Abstract
In the article entitled “Imaging Recommendations for Acute Stroke and Transient Ischemic Attack Patients: A Joint Statement by the American Society of Neuroradiology, the American College of Radiology and the Society of NeuroInterventional Surgery”, we are proposing a simple, pragmatic approach that will allow the reader to develop an optimal imaging algorithm for stroke patients at their institution.
Keywords: Stroke, thrombolysis, computed tomography, magnetic resonance imaging, catheter angiography
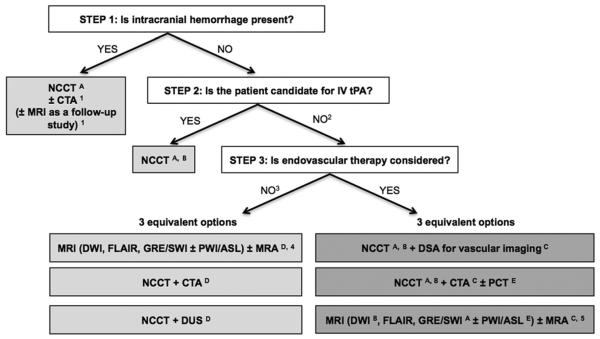
Stroke is a leading cause of death and disability worldwide. Imaging plays a critical role in evaluating patients suspected of having acute stroke and transient ischemic attack (TIA), especially before initiating treatment. Over the past few decades, major advances have occurred in stroke imaging and treatment, including FDA approval of recanalization therapies for treatment of acute ischemic stroke. A wide variety of imaging techniques have become available to assess vascular lesions and brain tissue status in patients with acute stroke. However, the practical challenge for physicians is to understand the multiple facets of these imaging techniques, including which imaging techniques to implement and how to optimally use them, given available resources at their local institutions. Important considerations include constraints of time and cost, access to imaging modalities, preferences of treating physicians, availability of expertise, and availability of endovascular therapy. The choice of which imaging techniques to use is influenced by both the time urgency for patient evaluation and the complexity of the literature on acute stroke imaging. Ideally, imaging algorithms should incorporate techniques that provide optimal benefit for patient outcomes without delaying treatment. Therefore, it is most practical and efficient to use a standardized imaging approach, with all relevant imaging studies conducted in as few sessions as possible (Fig. 1).
Fig 1.
Suggested imaging protocols for patients presenting with acute stroke symptoms on the basis of the clinical scenario and the therapeutic options available. Each of the gray boxes represents one imaging strategy. So as not to delay treatment, a standardized imaging approach should be used: one imaging strategy (gray box) should be selected, and all imaging studies belonging to this strategy should be performed up front in as few sessions as possible. 1To assess the etiology of the intracranial hemorrhage (CT angiography [CTA] for vascular pathologies, such as aneurysms, arteriovenous malformations, vasculopathies; MRI for vascular malformations and neoplastic and other pathologies associated with hemorrhage). 2Also if the patient is not a candidate for intravenous (IV) tissue plasminogen activator (tPA) (contraindication to tPA, outside time window for tPA) or if IV tPA failed or it is thought that it might fail. 3For patients who are outside the time window for acute reperfusion therapies (>4.5 hours at sites at which only IV tPA is being considered; >8 hours at sites at which endovascular therapy is considered) and for patients with transient ischemic attacks (TIAs), emphasis is on secondary prevention, and their imaging workup should be focused on vascular imaging (CTA, MR angiography [MRA], or doppler ultrasound [DUS]) to assess the carotid arteries as a possible cause of the ischemic stroke, with secondary prevention in mind. If MRA is performed, it makes sense to concurrently obtain MRI with diffusion-weighted imaging (DWI), fluid-attenuated inversion recovery (FLAIR), and gradient-recalled echo (GRE) or susceptibility-weighted imaging (SWI). Echocardiography should also be performed to assess for cardiac sources. 4If available, MRI or MRA is the preferred imaging modality for patients with TIA. 5At institutions at which MRI is available 24/7 and can be performed within a short time after admission. A: to assess for intracranial hemorrhage; B: to assess the extent of ischemic core; C: to assess the location and extent of the intravascular clot; D: to assess carotid atherosclerotic disease; E: to assess the extent of viable tissue. ASL = arterial spin-labeled; NCCT = noncontrast CT; PCT = perfusion CT; PWI = perfusion-weighted imaging.
