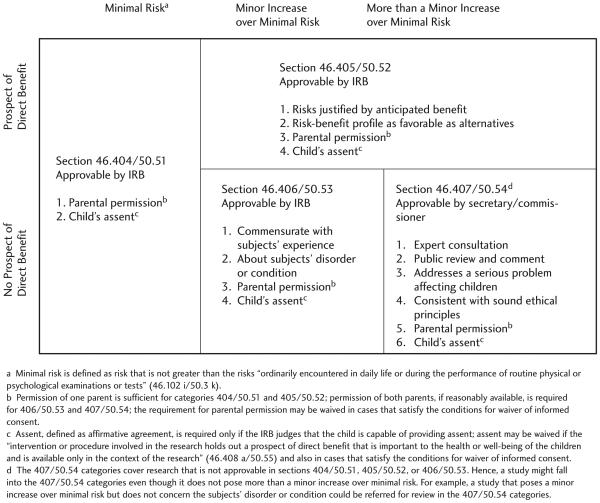
It is widely held that children should not participate in research that does not offer them the potential for benefit unless the risks are low.1 U.S. regulations attempt to implement this ethical standard by allowing institutional review boards (IRBs) to approve pediatric research that does not offer participants the prospect of “direct” benefit only when it poses minimal risk or poses a minor increase over minimal risk and satisfies several additional requirements (Figure 1).2 While these regulatory requirements preclude pediatric research that poses higher risks and does not offer a prospect of direct benefit, the regulations also include a fourth category for pediatric research that can be approved by the secretary of the Department of Health and Human Services (DHHS)3 or the commissioner of the Food and Drug Administration (FDA).4 This fourth category does not include an explicit limit on risks, raising the question of whether U.S. regulations allow children to be enrolled in research that poses higher risks and does not offer a prospect of direct benefit. To date, there has been little discussion of this question in the literature, leaving investigators, funders, and special reviewers with almost no guidance on whether U.S. regulations allow pediatric research that poses more than a minor increase over minimal risk and does not offer a prospect of direct benefit.
Figure 1.
U.S. Regulations for Pediatric Research and Associated Requirements
Some commentators assume that the U.S. regulations include an absolute upper limit on the level of risks allowed in pediatric research. For instance, in its report on the ethical conduct of research with children, the Institute of Medicine (IOM) states that, under U.S. regulations, research that does not offer a prospect of direct benefit and poses higher risks may be approved in adults but may not be approved in children. This view assumes that U.S. regulations include some upper limit on the level of risks allowed in pediatric research but not in research with adults.5 Similarly, the National Human Research Protections Advisory Committee (NHRPAC) argues that a minor increase over minimal risk is the upper limit on allowable risks in pediatric research that does not offer the prospect of direct benefit.6 In contrast, the Office for Human Research Protections (OHRP) points out that the 407/50.54 categories do not include an explicit upper limit on risks.7 Moreover, the Secretary’s Advisory Committee on Human Research Protections (SACHRP) noted that the absence of any explicit upper limit on risks implies that, in principle, U.S. regulations allow research with children that poses higher risks and does not offer them the prospect of direct benefit.8
The inclusion of the 407/50.54 categories in the regulations for pediatric research makes sense. They provide the flexibility to approve studies that are ethically appropriate and socially important but do not satisfy the mandated requirements for approval by an IRB. Recognizing the value of this regulatory flexibility, it is important to ensure that the requirements preclude inappropriate research. Perhaps the most important concern in this regard is the possibility that studies not offering a prospect of direct benefit and exposing children to high risks will be approved in the 407/50.54 categories. I argue here that this concern could be addressed by adopting a minor increase over minimal risk as the limit on risks in pediatric research. To maintain regulatory flexibility, exceptions could be allowed for research that poses higher risks but is still acceptable. Analysis suggests that there are two appropriate exceptions: 1) the children understand the research and agree to participate, and 2) the research offers participants the potential for appropriate nondirect benefits that justify the risks they face. This analysis suggests that any revisions to the U.S. regulations should consider adopting a limit of a minor increase over minimal risk, with these two exceptions, to the requirements on approval of studies in the 407/50.54 categories.9 In the meantime, those who review and approve studies in the 407/50.54 categories could adopt this approach as standard practice.
