Abstract
Importance
Most primary care clinicians lack the skills and resources to offer effective lifestyle and medication counseling to reduce coronary heart disease (CHD) risk. Thus, effective and feasible CHD prevention programs are needed for typical practice settings.
Objective
To assess the effectiveness, acceptability, and cost-effectiveness of a combined lifestyle and medication intervention to reduce CHD risk offered in counselor-delivered and web-based formats.
Design
Comparative effectiveness trial.
Setting
Five diverse family medicine practices in North Carolina.
Participants
Established patients, age 35–79, with no known cardiovascular disease, and at moderate to high risk for CHD -- 10 year Framingham Risk Score (FRS) ≥ 10%.
Intervention
Participants were randomized to counselor-delivered or web-based format, each including 4 intensive and 3 maintenance sessions. After randomization, both formats utilized a web-based decision aid showing potential CHD risk reduction associated with lifestyle and medication risk reducing strategies. Participants chose the risk reducing strategies they wished to follow.
Main Outcome and Measures
Outcomes were assessed at 4 and 12 months; the primary outcome was within group change in FRS at 4 month follow-up. Other measures included standardized assessments of blood pressure, blood lipids, lifestyle behaviors, and medication adherence. Acceptability and cost-effectiveness were also assessed.
Results
Of 2,274 screened patients, 385 were randomized (192 counselor; 193 web): mean age 62 years, 24% African American, and mean FRS 16.9%. Follow-up at 4 and 12 months was 91% and 87%, respectively. There was a sustained reduction in FRS at both 4 (primary outcome) and 12 month follow-up: for counselor, −2.3% (95% CI: −3.0% to −1.6%) and −1.9% (−2.8% to −1.1%) and for web, −1.5% (−2.2% to −0.9%) and −1.7%, (−2.6% to −0.8%) respectively. At 4 month follow-up, the adjusted difference in FRS between groups was −1.0% (95% CI −1.8% to −0.1%, p = 0.03) at 12 month follow-up, it was −0.6% (95% CI, −1.7% to 0.5%, p = 0.30). The 12 month costs from the payer perspective were $207 and $110 per person for the counselor and web interventions respectively.
Conclusions and Relevance
Both intervention formats reduced CHD risk through 12 month follow-up. The web format was less expensive.
Introduction
A healthy lifestyle1,2 and appropriate medications3–5 can substantially reduce the risk for coronary heart disease (CHD), yet getting patients to change their lifestyle and initiate and adhere to risk reducing medication can be difficult to achieve in clinical practice. In particular, most primary care clinicians lack the skills6,7 and resources8 to offer effective lifestyle and medication counseling to reduce CHD risk. Thus, to improve CHD prevention in primary care practices, where half of Americans are seen annually,9 clinicians need access to effective and feasible CHD prevention programs that could be implemented in their practice settings.
While many primary-care based programs to reduce CHD risk have been previously tested, these programs have limitations.10,11 Most have not jointly addressed lifestyle change and medication optimization and few have taken a patient-centered approach that informs patients about the relative merits of strategies to reduce CHD risk and encourages them to select their preferred risk reducing strategies. Further, few have been evaluated in comparative effectiveness studies12,13 that: 1) compare clinically relevant implementation strategies, 2) include a diverse population of participants, 3) include a heterogeneous selection of practices, and 4) collect data on a broad range of outcomes.
Given increasing evidence that supports the effectiveness of web-based interventions,14,15 we developed a combined lifestyle and medication intervention to reduce CHD risk and tested it in two formats: counselor-delivered and web-based. While the counselor intervention provides human interaction and the potential for a higher degree of tailoring, the web intervention offers greater reach, flexibility to patients in the timing and delivery of the intervention, and minimizes clinic staff demands and costs.16 In this paper, we report the results of a comparative effectiveness trial conducted to assess the effectiveness, acceptability, and cost-effectiveness of the intervention when offered in alternative formats.
METHODS
Study Overview
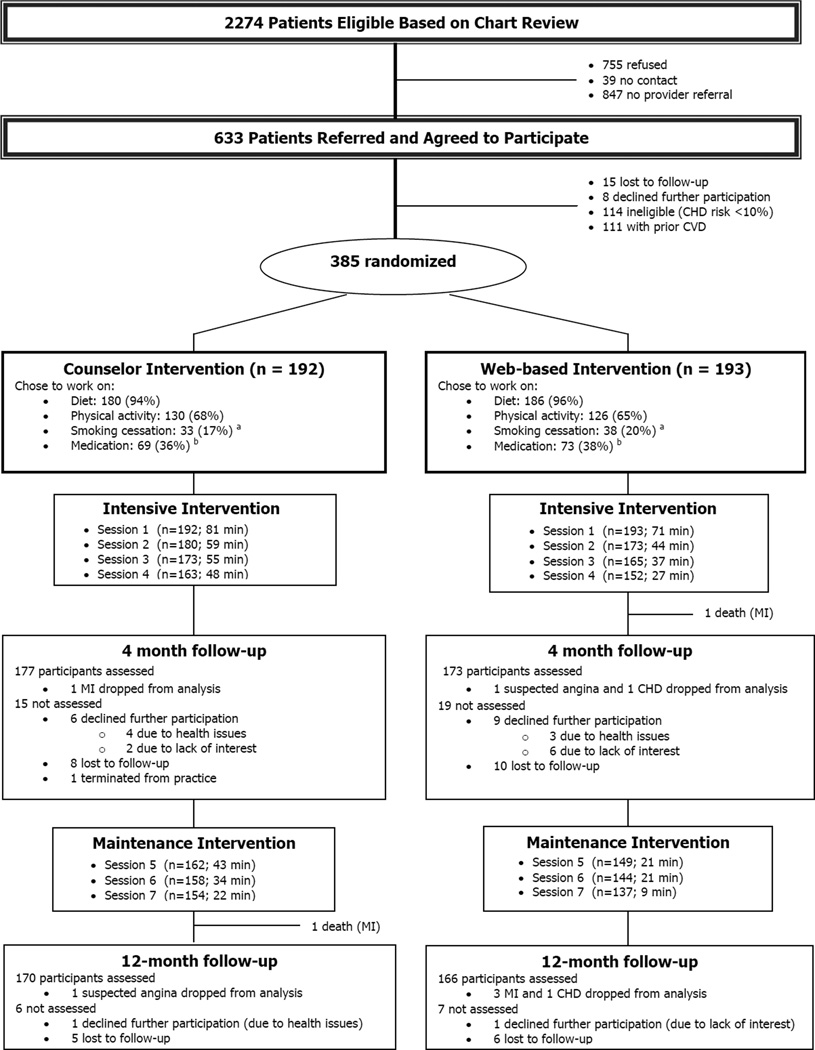
We conducted this study at 5 diverse family medicine practices located in central North Carolina. Our primary intent was to determine the comparative effectiveness of the two intervention formats on reducing CHD risk as assessed by the Framingham Risk Score (FRS).17 Participants were randomized to receive interventions similar in contact time, educational content, and individually tailored counseling, but different in format (Figure 1). Study outcomes were assessed at 4 and 12 months. Details of the study design, study practices, participant enrollment, and intervention components are described elsewhere.17 The University of North Carolina at Chapel Hill’s Institutional Review Board (IRB) approved and monitored this study, with data collected between January 31, 2011 and November 26, 2012.
Figure 1. Study flow diagram.
aNumber of smokers at baseline: 55 (counselor intervention) and 58 (web-based intervention)
bIncludes intent to start or increase blood pressure or cholesterol medication or start aspirin
Abbreviations: MI, myocardial infarction; CHD, coronary heart disease.
