Abstract
Background
Vitamin D, in addition to its established role in bone metabolism, may regulate the immune system and impact the outcome of allografts.
Methods
We identified 351 kidney allograft recipients who had serum levels of 25-hydroxyvitamin D (25[OH]D) measured within the first 30 days of transplantation. We evaluated the relationship between the circulating levels of 25(OH)D and acute cellular rejection (ACR), CMV disease, BKV nephropathy (BKVN), and kidney graft function.
Results
Vitamin D deficiency (circulating levels of 25(OH)D ≤20 ng/mL, defined using The Endocrine Society Clinical Practice 2011 Guideline) was observed in 216 (61.5%) of 351 kidney graft recipients. Vitamin D deficiency was more frequent in female recipients (P=0.007, Fisher’s Exact test) and African American recipients (P<0.001) and was less frequent in preemptive kidney graft recipients (P=0.002). Biopsy-confirmed ACR was more frequent in the vitamin D deficient group than in the sufficient group (10.2% vs. 3.7%, P=0.04). By multivariable Cox regression analysis, vitamin D deficiency was an independent risk factor for ACR (Hazard Ratio: 3.3, P=0.02). Vitamin D deficiency was not associated with CMV disease, BKVN, or kidney allograft function at 1 year. 1,25-dihydroxyvitamin D3 supplementation initiated within the first 90 days of transplantation was associated with a lesser incidence of ACR compared to no treatment with 1,25-dihydroxyvitamin D3 (5.1% vs. 13.0%, P=0.099).
Conclusions
Vitamin D deficiency is an independent risk factor for development of ACR within the first year of kidney transplantation and 1,25-dihydroxyvitamin D3 supplementation may help reduce the occurrence of ACR in the vitamin D deficient group.
Keywords: vitamin D, acute cellular rejection, vitamin D supplementation
INTRODUCTION
In addition to its role in calcium-phosphorous-bone metabolism, Vitamin D is considered to participate in a wide range of biologic processes, and vitamin D deficiency/insufficiency has been associated with all-cause mortality in humans (Reviewed in (1)). A role for vitamin D and its metabolites in regulating both innate immunity and adaptive immunity has also been reported. Administration of Vitamin D has been shown to be beneficial in experimental models of transplantation. 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) administration prevented acute rejection and prolonged survival in the ACI to Lewis rat renal transplant model (2), and also prevented chronic allograft nephropathy in the Fisher 344 to Lewis rat renal transplantation model (3). An allograft protective effect of 1,25(OH)2D3 has also been reported in orthotopic rat liver transplantation models (4, 5).
Mechanistic studies of vitamin D have elucidated pathways by which vitamin D may regulate immunity. Calcitriol inhibits both PHA-induced proliferation of human peripheral blood mononuclear cells (PBMC) and PHA- induced production of interleukin-2 by PBMC (6). Reichel and colleagues reported that vitamin D inhibits interferon-gamma production by human PBMC and enriched T cells at the pre-translational level (7). Oral calcitriol treatment of stable kidney graft recipients has been associated with a decreased display of HLA-DR and the co-stimulatory molecules CD28, CD40, and CD86 on the surface of peripheral blood leukocytes (8). In a randomized controlled trial of 19 recipients of living donor kidney grafts, calcitriol treatment of kidney donors and recipients was associated with an increased percentage of CD3+CD4+CD25+ cells in peripheral blood compared to the untreated group (9). Application of 1,25(OH)2D3 to the skin of mice is associated with alterations in the phenotype of CD11c+ dendritic cells and enhanced regulatory activity of CD4+CD25+ cells in draining lymph nodes (10).
Although previous studies have suggested that vitamin D deficiency has an adverse effect on allograft outcomes, there has been heterogeneity in the thresholds used to classify patients as vitamin D deficient or sufficient. Kim and colleagues defined vitamin D deficiency as circulating levels less than 10 ng/mL of 25(OH)D and reported lower eGFR in vitamin D deficient kidney graft recipients (11). Bienaimé et al. used a level of 15 ng/mL and reported that vitamin D is an independent risk factor for lower measured GFR and interstitial fibrosis and tubular atrophy (12).
The lack of uniformity in cutoff points for Vitamin D deficiency has been recently addressed by the 2011 guidelines developed by the Task Force of the Endocrine Society, which recommends measuring circulating levels of 25(OH)D rather than levels of 1,25-dihydroxyvitamin D [1,25(OH)2D] for assessing vitamin D status and defines vitamin D deficiency as circulating levels less than 20 ng/mL (13). Herein, we adapted the Endocrine Society’s diagnostic guideline and investigated the association between post-transplant vitamin D status and the incidence of acute cellular rejection, CMV disease, and BK virus nephropathy during the first year of transplantation and kidney allograft function at 1 year in our single center study of 351 kidney graft recipients.
RESULTS
One thousand two hundred and eleven patients received a kidney allograft at our center during the period January 2005 to December 2010, and the baseline characteristics are listed in Table 1A. Among the 1211 patients, 351 had circulating levels 25(OH)D measured within the first 30 days of transplantation and the remaining 860 did not have levels measured during the same period. Variables such as age of the recipients, gender, type of donor graft (living donor vs. deceased donor), prior history of transplantation, or induction therapy with anti-thymocyte globulin were not different between the two groups. African American race (P<0.01) and diabetes mellitus (P=0.06) were, however, over represented in the group without measured levels of vitamin D. The incidence of biopsy confirmed acute cellular rejection (ACR) was higher in the 25(OH)D measured cohort compared to the 25(OH)D unmeasured cohort (7.7% vs. 4.3%, P=0.02, Fisher’s Exact Test) and the incidence of CMV disease and that of BKV nephropathy were not different between the two groups.
Table 1A.
