamine (Gln), N-acetylaspartate (NAA), and myo-inositol (mI), and their relationship to an in vivo spectrum acquired using the same parameters. The unobstructed glutamate peak remains visible at 2.35ppm using TE-Averaged 1H-MRSI (vertical rectangle). (B) Positioning of the PRESS box (upper left), as well as a TE-Averaged 1HMRSI spectral array from a normal subject (lower left). Representative spectra from white matter (upper right) and gray matter (lower right) are shown from voxels shaded in the spectral array. (C) Glutamate estimation plotted as a function of percentage white matter per voxel in a single healthy control. Concentration estimates for gray and white matter are derived from the end points of the linear fit (y-intercepts, circles).
The spectroscopic data were acquired on the same 3T GE Excite scanner as the structural MRI, immediately following the acquisition of the anatomical images but prior to the administration of the contrast agent. The resulting coil combination data were TE-averaged, and the metabolic concentration of Glu and NAA were obtained using the LCmodel quantification algorithm. Because of the short repetition time (TR = 1.0 second), concentration estimates were corrected for T1 relaxation times derived from both WM and GM compartments using the same methods and phantom basis sets described elsewhere.15 The NAA T1 relaxation times used were 1.03 and 1.21 seconds in WM and GM, respectively. Glu T1 relaxation times used were 0.67 and 0.88 seconds in WM and GM, respectively. Individual NAA T2 relaxation times in MS WM and GM were measured directly from the TE-Average multiecho sequence at each time point. Glu T2s were not available. As this is a longitudinal spectroscopy study, we considered it important to acquire spectra from the same location for each patient at each time point. We used in-house software, now commercially available, that estimates the location of the PRESS box in 3 orthogonal planes from the baseline 3DIRSPGR. The coregistration software provides the MRI technician with the location information (right/left, anterior/posterior, superior/inferior) to assure a sufficient overlap between the PRESS boxes with an accuracy of 96 to 98%.15
Metabolite Estimates in Normal-Appearing GM and NAWM
On a per-subject basis, metabolite concentrations were estimated for each voxel within the 2D spectroscopic grid using LCmodel (see Fig, 1B). In brief, nonbrain regions were removed from the anatomical images using an automated brain extraction tool (fsl.fmrib.ox.ac.uk/fsl/fslwiki/BET) that is part of FSL.21 T1-weighted images were segmented into nonlesional GM, WM, and cerebrospinal fluid compartments using FAST, Functional Magnetic Resonance Imaging of the Brain's Automated Segmentation Tool (fsl.fmrib.ox.ac.uk/fsl/fslwiki/FAST),22 and T1 lesion masks.16 After segmentation, the GM and WM maps were regridded to the spectroscopic resolution and convolved with the point-spread function for spectroscopic imaging from which the percentage GM and WM content were estimated for each spectroscopic voxel. By modeling metabolite concentrations and magnetic resonance (MR) relaxation parameters as a linear function of WM content, normal-appearing GM and WM NAA and Glu estimates were extrapolated from the end points of a linear regression fit to all voxels within the PRESS box (see Fig, 1C).23 Spectroscopic voxels were included in the linear fit only if their concentration estimates had Cramer–Rao bounds within a threshold of 15% for Glu and 5% for NAA.15 Metabolite estimates outside these bounds did not contribute to reported values. The overall scan–rescan estimates of coefficients of variation of this method were 5% and 13% for NAA and Glu, respectively.15
Brain Volume Measurements
Annual percentage whole brain volume change was calculated from 3D-IRSPGR images using Structural Image Evaluation Using Normalization of Atrophy (SIENA), a fully automated method of longitudinal brain change analysis (fsl.fmrib.ox.a-c.uk/fsl/fslwiki/SIENA).24 Output is converted into percentage brain volume change (PBVC) per year between pairs of scans. For use as a baseline covariate, we also calculated normalized brain parenchymal volume from 3D-IRSPGR images using SIENA/X (fsl.fmrib.ox.ac.uk/fsl/fslwiki/SIENA).24 T1 lesion masks were derived from manual segmentation of T1-visible WM lesions on the 3D-IRSPGR using methods described previously.25,26 T1 lesion masks were incorporated into the SIENA/X program to prevent voxel misclassification errors. In addition, T1 lesion volume was used as a baseline covariate.
Clinical Outcomes
Neurologic evaluations were performed annually, including the Expanded Disability Status Scale (EDSS)27 and the Multiple Sclerosis Functional Composite (MSFC).28 MSFC testing included 9-Hole Peg Test, Paced Auditory Serial Addition Test-3 (PASAT), and Timed 25-Foot Walk.