Participants, Enrollment, and Randomization
Participants were established patients (i.e., had at least one office visit in the last 2 years), age 35–79, with no known cardiovascular disease (CVD), who were at moderate to high risk for CHD (≥ 10% 10-year risk of angina, myocardial infarction, or CHD death) based on their FRS. Participants were identified by chart reviews of patients scheduled for routine office visits, supplemented by referrals from clinicians and self-referrals based on word-of-mouth or in response to waiting room flyers. As an initial eligibility screen, the FRS was calculated using risk factors assessed by chart review (age, blood pressure, total cholesterol, high density lipoprotein cholesterol (HDL-C), diabetes, smoking, aspirin use, and left ventricular hypertrophy).17 Diabetes was included in the FRS and was not considered a CVD equivalent. Because aspirin was not accounted for by the Framingham risk equation, we modelled its effect on CHD risk using a 23% risk reduction for men and 0% reduction for women.3 Those with a FRS ≥ 10% were further evaluated by their primary care clinicians who 1) determined if the patient should be excluded for a variety of previously described17 medical conditions and 2) approved participation in the overall and physical activity component of the study.
Patients screened as eligible attended an enrollment visit, during which study staff obtained written informed consent, confirmed inclusion criteria, screened participants for potential bleeding risk associated with aspirin, re-assessed smoking status, assessed blood pressure using a standard protocol, and obtained a blood sample for study lab assessments. Participants’ FRS were re-calculated based on this standardized assessment and, if ≥ 10%, they were contacted for the baseline telephone survey. Those completing this survey were invited to the first intervention visit, where they were randomized, as previously described.17
Intervention
Both intervention formats began with a web-based decision aid, followed by the counseling program. As described elsewhere,17 the intervention was based on previously developed and tested lifestyle and medication interventions revised to be consistent with the latest evidence on CHD risk reduction.
Decision Aid
The decision aid 1) calculated participants’ 10-year FRS, 2) educated participants about their CHD risk factors and the pros and cons of risk-reducing strategies, and 3) showed participants how much their CHD risk might be reduced by one or more of the following: changes in diet, increased physical activity, smoking cessation, initiation of aspirin (for men only), or initiation or intensification of statins or hypertension medication. The following risk reduction estimates were used: 20–40% for diet,1,17–21 10–20% for physical activity,22,23 50% for smoking cessation, and 20–30% depending on type of medication (statins, blood pressure medication, and aspirin for men).24 For women who indicated an interest in aspirin, the decision aid provided information on stroke risk and the potential reduction in stroke with aspirin of 23%. Participants navigated the decision aid with the assistance of the health counselor and were encouraged to choose the risk reducing strategies they wished to focus on as part of this program.
Counseling
Both formats included 7 counseling sessions: 4 during a 4 month intensive phase (each about 45–60 minutes at monthly intervals) followed by 3 during an 8 month maintenance phase (each about 15–30 minutes at 2 month intervals). Counseling was tailored to choice of risk reducing strategy; diet, physical activity, medications, or any combination. To standardize counseling, the sequence, educational content, and tailoring of the counselor and web formats was the same. Specifically, both formats used the same set of questions to assess baseline habits and barriers. Additionally, counseling sessions included identical educational content (including graphics) that were presented in a 3 ring binder for counselor format and on a sequence of web pages for web format. Finally, the counselor and interactive web progam used the same process to select tailored goals and list first steps. For the counselor format, these goals were checked on a sheet; for the web format, they were printed.
Dietary counseling focused on improving carbohydrate and fat quality; physical activity counseling focused on walking 7,500 steps or 30 minutes on 5 days each week; and medication counseling focused on understanding medication instructions, planning ahead for refills, and encouragement to partner with clinician to make good decisions about medications to reduce CHD risk. All participants received a cook book, a pedometer for self-monitoring, and a guide with information on local resources promoting healthy eating and physical activity.. The initial visit was conducted at the clinic, where the counselor could assist participants with the web program, if needed. Subsequent visits were conducted at the clinic or remotely (by phone for counseling arm or computer for web arm). Counseling was conducted by trained health counselors, as previously described.17 Requests for medication initiation or intensification were routed to participants’ providers for approval.
Outcomes and Measures
Study measures addressed effectiveness, acceptability, and cost-effectiveness and were assessed by trained research staff at participating practices and by phone. The primary effectiveness measure was within group change in FRS at 4 month follow-up. The FRS was calculated using a well-validated Framingham risk equation25 with input of relevant risk factor data measured in a standardized fashion and baseline age used for follow-up assessments. Pre-specified secondary effectiveness outcomes included between group changes in FRS and change in dietary intake, physical activity, smoking, medication adherence, blood pressure, blood lipids, and health related quality of life. In addition, an analysis of moderators of outcomes was also planned.
Weight, blood pressure, total cholesterol, HDL-C, directly measured low density lipoprotein cholesterol (LDL-C), hemoglobin A1c (A1c), high sensitivity C reactive protein (hsCRP), alanine aminotransferase (ALT), creatinine, and plasma carotenoids26 were assessed at baseline, 4 and 12 months as previously described.17 At the first counseling visit, numeracy,27 literacy,28 and medication adherence29 were assessed using validated instruments. At follow-up visits, aspirin use was assessed by serum thromboxane level and smoking by NicAlert urine test, as previously described.17
The following measures were assessed by telephone at baseline and in-person at 4 and 12 month follow-up: medication use, fruit and vegetable intake,30 dietary fat quality,31 physical activity,32,33 and quality of life (SF-12, Quality Metric, Inc., Lincoln, RI). Medication adherence29 and acceptability of the interventions were assessed in-person at 4 and 12-month follow-up.
Process measures were collected at intervention sessions, by counselor or the web-program. Participants were advised to wear an Omron HJ-720ITC pedometer (Omron Healthcare, Bannockburn, IL) during the week before study measurement visits. Steps were assessed by averaging at least 3 days of 500 or more steps/day during the week prior to the visit. Assessment of costs for the cost-effective analysis are described in the Appendix.
Sample size
Sample size was based on the hypothesis that both interventions would reduce the FRS by at least 1.5 percentage points (absolute risk reduction of 1.5%). Using a one-sided test, a standard deviation of 3.1 units,24 an α = 0.05, and an expected 10% attrition, a sample of 225 participants in each arm would provide > 99% power to detect a within group reduction in FRS of 1.5 percentage points. This sample size would additionally provide 85% power to detect a 0.9 percentage point difference in FRS between the counselor and web arms (two-sided test).
Analysis
We summarized baseline sample characteristics using descriptive statistics and compared groups using chi-square and t-tests. The primary outcome analysis was conducted using an intention-to-treat approach with a paired t-test (1-sided) for changes in FRS within each intervention arm. Additionally, for the primary outcome, we used multiple approaches for imputing missing data including last observations carried forward and multiple imputation methods.17
Secondary outcomes were examined using paired t-tests or McNemar’s tests for within group comparisons (2-sided tests). Additional analyses were conducted to compare the mean changes in FRS and other outcomes between arms using a simple t-test and a multivariable analysis of covariance model (ANCOVA) adjusting for the baseline value of the outcome, practice, and additional variables deemed relevant to behavior change a priori (age, race, educational achievement, and BMI) or that differed between intervention groups at baseline (p < 0.10). In addition, we conducted longitudinal analyses with FRS data from all 3 time points using generalized linear mixed models that included time, study groups, and time by study group interaction as fixed and participants as random effects along with site and the full set of covariates as fixed effects. To assess potential moderators of change in FRS, we used linear regression models that included the baseline FRS, the potential moderator of interest, and study arm by potential moderator interaction term.
For cost-effectiveness, we assessed the incremental cost effectiveness ratio (ICER) of each intervention from the payer, participant, and societal perspectives, as described in the Appendix. We calculate the ICER per 1 absolute percentage point reduction in CHD risk and per quality adjusted life year (QALY) gained at 12 months. We calculate QALY gained in one year by converting SF-12 scores into a health related quality of life weight using a well-defined algorithm.34 Because our analysis considers only a one year time horizon, this weight is equivalent to QALYs saved over this time period. We then report incremental cost-effectiveness per QALY gained and compare these ratios to common thresholds of cost-effectiveness. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC) and Stata version 12 (StataCorp, College Station, TX) with p ≤ .05 considered significant.
RESULTS
Enrollment and Baseline Characteristics of Participants
As depicted in Figure 1, of 2274 patients eligible to be screened for the study, 633 agreed to participate. Of these, 114 were ineligible because their FRS calculated using standardized measures was less than 10%, 111 took part in another intervention for those with known CVD as described elsewhere,17 23 were lost to follow-up or declined participation and 385 participants took part in this study.