Baseline Characteristics of the Kidney Graft Recipients, Stratified by Vitamin D Measurement Status
| Recipient characteristics | All Transplant Recipients | Vitamin D Measured Group | Vitamin D Unmeasured Group | P Valuea |
|---|---|---|---|---|
| N=1211 | N=351 | N=860 | ||
| Age (mean±SD) | 52.5±13.6 | 52.3±13.6 | 52.6±13.6 | 0.82 |
| Female Gender, N | 472 (39.0%) | 130 (37.0%) | 342 (39.8%) | 0.40 |
| African American, N | 336 (27.7%) | 78 (22.2%) | 258 (30.0%) | 0.01 |
| Diabetes Mellitus, N | 375 (31.0%) | 95 (27.1%) | 280 (32.6%) | 0.06 |
| Deceased Donor Transplantation, N | 648 (53.5%) | 177 (50.4%) | 471 (54.8%) | 0.18 |
| Prior Kidney Transplantation, N | 134 (11.1%) | 38 (10.8%) | 96 (11.2%) | 0.92 |
| Anti-thymocyte Globulin Induction, N | 1044 (86.2%) | 303 (86.3%) | 741 (86.2%) | 0.99 |
| ACR Within the First Year, N | 64 (5.3%) | 27 (7.7%) | 37 (4.3%) | 0.02 |
| BK Virus Nephropathy Within the First Year, N | 21 (1.7%) | 5 (1.4%) | 16 (1.9%) | 0.81 |
| CMV Disease Within the First Year, N | 62 (5.1%) | 21 (6.0%) | 41 (4.8%) | 0.39 |
Wilcoxon-Rank Sum test was used to compare continuous variables and Fisher’s Exact test was used to compare categorical variables.
Circulating Levels of 25-hydroxyvitamin D in the 351 Kidney Allograft Recipients with Levels Measured within 30 Days of Transplantation
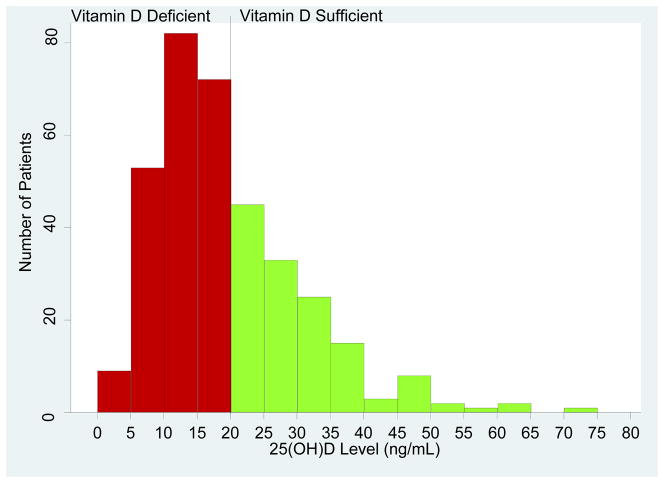
The mean (±SD) 25(OH)D level in the our study cohort of 351 kidney graft recipients was 20.2±11.1 ng/mL. Figure 1 illustrates the distribution of 25(OH)D levels among all 351 patients. Using the Endocrine Society Consensus guidelines (13) classification for vitamin D deficiency (circulating levels of 25(OH) D less than or equal to 20 ng/mL) or sufficiency (circulating levels of 25(OH)D greater than 20 ng/mL), 216 (61.5%) of the 351 patients were classified as vitamin D deficient and the remaining 135 patients (38.5%) were classified as vitamin D sufficient.
Figure 1. Distribution of the 25(OH)D Levels Among the Study Cohort of 351 Kidney Transplant Recipients.
Each bar on the X axis represents an interval of 5 ng/mL and ranges from 0 ng/mL to 80 ng/mL. The y-axis is the number of subjects corresponding to each bar. As per the Endocrine Society’s Clinical Practice Guidelines (13), a circulating level of 20 ng/mL was used as the threshold to classify the kidney graft recipients as vitamin D deficient (red bars) and vitamin D sufficient (green bars). The black vertical line in the frequency histogram is the Endocrine Society’s Clinical Practice Guidelines’ threshold. Two hundred and sixteen (61.5%) of the 351 kidney graft recipients were vitamin D deficient and the remaining 135 (38.5%) were vitamin D sufficient.
Pre-transplant characteristics of the kidney graft recipients, stratified by 25(OH)D levels, are summarized in Table 1B. The vitamin D deficient group was more likely to be female (43% vs. 28%, P=0.007, Fisher’s Exact test), African American (29% vs. 11%, P<0.001), and have diabetes (31% vs. 22%, P=0.07) than the vitamin D sufficient group. The vitamin D sufficient group was more likely to have a preemptive kidney transplantation (31% vs. 17%, P=0.002).
Table 1B.
Baseline Characteristics of the 351 Kidney Allograft Recipients With Measured Vitamin D, Stratified by Vitamin D Status
| Recipient characteristics | Vitamin D Sufficient Group >20 ng/mL | Vitamin D Deficient Group ≤20 ng/mL | P Valuea |
|---|---|---|---|
| N=135 | N=216 | ||
| Age (mean±SD) | 52.5±14.3 | 52.1±13.1 | 0.69 |
| Female Gender, N | 38 (28.2%) | 92 (42.6%) | 0.007 |
| African American Race, N | 15 (11.1%) | 63 (29.2%) | <0.001 |
| Diabetes Mellitus, N | 29 (21.5%) | 66 (30.6%) | 0.07 |
| Deceased Donor Transplantation, N | 69 (51.1%) | 108 (50.0%) | 0.91 |
| Preemptive Kidney Transplantation, N | 42 (31.1%) | 36 (16.7%) | 0.002 |
| Prior Kidney Transplantation, N | 12 (8.9%) | 26 (12.0%) | 0.38 |
| Antithymocyte Globulin Induction, N | 115 (85.2%) | 188 (87.0%) | 0.64 |
| Corticosteroid Maintenanceb, N | 23 (17.0%) | 50 (23.2%) | 0.18 |
| 25(OH)D (ng/mL) (mean±SD) | 31.2±9.6 | 13.3±4.2 | |
| Winter 25(OH)Dc (ng/mL) (mean±SD) | 32.6±11.2 | 13.6±4.0 | |
| Summer 25(OH)Dd (ng/mL) (mean±SD) | 30.2±7.9 | 13.0±4.3 | |
| Winter 25(OH)D Measurement, N | 60 (44.4%) | 85 (39.4%) | 0.37 |
| Summer 25(OH)D Measurement, N | 75 (55.6%) | 131 (60.7%) | 0.37 |
| Serum Calcium (mg/dL) (mean±SD) | 9.2±0.9 | 9.0±1.0 | 0.06 |
| Serum Phosphorus (mg/dL) (mean±SD) | 2.6±1.1 | 2.7±1.1 | 0.63 |
| Serum Alkaline Phosphatase (IU/L) (mean±SD) | 112±73 | 123±104 | 0.96 |
| Serum PTHe (pg/mL) (mean±SD) | 238±272 | 321±368 | <0.001 |
Wilcoxon-Rank Sum test was used to compare continuous variables and Fisher’s Exact test was used to compare categorical variables.