Statistical Analysis
All statistical analyses were performed using R (www.r-project.org). Linear mixed-effects modeling formed the core of statistical analysis.29 All reported mixed-effects models were fitted using restricted maximum likelihood estimation (maximum likelihood estimated models were only used when comparing models with different sets of fixed effects). Generalized linear models were fit using maximum likelihood. Linear mixed-effects models were initially fit with random (subject-specific) intercepts and slopes, but these generally did not converge. Diagnostic plots indicated that this was due to a lack of identifiability between residual error and random variation in slopes. We therefore fit models that included random effects for the intercept only.
The primary predictors of interest were baseline and sustained elevation of Glu[NAWM], Glu[GM], Glu/NAA[NAWM], and Glu/NAA[GM]. Patients were considered to have a sustained elevation of Glu if their Glu concentration was >2 standard deviations above the mean Glu concentration in the healthy control sample at 2 consecutive time points (12-month sustained). The presence or absence of sustained elevation of Glu was modeled as a binary predictor in a linear mixed-effects model.
The first hypothesis was that in vivo Glu would predict the loss of neuroaxonal integrity over time, as measured by decline in NAA. This was tested by modeling baseline Glu as a predictor of NAA decline, separately for WM and GM, with estimation and hypothesis testing focused on annual rate of change in NAA (mM/yr). Glu was also examined as a predictor of PBVC per year, measured with SIENA, as well as EDSS, MSFC, and PASAT. EDSS, MSFC, and PASAT were modeled as continuous variables. For SIENA, we adopted a model30 accommodating the inherent correlation between all pairs of change scores. However, we only modeled PBVC measurements between subsequent pairs of time points (ie, baseline–year 1, year 1–year 2, year 2–year 3, year 3–year 4) to create a slope for each patient rather than changes between all time point pairs because of the nonadditivity of percentage changes across multiple intervals.
Covariates in all models included age, sex, disease duration, baseline normalized brain parenchymal volume, baseline T1 lesion volume (from 3D-IRSPGR), EDSS (except when modeling clinical outcomes), and the presence of contrast-enhancing lesions. In linear mixed-effects models, each baseline predictor was modeled with an interaction with time (in addition to their main effects) to allow an association with longitudinal change in the outcome. In these models, the primary interest was in detecting statistically significant time by predictor interactions, that is, modifiers of the rate of change in out come (conditional t test). Models were fit for all predictor and outcome combinations, and each model was fit using 2 approaches: (1) a simple model that included only the primary predictor of interest (plus interaction with time in linear mixed-effects models), and (2) with aforementioned covariates added to the model. In the logistic regression models, the focus was on detecting a statistically significant effect of the predictor (Wald test). In all models, the estimated direction and size of the effect for the main variable of interest (Glu concentration or Glu/NAA ratio) remained approximately consistent regardless of whether covariates were added to the model (indicating robustness to precise model specification). We therefore only report 1 set of results here, namely those from the full models that include the additional covariates.
All hypothesis tests were performed at a nominal α = 0.05 significance level (ie, without adjustment for multiple comparisons). Although the need to adjust for multiple comparisons is controversial, it is most appropriate when several independent tests are conducted on 1 population. In the present analysis, independence cannot be assumed; rather, there are correlations and clear biologic relationships among many of the variables that reinforce rather than detract from each another. Thus, adjustment for multiple comparisons would be counterproductive in this situation. We rely on scientific judgment rather than formal adjustment methods to indicate where caution is warranted despite findings with p < 0.05.31,32
Motivation for Glu/NAA Ratio Predictor
The lack of a clear longitudinal effect of Glu or NAA alone on structural or clinical effects in this large data set prompted the consideration of baseline Glu/NAA ratio as a predictor so that baseline Glu concentrations would be in some sense adjusted for the amount of neuronal dysfunction present. Because Glu is stored in neurons, which are known to have reduced function/ integrity in MS,33 we considered it biologically plausible that loss or dysfunction of neurons may be associated with lower total Glu concentrations. We hypothesized that a predictor that appropriately combines Glu and NAA would have increased predictive power over Glu or NAA concentrations alone.
Results
Demographics of the Subjects
Baseline demographics and clinical data for subjects included in this study are given in Table 1.
TABLE 1.
Baseline Demographics
| Variable | Patients, n = 343 | Controls, n = 42 |
|---|---|---|
| Sex | ||
| Female | 234 | 26 |
| Male | 109 | 16 |
| Age, yr | 43.2 ± 9.6 | 40.5 ± 10.0 |
| Disease duration, yr | 9.1 ± 8.0 | — |
| Clinical subtype | ||
| CIS | 49 | — |
| RRMS | 263 | — |
| SPMS | 20 | — |
| PPMS | 11 | — |
| EDSS | 2 (0–7.5) | — |
| MSFC, z score | 0.20 ± 0.59 | — |
| Subjects on DMT | 226 | — |
Number for categorical variables, mean ± standard deviation for continuous variables, and median (range) for EDSS. CIS = clinically isolated syndrome; DMT = disease-modifying therapy; EDSS = Expanded Disability Status Scale; MSFC = Multiple Sclerosis Functional Composite; PPMS = primary progressive multiple sclerosis; RRMS = relapsing-remitting multiple sclerosis; SPMS = secondary progressive multiple sclerosis.