Table 1 reports baseline characteristics of study participants. The mean age was 62 years, 24% were African American, 32% were employed full time, and 88% had health insurance. Overall, the sample was at high risk for CHD: 86% had current or previous high blood pressure, 85% had current or previous high blood cholesterol, 61% had diabetes, and the mean FRS was 16.9%. Also, two-thirds of participants reported they were comfortable or very comfortable using a computer.
Table 1.
Baseline Participant Characteristics
| Characteristic | Total Sample (n=385) |
Counselor Group (n =192) |
Web Group (n =193) |
P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SE) | 62 (0.4) | 63 (0.5) | 62 (0.6) | 0.09 |
| Female sex, No. (%) | 186 (48) | 102 (53) | 84 (43) | 0.06 |
| Race, No. (%) | 0.49 | |||
| African American | 92 (24) | 43 (22) | 49 (25) | |
| White | 292 (76) | 150 (78) | 142 (74) | |
| Total household income, No. (%) | 0.68 | |||
| < $20,000 | 87 (23) | 39 (20) | 48 (25) | |
| $20–39,999 | 112 (29) | 61 (32) | 51 (26) | |
| $40–69,999 | 70 (18) | 33 (17) | 37 (19) | |
| $70–99,999 | 37 (10) | 19 (10) | 18 (9) | |
| $100,000 or more | 28 (7) | 12 (6) | 16 (8) | |
| Currently employed full time, No. (%) | 125 (32) | 63 (33) | 62 (32) | 0.88 |
| Health insurance, No. (%)a | 0.24 | |||
| Commercial | 248 (64) | 125 (65) | 123 (64) | |
| Medicare | 62 (16) | 35 (18) | 27 (14) | |
| Medicaid | 29 (7) | 15 (8) | 14 (7) | |
| No insurance | 46 (12) | 17 (9) | 29 (15) | |
| Education, No. (%) | 0.68 | |||
| Less than high school | 68 (18) | 37 (19) | 31 (16) | |
| High school | 144 (37) | 69 (36) | 75 (39) | |
| College graduate or advanced degree | 173 (45) | 86 (45) | 87 (45) | |
| Less than 7–8th grade reading level, No. (%) | 53 (14) | 23 (12) | 30 (15) | 0.31 |
| Risk factors for CHD, No. (%) | ||||
| High blood pressure | 332 (86) | 166 (86) | 166 (86) | 0.90 |
| High blood cholesterol | 326 (85) | 162 (84) | 164 (85) | 0.87 |
| Current smoker | 113 (29) | 55 (29) | 58 (30) | 0.76 |
| Diabetes | 236 (61) | 124 (65) | 112 (58) | 0.19 |
| Medication use relevant to CHD risk reduction, No. (%) | ||||
| Taking blood pressure medicine | 289 (75) | 144 (75) | 145 (75) | 0.85 |
| Taking cholesterol medicine | 236 (61) | 119 (62) | 117 (61) | 0.78 |
| Taking aspirin (limited to males) | 84 (42) | 38 (42) | 46 (42) | 0.99 |
| Factors affecting medication and lifestyle adherence, No. (%) | ||||
| Prescription drug plan | 335 (87) | 173 (90) | 162 (84) | 0.07 |
| Number of medications per day | 0.27 | |||
| None | 10 (3) | 6 (3) | 4 (2) | |
| 1–2 | 42 (11) | 18 (9) | 24 (12) | |
| 3–5 | 144 (37) | 69 (35) | 75 (39) | |
| 6–9 | 130 (34) | 74 (38) | 56 (29) | |
| 10 or more | 60 (16) | 26 (13) | 34 (18) | |
| Living with spouse or someone like spouse | 263 (68) | 124 (65) | 139 (72) | 0.12 |
| Comfort with computer | 255 (66) | 128 (67) | 127 (66) | 0.86 |
| Other outcomes, mean (SE) | ||||
| Framingham Risk Scoreb | 16.9 (0.3) | 16.9 (0.4) | 16.9 (0.4) | 0.60 |
| Weight in kg | 96 (1.1) | 95 (1.6) | 97 (1.5) | 0.26 |
| BMI, kg/m2 | 33 (0.4) | 33 (0.5) | 34 (0.5) | 0.71 |
| Systolic blood pressure | 134 (0.9) | 134 (1.2) | 134 (1.3) | 0.68 |
| Diastolic blood pressure | 79 (0.6) | 78 (0.7) | 80 (0.9) | 0.10 |
| Total cholesterol | 194 (2.4) | 197 (3.4) | 190 (3.3) | 0.14 |
| HDL-C | 41 (0.5) | 42 (0.8) | 39 (0.7) | 0.03 |
| LDL-C | 122 (2.0) | 125 (2.9) | 119 (2.8) | 0.26 |
| Hgb A1c | 6.9 (0.1) | 7.0 (0.1) | 6.8 (0.1) | 0.07 |
| hsCRP | 4.7 (0.5) | 4.9 (0.6) | 4.6 (0.8) | 0.29 |
| Fruit and vegetables servings/day | 4.1 (0.1) | 4.1 (0.1) | 4.1 (0.1) | 0.56 |
| Minutes of walking per week | 77 (8.3) | 74 (11.3) | 79 (12.0) | 0.63 |
| Steps per day (pedometer, n = 253) | 4691 (168.4) | 4604 (225.6) | 4776 (250.0) | 0.71 |
| Morisky medication adherence scale, No. (%) | 0.75 | |||
| Low adherence | 94 (27) | 49 (29) | 45 (26) | |
| Medium adherence | 136 (39) | 65 (39) | 71 (40) | |
| High adherence | 114 (33) | 54 (32) | 60 (34) | |
Categorized as commercial if participant had commercial and other insurance
Framingham risk scores calculated as percent chance of developing angina, myocardial infarction, or coronary heart disease death over a 10 year time frame
Abbreviations: CHD, coronary heart disease; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Hgb A1c, hemoglobin A1c; hsCRP, high sensitivity C-reactive protein.
Participants Choice of Risk Reducing Strategies, Intervention Participation, and Follow-up Rates
As noted in Figure 1, after viewing the decision aid, 366 (95%) participants elected to work on improving their diet, 256 (66%) chose to work on increasing their physical activity, 71 (18%) decided to work on smoking cessation, and 142 (37%), chose to start or increase blood pressure or cholesterol medication or start aspirin.. Follow-up rates at 4 and 12 months were 91% and 87%, respectively Those who did not return for follow-up at 4 months were more likely to be white, younger, walk fewer minutes each week and at 12 months, consume less fruit and vegetables and be less adherent to medications (p <0.05 for comparisons).
Study Outcomes
Change in study outcomes from baseline to follow-up, by treatment arm, are shown in Table 2. For the FRS, there was a statistically significant and sustained reduction at both 4 (primary outcome) and 12 month follow-up for participants in both study groups. For the counselor group, the change was −2.3% and −1.9% at 4 and 12 months, respectively. For the web group, it was −1.5% and −1.7%, respectively. When values of no change and multiple imputations methods were used to impute missing FRS scores, results did not change appreciably.
Table 2.
