Abstract
Background and Purpose
Angiographic revascularization grading after intra-arterial stroke therapy is limited by poor standardization, making it unclear which scale is optimal for predicting outcome. Using recently standardized criteria, we sought to compare the prognostic performance of 2 commonly used reperfusion scales.
Methods
Inclusion criteria for this multicenter retrospective study were acute ischemic stroke attributable to middle cerebral artery M1 occlusion, intra-arterial therapy, and 90-day modified Rankin scale score. Post–intra-arterial therapy reperfusion was graded using the Thrombolysis in Myocardial Infarction (TIMI) and Modified Thrombolysis in Cerebral Infarction (mTICI) scales. The scales were compared for prediction of clinical outcome using receiver-operating characteristic analysis.
Results
Of 308 patients, mean age was 65 years, and median National Institutes of Health Stroke Scale score was 17. The mean time from stroke onset to groin puncture was 305 minutes. There was no difference in the time to treatment between patients grouped by final TIMI (ie, 0 versus 1 versus 2 versus 3) or mTICI grades (ie, 0 versus 1 versus 2a versus 2b versus 3). Good outcome (modified Rankin scale, 0–2) was achieved in 32.5% of patients, and mortality rate was 25.3% at 90 days. There was a 6.3% rate of parenchymal hematoma type 2. In receiver-operating characteristic analysis, mTICI was superior to TIMI for predicting 90-day modified Rankin scale 0 to 2 (c-statistic: 0.74 versus 0.68; P<0.0001). The optimal threshold for identifying a good outcome was mTICI 2b to 3 (sensitivity 78.0%; specificity 66.1%).
Conclusions
mTICI is superior to TIMI for predicting clinical outcome after intra-arterial therapy. mTICI 2b to 3 is the optimal biomarker for procedural success.
Keywords: acute ischemic stroke, endovascular, intra-arterial therapy, modified TICI, revascularization, TIMI
Early revascularization is associated with improved outcomes after acute ischemic stroke.1 For this reason, the rate of device-specific revascularization has been used as a surrogate end point for US Food and Drug Administration clearance.2–4 However, a major problem is the lack of a standardized approach to cerebral angiographic revascularization grading.5 The 2 most commonly used cerebral angiographic revascularization grading systems are the Thrombolysis in Myocardial Infarction (TIMI) and Modified Thrombolysis in Cerebral Infarction (mTICI) scales. Moreover, these scales have been used in different studies to grade either recanalization of the primary arterial occlusive lesion or reperfusion of the distal tissue bed.6,7 This heterogeneous approach may explain, in part, why devices that have shown high revascularization rates in previous studies have failed to produce clinical benefits in recent trials.8,9
To address this issue, an expert panel drafted a consensus recommendation statement to standardize various aspects of angiographic revascularization grading.10 Among these recommendations, reperfusion scales were supported for the primary measurement of procedural success.
Using the operational definition of reperfusion that was adopted by the panel, the aims of this study were to compare the TIMI and mTICI scales to determine whether one is superior for predicting clinical outcome after intra-arterial therapy (IAT), and subsequently to identify an optimal threshold for use as a surrogate end point in future IAT trials.
Methods
In this multicenter retrospective study, clinical, imaging, and treatment data were collected from the participating centers for statistical analysis at the coordinating center. Inclusion criteria were acute ischemic stroke attributable to middle cerebral artery M1 segment (ie, main stem proximal to the bifurcation) occlusion, intra-arterial therapy, and available 90-day modified Rankin scale (mRS) score. Thirty-one patients (10% of this study cohort) from a single center were included in a previous study that performed a distinct analysis comparing interobserver agreement between the modified and original thrombolysis in cerebral infarction scales.11 Institutional review board approval was obtained at all centers for this Health Insurance Portability and Accountability Act–compliant study.
Revascularization Grading Methodology
Neurointerventionists and stroke neurologists with significant clinical and research experience in IAT convened in Chicago in January 2012 for a full-day round-table meeting with the purpose of standardizing cerebral angiographic revascularization grading methodology. The expert panel comprised stroke neurologists and neurointerventionists from neuroradiology, neurology, and neurosurgery. The consensus recommendations, which are published concurrently, supported tissue-level reperfusion as the primary measure of successful or effective revascularization.10 The operational criteria for angiographic tissue reperfusion was defined as restoration of capillary-level contrast opacification as evidenced by a capillary blush (ie, tissue staining) achieved in an antegrade fashion (Figures 1 and 2).
Figure 1.