Glu in NAWM and GM: Patients versus Healthy Controls
Table 2 shows baseline metabolite concentrations for MS subjects and healthy controls, and a comparison of each metabolite between all MS patients and controls. Glu and NAA concentrations were normally distributed in both GM and WM. Glu[NAWM] was statistically significantly higher, and NAA[NAWM] and NAA[GM] were statistically significantly lower, in MS patients compared to healthy controls. The estimated difference in Glu[GM] between MS patients and healthy controls was small and not statistically significant.
TABLE 2.
Metabolite Concentrations at Baseline
| Metabolite | Patients, n = 343 | Controls, n = 42 | Mean Differencea (95% CI) | p a |
|---|---|---|---|---|
| Glutamate[NAWM] | ||||
| All MS | 9.29 ± 1.70 | 8.44 ± 1.01 | 0.85 (0.49 to 1.21) | <0.001 |
| CIS | 9.38 ± 1.19 | |||
| RRMS | 9.32 ± 1.79 | |||
| SPMS | 9.12 ± 1.18 | |||
| PPMS | 8.36 ± 1.92 | |||
| Glutamate[GM] | ||||
| All MS | 10.79 ± 2.04 | 10.80 ± 1.70 | –0.015 (–0.58 to 0.55) | 0.96 |
| CIS | 10.84 ± 2.31 | |||
| RRMS | 10.82 ± 2.02 | |||
| SPMS | 10.59 ± 2.0 | |||
| PPMS | 10.15 ± 1.47 | |||
| NAA[NAWM] | ||||
| All MS | 9.90 ± 1.38 | 11.39 ± 1.31 | –1.49 (–1.91 to –1.07) | <0.001 |
| CIS | 10.11 ± 1.10 | |||
| RRMS | 9.89 ± 1.41 | |||
| SPMS | 9.83 ± 1.70 | |||
| PPMS | 9.25 ± 1.11 | |||
| NAA[GM] | ||||
| All MS | 8.83 ± 1.56 | 9.58 ± 1.70 | –0.75 (–1.29 to –0.22) | <0.001 |
| CIS | 8.70 ± 1.28 | |||
| RRMS | 8.89 ± 1.61 | |||
| SPMS | 8.57 ± 1.62 | |||
| PPMS | 8.32 ± 1.61 | |||
| Glu/NAA[NAWM] | ||||
| All MS | 0.94 ± 0.15 | 0.75 ± 0.10 | 0.19 (0.16 to 0.22) | <0.001 |
| CIS | 0.93 ± 0.08 | |||
| RRMS | 0.95 ± 0.16 | |||
| SPMS | 0.94 ± 0.12 | |||
| PPMS | 0.90 ± 0.16 | |||
| Glu/NAA[GM] | ||||
| All MS | 1.23 ± 0.18 | 1.16 ± 0.20 | 0.07 (0.01 to 0.14) | 0.022 |
| CIS | 1.25 ± 0.21 | |||
| RRMS | 1.23 ± 0.17 | |||
| SPMS | 1.26 ± 0.24 | |||
| PPMS | 1.25 ± 0.21 |
Mean concentrations ± standard deviations, given in millimoles.
Mean difference in millimoles and p values are given for the comparison between all MS patients versus controls using a Student t test.
CI = confidence interval; CIS = clinically isolated syndrome; Glu = glutamate; GM = gray matter; MS = multiple sclerosis; NAA = N-acetylaspartate; NAWM = normal-appearing white matter; PPMS = primary progressive multiple sclerosis; RRMS = relapsing–remitting multiple sclerosis; SPMS = secondary progressive multiple sclerosis.
Glu as a Predictor of NAA Decline
Parameter estimates, 95% confidence intervals (CIs), and p values obtained from multiple predictor models with Glu as the predictor and NAA as the outcome are given in Table 3. The mean follow-up time in this portion of the analysis was 2.2 years. Baseline Glu[NAWM] was a statistically significant predictor of longitudinal decline in NAA[GM], with an annualized rate of change adjustment in NAA[GM] of –0.06mM/yr (95% CI = –0.02 to –0.11mM/yr; p = 0.005). That is to say, for each 1mM increase in baseline Glu[NAWM], there is an estimated additional loss of 0.06mM/yr of NAA[GM], with a 95% CI of 0.02 to 0.11mM/yr. This effect of Glu on NAA decline occurs in addition to the effect of time (this interpretation applies to all of the results given in this work). Baseline Glu[GM] was a statistically significant predictor of longitudinal decline in both NAA[GM] and NAA[NAWM]. For each additional millimole in baseline Glu[GM], there is an estimated change of 20.05mM/yr in NAA[GM] (95% CI = –0.02 to –0.11mM/yr; p = 0.003), as well as an estimated change of –0.03mM/ yr in NAA[NAWM] (95% CI = –0.002 to –0.07mM/yr; p = 0.038). Baseline Glu[NAWM] was not a statistically significant predictor of longitudinal decline of NAA[NAWM], with an estimated change of –0.03mM/yr (95% CI = –0.07 to 0.01mM/yr; p = 0.12). However, the estimated effect was in the expected direction, and the magnitude was comparable with the other significant results.