Study outcomes: change from baseline by study group at 4 and 12 month follow-up. Data are for returnees, except as noted.
| 4 months | 12 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | n | Baseline | 4 months | Change (4 months minus baseline, 95% CI) |
P- value |
n | Baseline | 12 months | Change (12 months minus baseline, 95% CI) |
P-value |
| Framingham Risk Scorea: primary study outcome is change in risk score at 4 month follow-up -- mean (SE) | ||||||||||
| Counselor | 176 | 17.0 (0.5) | 14.7 (0.4) | −2.3 (–3.0 to −1.6) | <.001 | 168 | 17.1 (0.5) | 15.1 (0.5) | −1.9 (−2.8 to −1.1) | <.001 |
| Web | 171 | 16.8 (0.4) | 15.2 (0.5) | −1.5 (−2.2 to −0.9) | <.001 | 160 | 16.7 (0.4) | 15.0 (0.5) | −1.7 (−2.6 to −0.8) | <.001 |
| Framingham Risk Score: no change from baseline imputed for those lost to follow-up - - mean (SE) | ||||||||||
| Counselor | 191 | 17.0 (0.4) | 14.8 (0.4) | −2.1 (−2.7 to −1.5) | <.001 | 189 | 16.9 (0.4) | 15.2 (0.5) | −1.7 (−2.5 to −1.0) | <.001 |
| Web | 190 | 16.9 (0.4) | 15.6 (0.4) | −1.4 (−1.9 to −0.8) | <.001 | 186 | 16.8 (0.4) | 15.4 (0.4) | −1.5 (−2.2 to −0.7) | <.001 |
| Components of the Framingham Risk Score Assessed at Follow-up | ||||||||||
| Systolic blood pressure -- mean (SE) | ||||||||||
| Counselor | 176 | 134 (1.2) | 131 (1.2) | −3.0 (−5.5 to −0.5) | .02 | 169 | 134 (1.3) | 131 (1.4) | −3.2 (−6.0 to −0.4) | .02 |
| Web | 170 | 134 (1.4) | 133 (1.3) | −1.2 (−4.0 to 1.6) | .39 | 164 | 134 (1.4) | 133 (1.5) | −0.9 (−3.9 to 2.0) | .53 |
| Total cholesterol, mg/dL, -- mean (SE) | ||||||||||
| Counselor | 177 | 196 (3.5) | 188 (3.5) | −8.4 (−13.3 to −3.5) | <.001 | 170 | 196 (3.6) | 188 (3.6) | −7.6 (−12.6 to −2.6) | .003 |
| Web | 173 | 191 (3.6) | 187 (2.9) | −4.1 (−9.4 to 1.3) | .13 | 166 | 190 (3.6) | 187 (3.2) | −3.6 (−10.1 to 3.0) | .28 |
| HDL-C, mg/dL -- mean (SE) | ||||||||||
| Counselor | 177 | 41 (0.8) | 43 (0.8) | 1.4 (0.4 to 2.3) | .004 | 170 | 41 (0.8) | 42 (0.8) | 0.4 (−0.6 to 1.5) | .42 |
| Web | 173 | 40 (0.8) | 42 (0.8) | 1.8 (0.7 to 2.8) | .001 | 166 | 40 (0.8) | 42 (0.9) | 2.2 (1.0 to 3.3) | <.001 |
| Smoking -- no. (%) | ||||||||||
| Counselor | 177 | 48 (27%) | 39 (22%) | −5% (−9% to −1%) | .01 | 170 | 45 (26%) | 33(19%) | −7% (−11% to −3%) | .001 |
| Web | 173 | 50 (29%) | 43(25%) | −4% (−8% to −.3%) | ..03 | 166 | 48 (29%) | 39 (23%) | −5% (−10% to −1%) | .01 |
| Dietary intake | ||||||||||
| Fat quality screener scoreb -- mean (SE) | ||||||||||
| Counselor | 177 | 4.8 (0.2) | 4.1 (0.1) | −0.8 (−1.1 to −0.4) | <.001 | 170 | 4.9 (0.2) | 4.3 (0.1) | −0.6 (−1.0 to −0.2) | .001 |
| Web | 173 | 5.1 (0.2) | 4.2 (0.1) | −0.9 (−1.3 to −0.6) | <.001 | 166 | 5.1 (0.2) | 4.3 (0.1) | −0.8 (−1.1 to −0.4) | <.001 |
| Fruit and vegetable servings per day -- mean (SE) | ||||||||||
| Counselor | 177 | 4.2 (0.1) | 4.5 (0.1) | 0.3 (0.1 to 0.6) | .001 | 170 | 4.2 (0.1) | 4.5 (0.1) | 0.3 (0.1 to 0.5) | .01 |
| Web | 173 | 4.0 (0.1) | 4.2 (0.1) | 0.2 (0.0 to 0.5) | .04 | 166 | 4.1 (0.1) | 4.3 (0.1) | 0.3 (0.0 to 0.5) | .02 |
| Carotenoid index, mcg/dLc -- mean (SE) | ||||||||||
| Counselor | 152 | 35.4 (1.9) | 35.6 (1.7) | 0.2 (−2.2 to 2.7) | .41 | 140 | 34.8 (1.9) | 36.8 (1.9) | 2.0 (−0.6 to 4.7) | .07 |
| Web | 147 | 34.6 (1.7) | 34.4 (1.6) | −0.2 (−2.3 to 1.9) | .74 | 142 | 34.4 (1.7) | 34.6 (1.7) | 0.2 (−2.1 to 2.4) | .99 |
| Physical activity | ||||||||||
| Questionnaire—total walk time -- mean (SE) | ||||||||||
| Counselor | 177 | 77 (12) | 132 (19) | 55 (14 to 96) | .009 | 170 | 78 (12) | 95 (9) | 17 (−10 to 45) | .22 |
| Web | 173 | 83 (13) | 113(13) | 30 (0 to 61) | .06 | 166 | 84 (13) | 143 (24) | 59 (8 to 110) | .02 |
| Pedometer, total steps assessed during prior week -- mean (SE) | ||||||||||
| Counselor | 59 | 4767 (292) | 5657 (390) | 889 (274 to 1505) | .005 | 62 | 4619 (317) | 5254 (364) | 635 (135 to 1135) | .01 |
| Web | 62 | 4783 (344) | 5317 (339) | 533 (92 to 974) | .02 | 58 | 4996 (350) | 5176 (365) | 180 (−358 to 719) | .50 |
| Medication use | ||||||||||
| Morisky questionnaire for medication adherence—high adherence -- no. (%) | ||||||||||
| Counselor | 152 | 49 (32%) | 69 (45%) | 13% (4% to 22%) | .006 | 145 | 49 (34%) | 80 (55%) | 21% (11% to 32%) | <.001 |
| Web | 153 | 57(37%) | 86 (56%) | 19% (10% to 28%) | <.001 | 147 | 55 (37%) | 82 (56%) | 18% (8% to 28%) | <.001 |
| Taking blood pressure medication- - no. (%) | ||||||||||
| Counselor | 177 | 133 (75%) | 141 (80%) | 5% (0.1% to 9%) | .05 | 170 | 127 (75%) | 141 (83%) | 8% (4% to 13%) | <.001 |
| Web | 173 | 132 (76%) | 134 (77%) | 1% (−2% to 4%) | .48 | 166 | 127 (77%) | 131 (79%) | 2% (−2% to 6%) | .25 |
| Taking cholesterol medication -- no. (%) | ||||||||||
| Counselor | 177 | 110(62%) | 119 (67%) | 5% (−0.4% to 1%) | .07 | 170 | 106 (62%) | 113 (66%) | 4% (−2% to 10%) | .19 |
| Web | 173 | 105 (61%) | 104 (60%) | −0.6% (−6% to 5%) | .84 | 166 | 101 (61%) | 98 (59%) | −2% (−8% to 5%) | .59 |
| Taking aspirin -- no. (%) | ||||||||||