Kidney graft recipients maintained on corticosteroid immunosupression after post transplantation day 4.
Winter 25(OH)D measurement was defined as any measurement that occurred from the Southward equinox (September 23) to the Northward equinox (March 20).
Summer 25(OH)D measurement was defined as any measurement that occurred from the Northward equinox (March 21) to the Southward equinox (September 22).
PTH values were available in 132 of 135 in the vitamin D Sufficient Group and in 203 of 216 in the vitamin D Deficient Group.
Serum PTH levels were significantly higher in the vitamin D deficient group compared to the sufficient group (321±368 vs. 238±272 pg/mL, P<0.001, Wilcoxon-Rank Sum test), The vitamin D sufficient group had a marginally higher serum calcium (9.2±0.9 vs. 9.0±1.0, P=0.06) than the deficient group.
Age at the time of transplantation, organ donor type (deceased donor vs. living donor), history of prior kidney transplantation, timing of 25(OH)D measurement (winter vs. summer), serum phosphorus, and serum alkaline phosphatase were not associated with vitamin D status (Table 1B).
Among the 351 kidney graft recipients, 303 received anti-thymocyte induction therapy while the remaining 47 patients receiving anti-CD25 monoclonal antibody therapy (1 patient received alefacept induction); 73 patients received maintenance corticosteroids while 271 did not receive maintenance corticosteroids (corticosteroids stopped on day 4 post-transplantation). The vitamin D deficient patients were not significantly different from the vitamin D sufficient patients in terms of anti-thymocyte induction therapy or corticosteroid maintenance therapy (Table 1B).
Vitamin D Status and Biopsy Confirmed Acute Cellular Rejection
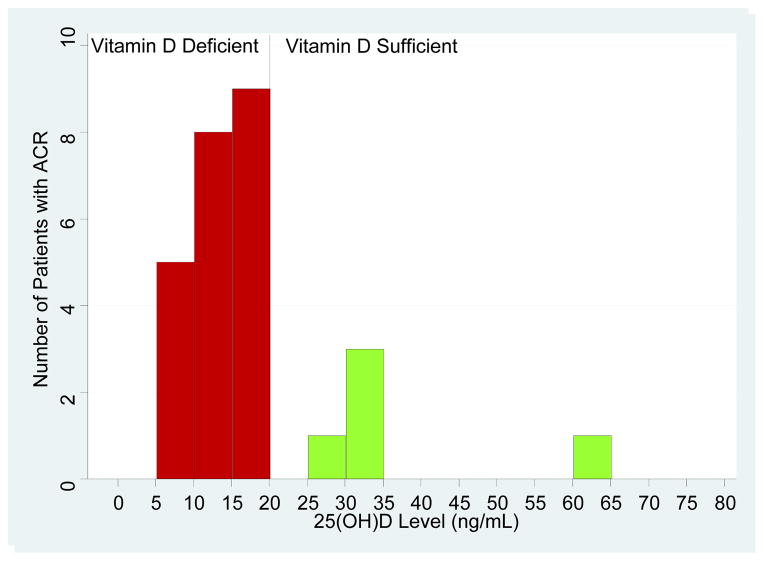
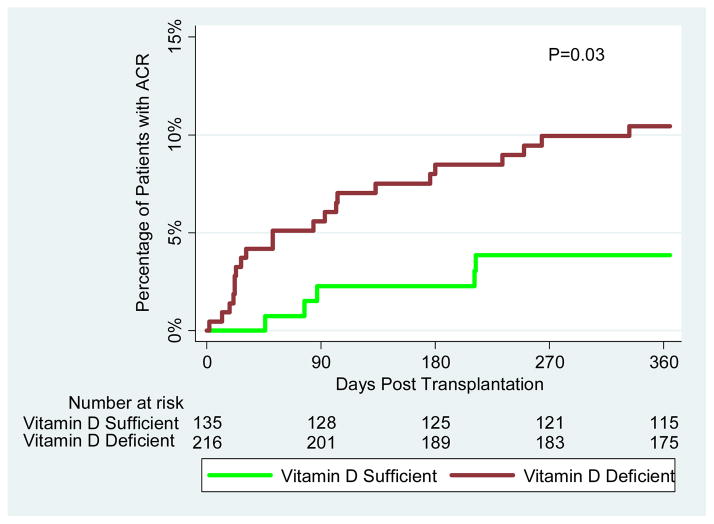
Biopsy confirmed acute cellular rejection (ACR) within the first year of kidney transplantation occurred in 27 (7.7%) of the 351 kidney graft recipients evaluated for vitamin D status. ACR was significantly more frequent in the vitamin D deficient group (N=22, 10.2%) than in the sufficient group (N=5, 3.7%) (P=0.04, Fisher’s Exact Test). The number of patients with ACR by circulating levels of 25(OH)D intervals is shown in Figure 2A. Kaplan-Meier analysis of time to development of ACR demonstrated that patients with vitamin D deficiency were at greater risk of developing ACR within the first year of transplantation compared to those without the deficiency (Figure 2B; log rank test: P=0.03).
Figure 2. Vitamin D Status and Acute Cellular Rejection.
(A) Each bar on the X-axis represents an interval of 5 ng/mL of circulating levels of 25(OH)D and the levels ranges from 0 ng/mL to 80 ng/mL. The y-axis is the number of patient who developed ACR in each cohort represented by the corresponding interval. In the first 12-months of transplantation, twenty two of the 216 vitamin D deficient patients (10.2%) experienced an episode of biopsy conformed ACR and 5 (3.7%) of the 135 vitamin D sufficient patients developed ACR (P=0.04 by Exact Fisher’s Test). (B) The red curve represents vitamin D deficient patients (circulating levels of 25(OH)D, ≤20 ng/mL) and the green curve represents vitamin D sufficient patients (circulating levels of 25(OH)D, >20 ng/mL). The number of days post-transplantation is represented on the x-axis and the proportion of patients with ACR is represented on the y-axis. The number of kidney graft recipients at risk for ACR is provided below the corresponding time points. The Kaplan-Meier curves were compared using a log-rank test.