The 407/50.54 Categories
The DHHS and FDA regulations include four categories for pediatric research. The first three allow IRBs to approve the enrollment of children in research that poses minimal risk (section 404/50.51), research that poses a minor increase over minimal risk (section 406/50.53), and research that offers the prospect of direct benefit (section 405/50.52). The fourth category allows for the approval of studies that are not approvable by an IRB.
The regulations include substantive requirements on studies that are approvable by IRBs in the first three categories. Many of these requirements—e.g., parents must give permission and children who are capable must give their assent— help to provide important protection for children. At the same time, there may be good reasons that a particular study does not satisfy all of the requirements for approval by an IRB. Inclusion of the 407/50.54 categories in the regulations—categories for research that is not approvable by an IRB—provides flexibility for expert committees to review these studies and recommend approval of those that are ethically appropriate and scientifically important.10
To consider one example, the regulations stipulate that IRBs may approve pediatric research that poses greater than minimal risk and offers the prospect of direct benefit only when “the relation of the anticipated benefit to the risk is at least as favorable to the subjects as that presented by available alternative aproaches.”11 As a general rule, this requirement makes sense. It protects ill children from being enrolled in studies providing substantially inferior treatment. Yet, there may be some studies that do not satisfy this requirement but are nonetheless appropriate. For example, to address costs, it can be important to study interventions that are slightly less favorable than available alternatives but that are significantly less expensive. This research can be vital to making health care available to all children. The 407/50.54 categories provide the flexibility to allow for special approval of this type of research when appropriate.
To realize this flexibility in a way that still protects children, the 407/50.54 categories rely primarily on procedural protections, with a few substantive requirements. Specifically, studies submitted for approval in the 407/50.54 categories must undergo public review and evaluation by a panel of experts. The studies may be approved by the secretary of DHHS or the commissioner of FDA only if they satisfy the following requirements: 1) the research presents a reasonable opportunity to further understand, prevent, or alleviate a serious problem affecting the health or welfare of children; 2) the research will be conducted in accordance with sound ethical principles; and 3) adequate provisions are made for soliciting the assent of children and the permission of their parents or guardians. The fact that these requirements do not include an upper limit on risks raises the question of whether U.S. regulations allow children to be enrolled in research that poses higher risks and does not offer a prospect of direct benefit.
The National Commission’s Recommendations
The U.S. regulations governing pediatric research are based on the recommendations of the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research.12 The National Commission explicitly endorsed pediatric research that does not offer a prospect of direct benefit when the risks are “minimal,” which the Commission (and later the regulations) defined as risks that do not exceed those that children ordinarily encounter in daily life or during routine examinations. This conclusion led to discussion over whether regulations should allow pediatric research that does not offer the prospect of direct benefit and poses greater than minimal risk. The Commission’s final report states that this question raised the “most difficult ethical issues” it faced in four years of deliberation on all aspects of clinical research.13
Several members of the Commission argued that the level of risks children ordinarily encounter in daily life or during routine examinations should be the upper limit on pediatric research that does not offer a prospect of direct benefit. The Commission ultimately decided (with two members dissenting) that research posing greater than minimal risk and not offering the prospect of direct benefit could be justified.14 However, the Commission held that such research is acceptable only when the risks do not exceed “a minor increase over minimal.”15 While the Commission did not define a “minor increase” over minimal risk, the general idea is that, in exceptional circumstances, it can be appropriate to expose children to risks that are slightly greater than the risks children ordinarily encounter in daily life or during routine examinations.
Since the National Commission’s report, there has been substantial discussion and debate about the federal levels of minimal risk and minor increase over minimal risk and about whether they offer appropriate protection for pediatric research participants.16 There has been concern that defining minimal risks in terms of the risks of daily life creates the possibility of exploiting children who happen to face greater risks in their daily lives.17 To address this concern, it has been argued that minimal risk should be defined in terms of the risks present in the daily lives and routine examinations of average, healthy children.18 Others argue that the minimal risk standard should be limited to the risks posed by routine examinations for healthy children.19 Still others propose defining minimal risk based on the risks present in appropriate charitable activities for children20 or the risks deemed acceptable by a scrupulous parent.21 Recognizing these variations and the existence of a few exceptions,22 there is general agreement that it can be acceptable to enroll children in research that poses minimal risk.