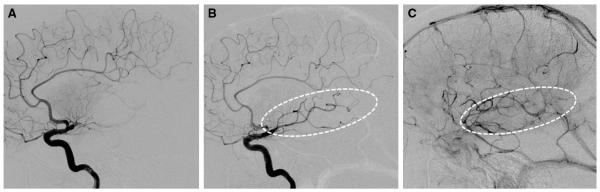
Example of Modified Thrombolysis in Cerebral Infarction 2a (mTICI 2a). Lateral projection images of M1 occlusion at baseline (A), post-treatment early arterial (B), and late parenchymal-venous (C) phases demonstrate restoration of antegrade flow into the inferior division branches, which produces a capillary blush in <50% of the middle cerebral artery territory (dotted ovals).
Figure 2.
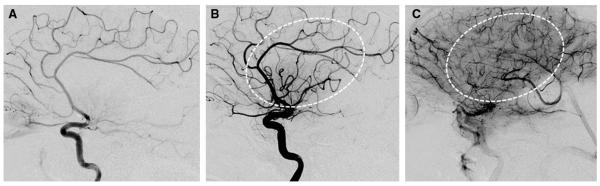
Example of Modified Thrombolysis in Cerebral Infarction 2b (mTICI 2b). Lateral projection images of M1 occlusion at baseline (A), post-treatment early arterial (B), and late parenchymal (C) phases demonstrate restoration of antegrade flow into the dominant superior division branches, which produces a capillary blush in >50% of the middle cerebral artery territory (dotted ovals).
This operational definition was used by the local investigators to grade angiographic results after IAT using the TIMI and mTICI scales (Table). The only difference between these scales is that partial reperfusion (TIMI 2) of the target downstream ischemic territory in the TIMI scale is further subdivided as minor (mTICI 2a: less than half of the target territory reperfused) or major (mTICI 2b: half or greater reperfused) in the mTICI scale (Figures 1 and 2). Only M1 occlusions were included in this analysis to simplify estimation of the target downstream territory (ie, entire middle cerebral artery territory for M1 occlusion). For patients with tandem cervical internal carotid artery lesions, only the intracranial occlusion was used to determine the downstream territory.
Table.
TIMI and mTICI Scales
| TIMI | Angiographic Criteria | mTICI | Angiographic Criteria |
|---|---|---|---|
| 0 | No perfusion | 0 | No perfusion |
| 1 | Minimal flow past the occlusion with little to no perfusion | 1 | Minimal flow past the occlusion with little to no perfusion |
| 2 | Antegrade partial perfusion of the downstream ischemic territory | 2a | Antegrade partial perfusion of less than half of the downstream ischemic territory |
| 2b | Antegrade partial perfusion of half or greater of the downstream ischemic territory | ||
| 3 | Antegrade complete perfusion of the downstream ischemic territory | 3 | Antegrade complete perfusion of the downstream ischemic territory |
Perfusion signifies capillary opacification or blush angiographically. mTICI indicates modified thrombolysis in cerebral infarction; and TIMI, thrombolysis in myocardial infarction.
Statistical Analysis
Clinical, imaging, and treatment data were summarized using standard descriptive statistics. Comparison of TIMI and mTICI for predicting good outcome (90-day mRS, 0–2) was performed using receiver-operating characteristic analysis. The method of DeLong et al12 was used to compare the c-statistic. The optimal score threshold for the superior scale was then determined weighing sensitivity and specificity equally. A similar analysis was performed for 90-day mRS 0 to 3 and for mortality. The proportions of good outcome were compared across the mTICI grades using the χ2 test. Statistical significance was set at P<0.05, and analyses were performed using MedCalc software (version 12.4.0.0, Ostend, Belgium).
Results
Six centers contributed 308 patients to this study. The mean age was 65.1±16.5 years. There were 167 (54.2%) women. Strokes involved the left hemisphere in 155 (50.3%) patients. The median admission National Institutes of Health Stroke Scale score was 17 (interquartile range, 15–20). Hypertension was seen in 65.1%, diabetes mellitus in 17.6%, hyperlipidemia in 42.5%, and coronary disease in 29.8% of patients.