Independent variables that were associated with development of ACR using univariate Cox Regression analysis (P<0.10) included vitamin D deficiency, antithymocyte globulin induction therapy, deceased donor kidney transplantation, and female gender. On the other hand, African American race, preemptive transplantation, prior transplantation, corticosteroid maintenance, and PTH levels were not associated with ACR in the first year after transplantation (Table 2A). After adjustment for variables with a P<0.10, the multivariable analysis confirmed that vitamin D deficiency was an independent risk factor for ACR (HR: 3.3, 95% confidence intervals (CI): 1.2–8.7, P=0.02). The use of anti-thymocyte globulin induction therapy was associated with a reduced risk of ACR and deceased donor transplantation with an increased risk, as aniticipated (Table 2A).
Table 2A.
Multivariable Cox Regression Analysis for the Risk Factors Associated with ACR
| Characteristic | N (%) | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Vitamin D Deficiency (25(OH)D levels ≤20 ng/mL) | 216 (61.5%) | 2.84 | 1.08–7.51 | 0.04 | 3.26 | 1.23–8.65 | 0.02 |
| Age (per year) | 351 (100.0%) | 1.00 | 0.97–1.02 | 0.75 | |||
| Female Gender | 130 (37.0%) | 0.47 | 0.19–1.16 | 0.09 | 0.45 | 0.18–1.12 | 0.09 |
| African American Race | 78 (22.2%) | 1.23 | 0.52–2.90 | 0.64 | |||
| Deceased Donor Transplantation | 177 (50.4%) | 2.00 | 0.90–4.45 | 0.09 | 2.31 | 1.02–5.22 | 0.05 |
| Preemptive Transplantation | 78 (22.2%) | 0.77 | 0.29–2.04 | 0.60 | |||
| Prior Transplantation | 38 (10.8%) | 1.98 | 0.75–5.22 | 0.17 | |||
| Antithymocyte Globulin Induction | 303 (86.3%) | 0.44 | 0.19–1.04 | 0.06 | 0.39 | 0.16–0.94 | 0.04 |
| Corticosteroid Maintenance | 73 (20.8%) | 0.86 | 0.32–2.26 | 0.75 | |||
| PTH (per 10 ng/mL)a | 335 (95.5%) | 1.00 | 0.99–1.01 | 0.88 | |||
Multivariable Cox regression was used to determine whether vitamin D deficiency was associated with ACR within the first year of transplantation. Characteristics associated with ACR in a univariate Cox regression analysis (P<0.10) were included in the multivariable analysis for the identification of independent risk factors associated with ACR. Hazard ratios (HR) with 95% confidence interval (CI) and P value are reported. Highlighted in bold are the variables associated with ACR with a P value of less than 0.10.
PTH was available only in 335 transplant recipients. Univariate analysis was performed for this factor based on the 335 transplant recipients.
Vitamin D Supplementation and ACR in the Vitamin D Deficient Group
The decision to treat patients with Vitamin D and the choice of Vitamin D preparation prescribed were determined by each patients’ treating physicians and were not standardized in this cohort. Vitamin D therapy was initiated within the first 90 days of transplantation in 133 of 216 vitamin D deficient patients and was not initiated in the remaining 83 patients (38%). Among these 133 given vitamin D supplementation, 28 patients received 1,25(OH)2D3 alone, 50 received 1,25(OH)2D3 and 25(OH)D, and 55 received 25(OH)D supplementation alone; 83 received neither 1,25(OH)2D3 nor 25(OH)D supplementation during the first 90 days of transplantation.
Among the 78 patients who received 1,25(OH)2D3, the incidence of ACR during the first year of transplantation was 5.1% compared with 14.5% in the 55 patients who received 25(OH)D only and 12.0% in the 83 patients who received neither 1,25(OH)2D3 nor 25(OH)D. Thus, in the 25(OH)D deficient group, exposure to 1,25(OH)2D3 was associated with a lower rate of ACR compared to those not treated with 1,25(OH)2D3 (5.1% vs. 13.0%, P=0.099). In the 78 patients who received 1,25(OH) 2D3, all 4 patients who developed ACR did receive 1,25(OH) 2D3 prior to the ACR event.
Table 2B shows the impact of supplementation with 1,25(OH)2D3 on the risk of ACR. In complete agreement with the notion that vitamin D deficiency is a risk factor for ACR, the hazard ratio for ACR increased from 3.3 (95% CI, 1.2 to 8.7, P=0.02) observed in the entire group of vitamin D deficient patients to 4.4 (95% CI: 1.6–11.8, P=0.004) in the subset of vitamin D deficient not treated with 1,25(OH)2D3 and the hazard ratio decreased to 1.5 (95% CI, 0.4–5.8, P=0.52) in the subset of vitamin D deficient treated with 1,25(OH)2D3.
Table 2B.
Multivariable Cox Regression Analysis for Development of ACR with Vitamin D Deficient Patients Stratified by Treatment With or Without 1,25(OH)2D3
| Characteristic | N (%) | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Vit D Group | |||||||
| Vit D Sufficient (Reference) | 135 (38.5%) | 1.00 | ------------ | ------ | 1.00 | ------------ | ------ |
| Vit D Deficient and no 1,25 Txa | 138 (39.3%) | 3.67 | 1.36–9.88 | 0.01 | 4.36 | 1.61–11.8 | 0.004 |
| Vit D Deficient and 1,25 Txb | 78 (22.2%) | 1.42 | 0.38–5.27 | 0.61 | 1.54 | 0.41–5.75 | 0.52 |
| Age (per year) | 351 (100.0%) | 1.00 | 0.97–1.02 | 0.75 | |||
| Female Gender | 130 (37.0%) | 0.47 | 0.19–1.16 | 0.09 | 0.44 | 0.18–1.11 | 0.08 |
| African American Race | 78 (22.2%) | 1.23 | 0.52–2.90 | 0.64 | |||
| Deceased Donor Transplantation | 177 (50.4%) | 2.00 | 0.90–4.45 | 0.09 | 2.48 | 1.09–5.60 | 0.03 |
| Preemptive Transplantation | 78 (22.2%) | 0.77 | 0.29–2.04 | 0.60 | |||
| Prior Transplantation | 38 (10.8%) | 1.98 | 0.75–5.22 | 0.17 | |||
| Antithymocyte Globulin Induction | 303 (86.3%) | 0.44 | 0.19–1.04 | 0.06 | 0.40 | 0.17–0.97 | 0.04 |
| Corticosteroid Maintenance | 73 (20.8%) | 0.86 | 0.32–2.26 | 0.75 | |||
| PTH (per 10 ng/mL)c | 335 (95.5%) | 1.00 | 0.99–1.01 | 0.88 | |||
Multivariable Cox regression was used to determine whether vitamin D deficiency was associated with ACR within the first year of transplantation. Characteristics associated with ACR in a univariate Cox regression analysis (P<0.10) were included in the multivariable analysis for the identification of independent risk factors associated with ACR. Hazard ratios (HR) with 95% confidence interval (CI) and P value are reported. Highlighted in bold are the variables associated with ACR with a P value of less than 0.10.