TABLE 3.
Glutamate and Sustained Elevation of Glutamate as Predictors of NAA Decline (N1 = 343 and N2 = 211, respectively)
| Outcome | ||
|---|---|---|
| Predictor | NAA[NAWM] | NAA[GM] |
| Glu[NAWM] | –0.03 (–0.07 to 0.01) p = 0.12 | –0.06 (–0.11 to –0.02) p = 0.004a |
| Glu[GM] | –0.03 (–0.07 to –0.002) p = 0.038a | –0.05 (–0.08 to –0.02) p = 0.003a |
| Sustained elevation Glu[NAWM] | –0.28 (–0.41 to –0.15) p < 0.001a | –0.15 (–0.30 to 0.004) p = 0.056a |
| Sustained elevation Glu[GM] | 0.21 (–0.14 to 0.56) p = 0.24 | –0.07 (–0.46 to 0.33) p = 0.75 |
Parameter estimate for the predictor by time interaction and 95% confidence intervals are given for each model.
Mean follow-up time was 2.2 years for NAA.
Models in which the predictor by time interaction was statistically significant at α = 0.05.
Glu = glutamate; GM = gray matter; NAA = N-acetylaspartate; NAWM = normal-appearing white matter.
Sustained Elevation of Glu as a Predictor of NAA Decline
Sustained elevation of Glu[NAWM] was a statistically significant predictor of decline in NAA[NAWM]; the presence of a sustained elevation of Glu[NAWM] was associated with an estimated change of –0.28mM/yr in NAA[NAWM] (95% CI = –0.15 to 20.41mM/yr; p < 0.001) compared to patients who did not have a sustained elevation of Glu[NAWM]. The presence of a sustained elevation of Glu[NAWM] was associated with an estimated change of 20.15mM/yr in NAA[GM] (95% CI = –0.004 to –0.30mM/yr; p = 0.056) compared to patients who did not have a sustained elevation of Glu[NAWM]. Sustained elevation of Glu[GM] was not a statistically significant predictor of NAA decline in either the WM or the GM, with CIs extending widely on both sides of zero and hence leading to inconclusive interpretation (see Table 3).
Glu and Sustained Elevation of Glu as Predictors of Annualized PBVC, MSFC, PASAT, and EDSS Decline
Neither Glu[NAWM], Glu[GM], the sustained elevation of Glu[NAWM], nor the sustained elevation of Glu[GM] were statistically significant predictors of linear PBVC after 2.8 years mean follow-up, or MSFC or EDSS worsening after 3.8 years mean follow-up. The results from this portion of the analysis were inconclusive; some estimates of the longitudinal effect of Glu on the outcome were in the expected direction, and others were not, making it difficult to interpret any overall pattern in the results. The single exception was that the sustained elevation of Glu[GM] was a statistically significant predictor of PASAT decline. The presence of a sustained elevation of Glu[GM] was associated with an additional change of –1.2 points/yr on the raw PASAT score (95% CI = –0.003 to –2.4 points/yr; p = 0.0496), compared to patients who did not have a sustained elevation of Glu[GM]. This finding was not congruent with other results in this portion of the analysis.
Glu/NAA Ratio as a Predictor of Annualized PBVC, MSFC, PASAT, and EDSS Decline
Parameter estimates, 95% CIs and p values obtained from multiple predictor models with baseline Glu/NAA as the primary predictor of interest and PBVC or linear change in clinical metrics as outcome are given in Table 4. These estimates and CIs are expressed as change in outcome for every 0.1U change in Glu/NAA ratio. The mean follow-up time for PBVC and clinical metrics was 2.8 and 3.8 years after the baseline Glu/NAA, respectively.
TABLE 4.
Glu/NAA as a Predictor of Clinical Outcomes (N = 211)a
| Predictor | ||
|---|---|---|
| Longitudinal Outcomec,d | Glu/NAA[NAWM]b | Glu/NAA[GM]b |
| Annualized PBVC | 0.33 (0.13, 0.52) p=0.002 | 0.06 (−0.08, 0.20) p=0.42 |
| MSFC | 0.009 (0.004, 0.014) p<0.001 | –0.003 (–0.007, 0.001) p=0.11 |
| PASAT | 0.17 (0.07, 0.27) p<0.001 | 0.004 (–0.07, 0.08) p=0.92 |
| EDSS | 0.001 (–0.02, 0.02) p=0.92 | –0.007 (−0.02, 0.01) p=0.29 |
NAAA = N-acetylaspartate, PBVC = Percent Brain Volume Change, MSFC = Multiple Sclerosis Functional Composite, PASAT = Paced Auditory Serial Addition Test, EDSS = Expanded Disability Status Score, NAWM = Normal Appearing White Matter, GM = Grey Matter.