Before IAT, intravenous tissue-type plasminogen activator (IV) was administered to 59.1% (176 of 298) of patients. The mean time from stroke onset to groin puncture was 305±140 minutes (n=279). Angioplasty or stenting of a tandem cervical internal carotid artery lesion was performed in 19 (6.2%) cases. The distribution of final mTICI scores was 15.3% for score 0, 7.8% for 1, 28.2% for 2a, 31.5% for 2b, and 17.2% for 3. By definition, the percentages for TIMI scores 0, 1, and 3 were the same; the rate of TIMI 2 was 60.4% (combined mTICI 2a+2b). There was no difference in the time to treatment for patients grouped by final TIMI (ie, 0 versus 1 versus 2 versus 3) or mTICI grades (ie, 0 versus 1 versus 2a versus 2b versus 3). Nontarget emboli were reported in 4.6% (7 of 152).
Good outcome was achieved in 100 (32.5%) patients. Seventy-eight (25.3%) patients were dead at 90 days. There was a 6.3% (18 of 286) rate of parenchymal hematoma type 2. Among patients who achieved either partial or complete reperfusion (TIMI 2–3 or mTICI 2a–3), earlier time to reperfusion was significantly associated with good outcome (mean 339 versus 424 minutes for good versus poor outcome, respectively; P<0.0001).
Prediction of Clinical Outcome: TIMI Versus mTICI
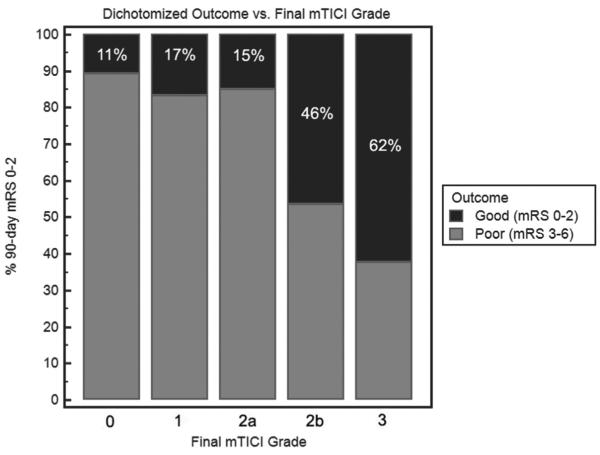
Adjusting for age, baseline National Institutes of Health Stroke Scale score, time to groin puncture, and time to reperfusion, both TIMI and mTICI were independent predictors of good outcome when entered separately in the logistic regression model. In receiver-operating characteristic analysis, mTICI was superior to TIMI for predicting 90-day mRS 0 to 2 (c-statistic: 0.74 versus 0.68 for mTICI versus TIMI; P<0.0001 for comparison). The optimal mTICI score threshold for identifying a good outcome was mTICI 2b to 3 (sensitivity 78.0%, specificity 66.1%). The proportions of good outcome for each mTICI grade are shown in Figure 3. In stepwise comparison, the only significant difference was seen between mTICI 2a to 2b. Similarly, mTICI was superior to TIMI for predicting 90-day mRS 0 to 3 (c-statistic: 0.73 versus 0.68; P=0.0002) and mortality (c-statistic: 0.68 versus 0.63; P=0.001).
Figure 3.
Proportion of good outcomes by mTICI grade (P<0.0001 for overall comparison). In successive stepwise comparison, the only significant difference was between mTICI 2a and 2b (P<0.0001); there was a trend for the difference between mTICI 2b and 3 (P=0.09). mRS indicates modified Rankin scale; and mTICI, modified thrombolysis in cerebral infarction.
Discussion
mTICI is superior to TIMI for predicting functional outcome and mortality after intra-arterial therapy of middle cerebral artery M1 occlusions, supporting its use as the standard reperfusion grading scale. Furthermore, mTICI 2b to 3 (ie, substantial reperfusion in greater than half of the target downstream territory) is the optimal threshold for predicting 90-day independence and should be used as the target angiographic end point for technical success, instead of the traditional end point of TIMI 2 to 3 (ie, mTICI 2a–3).
The use of TIMI 2 to 3 rate as the historical benchmark for device effectiveness may help to explain the heterogeneous clinical response to reperfusion seen in previous device trials.2,13 The present results suggest that mTICI 2a (minor reperfusion) yields little to no improvement in the proportion of good outcomes compared with mTICI 0 to 1 (Figure 3). Therefore, clinical outcomes in the TIMI 2 to 3 group are strongly dependent on the percentage of mTICI 2a results therein. In addition, the limitation of this end point has been highlighted by the Interventional Management of Stroke (IMS) III and Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) trials.8,9 They failed to demonstrate superiority of IAT despite high TIMI 2 to 3 rates (≈70–75%). The negative results are better explained by the low rates of mTICI 2b to 3 (27% in MR RESCUE and 40% in IMS III), which are well below the 68% rate reported by TREVO 2 (using a variant TICI 2b threshold of >67% reperfusion).4
Future studies should evaluate the inter-rater reliability of the mTICI scale. However, data from Arnold et al14 suggest that agreement should be high (κ=0.95 for <50% versus >50% reperfusion). Moreover, mTICI should be studied in intracranial internal carotid artery occlusions where the target downstream territory may be variably defined. It is anticipated that further refinements may increase prognostic accuracy, such as incorporating speed of reperfusion into the grading criteria. Consistent with previous studies,15,16 earlier time to reperfusion was associated with better outcomes in this cohort. Additionally, future work should investigate whether a different cut point for mTICI 2b (eg, 67% versus 50%) may provide a better prediction of clinical outcome.