There has been significantly less discussion of whether it can be appropriate to enroll children in research that poses a “minor increase” over minimal risk. Some commentators argue that pediatric research not offering the prospect of direct benefit should be prohibited if it poses greater than minimal risk. Others agree with the National Commission that research in this category can be justified in some cases.23 This position is defended on the grounds that, in exceptional circumstances, it can be acceptable to expose children to risks that are slightly greater than those typically allowed in daily life.
The literature suggests very wide agreement that children can be enrolled in research that does not offer a prospect of direct benefit when it poses at most a minor increase over minimal risk. Yet, current U.S. regulations do not explicitly prohibit research that poses greater risks from being approved in the 407/50.54 categories. The regulations do include a requirement that research approved in the 407/50.54 categories must be consistent with “sound ethical principles.” The principle that children should not be exposed to high risks without the potential for benefit seems the soundest ethical principle in pediatric research. Thus, one might conclude—as the IOM, NHRPAC, and others have—that, in effect, current federal regulations on pediatric research include an upper limit on risks. Is this approach sufficient to protect pediatric research subjects?
Analysis of the Current Situation
While the 407/50.54 categories do not include an explicit upper limit on risks, they do include requirements for expert input, as well as public review and comment. One might assume that these procedural requirements, together with the requirement that research approved in these categories must be consistent with sound ethical principles, are sufficient to protect children from being exposed to high risks without the potential for benefit. This view gains support from the fact that the 407/50.54 categories are rarely used to approve pediatric research studies. In addition, there do not appear to be any documented cases in which the absence of an explicit upper limit on risks has resulted in children being harmed.
These considerations suggest that it may not be unreasonable to hope that current regulations are sufficient to protect pediatric research subjects. However, current debate over whether higher risk research is allowed under U.S. regulations suggests that the requirement for consistency with sound ethical principles may not be sufficient to clearly protect children from higher risks. This concern is underscored by debate within one of the expert panels charged with reviewing a study for possible approval in the 407/50.54 categories. The panel was charged with reviewing a study that proposed to give granuclocyte colony stimulating factor (G–CSF) to healthy children who were acting as bone marrow donors for their affected siblings.24 The G-CSF was used to increase the number of stem cells in the donor’s blood that could be collected for the transfusion. The healthy donors were regarded as research subjects because they were receiving the G-CSF. The panel found that the proposed study posed more than a minor increase over minimal risk to the donors. This finding led to debate within the panel over whether U.S. regulations allow pediatric research that poses more than a minor increase over minimal risk and does not offer a prospect of direct benefit. One committee member argued that this level of risk is excessive and that therefore the study was not consistent with sound ethical principles. Other committee members responded that pediatric research that poses more than a minor increase over minimal risk is not precluded by the regulations governing the 407/50.54 categories.
We should not be surprised to find disagreement over whether pediatric research that poses more than a minor increase over minimal risk is consistent with sound ethical principles. For the past 30 years, there has been extensive debate over what levels of pediatric research risks are consistent with sound ethical principles, with commentators endorsing a range of positions. Some commentators argue that sound ethical principles preclude pediatric research that poses any risks and does not offer a prospect of clinical benefit.25 As we have seen, many commentators argue that minimal risk pediatric research, and perhaps minor increase over minimal risk research, can be acceptable. Finally, some widely endorsed ethical theories, such as Utilitarianism, imply that risks greater than a minor increase over minimal, including possibly very high risks, can be justified by sufficient benefits to future patients.
The fact that current regulations have not resulted in any documented cases of children being exposed to excessive risks is important. Yet, this debate over whether greater risks should be allowed suggests the need for explicit risk limits. We should not wait until some children are seriously harmed to ensure that existing regulations are adequate. In addition, it is important to provide clarity that advances in pediatric medicine are not being won at the cost of exposing some children to excessive risks and to be publicly accountable for this. These considerations suggest the need for an explicit risk limit to prospectively protect all children from excessive risks and to provide reassurance that children are being so protected.