Parameter estimate for the predictor by time interaction and 95% confidence intervals are given for each model; models in which the predictor by time interaction was statistically significant at alpha = 0.05 are bolded.
The value given in the table reflects the effect of a 10% change in Glu/NAAA.
PBVC is modeled as percent brain volume change per year. MSFC, PASAT and EDSS are modeled as continuous variables.
Mean follow up time was 2.8 years after Glu/NAAA measurement for PBVC and 3.8 years after Glu/NAAA measurement for clinical outcomes.
Glu/NAA[NAWM] was a statistically significant predictor of longitudinal decline in brain volume. Each 0.1U increase in baseline Glu/NAA[NAWM] was associated with an additional –0.33% brain volume per year (95% CI = –0.13 to –0.52%/yr; p = 0.001). Baseline Glu/ NAA[GM] was not a statistically significant predictor of brain volume change. Each 0.1U increase in baseline Glu/NAA[GM] was associated with an additional –0.06% brain volume/yr (95% CI, –0.08 to 0.20%/yr; p 5 0.42). This estimate is in the expected direction, but CIs include the possibility of no effect of Glu/NAA[GM] on the annual rate of brain volume loss.
Glu/NAA[NAWM] was a statistically significant predictor of linear decline in MSFC z scores over time; for each 0.1U increase in baseline Glu/NAA[NAWM], there was an additional change of –0.009 in the MSFC z score per year (95% CI = –0.004 to –0.014U/yr; p < 0.001). In addition, Glu/NAA[NAWM] was a statistically significant predictor of longitudinal PASAT decline; for each 0.1U increase in baseline Glu/NAA[NAWM], there was an additional change of –0.17 points on raw PASAT scores per year (95% CI = –0.07 to –0.27 points/yr; p < 0.001). Glu/NAA[NAWM] was not a statistically significant predictor of longitudinal EDSS decline. Each 0.1U increase in baseline Glu/NAA[NAWM] was associated with an additional change in EDSS of –0.0008 points/yr (95% CI = –0.02 to 0.02 points/yr; p = 0.9245). Although the effect is in the expected direction, the magnitude is very small, and the CIs include the possibility of no effect of Glu/NAA[NAWM] on EDSS over time.
Discussion
In this work, we used a multivoxel spectroscopy pulse sequence that provides unobstructed Glu signal detection, allowing estimation of Glu concentration in NAWM and GM in a large, prospectively collected cohort of MS patients. We hypothesized that increased brain Glu would lead to a cascade of detrimental effects, starting with neuroaxonal dysfunction, followed by decreased brain volume, and ultimately, worsening clinical outcomes. Consistent with our hypothesized sequence of events, our data indicate that higher Glu concentrations increase the rate of NAA decline over 2 years, and higher Glu/NAA ratio in NAWM increases the rate of brain volume loss over 3 years, and MSFC and PASAT decline over 4 years. These results suggest that Glu plays an important role in markers of disease progression in MS, although contributions from other mediators associated with inflammation (such as reactive oxygen species34–36) are not excluded.
Strengths of our study include the 5-year prospective study design, the large sample size, the application of a multivoxel Glu spectroscopy method to MS, and a hypothesis-driven approach to the analysis. Moreover, the statistical methods yielded robust findings that demonstrate effects of Glu on NAA and Glu/NAA on brain volume, MSFC, and PASAT occurring in addition to the effect of time (and other covariates including relevant brain MRI metrics and patient demographics). In other words, these results demonstrate the independent relationship between Glu, and by inference Glu toxicity, and our markers of neurodegeneration.
A better understanding of the pathophysiology underlying MS progression is paramount in accelerating the development of new therapeutic agents targeting permanent CNS injury. Disease heterogeneity is likely broad in MS and could involve several different biological processes and pathways. Our data presented here support a potential bridge between inflammatory and neurotoxicity events mediated by Glu. Across all outcomes, Glu and Glu/NAA were stronger predictors in NAWM than in GM. Although current proton MR spectroscopy does not differentiate between intracellular and extracellular Glu, the excess Glu that we detected in MS subjects is likely to be driven by intracellular Glu; in MS, histopathological data indicate that there is an excess of activated macrophages/microglia in the NAWM.37 Such cells carry Glu, and can release their Glu content into the extracellular compartment,38 where there is defective Glu uptake due to decreased excitatory amino acid transporter expression on the surface of oligodendrocytes.3 Additionally, it has been elegantly demonstrated that WM axons can express functional kainate receptors that recapitulate postsynaptic glutamatergic synapses in the GM. These Glu receptors internalize calcium and initiate the oxidative cascade. This is likely to be a relevant mechanism of axonal compromise and degeneration in the WM of MS subjects.39,40 Oxidative stress is a downstream effect of excess Glu that leads to the overproduction of reactive oxygen species (ROS), which have been shown to damage myelin,34 oligodendrocytes,35 and mitochondria.36 Glutathione is an antioxidant that is essential for protecting against ROS. In MS, several glutathione S-transferase polymorphisms have been associated with more severe long-term disability in Northern European Caucasians.41 Interestingly, in vivo MR spectroscopic imaging of glutathione at 7T has demonstrated a reduction of glutathione in the GM of MS patients compared with healthy controls, as well as in an MS lesion.42 These data suggest that MS patients may lack the ability to produce sufficient glutathione in the presence of a Glu stimulus.