| Counselor | 177 | 76(43%) | 104 (59%) | 16% (10% to 22%) | <.001 | 170 | 72 (42%) | 104 (61%) | 19% (12% to 26%) | <.001 |
| Web | 173 | 84 (48%) | 111 (64%) | 16% (9% to 22%) | <.001 | 166 | 80 (48%) | 105 (63%) | 15% (7% to 23%) | <.001 |
| Other outcomes | ||||||||||
| Diastolic blood pressure -- mean (SE) | ||||||||||
| Counselor | 176 | 80 (0.7) | 77 (0.8) | −1.4 (−2.8 to 0.0) | .06 | 169 | 78 (0.8) | 77 (0.8) | −1.0 (−2.5 to 0.4) | .17 |
| Web | 170 | 80 (1.0) | 79 (0.9) | −0.8 (−2.4 to 0.8) | .34 | 164 | 80 (1.0) | 79 (1.0) | −0.2 (−1.8 to 1.4) | .81 |
| LDL-C, mg/dL, -- mean (SE) | ||||||||||
| Counselor | 177 | 124 (3.0) | 120 (3.2) | −4.5 (−9.1 to 0.1) | .05 | 170 | 124 (3.1) | 119 (3.1) | −4.9 (−9.7 to −0.1) | .04 |
| Web | 173 | 120 (3.0) | 118 (2.6) | −1.2 (−5.5 to 3.0) | .57 | 166 | 119 (3.0) | 117 (2.9) | −2.0 (−7.0 to 3.0) | .43 |
| Weight in kg -- mean (SE) | ||||||||||
| Counselor | 177 | 95 (1.7) | 95 (1.7) | −0.5 (−1.0 to 0.0) | .04 | 170 | 95 (1.7) | 94 (1.7) | −1.0 (−1.7 to −0.2) | .01 |
| Web | 173 | 97 (1.6) | 96 (1.5) | −1.1 (−1.6 to −0.5) | <.001 | 166 | 97 (1.6) | 95 (1.6) | −1.5 (−2.3 to −0.8) | <.001 |
| HgbA1c (overall) -- mean (SE) | ||||||||||
| Counselor | 176 | 7.0 (0.1) | 6.8 (0.1) | −0.3 (−0.4 to −0.1) | .002 | 170 | 7.0 (0.1) | 6.9 (0.1) | −0.2 (−0.4 to 0.0) | .03 |
| Web | 173 | 6.8 (0.1) | 6.7 (0.1) | 0.0 (−0.1 to 0.1) | .41 | 166 | 6.7 (0.1) | 6.8 (0.1) | 0.1 (0.0 to 0.2) | .28 |
| HgbA1c for those with diabetes -- mean (SE) | ||||||||||
| Counselor | 111 | 7.6 (0.1) | 7.3 (0.1) | −0.3 (−0.6 to −0.1) | .01 | 107 | 7.6 (0.1) | 7.4 (0.1) | −0.2 (−0.5 to 0.0) | .10 |
| Web | 98 | 7.4 (0.1) | 7.3 (0.1) | −0.1(−0.3 to 0.1) | .31 | 96 | 7.4 (0.1) | 7.5 (0.1) | 0.1 (−0.1 to 0.3) | .46 |
| hsCRP -- mean (SE) | ||||||||||
| Counselor | 177 | 4.7 (0.6) | 3.6 (0.3) | −1.1 (−2.1 to −0.1) | .03 | 170 | 4.5 (0.6) | 5.0 (0.9) | 0.5 (−1.1 to 2.1) | .52 |
| Web | 173 | 4.7 (0.9) | 3.8 (0.4) | −0.9 (−2.5 to 0.6) | .25 | 166 | 4.8 (0.9) | 4.2 (0.5) | −0.5 (−2.3 to 1.3) | .57 |
| QOL, SF-12 Mental composite-- mean (SE) | ||||||||||
| Counselor | 177 | 52 (0.4) | 53 (0.4) | 0.5 (−0.5 to 1.5) | .32 | 170 | 52 (0.4) | 53 (0.4) | 0.3 (−0.7 to 1.3) | .60 |
| Web | 173 | 53 (0.5) | 52 (0.4) | −0.3 (−1.2 to 0.7) | .61 | 166 | 53 (0.5) | 53 (0.4) | 0.1 (−0.9 to 1.2) | .83 |
| QOL, SF-12 Physical composite -- mean (SE) | ||||||||||
| Counselor | 177 | 43 (0.9) | 45 (0.9) | 2.4 (1.0 to 3.7) | <.001 | 170 | 43 (0.9) | 45 (0.9) | 2.2 (0.7 to 3.6) | .003 |
| Web | 173 | 42 (0.9) | 44 (0.9) | 1.6 (0.3 to 3.0) | .02 | 166 | 42 (0.9) | 45 (0.9) | 2.2 (0.6 to 3.8) | .01 |
Framingham risk scores calculated as percent chance of developing angina, myocardial infarction, or coronary heart disease death over a 10 year time frame for those who did not developed cardiovascular disease from baseline to follow-up.
A lower score indicates improved fat quality.
Carotenoid index, calculated as the sum of α-carotene, β-carotene, β-cryptoxanthin, and zeaxanthin. Data presented are for nonsmokers. A higher index indicates greater fruit and vegetable consumption. Statistical tests performed on log transformed data.
Abbreviations: HCL-C, high density lipoprotein cholesterol; RESIDE, RESIDential Environment Project; LDL-C, low density lipoprotein Cholesterol; HgbA1c, hemoglobin A1c; hsCRP, high sensitivity C-reactive protein; QOL, quality of life.
In both groups, all components of the FRS changed in the direction of decreased risk and the majority of changes were statistically significant and maintained from 4 to 12 month follow-up. Likewise, most changes in diet and physical activity were in the direction of decreased risk and sustained over time. Moreover, there were substantial increases in appropriate use of and adherence with medication to reduce CHD risk. Other statistically significant outcomes of note include slight weight loss at 12 months, a reduction in A1c in the counselor group, and a sustained improvement in the physical component measure of quality of life in both groups.
Self-reported results for tobacco cessation and aspirin use at follow-up were confirmed by biomarkers. Of 23 smokers who reported cessation, 18 (78%) were confirmed by urine cotinine testing and of 425 participants who reported aspirin use, 319 (75%) had serum thromboxane levels consistent with aspirin use.
The difference in study outcomes between treatment arms are shown in Table 3. At 4 month follow-up, the adjusted change (SE) in FRS was −2.4% (0.3) for counselor and −1.4% (0.3) for web, difference −1.0% (95% CI −1.8% to −0.1%, p = 0.03). At 12 month follow-up, the adjusted change (SE) in FRS −2.1% (0.4) for counselor and −1.5% (0.4) for web, difference −0.6% (95% CI, −1.7% to 0.5%, p = 0.30). When change in FRS was assessed by longitudinal analysis, there was no significant time by group interaction (p = 0.27) and within and between group comparisons were similar to analyses at each time point.
Table 3.
Comparison of outcomes between study group at 4 and 12 months.a Data are for returnees.