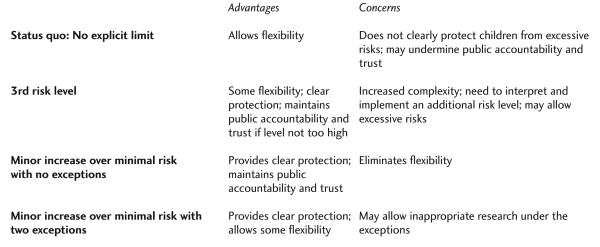
The conclusion that more explicit guidance on allowable risks should be incorporated in the 407/50.54 categories is underscored by recent discussion from the Presidential Commission for the Study of Bioethical Issues.26 The commission has been charged with assessing the ethics of conducting studies with children to test vaccines for anthrax. A good deal of the discussion focused on uncertainty regarding what level of risks is acceptable in the 407/50.54 categories. Answering these questions prospectively and not waiting until children are exposed to high risks is especially important given the possibility that these trials might enroll thousands of children. There are at least three options for how the current research regulations might be revised to more clearly protect pediatric subjects from high risks: 1) incorporate a third risk level, 2) mandate minor increase over minimal risk as the upper limit with no exceptions, or 3) mandate minor increase over minimal risk as the upper limit but allow some exceptions (Figure 2).
Figure 2.
Four Options for Risk Limitations in the 407/50.54 Categories
Establish a Third Risk Level
Establishing a third risk level for the 407/50.54 categories would allow some pediatric subjects to be exposed to risks greater than a minor increase over minimal, without allowing any pediatric subjects to be exposed to very high risks (risks that exceed the third risk level). This approach has the virtue of providing increased flexibility while making clear that there is an upper limit on the risks to which children may be exposed. However, this proposal faces two problems, one theoretical and one practical. At a theoretical level, this approach would need to provide an argument for why it can be acceptable to expose children to higher risks. The fact that parental permission is required represents an important protection. However, there are limits on the extent to which we allow parents to expose their children to risks.27 If we understand minimal risks as those risks that are typically acceptable for children, and a minor increase over minimal risk as risk that can be acceptable for children in extraordinary circumstances, it is unclear how we might justify even greater risks for children in general.
At a practical level, there is already significant debate and uncertainty regarding the interpretation and implementation of the two existing risk levels. Adding a third risk level would likely increase this confusion. Thus, even if one could identify a compelling theoretical justification for allowing risks greater than a minor increase over minimal in some cases, it would remain unclear whether this approach could be implemented in a way that ensured children are not exposed to even higher risks in practice.
Minor Increase over Minimal Risk with No Exceptions
To avoid the confusion and ethical concern involved with establishing a third risk level, an alternative approach would be to explicitly adopt a minor increase over minimal risk as the limit on risks for studies approvable in the 407/50.54 categories. This approach is consistent with the recommendations of the National Commission, as well as the views of many commentators. This approach has the advantage of providing clear protection for children, thus providing assurance that advances in pediatric medicine are not being won at the cost of exposing some children to excessive risks.
Whether the loss of flexibility with this approach is a problem depends on whether there are cases in which it can be acceptable to expose children to even greater than a minor increase over minimal risk in the context of research that does not offer a prospect of direct benefit. If there are no appropriate exceptions, a minor increase over minimal risk should be incorporated as the absolute upper limit. If there are appropriate exceptions, the regulations could be more flexible, mandating that a minor increase over minimal risk is the upper limit unless the research satisfies a justified exception. Analysis suggests there are at least two cases in which it can be appropriate to expose pediatric research subjects to more than a minor increase over minimal risk in the context of research that does not offer a prospect of direct benefit.
Minor Increase over Minimal Risk, with Exceptions
Pediatric research merits strict limits on risks because children are unable to give informed consent. Yet, empirical data find that many teenagers under the age of legal majority are able to understand a good deal about research.28 These data suggest that it may be acceptable, in some cases, to allow teenagers who understand the research in question to be exposed to risks greater than a minor increase over minimal, provided they give assent and their parents provide permission.29 This approach is consistent with the principle that although children are generally too young to understand research and thus should not be exposed to greater than a minor increase over minimal risk, when children can understand research, there is no need for a special limit on risks. Thus, they may be exposed to risks up to those that are regarded as acceptable for competent adults.