Injured WM and axonal degeneration in the brain can impact brain volume. Brain atrophy will eventually lead to clinical symptoms, particularly cognitive dysfunction. It is perhaps not surprising that neither Glu nor Glu/NAA was predictive of EDSS decline, as the EDSS is dominated by ambulation and spinal cord injury, which are not evaluated by brain Glu measurements. An alternative explanation for our findings could be that Glu and Glu/NAA were stronger predictors in the WM because there is typically a higher signal-to-noise ratio in the WM in MR spectroscopy compared to the relatively thin cortical GM, where partial voluming plays a prominent role. Of note, in a case–control study using a single-echo, single-voxel spectroscopy technique and specifically focusing on MS GM, Muhlert et al recently reported a reduction of Glu plus glutamine and Glu in MS patients compared to healthy individuals.17 In our study we did not see such a reduction in [Glu]GM when comparing all MS patients to healthy controls. This may have been due to differences in acquisition and metabolite quantification methods, including how our method addressed partial voluming. Interestingly, when adjusting [Glu] for [NAA] in GM, we find an increase in the Glu/ NAAGM in MS patients compared to healthy controls (see Table 2). Further studies specifically focusing on the Glu/NAA[GM] ratio between MS subtypes would be of interest.
We consider the biological interpretation of Glu/ NAA to be different than that of the NAA/Cr ratio, another metabolite ratio that has been used in MS research for >2 decades. In the NAA/Cr ratio, creatine is used as an internal reference, whereas in the Glu/NAA ratio, both metabolites may reflect linked pathological processes of CNS injury. Glu is stored in neurons, which are known to have reduced function/integrity in multiple sclerosis.33 As such, we consider it biologically plausible that NAA and Glu concentrations may not be independent in MS, and that a predictor that adjusts Glu concentrations for the amount of neuronal dysfunction present would have increased predictive power over either metabolite alone. Although it has been shown that NAA concentrations in the NAWM can fluctuate over time, particularly in early relapsing–remitting MS,43 neither NAA nor Glu alone predicted brain volume decline or clinical outcomes in our cohort. This suggests that our findings were not simply a function of fluctuating NAA levels, and supports our interpretation that Glu/NAA may be a more biologically relevant predictor than either metabolite alone.
Our study has limitations. This study was conducted at a single site. Although prospective, our study is observational. Our sample size is relatively large, but the study design did not include a separate group of patients for replication. Results from other data sets or groups would need to replicate our findings. Finally, our cohort included a relatively low number of patients with primary and secondary progressive MS, so our findings are mostly representative of patients with relapsing–remitting MS.
In conclusion, the combined presence of an elevation of Glu and a reduction of NAA in NAWM is predictive of tissue loss and clinical worsening in MS. This provides further evidence that Glu biology is a relevant pathway of disease progression in MS, and adds to our prior work showing that the presence of a higher number of module-specific Glu-associated genomic variants is correlated with faster rates of NAA decline and brain volume loss.16 Therefore, the development of MS therapies targeting Glu biology as a therapeutic goal could be worthwhile to slow down disease progression.
Acknowledgment
This research was supported by a grant from the National Multiple Sclerosis Society (RG#3517; D.P.) for MRI/MR spectroscopy postprocessing, the NIH National Institute of Neurologic Disorders and Stroke (NINDS R01NS062885; D.P.) for MRI/MR spectroscopy postprocessing, and industry-sponsored research grants from GlaxoSmithKline and Biogen Idec for clinical and conventional MRI data collection. This investigation was also supported by a Sylvia Lawry Physician Fellowship Award from the National Multiple Sclerosis Society (FP1778-A-1; C.J.A.).
We thank our patients who generously agreed to serve as study participants, the MS clinicians at the UCSF Multiple Sclerosis Center for referring to the EPIC Study, and the UCSF research coordinators for facilitating data collection.