Vit D Deficient and no 1,25 Tx represents the graft recipients who are Vitamin D deficient and did not receive 1,25(OH)2D3 supplementation.
Vit D Deficient and 1,25 Tx represents the graft recipients who are Vitamin D deficient and received 1,25(OH)2D3 supplementation.
PTH was available only in 335 transplant recipients. Univariate analysis was performed for this factor based on the 335 transplant recipients.
1,25(OH)2D3 supplementation was also prescribed in 37 of the 135 vitamin D sufficient patients. Only 5 patients in the vitamin D sufficient group developed ACR and this low incidence of ACR precluded meaningful analysis of the impact of active vitamin D supplementation in the vitamin D sufficient cohort.
Vitamin D Status and Post-Transplant Viral Infections
The incidence of CMV disease and BK virus associated nephropathy during the first year of transplantation was similar in the vitamin D deficient group and the sufficient group. CMV disease occurred in 13 patients (6.0%) in the deficient group compared to 8 patients (5.9%) in the sufficient group during the first year of transplantation (P=0.99). When stratified by CMV donor/recipient antibody status, there was no significant difference in CMV incidence between the vitamin D deficient group and the vitamin D sufficient group (Supplemental Digital Content [SDC] Table S1).
Our center utilizes a CMV prophylaxis strategy rather than a CMV screening strategy and 345 of the 351 transplant recipients received valgancyclovir prophylaxis during the first 6 months of transplantation and only 6 transplant recipients received acyclovir. The almost universal use of CMV prophylaxis precluded meaningful analysis of the association between prophylaxis and ACR.
BK virus associated nephropathy occurred in 3 (1.4%) in the deficient group compared to 2 (1.5%) in the sufficient group during the first year of transplantation (P=0.99). Two hundred and forty-six of 351 transplant recipients had a BK virus level measured during the first year of transplantation. BK viremia based on a definition of >1000 copies/mL was not significantly different between those that were 25(OH)D sufficient and those that were 25(OH)D deficient (18.6% vs. 13.1%, P=0.23).
Vitamin D Status and Allograft Function
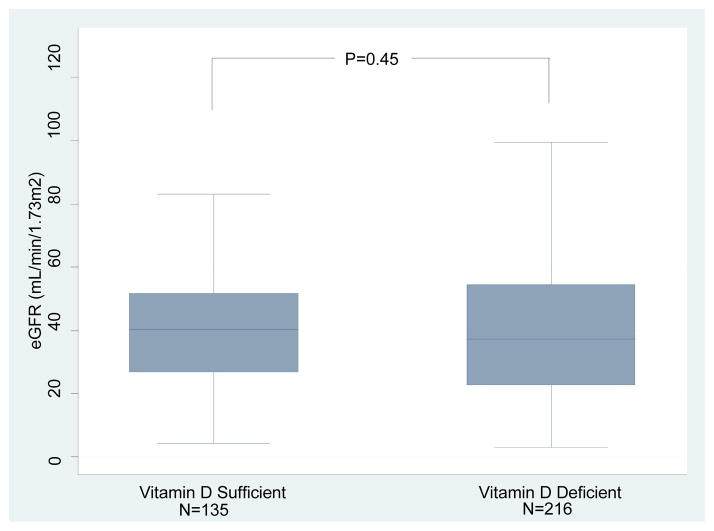
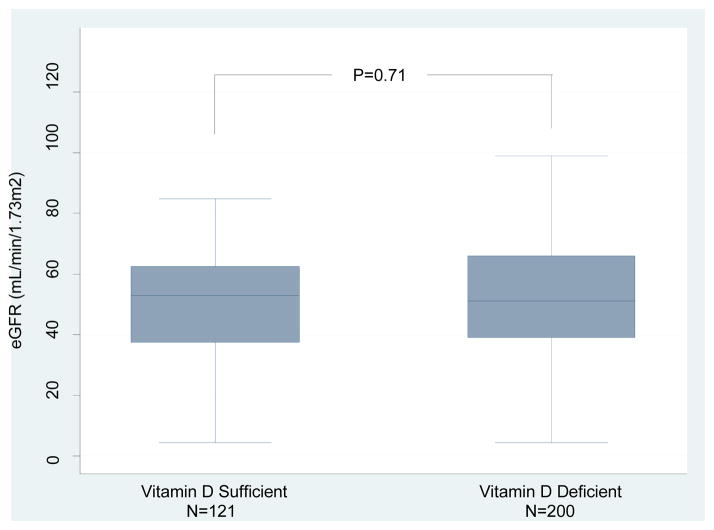
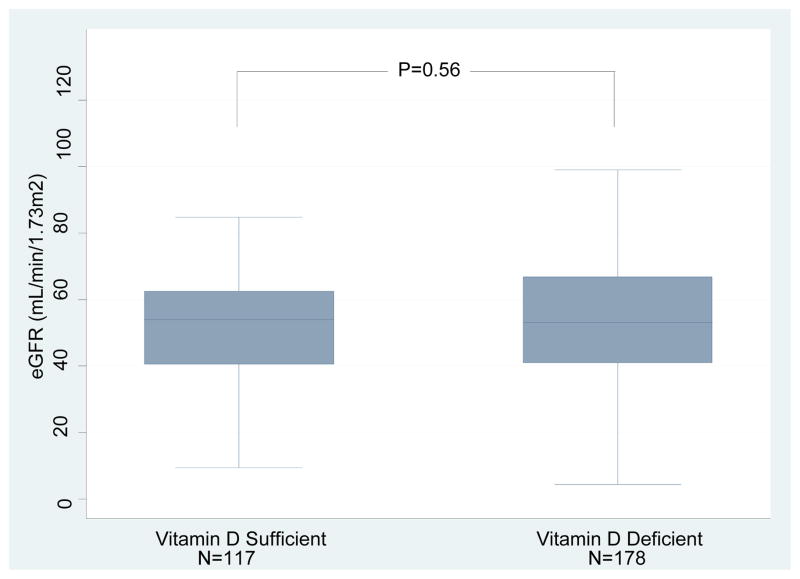
Box and whisker plots of the eGFR measured at the time of measurement of 25(OH)D levels in the vitamin D sufficient or deficient group are shown in Figure 3A; the mean (±SD) eGFR was 39.8±17.8 mL/min/1.73m2 in the sufficient group and 39.3±21.8 mL/min/1.73m2 in the deficient group (P=0.45, Wilcoxon-Rank Sum test). Serum creatinine levels at 1 year after transplantation were available in 321 of the 351 kidney graft recipients evaluated for vitamin D status. At 1 year post-transplantation, eGFR was 50.9±18.5 mL/min/1.73m2 in the vitamin D sufficient group and 52.6±21.7 mL/min/1.73m2 in the deficient group at 1 year (P=0.71, Figure 3B). We repeated the analysis after excluding the kidney graft recipients who had an episode of ACR within 12 months of transplantation. eGFRs was again not significantly different between the vitamin D sufficient group and the deficient group (52.1±17.4 mL/min/m2 vs. 53.9±21.7 mL/min/m2, respectively, P=0.56) (Figure 3C).