Second, IRBs may approve pediatric research that poses greater than a minor increase over minimal risk only when it offers a prospect of direct benefit. While there is some debate, direct benefits are typically understood as potential benefits resulting from receiving the intervention under study.30 This definition of direct benefits precludes investigators from adopting inappropriate practices, such as justifying ever-increasing risks to pediatric research subjects simply by offering them or their parents more money. To implement this protection, it seems reasonable to prohibit IRBs from approving pediatric research posing greater than a minor increase over minimal risk and not offering the prospect for direct benefit. At the same time, there may be rare cases in which the potential for nondirect benefits can justify greater risks. Investigators sometimes include monitoring procedures that offer research participants an important potential for clinical benefit. Under the definition of direct benefits as those resulting from the intervention being tested, these benefits would not qualify as “direct.” However, if the potential benefits from the monitoring procedure are significant enough and they are not available outside the research context, they might justify enrolling children in a study that poses more than a minor increase over minimal risk.
A second example comes out of the expert panel that reviewed the G–CSF study. The panel determined that the proposed study posed greater than a minor increase over minimal risk to the children who were donating bone marrow to their affected siblings. The panel further found that participation in the study offered the donors the potential for psychological benefits in the form of helping an ill sibling.31 The panel recommended approval of the study on these grounds. This ruling suggests that there might be cases in which it can be appropriate to expose children to somewhat greater than a minor increase over minimal risk, provided the research offers subjects the potential for important benefits that do not qualify as “direct.”
Pediatric research that poses greater than a minor increase over minimal risk and does not offer a prospect of direct benefit raises important ethical concerns. For example, it can be difficult to determine whether riskier pediatric research offers participants the potential for sufficient and real psychological benefit. And a policy allowing pediatric research on the grounds that it offers the potential for psychological benefit may be especially susceptible to abuse. Prohibiting IRBs from approving riskier pediatric research on these grounds provides an important way to address these concerns. At the same time, it seems reasonable to allow an expert panel to consider recommending approval of such research after thorough review and public comment. Research in this category should be approved only when the nondirect benefits are important and are sufficient to justify the risks to subjects. When these conditions are satisfied, the fact that research offers the potential for (nondirect) benefits to participating children suggests there is no need for an upper limit on allowable risks. Given that these studies offer the potential for important nondirect benefits that justify the risks, they too do not violate the principle that children should be enrolled in higher risk research only when it offers them the potential for benefit.
The present analysis suggests that the best approach may be to explicitly adopt a minor increase over minimal risk as the upper limit on risks in the 407/50.54 categories but then to allow for approval of studies that satisfy these two exceptions. This approach establishes a clear limit on risks, thus providing assurance that children are being adequately protected, while allowing exceptions in the two instances that do not need a risk limit: when the children understand the study and agree to participate and when the study offers the potential for nondirect benefits that justify the risks. If future research identifies additional appropriate exceptions, they could be added to the list. In this way, the present approach maintains flexibility while still protecting children.
Conclusion
The National Commission, as well as a number of commentators and groups, have argued that children should not be enrolled in research that poses greater than a minor increase over minimal risk and does not offer the prospect of direct benefit. However, U.S. regulations do not include an explicit limit on risks in the 407/50.54 categories. This situation raises concern that current regulations may not provide sufficient protection for pediatric research subjects.
To address this concern, a risk limit of a minor increase over minimal could be added as a fourth requirement on studies approvable in the 407/50.54 categories, with exceptions allowed in two cases: participating children understand the research and assent, or enrollment offers participating children the potential for important nondirect benefits that justify the risks they face. This approach helps to maintain the flexibility in the 407/50.54 categories to approve studies that are ethically appropriate and scientifically important but that do not satisfy all the requirements mandated for IRB approval. At the same time, this approach protects children who cannot understand the research from being exposed to risks greater than a minor increase over minimal that are not justified by a potential for benefit. Any future revisions to the U.S. regulations could add this requirement to existing regulations. In the meantime, 407/50.54 panels should consider adopting this approach as part of their standard practice.