Study limitations include its retrospective design and angiographic grading by local site investigators. Clearly, our results should be validated in a prospective data set, preferably using core laboratory adjudication. Nevertheless, our findings suggest that the superiority of mTICI is generalizable to diverse centers and readers. An additional limitation is the relatively long time from stroke onset to groin puncture (mean ≈5 hours). However, mTICI was superior to TIMI in both patients treated within 4.5 hours and those treated after this time (both P=0.01 for the difference in c-statistic). The strengths of this analysis include a study population that is homogeneous for occlusion level and the use of standardized reperfusion grading criteria, which is a major advantage over the previous literature.
Acknowledgments
The authors appreciate the efforts of the Cerebral Angiographic Revascularization Grading Collaborators, whose standardized grading criteria were utilized in this study.
Sources of Funding None.
Disclosures Dr Yoo receives research funding from the National Institutes of Health (NIH), Penumbra Inc, and Remedy Pharmaceuticals. Dr Prabhakaran receives research funding from Genentech. Dr Linfante receives consulting fees and expenses from Stryker and Codman Neurovascular. Dr Liebeskind receives consulting fees and expenses from Stryker and Covidien. Dr Khatri receives research funding from NIH, Penumbra Inc, and Genentech. Dr Kallmes receives research funding from MicroVention, Codman Neurovascular, Sequent, and Covidien, and receives consulting fees and expenses from Codman Neurovascular and Covidien. Dr Dabus receives consulting fees and expenses from Covidien, Codman Neurovascular, and Reverse Medical. Dr Zaidat is an equity shareholder in Galaxy Therapeutics and receives consulting fees and expenses from Stryker and Gore Medical.
References
- 1.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 2.Penumbra Pivotal Stroke Trial Investigators The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761–2768. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 3.Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, et al. SWIFT Trialists Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 4.Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, et al. TREVO 2 Trialists Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomsick T. TIMI, TIBI, TICI: I came, I saw, I got confused. AJNR Am J Neuroradiol. 2007;28:382–384. [PMC free article] [PubMed] [Google Scholar]
- 6.Soares BP, Chien JD, Wintermark M. MR and CT monitoring of recanalization, reperfusion, and penumbra salvage: everything that recanalizes does not necessarily reperfuse! Stroke. 2009;40(suppl 3):S24–S27. doi: 10.1161/STROKEAHA.108.526814. [DOI] [PubMed] [Google Scholar]
- 7.Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T, IMS-I Investigators Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. 2005;36:2400–2403. doi: 10.1161/01.STR.0000185698.45720.58. [DOI] [PubMed] [Google Scholar]
- 8.Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Interventional Management of Stroke (IMS) III Investigators Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, et al. MR RESCUE Investigators A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suh SH, Cloft HJ, Fugate JE, Rabinstein AA, Liebeskind DS, Kallmes DF. Clarifying differences among thrombolysis in cerebral infarction scale variants: is the artery half open or half closed? Stroke. 2013;44:1166–1168. doi: 10.1161/STROKEAHA.111.000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 13.Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, et al. Multi MERCI Investigators Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 14.Arnold M, Nedeltchev K, Remonda L, Fischer U, Brekenfeld C, Keserue B, et al. Recanalisation of middle cerebral artery occlusion after intra-arterial thrombolysis: different recanalisation grading systems and clinical functional outcome. J Neurol Neurosurg Psychiatry. 2005;76:1373–1376. doi: 10.1136/jnnp.2004.055160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA, IMS I and II Investigators Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73:1066–1072. doi: 10.1212/WNL.0b013e3181b9c847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazighi M, Serfaty JM, Labreuche J, Laissy JP, Meseguer E, Lavallée PC, et al. RECANALISE Investigators Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol. 2009;8:802–809. doi: 10.1016/S1474-4422(09)70182-6. [DOI] [PubMed] [Google Scholar]