Figure 3. Box and Whisker Plots of eGFR in kidney graft recipients classified as 25(OH)D sufficient or deficient.
(A) Box and Whisker Plots of eGFR (mL/min/1.73m2) at time of 25(OH)D measurement for the vitamin D sufficient group and vitamin D deficient group. (B) Box and Whisker Plots of eGFR (mL/min/1.73m2) at 1 year for the vitamin D sufficient group and vitamin D deficient group (N=321 out of 351 with available 1 year eGFR). (C) Box and Whisker Plots of eGFR (mL/min/1.73m2) at 1 year for the vitamin D sufficient group and vitamin D deficient group (N=295 out of 351 with available 1 year eGFR and without development of ACR within the first year). In each box plot, the horizontal line represents the median with the edges of the box representing the 25th and 75th percentiles. The top whisker represents the 75th percentile value plus 1.5 times the interquartile range and the bottom whisker represents the 25th percentile value minus 1.5 times the interquartile range. P values were calculated using the Wilcoxon-Rank Sum test.
DISCUSSION
Using the Endocrine Society Clinical Practice guidelines to characterize vitamin D deficiency, we found that a circulating level of 25(OH)D less than 20ng/mL is an independent risk factor for biopsy confirmed ACR in kidney graft recipients in the first year post transplantation even after controlling for timing of follow up and important potential confounders such as type of induction therapy, donor organ type, and gender.
Our demonstration that vitamin D deficiency is associated with ACR confirms and extends earlier reports. Ma and colleagues performed a cross sectional study of 94 kidney graft recipients and reported that patients who had a history of acute rejection had a significantly lower level of 25(OH)D than those who did not have a history of acute rejection (14). Kim et al. examined the 25(OH)D levels prior to kidney transplantation and reported that patients with 25(OH)D deficiency, defined as less than 10 ng/mL, had a higher incidence of biopsy proven acute rejections than those with levels greater than 10ng/mL (11). Different cutoff points for vitamin D deficiency were used in these studies, and the impact of important confounders such as therapy, donor organ type, and age were not addressed.
The association between 25(OH)D levels and ACR has been noted in recipients of organs other than kidneys. In a study of 133 liver transplant recipients, pre-transplant circulating levels less than 5 ng/mL of 25(OH)D levels were independently associated with moderate-to-severe ACR episodes within 2 months post transplantation (15). In a study of 102 lung transplant recipients, those with 25(OH) D levels less than 30ng/mL had more episodes of ACR and more aggressive ACR during the first year post transplantation than those with levels greater than 30ng/mL (16).
The clinical impact of vitamin D supplementation in clinical transplantation has not been clearly established. Courbebaisse and colleagues retrospectively studied 64 kidney transplant recipients with 25(OH)D levels less than 30 ng/mL who were treated with 25(OH)D at 3 months post transplantation and did not find an association between 25(OH)D treatment and acute rejection or allograft function at 1 year (17). A case control study of 26 transplant recipients treated with calcitriol and 50 transplant recipients not treated with calcitriol demonstrated improved graft survival and a trend towards fewer acute rejection episodes in the calcitriol treated group (18). Sezer and colleagues reported that transplant recipients (n=57) who were treated with calcitriol for osteoporosis had improved renal function and a lower requirement for pulse dose steroids at 2 years compared to untreated control patients (n=53) (19).
Our study included 216 patients with vitamin D deficiency and who received either no vitamin D treatment; 25(OH)D treatment alone; or 1,25(OH) 2D3 with or without 25(OH)D within the first 90 days of transplantation. Interestingly, we found that those who were treated with 1,25(OH)2D3 supplementation initiated within the first 90 days of transplantation had the lowest incidence of ACR compared to those who did not receive 1,25(OH)2D3 supplementation. It should be noted, however, that treatment with active vitamin D consisted of calcitriol, doxecalciferol, or hectoral and it is possible that the type of 1,25(OH)2D3 administered may have differential immunological effects. Nevertheless, our observations that the hazard ratio for ACR increased from 3.3 observed in the entire group of vitamin D deficient patients to 4.4 in the subset of vitamin D deficient not treated with 1,25(OH)2D3 and the hazard ratio decreased to 1.5 in the subset of vitamin D deficient treated with 1,25(OH)2D3 is in accord with vitamin D deficiency being a risk factor for ACR and consistent with experimental evidence that 1,25(OH)2D3 prolongs allograft survival in a number of experimental models of transplantation (2–5). Possible mechanisms for the allograft protective role of 1,25(OH)2D3 include: decreasing T cell production of inflammatory mediators such as IL-2 and interferon-γ (6, 7), downregulating the immunogenic activity of dendritic cells activity (20), and upregulating the immunoprotective activity of T regulatory cells (9, 10).