Footnotes
Disclaimer
The views expressed are the author’s own. They do not represent the position or policy of the NIH, the DHHS, or the U.S. government. This work was supported by NIH intramural funds.
References
- 1.Tait AR. Weighing the risks and benefits of pediatric research: A risky business. Archives of Pediatric and Adolescent Medicine. 2010;164:579–581. doi: 10.1001/archpediatrics.2010.58. [DOI] [PubMed] [Google Scholar]; Ross LF, Nelson RM. Pediatric research and the federal minimal risk standard. JAMA. 2006;295(7):759–760. doi: 10.1001/jama.295.7.759-a. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services Protection of Human Subjects. 45 CFR 46. [Google Scholar]; U.S. Food and Drug Administration Protection of Human Subjects. 21 CFR 50. [Google Scholar]
- 3. See ref. 2, 45 CFR 46.406.
- 4. See ref. 2, 21 CFR 50.54.; Field MJ, Behrman RE. Ethical Conduct of Clinical Research Involving Children. National Academies Press; Washington DC: 2004. p. 145. [PubMed] [Google Scholar]
- 5.Field, Behrman 2004. See ref. 4.
- 6.Final report to NHRPAC from Children’s Workgroup. Clarifying Specific Portion of 45 CFR 46 Subpart D that Governs Children’s Research. http://www.hhs.gov/ohrp/archive/nhrpac/documents/nhrpac16.pdf.
- 7.U.S. Department of Health and Human Services Children Involved as Subjects in Research: Guidance on the HHS 45 CFR 46.407 (“407”) Review Process. 2005 May 26; http://www.hhs.gov/ohrp/policy/populations/guidance_407process.html.
- 8.Algorithm for Subpart D Analysis. http://www.hhs.gov/ohrp/sachrp/appendixa.pdf.; SACHRP letter to HHS secretary on children’s research. http://www.hhs.gov/ohrp/sachrp/pages3-7from08112004sec.pdf.
- 9.U.S. Department of Health and Human Services Advance Notice of Proposed Rule-making: Human Subjects Research Protections: Enhancing Protections for Research Subjects and Reducing Burden, Delay, and Ambiguity for Investigators. http://www.gpo.gov/fdsys/pkg/FR-2011-07-26/html/2011-18792.htm. [Google Scholar]
- 10. The U.S. regulations include an analogous category for research with pregnant women, fetuses, and neonates, 45 CFR 46.207, but not for research with prisoners.
- 11. 45 CFR 46.405(b)
- 12.National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research . Report and Recommendations on Research Involving Children. U.S. Department of Health, Education and Welfare; 1977. DHEW publication (05)77-0004. [Google Scholar]; Jonsen AR. Research involving children: Recommendations of the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. Pediatrics. 1978;62:131–136. [PubMed] [Google Scholar]
- 13.National Commission See ref. 12.
- 14.National Commission See ref. 12.
- 15.National Commission See ref. 12.