| Outcome | 4 months | 12 months | ||||||
|---|---|---|---|---|---|---|---|---|
| Continuous variables: Crude and adjustedb difference in change of outcome between study groups (change for counselor minus change for web)c | ||||||||
| Crude | Adjusted | Crude | Adjusted | |||||
| Mean (95% CI) | p | Mean (95% CI) | p | mean (95% CI) | p | mean (95% CI) | p | |
| Framingham Risk Scored | −0.8 (−1.7 to 0.1) | 0.09 | −1.0 (−1.8 to −0.1) | 0.03 | −0.2 (−1.4 to 1.0) | 0.72 | −0.6 (−1.7 to 0.5) | 0.30 |
| Components of the Framingham Risk Score | ||||||||
| Systolic blood pressure, mmHg | −1.8 (−5.5 to 1.9) | 0.35 | −1.5 (−4.5 to 1.5) | 0.33 | −2.3 (−6.3 to 1.7) | 0.26 | −2.6 (−6.1 to 0.9) | 0.14 |
| Total cholesterol, mg/dL, | −4.3 (−11.5 to 2.9) | 0.24 | −2.9 (−9.2 to 3.3) | 0.36 | −4.1 (−12.2 to 4.1) | 0.33 | −2.4 (−9.8 to 5.1) | 0.53 |
| HDL-C, mg/dL | −0.4 (−1.8 to 0.9) | 0.54 | −0.4 (−1.7 – 1.0) | 0.58 | −1.7 (−3.3 to −0.2) | 0.03 | −1.5 (−3.0 to −0.0) | 0.05 |
| Dietary Intake | ||||||||
| Fat Quality Screener Scoree | 0.2 (−0.3 to 0.7) | 0.46 | 0.03 (−0.3 to 0.4) | 0.87 | 0.2 (−0.3 to 0.7) | 0.51 | 0.02 (−0.4 to 0.3) | 0.93 |
| Fruit and Vegetable servings/d | 0.1 (−0.2 to 0.4) | 0.46 | 0.3 (0.0 to 0.5) | 0.05 | 0.02 (−0.3 to 0.3) | 0.91 | 0.1 (−0.1 to 0.4) | 0.31 |
| Carotenoid Indexf | 0.4 (−2.9 to 3.7) | 0.43 | 0.2 (−2.6 to 3.1) | 0.47 | 1.9 (−1.6 to 5.4) | 0.20 | 2.3 (−0.9 to 5.6) | 0.12 |
| Physical Activity | ||||||||
| RESIDE questionnaire—total walk time (minutes per week) | 25.1 (−25.9 to 76.1) | 0.33 | 31.4 (−10.4 to 73.3) | 0.14 | −41.7 (−99.1 to 15.6) | 0.15 | −39.6 (−85.6 to 6.5) | 0.09 |
| Pedometer, steps per day in prior week | 356 (−386 to 1099) | 0.34 | 339 (−376 to 1054) | 0.35 | 455 (−266 to 1176) | 0.21 | 592 (−100 to 1284) | 0.09 |
| Other Outcomes | ||||||||
| Diastolic blood pressure, mmHg | −0.6 (−2.7 to 1.5) | 0.59 | −1.4 (−3. to 0.5) | 0.14 | −0.8 (−3.0 to 1.3) | 0.44 | −1.6 (−3.4 to 0.3) | 0.10 |
| LDL-C, mg/dL, | −3.3 (−9.5 to 2.9) | 0.30 | −2.1 (−7.6 to 3.4) | 0.46 | −2.9 (−9.8 to 4.0) | 0.40 | −1.0 (−7.4 to 5.3) | 0.75 |
| Weight in kg | 0.5 (−0.2 to 1.3) | 0.13 | 0.6 (−0.1 to 1.3) | 0.10 | 0.6 (−0.4 to 1.6) | 0.27 | 0.8 (−0.2 to 1.8) | 0.13 |
| HgbA1c (all participants) | −0.2 (−0.4 to −0.02) | 0.03 | −0.1 (−0.3 to 0.04) | 0.14 | −0.2 (−0.4 to −0.04) | 0.02 | −0.2 (−0.3 to 0.00) | 0.05 |
| HgbA1c (those with diabetes) | −0.2 (−0.5 to 0.1) | 0.12 | −0.1 (−0.3 to 0.2) | 0.48 | −0.3 (−0.6 to 0.03) | 0.07 | −0.2 (−0.4 to 0.1) | 0.20 |
| hsCRP | −0.2 (−2.1 to 1.6) | 0.82 | −0.1 (−1.0 to 0.7) | 0.73 | 1.0 (−1.4 to 3.4) | 0.40 | 0.9 (−0.9 to 2.7) | 0.33 |
| QOL, SF−12 Mental composite | 0.8 (−0.6 to 2.1) | 0.28 | 0.3 (−0.9 to 1.4) | 0.62 | 0.1 (−1.3 to 1.6) | 0.84 | −0.5 (−1.5 to 0.6) | 0.38 |
| QOL, SF−12 Physical composite | 0.7 (−1.1 to 2.6) | 0.44 | 1.1 (−0.6 to 2.8) | 0.20 | 0.0 (−2.2 to 2.1) | 0.97 | 0.8 (−1.2 to 2.7) | 0.44 |
| Categorical variables--medication use: Crude difference in percentage at 4 and 12 month follow-up (percentage for counselor minus percentage for web) | ||||||||
| mean (95% CI) | P | mean (95% CI) | P | |||||
| smoking | −1% (−6% to 4%) | 0.69 | −2% (−8% to 4%) | 0.59 | ||||
| Taking aspirin | 0% (−9% to 10%) | 0.95 | 4% (−7% to 14%) | 0..48 | ||||
| Morisky questionnaire for medication adherence—high adherence | −6% (−18% to 7%) | 0.36 | 3% (−11% to 17%) | 0.68 | ||||
| Taking blood pressure medication | 3% (−2% to 9%) | 0.22 | 6% (−.2% to 12%) | 0.06 | ||||
| Taking cholesterol medication | 6% (−2% to 14%) | 0.16 | 6% (−3% to 15%) | 0.20 | ||||
See Table 2 for the number of participants with followed data used to calculate the difference scores in this table.
Adjusted for baseline value of variable, age, sex, race (white vs. other), BMI, prescription drug plan, HDL-C, HgA1c, and study site
A negative difference score indicates the value of the outcome is lower in the counselor group.
Framingham risk scores calculated as percent chance of developing angina, myocardial infarction, or coronary heart disease death over a 10 year time frame for those who did not developed cardiovascular disease from baseline to follow-up.
A lower score indicates improved fat quality.
Carotenoid index, calculated as the sum of α-carotene, β-carotene, β-cryptoxanthin, and zeaxanthin. Data presented are for non-smokers. A higher index indicates greater fruit and vegetable consumption. Statistical tests performed on log transformed data.
Abbreviations: HCL-C, high density lipoprotein cholesterol; RESIDE, RESIDential Environment Project; LDL-C, low density lipoprotein Cholesterol; HgbA1c, hemoglobin A1c; hsCRP, high sensitivity C-reactive protein; QOL, quality of life.
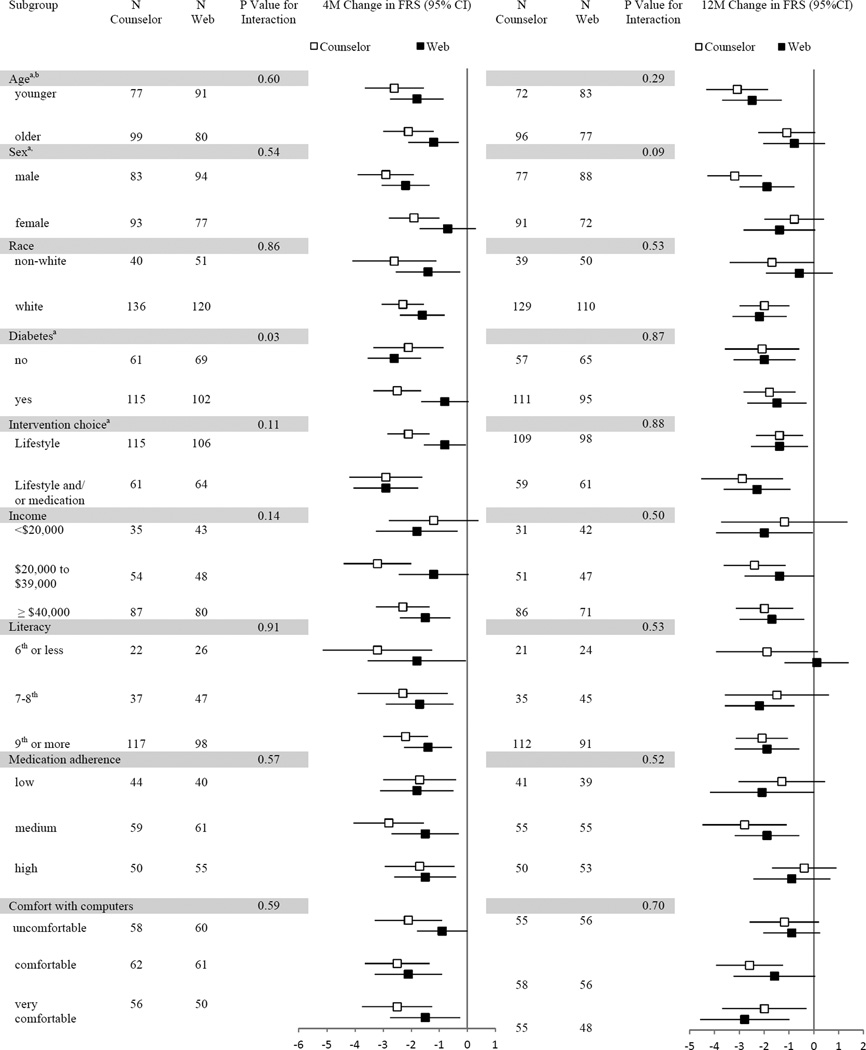
Sub-group analysis
Figure 2 shows the change in FRS at 4 and 12 month follow-up stratified on selected baseline variables. Assessing change in FRS by subgroups, without regard to treatment arm, the intervention was significantly more effective at 4 and 12 months among younger participants (P = .05 and <.001). In addition, at 4 month follow-up, the intervention was more effective among males (P = .04), those without diabetes (P = .02), and those choosing lifestyle and medication (P = .01). We noted little difference in the effectiveness of the counselor-delivered vs. web-based interventions when change in FRS was assessed by treatment arm and subgroups. At 4 month follow-up, there were a larger improvement in FRS among participants with diabetes in the counselor group (P for interaction = 0.03).