IMAGING PATIENTS PRESENTING WITH ACUTE STROKE SYMPTOMS
The initial step in the evaluation of patients with symptoms of acute stroke is to differentiate between hemorrhagic and ischemic stroke (Fig. 1). For patients with acute ischemic stroke who are candidates for intravenous (IV) tissue plasminogen activator (tPA), noncontrast CT (NCCT) of the head should be performed to determine eligibility for treatment. IV tPA can then be initiated without waiting for further imaging. In patients under consideration for endovascular therapy, 3 imaging options may be used: (1) NCCT followed immediately by digital subtraction angiography for vascular assessment, (2) NCCT plus CT angiography (CTA) with or without perfusion CT, or (3) MRI plus MR angiography (MRA) with or without perfusion MR at institutions that can offer MRI 24/7 without delaying treatment. In patients who are not candidates for IV or endovascular therapy, and in patients with TIAs, vascular imaging is recommended to guide management for secondary prevention of future stroke.
IMAGING PATIENTS WITH INTRACRANIAL HEMORRHAGE
If intraparenchymal hemorrhage is present, as occurs in 15% of all strokes, the imaging evaluation in the acute phase may include CTA of the intracranial arteries for evaluation of an underlying vascular malformation or aneurysm [1–3]. MRI without and with contrast is sometimes obtained to assess for an underlying neoplastic or vascular mass or associated microhemorrhages that may suggest amyloid angiopathy, multiple cavernous malformations, or septic emboli, among other etiologies. In the acute phase, the sensitivity of MRI may be limited by mass effect from the hematoma and the complex MRI signal of blood products that may obscure subtle enhancing lesions; however, its sensitivity is improved in the subacute phase once the hematoma has been resorbed [1–3].
IMAGING PATIENTS WITH ACUTE ISCHEMIC STROKE WHO ARE CANDIDATES FOR IV THROMBOLYSIS
Treatment options are considered for patients with acute ischemic stroke without intracranial hemorrhage present on imaging. FDA guidelines for the administration of IV thrombolysis include imaging to exclude intracranial hemorrhage and its interpretation by a physician with appropriate expertise. Completion of this initial imaging within 45 min of the patient admission to the emergency department is a CMS Hospital Outpatient Quality Reporting Program measure [4–6]. There is strong evidence supporting the use of IV tPA as a recanalization therapy to improve clinical outcomes during the 0-hour to 3-hour time window [7–9] and during the 3-hour to 4.5-hour time window [10–12]. This benefit is despite an increased risk for symptomatic intracranial hemorrhage after infusion. Overall, there is strong evidence supporting the timely use of NCCT or MRI of the brain to exclude hemorrhage in patients with the clinical diagnosis of stroke and before initiating IV thrombolytic therapy [7,13]. The primary goals of imaging during the 0-hour to 4.5-hour time window are to exclude the presence of intracranial hemorrhage and assess the presence and extent of ischemic changes. The presence of intracranial hemorrhage (excluding microbleeds) is an absolute contraindication to administering IV thrombolytic therapy. The presence of a large acute hypodensity on NCCT increases the risk for hemorrhagic transformation after thrombolytic therapy. This is considered a relative, not absolute, contraindication for IV tPA. MR diffusion-weighted imaging (DWI) may be obtained for a more definitive estimate of the extent of ischemia, only if this does not delay IV thrombolysis [9,14,15].
IMAGING PATIENTS WITH ACUTE ISCHEMIC STROKE WHO ARE CANDIDATES FOR ENDOVASCULAR REVASCULARIZATION
There is limited evidence supporting the use of intra-arterial thrombolytic agents up to 6 hours after symptom rest. Also, the evidence supporting improved clinical outcomes with first-generation mechanical embolectomy devices up to 8 hours after symptom onset, compared with standard medical care, has recently been challenged by the results of the Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy [16], Interventional Management of Stroke III [17], and Intra-Arterial Versus Systemic Thrombolysis for Acute Ischemic Stroke Expansion [18] trials.
There are 3 major imaging strategies (and numerous combinations of these 3 strategies) used in patients with acute ischemic stroke who are considered for endovascular revascularization therapy, with different underlying rationales (Fig. 1). There is currently no definitive evidence supporting 1 strategy over the other. Some believe that more imaging provides additional, clinically relevant information, whereas others are concerned about the time delay resulting from the additional imaging and the potential delay to recanalization it may cause. The choice of imaging implemented may depend on physician preference and logistic factors (such as whether advanced imaging, especially MRI, can be performed quickly and on a 24/7 basis). In considering the underlying rationale for endovascular therapy, additional imaging may be more justified in patients within the 4.5-hour to 8-hour time window. In patients with contraindications to IV tPA within the 0-hour to 4.5-hour time window and in patients considered for endovascular therapy after IV tPA failure, imaging the volume of the infarct may be sufficient.