- 16.Kopelman LM. Minimal risk as an international ethical standard in research. Journal of Medicine and Philosophy. 2004;29:351–378. doi: 10.1080/03605310490500545. [DOI] [PubMed] [Google Scholar]; Rid A, Emanuel EJ, Wendler D. Evaluating the risks of clinical research. JAMA. 2010;304(13):1472–1479. doi: 10.1001/jama.2010.1414. [DOI] [PubMed] [Google Scholar]; Ross LF, Nelson RM. Pediatric research and the federal minimal risk standard. JAMA. 2006;295(7):759. doi: 10.1001/jama.295.7.759-a. author reply 759–760. [DOI] [PubMed] [Google Scholar]; Kopelman LM, Murphy TF. Ethical concerns about federal approval of risky pediatric studies. Pediatrics. 2004;113:1783–1789. doi: 10.1542/peds.113.6.1783. [DOI] [PubMed] [Google Scholar]; Ross LF. Children in Medical Research: Access versus Protection. Oxford University Press; New York: 2006. [Google Scholar]
- 17.Fisher CB, Kornetsky SZ. SACHRP recommendations for review of children’s research requiring DHHS secretary’s approval. IRB: Ethics & Human Research. 2005;27(3):1–7. [PubMed] [Google Scholar]
- 18.Fisher CB, Kornetsky SZ, Prentice ED. Determining risk in pediatric research with no prospect of direct benefit: Time for a national consensus on the interpretation of federal regulations. American Journal of Bioethics. 2007;7:5–10. doi: 10.1080/15265160601171572. [DOI] [PubMed] [Google Scholar]
- 19.Resnik DB. Eliminating the daily risks standard from the definition of minimal risk. Journal of Medical Ethics. 2005;31:35–38. doi: 10.1136/jme.2004.010470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wendler D. Protecting subjects who cannot give consent: Toward a better standard for “minimal” risks. Hastings Center Report. 2005;35(5):37–43. [PubMed] [Google Scholar]
- 21.Nelson RM, Ross LF. In defense of a single standard of research risk for all children. Journal of Pediatrics. 2005;147:565–566. doi: 10.1016/j.jpeds.2005.08.051. [DOI] [PubMed] [Google Scholar]; Freedman B, Fuks A, Weijer C. In loco parentis: Minimal risk as an ethical threshold for research upon children. Hastings Center Report. 1993;23(2):13–9. [PubMed] [Google Scholar]
- 22.Grimes v. Kennedy Krieger Institute, Inc. 782 A.2d 807 (Md. Ct. of App. 2001, reconsideration denied, Oct. 11, 2001); Lenk C, Radenbach K, Dahl M, Wiesemann C. Non-therapeutic research with minors: How do chairpersons of German research ethics committees decide? Journal of Medical and Ethics. 2004;30:85–87. doi: 10.1136/jme.2003.005900. Claudia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedman, et al. 1993. See ref. 21.
- 24.Pediatric Advisory Committee Children’s Oncology Group protocol ASCT0631: A phase III randomized trial of granulocyte colony stimulating factor (G-CSF) stimulated bone marrow vs. conventional bone marrow as a stem cell source in matched sibling donor transplantation. 2008 Dec 9; http://www.fda.gov/ohrms/dockets/ac/08/transcripts/2008-4406t-01.pdf. [Google Scholar]
- 25.Ramsey P. The enforcement of morals: Nontherapeutic research on children: A reply to Richard McCormick. Hastings Center Report. 1976;6(4):21–30. [PubMed] [Google Scholar]; Sammons HM, Malhotra J, Choonara I, Sitar DS, Matsui D, Rieder MJ. Survey of pediatricians in Canada and Europe. European Journal of Clinical Pharmacology. 2007;63:431–436. doi: 10.1007/s00228-007-0281-9. [DOI] [PubMed] [Google Scholar]
- 26.Presidential Commission for the Study of Bioethical Issues Meeting ten; Washington, D.C.. Aug. 2012. http://bioethics.gov/cms/meeting-10. [Google Scholar]
- 27.Coleman DL. The legal ethics of pediatric research. Duke Law Journal. 2007;57:517–624. [PubMed] [Google Scholar]
- 28.Ondrusek N, Abramovitch R, Pencharz P, Koren G. Empirical examination of the ability of children to consent to clinical research. Journal of Medical Ethics. 1998;24:158–165. doi: 10.1136/jme.24.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]; Weithorn LA, Campbell S. The competency of children and adolescents to make informed treatment decisions. Child Development. 1982;53:1589–1598. [PubMed] [Google Scholar]; Susman EJ, Dorn LD, Fletcher JC. Participation in biomedical research: The consent process as viewed by children, adolescents, young adults, and physicians. Journal of Pediatrics. 1992;12:547–552. doi: 10.1016/s0022-3476(05)81142-4. [DOI] [PubMed] [Google Scholar]
- 29.Ackerman TF. Moral duties of parents and nontherapeutic clinical research procedures involving children. Bioethics Quarterly. 1980;2:94–111. doi: 10.1007/BF00915263. [DOI] [PubMed] [Google Scholar]
- 30.King N. Defining and describing benefit appropriately in clinical trials. Journal of Law, Medicine, and Ethics. 2000;28:332–343. doi: 10.1111/j.1748-720x.2000.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 31. See ref. 24.