Figure 2. Change in Framingham Risk Score, stratified on selected baseline variables, shown by treatment arm at 4 and 12 month follow-up.
aAt 4 month follow-up, P-value ≤ .05 for comparison of FRS between sub-groups with web and counselor groups combined; bat 12 month follow-up, P-value ≤ .05 for same comparison.
Adverse Outcomes
There were no reported adverse side effects related to dietary change or increased physical activity. Deaths due to CHD and newly diagnosed CHD are noted in Figure 1. There were no other deaths during follow-up. In addition, there was no material change in ALT or creatinine from baseline to follow-up.
Acceptability
Both counselor and web formats were well received. At 4 month follow-up, among 177 counselor participants completing the acceptability survey, 137 (77%) strongly agreed and 36 (20%) agreed that they would recommend this program to others. Similarly, among 173 web participants, 128 (74%) strongly agreed and 42 24%) agreed with this statement. At 12 month follow-up, among 170 counselor and 166 web participants completing the survey, 166 (98%) counselor and 161 (97%) web participants would recommend or strongly recommend this program to others.
Cost Effectiveness
At 12 months, the costs per participant from the payer perspective were $207 (SE 3.4) and $110 (SE: 3.5) for the Counselor and Web interventions respectively (p<0.001). From the payer perspective, the incremental cost-effectiveness ratio for the less expensive Web intervention, compared to no intervention, was $73 per percentage point reduction in CHD risk and $2,973 per QALY gained, which is considered very cost-effective based on common benchmarks35. Additional results are reported in the Appendix.
Sensitivity Analysis
A limited sensitivity analysis was conducted (Table 4) to assess change in 10-year risk for CHD as calculated with the Adult Treatment Panel (ATP) III risk calculator36 (which calculates MI and CHD death) and the Framingham risk calculator used for this study25 without including a term for aspirin. Overall, results were similar, with significant reductions in estimated CHD risk in both groups at 4 and 12 month follow-up.
Table 4.
Sensitivity Analysis: Change in 10-Year Risk for Coronary Heart Disease as assessed by Adult Treatment Panel (ATP) III and Framingham Risk Equations without Term for Aspirin.
| Risk Calculator | Study Group | N | Baseline Mean (SE) |
Follow-up Mean (SE) |
Change in Mean (95% CI) |
P-value |
|---|---|---|---|---|---|---|
| 4 Month Outcomes | ||||||
| ATPIII | Counselor | 176 | 10.6 (0.5) | 9.7 (0.5) | −0.9 (−1.4 to −0.4) | <.001 |
| Web | 171 | 10.9 (0.5) | 10.1 (0.5) | −0.7 (−1.2 to −0.3) | .002 | |
| FRS | Counselor | 176 | 18.0 (0.5) | 16.0 (0.5) | −1.9 (−2.6 to −1.3) | <.0001 |
| Web | 171 | 17.9 (0.5) | 16.8 (0.5) | −1.1 (−1.7 to −0.4) | .001 | |
| 12 Month Outcomes | ||||||
| ATPIII | Counselor | 168 | 10.5 (0.5) | 9.9 (0.5) | −0.6 (−1.2 --to 0.0) | .04 |
| Web | 160 | 11.0 (0.6) | 10.2 (0.6) | −0.8 (−1.4 to −0.1) | .02 | |
| FRS | Counselor | 168 | 17.9 (0.5) | 16.3 (0.5) | −1.7 (−2.5 to −0.9) | <.001 |
| Web | 160 | 17.9 (05) | 16.6 (0.5) | −1.3 (−2.2 to −0.4) | .006 | |
Discussion
In this comparative effectiveness trial, a combined lifestyle and medication intervention lowered predicted 10-year CHD risk within each treatment arm (pre-post change) at 4 and 12 months. This risk reduction was achieved by improvements in lifestyle, medication use, or both and mediated through improvements in blood pressure, blood lipids, cigarette smoking, and aspirin use. The intervention was highly acceptable to participants, and the web format was cost effective based on established benchmarks.
These findings reinforce increasing evidence suggesting web-based interventions can have an important role in clinical practice.14,37,38 In this study, the web-based intervention was equally effective to the counselor-delivered intervention at 12 month follow-up. This suggests web interventions could be used to fill important gaps in counselor availability and, where counselors are available, allow counselors to focus their efforts on harder to change behaviors, such as refractory lifestyle behaviors.37 Web interventions might also be used to reach populations who have limited access to the clinic.
This study has several limitations. It was designed as a comparative effectiveness trial, without a no-intervention control group. Thus, observed changes could be due in part to regression to the mean (though baseline screening included two sequential assessments of FRS), secular trends, or other factors. Though non-intervention factors may account for some of the observed change, we believe much of the change was due to intervention effects as the components of the current intervention have previously been compared to no-intervention control groups and have been shown to be effective.17 In a previous trial of a similar web-delivered medication intervention,24 the additional reduction in FRS between intervention and control groups at 3 month follow-up was 1.1 percentage points overall and 1.4 percentage points among a pre-specified subgroup of participants with a 10-year predicted risk >10%. In a previous trial of a similar counselor-delivered dietary intervention,39 there was a substantial increase in fruit and vegetable intake, confirmed by blood carotenoids.
Additional limitations include many secondary outcomes that were self-reported behaviors, which may be exaggerated due to social desirability reporting bias, though we did measure biomarker change for fruit and vegetable intake, aspirin use, and smoking cessation. Also, we present many comparisons in our secondary analysis, and some p-values may be significant by chance. Our follow-up interval was 12 months, and the intervention effects may attenuate over time. Further, our achieved sample size was somewhat less than our goal, decreasing power to detect between group differences. The generalizability of our findings may be limited to established, older patients who are at high risk for CHD. Finally, as lifestyle change may have beneficial effects on CHD risk independent of traditional risk factors,1,40 calculated change in FRS may underestimate intervention benefit.
In conclusion, the combined lifestyle and medication intervention tested in alternative formats yielded a substantial and sustained reduction in predicted 10-year CHD risk. Risk reduction was similar in both intervention formats at 12 month follow-up, though the web was less expensive to implement. Future research should assess the implementation and maintenance of high-quality evidence-based interventions in a broad selection of clinical settings. In addition, the lifestyle component of the interventions could be used, and should be studied, in non-clinical health promotion settings.
Supplementary Material
Acknowledgments
This research was supported by the U.S. Centers for Disease Control and Prevention (CDC), American Recovery and Reinvestment Act of 2009, Cooperative Agreement Number 1U48DP002658 and also supported in part by National Institutes of Health grant P30DK056350 to the University of North Carolina at Chapel Hill Nutrition Obesity Research Center. The content is solely the responsibility of the authors, who give special thanks to: 1) participating practices in the North Carolina Family Medicine Research Network (Cabarrus Family Medical, Kannapolis, NC; Caswell Family Medical Center, Yanceyville, NC; Dayspring Family Medicine, Eden, NC; Durham Family Practice, Durham, NC; Moncure Community Health Center, Moncure, NC; 2) the health counselors who delivered interventions at these sites (Kim Grimm, Beth Jenks, Taimur Khan, Lauren Martin), 3) Russell Tracy, Ph.D and Elaine Cornell at the Laboratory for Clinical Biochemistry Research at the University of Vermont, and 4) the study participants, whole willing participation made this study possible.