Earlier studies have also linked 25(OH)D deficiency to viral as well as bacterial infections in the general population (Reviewed in(1, 21)). The impact of vitamin D deficiency on infections in organ graft recipients, however, has not been fully characterized. In the current study, we did not find a significant association between vitamin D deficiency and two common post-transplant diseases - CMV disease and BK virus nephropathy. Our analysis of the relationship between vitamin D status and CMV disease and BK virus nephropathy, however, must be interpreted with caution in view of the relatively small number of events in our study cohort.
The mechanism for the association of vitamin D status and kidney graft function is not known. A large, single center study of 634 kidney graft recipients found that circulating levels less than 15 ng/mL of 25(OH)D at 3 months post transplantation is an independent risk factor for lower measured GFR and interstitial fibrosis and tubular atrophy at 1 year post transplantation (12). We did not find an association between vitamin D deficiency and 1 year allograft function as some retrospective studies have found (11, 12).
The strengths of our study design include the measurement of 25(OH)D status within the first month of transplantation, the use of a pre-specified definition of vitamin D deficiency (circulating levels of 25(OH)D less than 20 ng/mL) as defined by the Endocrine Society, and the availability of 1 year follow up data on all patients. Limitations of our study include the lack of serial 25(OH)D levels which may have fluctuated during the first year of transplantation, the potential for unmeasured confounders of the relationship between vitamin D status and renal function/allograft status, and the use of eGFR rather than iohexal or inulin to measure GFR. Because only 351 of the 1211 transplant recipients from January 2005 to December 2010 had measured 25(OH)D levels for analysis and the remaining 860 did not, a selection bias may have existed within our study. It should be noted, however, that there are no evidence-based guidelines for screening patients for vitamin D status in the post-transplantation period, and screening at the current time is primarily physician’s choice. The findings from this study that vitamin D deficiency is associated with ACR, and that supplementation with active vitamin D may reduce this risk suggests that issues related to screening vitamin D levels in the post-transplantation period and treatment in those found to be vitamin D deficient require evaluation in properly designed clinical trials. Additionally, the incidence of ACR was lower in the 860 patients without vitamin D measurement in the 30 days of transplantation compared to the 351 patients with vitamin D measurement during the same time interval. The basis for the lower incidence is difficult to elucidate in part due to retrospective cohort research design. It should be noted, however, that the 7.7% incidence of ACR in the group with vitamin D measurement is not higher than the incidence reported for recipients of kidney allografts in the US (22).
Despite these limitations, our observations, together, support the conduct of appropriately designed clinical trials to investigate the role of vitamin D supplementation in kidney graft recipients.
METHODS
Among the 1211 patients who received a kidney transplantation at the New York Presbyterian Hospital – Weill Cornell Medical Center during the period of January 2005 to December 2010, we identified 351 patients who had circulating levels of 25(OH)D measured within the first 30 days of transplantation and performed a retrospective cohort study to assess the impact of vitamin D status on renal allograft outcomes. The Institutional Review Board approved this study.
Vitamin D Measurement and Status
25(OH)D levels were measured using DiaSorin Liaison® quantitative chemiluminescent immunoassay (ARUP laboratories, Salt Lake City, UT). 25(OH)D deficiency was defined as a circulating level ≤20 ng/mL, and 25(OH)D sufficiency was defined as a level > 20 ng/mL, based on the Endocrine Society Clinical Practice guidelines (13). 25(OH)D supplementation was defined as 25(OH)D supplementation in the form of ergocalciferol or cholecalciferol initiated by the transplant physician within the first 90 days of transplantation. Among the 78 patients who received 1,25(OH)2D3, 65% received calcitriol, 27% received paricalcitol, and 8% received doxercalciferol. Despite this heterogeneity, we analyzed them as a single group of 1,25(OH)2D3 treated patients although it is possible that individual analogs may have differential benefits.
The median time to 25(OH)D supplementation was 22 days (IQR, from 14 to 35 days) and the median time to 1,25(OH)2D3 supplementation was 15 days (IQR, from 7 to 31 days). The no vitamin D supplementation group had no record of receiving either 25(OH)D or 1,25(OH)2D3 within the first 90 days of transplantation. Type of multivitamin use or diet was not routinely documented and was not included in the analysis.
Clinical Course and Outcomes
The kidney graft recipients were managed with the use of our center protocol. The recipients received induction therapy in the form of either antithymocyte globulin or IL-2 receptor antibody therapy, and received maintenance immunosuppressive therapy comprised of tacrolimus and mycophenolate mofetil or mycophenolic acid, with or without maintenance corticosteroids. ACR was biopsy confirmed in each instance.
Estimated GFR
eGFR at the time of vitamin D measurement and at 1 year post transplantation were calculated using the 4 variable MDRD equation. The value of eGFR at 1 year was determined using the creatinine measurement closest to 365 days post transplantation. eGFRs measured more than 90 days from day 365 post transplantation were excluded from the analysis.
Infections
Transplant recipient’s records were reviewed for occurrence of CMV disease and BK virus nephropathy (BKVAN) during the first year of transplantation. BKVAN diagnosis was based on kidney allograft biopsy showing SV40 positive staining. CMV disease diagnosis was based on a positive blood PCR or pp65 antigenemia and evidence of organ involvement. BK viremia was defined as greater than 1000 copies of BK virus DNA per mL of plasma.
Statistical Analysis
Categorical variables were compared using Fisher’s Exact tests, and continuous variables were compared using Wilcoxon Rank-Sum tests. Kaplan-Meier curves were constructed and compared using a log-rank test. To evaluate the risk factors associated with ACR, univariate Cox regression analysis was performed. Variables with a P value of <0.1 were included in a multivariable Cox proportional hazards regression to identify independent risk factors for ACR. Statistical analyses were performed using STATA 12.0 I/C (Statacorp, College Station, TX). For all analyses, we performed 2-sided tests and confidence intervals with type I error of 5%.
Supplementary Material
Acknowledgments
We thank all our colleagues at the Division of Nephrology and Hypertension, Division of Transplant Surgery, and Department of Transplantation Medicine and thank our patients at New York Presbyterian Hospital – Weill Cornell Medical Center and the Rogosin Institute for their kind help in conducting this study.