Footnotes
Authorship
D.P. was involved in study concept and design, data collection, standardized assessment of subjects, study conduct, data interpretation and analysis, literature search, and writing of the report. C.J.A. was involved in data interpretation and analysis, literature search, and writing of the report. J.K. was involved in data interpretation and analysis, and writing of the report. P.C. was involved in data interpretation and analysis. M.S. and S.J.N. were involved in study conduct, standardized assessment of MRI/MR spectroscopy processing, and writing of the report. D.T.O. was involved in MRI data processing and data collection. B.A.C. was involved in subject recruitment and the standardized clinical assessment of subjects. S.L.H. was involved in study design and conduct, and data collection.
Potential Conflicts of Interest
D.P.: consultancy, CNS Imaging Consultant. C.J.A.: board membership, Genzyme. M.S.: employment, Synarc. D.T.O.: consultancy, board membership, speaking fees, Acorda Therapeutics, Biogen Idec, Genzyme, Ipsen Pharmaceuticals, Teva Neuroscience. S.L.H. board membership, BioMarin.
References
- 1.Matute C, Domercq M, Sanchez-Gomez MV. Glutamate-mediated glial injury: mechanisms and clinical importance. Glia. 2006;53:212–224. doi: 10.1002/glia.20275. [DOI] [PubMed] [Google Scholar]
- 2.Werner P, Pitt D, Raine CS. Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann Neurol. 2001;50:169–180. doi: 10.1002/ana.1077. [DOI] [PubMed] [Google Scholar]
- 3.Pitt D, Nagelmeier IE, Wilson HC, Raine CS. Glutamate uptake by oligodendrocytes: implications for excitotoxicity in multiple sclerosis. Neurology. 2003;61:1113–1120. doi: 10.1212/01.wnl.0000090564.88719.37. [DOI] [PubMed] [Google Scholar]
- 4.Geurts JJ, Wolswijk G, Bo L, et al. Altered expression patterns of group I and II metabotropic glutamate receptors in multiple sclerosis. Brain. 2003;126:1755–1766. doi: 10.1093/brain/awg179. [DOI] [PubMed] [Google Scholar]
- 5.Sarchielli P, Greco L, Floridi A, Gallai V. Excitatory amino acids and multiple sclerosis: evidence from cerebrospinal fluid. Arch Neurol. 2003;60:1082–1088. doi: 10.1001/archneur.60.8.1082. [DOI] [PubMed] [Google Scholar]
- 6.Stover JF, Pleines UE, Morganti-Kossmann MC, et al. Neurotransmitters in cerebrospinal fluid reflect pathological activity. Eur J Clin Invest. 1997;27:1038–1043. doi: 10.1046/j.1365-2362.1997.2250774.x. [DOI] [PubMed] [Google Scholar]
- 7.Baranzini SE, Galwey NW, Wang J, et al. Pathway and network-based analysis of genome-wide association studies in multiple sclerosis. Hum Mol Genet. 2009;18:2078–2090. doi: 10.1093/hmg/ddp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med. 2000;6:67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- 9.Smith T, Groom A, Zhu B, Turski L. Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nat Med. 2000;6:62–66. doi: 10.1038/71548. [DOI] [PubMed] [Google Scholar]
- 10.Bolton C, Paul C. Glutamate receptors in neuroinflammatory demyelinating disease. Mediators Inflamm. 2006;2006:93684. doi: 10.1155/MI/2006/93684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basso AS, Frenkel D, Quintana FJ, et al. Reversal of axonal loss and disability in a mouse model of progressive multiple sclerosis. J Clin Invest. 2008;118:1532–1543. doi: 10.1172/JCI33464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurd R, Napapon S, Srinivasan R, et al. Measurement of brain glutamate using TE-averaged PRESS at 3T. Magn Reson Med. 2004;51:435–440. doi: 10.1002/mrm.20007. [DOI] [PubMed] [Google Scholar]
- 13.Bjatmar C, Battistuta J, Terada N, et al. N-acetylaspartate is an axon-specific marker of mature white matter in vivo: a biochemical and immunohistochemical study on the rat optic nerve. Ann Neurol. 2002;51:51–58. doi: 10.1002/ana.10052. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan R, Sailasuta N, Hurd R, et al. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3T. Brain. 2005;128:1016–1025. doi: 10.1093/brain/awh467. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan R, Cunningham C, Chen A, et al. TE-averaged two-dimensional proton spectroscopic imaging of glutamate at 3T. Neuroimage. 2006;30:1171–1178. doi: 10.1016/j.neuroimage.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 16.Baranzini SE, Srinivasan R, Khankhanian P, et al. Genetic variation influences glutamate concentrations in brains of patients with multiple sclerosis. Brain. 2010;133:2603–2611. doi: 10.1093/brain/awq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muhlert N, Atzori M, De Vita E, et al. Memory in multiple sclerosis is linked to glutamate concentration in grey matter regions. J Neurol Neurosurg Psychiatry. 2014 doi: 10.1136/jnnp-2013-306662. doi:10.1136/jnnp-2013-306662. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelletier D, Nelson SJ, Grenier D, et al. 3D echo planar (1)HRMS imaging in MS: metabolite comparison from supratentorial vs. central brain. Magn Reson Imaging. 2002;20:599–606. doi: 10.1016/s0730-725x(02)00533-7. [DOI] [PubMed] [Google Scholar]