Specific Role of Funding Agency
The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of interest: none
Financial disclosures: none
References
- 1.Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Appel LJ, Van Horn L. Components of a cardioprotective diet: new insights. Circulation. 2011;123(24):2870–2891. doi: 10.1161/CIRCULATIONAHA.110.968735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antithrombotic Trialists C, Baigent C, Blackwell L, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cholesterol Treatment Trialists C, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gueyffier F, Froment A, Gouton M. New meta-analysis of treatment trials of hypertension: improving the estimate of therapeutic benefit. J Hum Hypertens. 1996;10(1):1–8. [PubMed] [Google Scholar]
- 6.Ammerman A, DeVellis R, Carey T, et al. Physician-based diet counseling for cholesterol reduction: current practices, determinants, and strategies for improvement. Prev Med. 1993;22(1):96–109. doi: 10.1006/pmed.1993.1007. [DOI] [PubMed] [Google Scholar]
- 7.Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners. Prev Med. 1995;24(6):546–552. doi: 10.1006/pmed.1995.1087. [DOI] [PubMed] [Google Scholar]
- 8.Fineberg HV. The paradox of disease prevention: celebrated in principle, resisted in practice. JAMA. 2013;310(1):85–90. doi: 10.1001/jama.2013.7518. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Ambulatory care use and physician visits. [Accessed December 17, 2013]; http://www.cdc.gov/Nchs/fastats/docvisit.htm.
- 10.Ebrahim S, Beswick A, Burke M, Davey Smith G. Multiple risk factor interventions for primary prevention of coronary heart disease. Cochrane Database Syst Rev. 2006;(4):CD001561. doi: 10.1002/14651858.CD001561.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein MG, Whitlock EP, DePue J. Multiple behavioral risk factor interventions in primary care. Summary of research evidence. Am J Prev Med. 2004;27(2 Suppl):61–79. doi: 10.1016/j.amepre.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Slutsky JR, Clancy CM. Patient-centered comparative effectiveness research: essential for high-quality care. Arch Intern Med. 2010;170(5):403–404. doi: 10.1001/archinternmed.2010.5. [DOI] [PubMed] [Google Scholar]
- 13.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290(12):1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 14.Portnoy DB, Scott-Sheldon LA, Johnson BT, Carey MP. Computer-delivered interventions for health promotion and behavioral risk reduction: a meta-analysis of 75 randomized controlled trials, 1988–2007. Prev Med. 2008;47(1):3–16. doi: 10.1016/j.ypmed.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritterband LM, Tate DF. The science of internet interventions. Introduction. Ann Behav Med. 2009;38(1):1–3. doi: 10.1007/s12160-009-9132-5. [DOI] [PubMed] [Google Scholar]
- 16.Noell J, Glasgow RE. Interactive technology applications for behavioral counseling: issues and opportunities for health care settings. Am J Prev Med. 1999;17(4):269–274. doi: 10.1016/s0749-3797(99)00093-8. [DOI] [PubMed] [Google Scholar]
- 17.Sheridan SL, Draeger LB, Pignone MP, et al. Designing and implementing a comparative effectiveness study of two strategies for delivering high quality CHD prevention: Methods and participant characteristics for the Heart to Health study. Contemp Clin Trials. 2013;36(2):394–405. doi: 10.1016/j.cct.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esposito K, Maiorino MI, Di Palo C, Giugliano D, Campanian Postprandial Hyperglycemia Study G. Adherence to a Mediterranean diet and glycaemic control in Type 2 diabetes mellitus. Diabet Med. 2009;26(9):900–907. doi: 10.1111/j.1464-5491.2009.02798.x. [DOI] [PubMed] [Google Scholar]
- 19.Mozaffarian D. Effects of dietary fats versus carbohydrates on coronary heart disease: a review of the evidence. Curr Atheroscler Rep. 2005;7(6):435–445. doi: 10.1007/s11883-005-0060-y. [DOI] [PubMed] [Google Scholar]
- 20.Oh K, Hu FB, Manson JE, Stampfer MJ, Willett WC. Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the nurses' health study. Am J Epidemiol. 2005;161(7):672–679. doi: 10.1093/aje/kwi085. [DOI] [PubMed] [Google Scholar]
- 21.Pereira MA, O'Reilly E, Augustsson K, et al. Dietary fiber and risk of coronary heart disease: a pooled analysis of cohort studies. Arch Intern Med. 2004;164(4):370–376. doi: 10.1001/archinte.164.4.370. [DOI] [PubMed] [Google Scholar]
- 22.Hamer M, Chida Y. Active commuting and cardiovascular risk: a meta-analytic review. Prev Med. 2008;46(1):9–13. doi: 10.1016/j.ypmed.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Zheng H, Orsini N, Amin J, Wolk A, Nguyen VT, Ehrlich F. Quantifying the dose-response of walking in reducing coronary heart disease risk: meta-analysis. Eur J Epidemiol. 2009;24(4):181–192. doi: 10.1007/s10654-009-9328-9. [DOI] [PubMed] [Google Scholar]
- 24.Sheridan SL, Draeger LB, Pignone MP, et al. A randomized trial of an intervention to improve use and adherence to effective coronary heart disease prevention strategies. BMC Health Serv Res. 2011;11:331. doi: 10.1186/1472-6963-11-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121(1 Pt 2):293–298. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 26.Jilcott SB, Keyserling TC, Samuel-Hodge CD, Johnston LF, Gross MD, Ammerman AS. Validation of a brief dietary assessment to guide counseling for cardiovascular disease risk reduction in an underserved population. J Am Diet Assoc. 2007;107(2):246–255. doi: 10.1016/j.jada.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz LM, Woloshin S, Black WC, Welch HG. The role of numeracy in understanding the benefit of screening mammography. Ann Intern Med. 1997;127(11):966–972. doi: 10.7326/0003-4819-127-11-199712010-00003. [DOI] [PubMed] [Google Scholar]
- 28.Davis TC, Long SW, Jackson RH, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med. 1993;25(6):391–395. [PubMed] [Google Scholar]
- 29.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Block G, Gillespie C, Rosenbaum EH, Jenson C. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med. 2000;18(4):284–288. doi: 10.1016/s0749-3797(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 31.Kraschnewski JL, Gold AD, Gizlice Z, et al. Development and evaluation of a brief questionnaire to assess dietary fat quality in low-income overweight women in the southern United States. J Nutr Educ Behav. 2013;45(4):355–361. doi: 10.1016/j.jneb.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Giles-Corti B, Timperio A, Cutt H, et al. Development of a reliable measure of walking within and outside the local neighborhood: RESIDE's Neighborhood Physical Activity Questionnaire. Prev Med. 2006;42(6):455–459. doi: 10.1016/j.ypmed.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Jones SA, Evenson KR, Johnston LF, et al. Psychometric properties of the modified RESIDE physical activity questionnaire among low-income overweight women. J Sci Med Sport. doi: 10.1016/j.jsams.2013.12.007. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42(9):851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 35.Murray CJ, Evans DB, Acharya A, Baltussen RM. Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ. 2000;9(3):235–251. doi: 10.1002/(sici)1099-1050(200004)9:3<235::aid-hec502>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 36.Risk Assessment Tool for Estimating Your 10-year Risk of Having a Heart Attack. [Accessed March 16, 2014];2014 http://cvdrisk.nhlbi.nih.gov/calculator.asp.
- 37.Glasgow RE, Bull SS, Piette JD, Steiner JF. Interactive behavior change technology. A partial solution to the competing demands of primary care. Am J Prev Med. 2004;27(2 Suppl):80–87. doi: 10.1016/j.amepre.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 38.Linn AJ, Vervloet M, van Dijk L, Smit EG, Van Weert JC. Effects of eHealth interventions on medication adherence: a systematic review of the literature. J Med Internet Res. 2011;13(4):e103. doi: 10.2196/jmir.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keyserling TC, Samuel Hodge CD, Jilcott SB, et al. Randomized trial of a clinic-based, community-supported, lifestyle intervention to improve physical activity and diet: The North Carolina enhanced WISEWOMAN project. Prev Med. 2008;46(6):499–510. doi: 10.1016/j.ypmed.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 40.de Lorgeril M, Renaud S, Mamelle N, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994;343(8911):1454–1459. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.