The first strategy consists of going to the angiography suite immediately after the initial NCCT. The rationale for this approach is to minimize the door-to-recanalization time. In this setting, the vascular patency status is assessed on the digital subtraction angiography that precedes the therapeutic portion of the procedure, before lysis or removal of the clot. Collateral patterns can also be demonstrated, although infarct volume can be indirectly assessed only by attention to flow, parenchymal blush, and arterial to venous transit times. The second strategy consists of performing CTA to assess vascular patency, with or without perfusion imaging, to better characterize the site of occlusion and the ischemic tissue before making an endovascular treatment decision. The third strategy consists of using MRI or MRA, possibly with DWI and perfusion-weighted imaging, at institutions at which it can be performed quickly and on a 24/7 basis. The rationale for these latter approaches is that the extra time needed to perform this additional imaging may be justified by the information gathered and the implications for decision making [19,20]. Some studies have demonstrated that the extra time for imaging before treatment does not adversely affect outcomes [21–23].
IMAGING PATIENTS WITH ACUTE ISCHEMIC STROKE WHO ARE NOT CANDIDATES FOR IV OR ENDOVASCULAR THERAPY AND PATIENTS WITH TIAs
When acute revascularization therapy is not being considered, the role of imaging is focused primarily on diagnosis, prevention of immediate complications, and the identification of potentially treatable causes of future stroke. In patients with TIAs, multimodal MRI is preferred, and NCCT should be performed only if MRI is not available, as NCCT has limited utility in patients whose symptoms have resolved [24]. DWI can demonstrate lesions in approximately 40% of patients with TIAs [25–27], and DWI positivity in patients with TIAs is associated with a higher risk for recurrent ischemic events [28]. The distribution of DWI lesions can help with the determination of stroke etiology (scattered emboli in multiple territories indicative of a proximal embolic source [eg, cardiac], watershed distribution of lesions suggestive of hypoperfusion from carotid disease, etc) [29–31].
CTA or MRA of the intracranial and cervical arteries and duplex ultrasound for the cervical arteries are used to identify stenosis and/or occlusion [24] and to determine appropriate secondary prevention, such as extracranial carotid revascularization, for these patients. An appropriate evaluation for cardiac sources of TIA or stroke (eg, echocardiography) should also be performed.
IMAGING THE CERVICAL ARTERIES IN PATIENTS WITH ACUTE STROKE AND TIAs
In patients with acute stroke, vascular imaging should be performed to evaluate the mechanism of stroke and assess risk for future stroke [1]. Overall, vascular imaging with duplex ultrasound, CTA, MRA, or digital subtraction angiography has good agreement. Concordant results from at least two noninvasive imaging techniques can be used to determine treatment eligibility for revascularization procedures.
TAKE-HOME POINTS.
The primary goal of imaging patients with acute stroke symptoms is to distinguish between hemorrhagic and ischemic stroke.
Early identification of the stroke etiology or mechanism (carotid atherosclerotic disease or other treatable causes) is critical to treatment decisions and long-term management.
In acute stroke patients who are candidates for IV thrombolysis (0-hour to 4.5-hour time window), either noncontrast CT or MRI of the brain is recommended to exclude intracranial hemorrhage and determine the extent of ischemic changes.
In acute stroke patients who are candidates for endovascular therapy, vascular imaging (CTA, MRA, conventional angiography) is strongly recommended during the initial imaging evaluation.
In acute stroke patients, vascular imaging of the head and neck should be performed to evaluate the mechanism of stroke and assess risk of future stroke.
ACKNOWLEDGMENTS
We thank Judy Burleson, MHSA, director of metrics, ACR, and Christine Waldrip, RN, MHA, program manager, ACR Appropriateness Criteria®, for the support they provided in the preparation of this report.