J.L. is a recipient of a KL2 Scholars Award from the Weill Cornell Clinical and Translational Science Center. T.M. is a recipient of a career development K08-DK087824 from the National Institutes of Diabetic and Digestive and Kidney Diseases. This publication has been supported in part by the awards: KL2-TR-000458 (J.L.) from the National Center for Advancing Translational Sciences, National Institutes of Health, through the Weill Cornell Clinical and Translational Science Center, K08-DK087824 (T.M.) from the National Institutes of Diabetic and Digestive and Kidney Diseases, National Institutes of Health, and 2R37AI051652 (M.S.) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- 1,25(OH)2D3
1,25-dihydroxyvitamin D3
- ACR
Acute cellular rejection
- eGFR
estimated glomerular filtration rate as measured by the MDRD 4 variable equation
Footnotes
Author Contributions:
JL: Research design, data analyses, and writing of the manuscript. No conflict of interest.
DD: Research design. No conflict of interest.
PA: Research design and writing of the manuscript. No conflict of interest.
JL: Research design. No conflict of interest.
MS: Research design, data analyses, and writing of the manuscript. No conflict of interest.
TM: Research design, data analyses, and writing of the manuscript. No conflict of interest.
Parts of the information reported in this article were presented as an abstract at the American Transplant Congress 2013.
References
- 1.Pludowski P, Holick MF, Pilz S, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-A review of recent evidence. Autoimmun Rev. 2013 doi: 10.1016/j.autrev.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Redaelli CA, Wagner M, Gunter-Duwe D, et al. 1alpha,25-dihydroxyvitamin D3 shows strong and additive immunomodulatory effects with cyclosporine A in rat renal allotransplants. Kidney Int. 2002;61 (1):288. doi: 10.1046/j.1523-1755.2002.00101.x. [DOI] [PubMed] [Google Scholar]
- 3.Hullett DA, Laeseke PF, Malin G, Nessel R, Sollinger HW, Becker BN. Prevention of chronic allograft nephropathy with vitamin D. Transpl Int. 2005;18 (10):1175. doi: 10.1111/j.1432-2277.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang AB, Zheng SS, Jia CK, Wang Y. Role of 1,25-dihydroxyvitamin D3 in preventing acute rejection of allograft following rat orthotopic liver transplantation. Chin Med J (Engl) 2004;117 (3):408. [PubMed] [Google Scholar]
- 5.Redaelli CA, Wagner M, Tien YH, et al. 1 alpha,25-Dihydroxycholecalciferol reduces rejection and improves survival in rat liver allografts. Hepatology. 2001;34 (5):926. doi: 10.1053/jhep.2001.28705. [DOI] [PubMed] [Google Scholar]
- 6.Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol) J Clin Invest. 1984;74 (4):1451. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reichel H, Koeffler HP, Tobler A, Norman AW. 1 alpha,25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1987;84 (10):3385. doi: 10.1073/pnas.84.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmadpoor P, Ilkhanizadeh B, Ghasemmahdi L, Makhdoomi K, Ghafari A. Effect of active vitamin D on expression of co-stimulatory molecules and HLA-DR in renal transplant recipients. Exp Clin Transplant. 2009;7 (2):99. [PubMed] [Google Scholar]
- 9.Ardalan MR, Maljaei H, Shoja MM, et al. Calcitriol started in the donor, expands the population of CD4+CD25+ T cells in renal transplant recipients. Transplant Proc. 2007;39 (4):951. doi: 10.1016/j.transproceed.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Gorman S, Judge MA, Hart PH. Topical 1,25-dihydroxyvitamin D3 subverts the priming ability of draining lymph node dendritic cells. Immunology. 2010;131 (3):415. doi: 10.1111/j.1365-2567.2010.03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H, Kang SW, Yoo TH, et al. The impact of pretransplant 25-hydroxy vitamin D deficiency on subsequent graft function: an observational study. BMC Nephrol. 2012;13:22. doi: 10.1186/1471-2369-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bienaime F, Girard D, Anglicheau D, et al. Vitamin d status and outcomes after renal transplantation. J Am Soc Nephrol. 2013;24 (5):831. doi: 10.1681/ASN.2012060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96 (7):1911. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 14.Ma MK, Mok MM, Yung S, Tang CS, Chan TM. High prevalence of vitamin D insufficiency in southern Chinese renal transplant recipients. Ren Fail. 2012;34 (8):980. doi: 10.3109/0886022X.2012.706878. [DOI] [PubMed] [Google Scholar]
- 15.Bitetto D, Fabris C, Falleti E, et al. Vitamin D and the risk of acute allograft rejection following human liver transplantation. Liver Int. 2010;30 (3):417. doi: 10.1111/j.1478-3231.2009.02154.x. [DOI] [PubMed] [Google Scholar]
- 16.Lowery EM, Bemiss B, Cascino T, et al. Low vitamin D levels are associated with increased rejection and infections after lung transplantation. J Heart Lung Transplant. 2012;31 (7):700. doi: 10.1016/j.healun.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Courbebaisse M, Xu-Dubois YC, Thervet E, et al. Cholecalciferol supplementation does not protect against renal allograft structural and functional deterioration: a retrospective study. Transplantation. 2011;91 (2):207. doi: 10.1097/TP.0b013e318200ba37. [DOI] [PubMed] [Google Scholar]
- 18.O’Herrin JK, Hullett DA, Heisey DM, Sollinger HW, Becker BN. A retrospective evaluation of 1,25-dihydroxyvitamin D(3) and its potential effects on renal allograft function. Am J Nephrol. 2002;22 (5–6):515. doi: 10.1159/000065289. [DOI] [PubMed] [Google Scholar]
- 19.Sezer S, Uyar M, Arat Z, Ozdemir FN, Haberal M. Potential effects of 1,25-dihydroxyvitamin D3 in renal transplant recipients. Transplant Proc. 2005;37 (7):3109. doi: 10.1016/j.transproceed.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira GB, van Etten E, Verstuyf A, et al. 1,25-Dihydroxyvitamin D3 alters murine dendritic cell behaviour in vitro and in vivo. Diabetes Metab Res Rev. 2011;27 (8):933. doi: 10.1002/dmrr.1275. [DOI] [PubMed] [Google Scholar]
- 21.Lang PO, Samaras N, Samaras D, Aspinall R. How important is vitamin D in preventing infections? Osteoporos Int. 2013;24 (5):1537. doi: 10.1007/s00198-012-2204-6. [DOI] [PubMed] [Google Scholar]
- 22.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2011 Annual Data Report: kidney. Am J Transplant. 2013;13 (Suppl 1):11. doi: 10.1111/ajt.12019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.