- 19.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 20.Lublin FD, Reingold SC. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical trials of New Agents in Multiple Sclerosis. The clinical course of multiple sclerosis: results of an international survey. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 21.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 23.Hetherington HP, Pan JW, Mason GF, et al. Quantitative 1H spectroscopic imaging of human brain at 4.1 T using image segmentation. Magn Reson Med. 1996;36:21–29. doi: 10.1002/mrm.1910360106. [DOI] [PubMed] [Google Scholar]
- 24.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 25.Mowry EM, Beheshtian A, Waubant E, et al. Quality of life in multiple sclerosis is associated with lesion burden and brain volume measures. Neurology. 2009;72:1760–1765. doi: 10.1212/WNL.0b013e3181a609f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okuda DT, Srinivasan R, Oksenberg JR, et al. Genotype-pheno-type correlations in multiple sclerosis: HLA genes influence disease severity inferred by 1HMR spectroscopy and MRI measures. Brain. 2009;132:250–259. doi: 10.1093/brain/awn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 28.Rudick R, Antel J, Confavreux C, et al. Recommendations from the National Multiple Sclerosis Society Clinical Outcomes Assessment Task Force. Ann Neurol. 1997;42:379–382. doi: 10.1002/ana.410420318. [DOI] [PubMed] [Google Scholar]
- 29.McCulloch CE, Searle SR, Neuhaus JM. Generalized, linear, and mixed models. Wiley; Hoboken, NJ: 2008. [Google Scholar]
- 30.Frost C, Kenward MG, Fox NC. The analysis of repeated ’direct’ measures of change illustrated with an application in longitudinal imaging. Stat Med. 2004;23:3275–3286. doi: 10.1002/sim.1909. [DOI] [PubMed] [Google Scholar]
- 31.Bacchetti P. Peer review of statistics in medical research: the other problem. BMJ. 2002;324:1271–1273. doi: 10.1136/bmj.324.7348.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutta R, Trapp BD. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol. 2011;93:1–12. doi: 10.1016/j.pneurobio.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bongarzone ER, Pasquini JM, Soto EF. Oxidative damage to proteins and lipids of CNS myelin produced by in vitro generated reactive oxygen species. J Neurosci Res. 1995;41:213–221. doi: 10.1002/jnr.490410209. [DOI] [PubMed] [Google Scholar]
- 35.Merrill JE, Ignarro LJ, Sherman MP, et al. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993;151:2132–2141. [PubMed] [Google Scholar]
- 36.Lu F, Selak M, O'Connor J, et al. Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J Neurol Sci. 2000;177:95–103. doi: 10.1016/s0022-510x(00)00343-9. [DOI] [PubMed] [Google Scholar]
- 37.Allen IV, McKeown SR. A histological, histochemical and biochemical study of the macroscopically normal white matter in multiple sclerosis. J Neurol Sci. 1979;41:81–91. doi: 10.1016/0022-510x(79)90142-4. [DOI] [PubMed] [Google Scholar]
- 38.Piani D, Frei K, Do KQ, et al. Murine brain macrophages induced NMDA receptor mediated neurotoxicity in vitro by secreting glutamate. Neurosci Lett. 1991;133:159–162. doi: 10.1016/0304-3940(91)90559-c. [DOI] [PubMed] [Google Scholar]
- 39.Ouardouz M, Coderre E, Basak A, et al. Glutamate receptors on myelinated spinal cord axons: I. GluR6 kainate receptors. Ann Neurol. 2009;65:151–159. doi: 10.1002/ana.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouardouz M, Coderre E, Zamponi G, et al. Glutamate receptors on myelinated spinal cord axons: II. AMPA and GluR5 receptors. Ann Neurol. 2009;65:160–166. doi: 10.1002/ana.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mann CL, Davies MB, Boggild MD, et al. Glutathione S-transferase polymorphisms in MS: their relationship to disability. Neurology. 2000;54:552–557. doi: 10.1212/wnl.54.3.552. [DOI] [PubMed] [Google Scholar]
- 42.Srinivasan R, Ratiney H, Hammond-Rosenbluth KE, et al. MR spectroscopic imaging of glutathione in the white and gray matter at 7T with an application to multiple sclerosis. Magn Reson Imaging. 2010;28:163–170. doi: 10.1016/j.mri.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Tiberio M, Chard DT, Altmann DR, et al. Metabolite changes in early relapsing-remitting multiple sclerosis. A two year follow-up study. J Neurol. 2006;253:224–230. doi: 10.1007/s00415-005-0964-z. [DOI] [PubMed] [Google Scholar]