REFERENCES
- 1.Kidwell CS, Wintermark M. Imaging of intracranial haemorrhage. Lancet Neurol. 2008;7:256–67. doi: 10.1016/S1474-4422(08)70041-3. [DOI] [PubMed] [Google Scholar]
- 2.Huisman TA. Intracranial hemorrhage: ultrasound, CT and MRI findings. Eur Radiol. 2005;15:434–40. doi: 10.1007/s00330-004-2615-7. [DOI] [PubMed] [Google Scholar]
- 3.Hoggard N, Wilkinson ID, Paley MN, Griffiths PD. Imaging of haemorrhagic stroke. Clin Radiol. 2002;57:957–68. doi: 10.1053/crad.2002.0954. [DOI] [PubMed] [Google Scholar]
- 4.European Stroke Organisation (ESO) Executive Committee. ESO Writing Committee Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 5.Saver JL. Time is brain—quantified. Stroke. 2006;37:263–6. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 6.Wahlgren N, Ahmed N, Eriksson N, et al. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe Implementation of Thrombolysis in Stroke–Monitoring Study (SITS-MOST) Stroke. 2008;39:3316–22. doi: 10.1161/STROKEAHA.107.510768. [DOI] [PubMed] [Google Scholar]
- 7.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 8.Adams HP, Jr, Brott TG, Furlan AJ, et al. Guidelines for thrombolytic therapy for acute stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Circulation. 1996;94:1167–74. doi: 10.1161/01.cir.94.5.1167. [DOI] [PubMed] [Google Scholar]
- 9.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–25. [PubMed] [Google Scholar]
- 10.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 11.Bluhmki E, Chamorro A, Davalos A, et al. Stroke treatment with alteplase given 3.0–4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009;8:1095–102. doi: 10.1016/S1474-4422(09)70264-9. [DOI] [PubMed] [Google Scholar]
- 12.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 13.DeLaPaz RL, Wippold FJ, II, Cornelius RS, et al. ACR Appropriateness Criteria® on cerebrovascular disease. J Am Coll Radiol. 2011;8:532–8. doi: 10.1016/j.jacr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 14.von Kummer R, Meyding-Lamade U, Forsting M, et al. Sensitivity and prognostic value of early CT in occlusion of the middle cerebral artery trunk. AJNR Am J Neuroradiol. 1994;15:9–15. [PMC free article] [PubMed] [Google Scholar]
- 15.Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32:438–41. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- 16.Chelsea KS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–23. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–13. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DE-FUSE) study. Ann Neurol. 2006;60:508–17. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 20.Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11:860–7. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schellinger PD, Bryan RN, Caplan LR, et al. Evidence-based guideline: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;75:177–85. doi: 10.1212/WNL.0b013e3181e7c9dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breuer L, Schellinger PD, Huttner HB, et al. Feasibility and safety of magnetic resonance imaging-based thrombolysis in patients with stroke on awakening: initial single-centre experience. Int J Stroke. 2010;5:68–73. doi: 10.1111/j.1747-4949.2010.00410.x. [DOI] [PubMed] [Google Scholar]
- 23.Kohrmann M, Schellinger PD. Stroke-MRI: extending the time-window: recent trials and clinical practice. Int J Stroke. 2007;2:53–5. doi: 10.1111/j.1747-4949.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 24.Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. Stroke. 2009;40:2276–93. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 25.Kidwell CS, Alger JR, Di Salle F, et al. Diffusion MRI in patients with transient ischemic attacks. Stroke. 1999;30:1174–80. doi: 10.1161/01.str.30.6.1174. [DOI] [PubMed] [Google Scholar]
- 26.Coutts SB, Hill MD, Simon JE, Sohn CH, Scott JN, Demchuk AM. Silent ischemia in minor stroke and TIA patients identified on MR imaging. Neurology. 2005;65:513–7. doi: 10.1212/01.wnl.0000169031.39264.ff. [DOI] [PubMed] [Google Scholar]
- 27.Restrepo L, Jacobs MA, Barker PB, Wityk RJ. Assessment of transient ischemic attack with diffusion- and perfusion-weighted imaging. AJNR Am J Neuroradiol. 2004;25:1645–52. [PMC free article] [PubMed] [Google Scholar]
- 28.Cucchiara BL, Messe SR, Taylor RA, et al. Is the ABCD score useful for risk stratification of patients with acute transient ischemic attack? Stroke. 2006;37:1710–4. doi: 10.1161/01.STR.0000227195.46336.93. [DOI] [PubMed] [Google Scholar]
- 29.Kang DW, Chalela JA, Ezzeddine MA, Warach S. Association of ischemic lesion patterns on early diffusion-weighted imaging with toast stroke subtypes. Arch Neurol. 2003;60:1730–4. doi: 10.1001/archneur.60.12.1730. [DOI] [PubMed] [Google Scholar]
- 30.Wessels T, Wessels C, Ellsiepen A, et al. Contribution of diffusion-weighted imaging in determination of stroke etiology. AJNR Am J Neuroradiol. 2006;27:35–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Rovira A, Grive E, Rovira A, Alvarez-Sabin J. Distribution territories and causative mechanisms of ischemic stroke. Eur Radiol. 2005;15:416–26. doi: 10.1007/s00330-004-2633-5. [DOI] [PubMed] [Google Scholar]