Abstract
BACKGROUND
Current approaches to diagnosing testosterone deficiency do not consider the physiological consequences of various testosterone levels or whether deficiencies of testosterone, estradiol, or both account for clinical manifestations.
METHODS
We provided 198 healthy men 20 to 50 years of age with goserelin acetate (to suppress endogenous testosterone and estradiol) and randomly assigned them to receive a placebo gel or 1.25 g, 2.5 g, 5 g, or 10 g of testosterone gel daily for 16 weeks. Another 202 healthy men received goserelin acetate, placebo gel or testosterone gel, and anastrozole (to suppress the conversion of testosterone to estradiol). Changes in the percentage of body fat and in lean mass were the primary outcomes. Subcutaneous- and intraabdominal-fat areas, thigh-muscle area and strength, and sexual function were also assessed.
RESULTS
The percentage of body fat increased in groups receiving placebo or 1.25 g or 2.5 g of testosterone daily without anastrozole (mean testosterone level, 44±13 ng per deciliter, 191±78 ng per deciliter, and 337±173 ng per deciliter, respectively). Lean mass and thigh-muscle area decreased in men receiving placebo and in those receiving 1.25 g of testosterone daily without anastrozole. Leg-press strength fell only with placebo administration. In general, sexual desire declined as the testosterone dose was reduced.
CONCLUSIONS
The amount of testosterone required to maintain lean mass, fat mass, strength, and sexual function varied widely in men. Androgen deficiency accounted for decreases in lean mass, muscle size, and strength; estrogen deficiency primarily accounted for increases in body fat; and both contributed to the decline in sexual function. Our findings support changes in the approach to evaluation and management of hypogonadism in men.
Testosterone therapy is prescribed for millions of men each year, and the number is increasing rapidly. Prescription sales of testosterone increased by 500% in the United States between 1993 and 2000.1 Most testosterone prescriptions are written to treat non-specific symptoms, such as fatigue or sexual dysfunction, when accompanied by testosterone levels below the laboratory reference range. Currently, testosterone levels that are at least 2 SD below the mean value for healthy young adults are classified as low.1,2 Although convenient, this classification fails to consider the physiological consequences of specific testosterone levels.
More than 80% of circulating estradiol in men is derived from the aromatization of testosterone.3 Thus, as serum testosterone levels decline, there is a concomitant decline in serum estradiol levels.4–5 Nevertheless, the consequences of male hypogonadism are routinely attributed solely to androgen deficiency; the potential role of the concomitant decline in estrogens is typically ignored. It has become clear, however, that estrogen deficiency may be important in the pathogenesis of some consequences of male hypogonadism, such as bone loss.6–8 The potential role of estrogen deficiency in the pathogenesis of other consequences of hypogonadism, such as alterations in body composition or sexual function, is largely unknown. Information on the role of estrogens in male hypogonadism may help identify men at risk for specific manifestations of the condition and may provide a rationale for novel approaches to its management. We sought to determine the relative degree of testosterone deficiency, estradiol deficiency, or both at which undesirable changes in body composition, strength, and sexual function begin to occur and whether those changes are due to androgen deficiency, estrogen deficiency, or both.
METHODS
STUDY PARTICIPANTS
We recruited two cohorts of men who were 20 to 50 years of age and healthy. All the men had normal serum testosterone levels. Details of the eligibility criteria and study completion are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
STUDY DESIGN AND PROTOCOL
All participants received goserelin acetate (Zoladex, AstraZeneca), at a dose of 3.6 mg subcutaneously at weeks 0, 4, 8, and 12, to suppress endogenous gonadal steroids. Participants were then randomly assigned to receive 0 g (placebo), 1.25 g, 2.5 g, 5 g, or 10 g of a topical 1% testosterone gel (AndroGel, Abbott Laboratories) daily for 16 weeks. Participants in cohort 2 also received anastrozole (Arimidex, AstraZeneca) at a dose of 1 mg daily to block the aromatization of testosterone to estrogen. Participants were unaware of the study-group assignments.
Participants were seen every 4 weeks. At each visit, fasting blood samples were obtained to measure gonadal steroid levels, and questionnaires were administered to assess physical function, health status, vitality, and sexual function. At baseline and week 16, body fat and lean mass were assessed by means of dual-energy x-ray absorptiometry (DXA); subcutaneous- and intraabdominal-fat areas and thigh-muscle area were measured by means of computed tomography (CT); and lower-extremity strength was determined by means of a leg press. Data on bone homeostasis (bone-turnover markers and bone mineral density), risk factors for cardiovascular disease (blood pressure, lipids, and insulin sensitivity), and levels of leptin and prostate-specific antigen were also collected but are not included in the present report.
The study was approved by the institutional review board of Partners Healthcare. All participants provided written informed consent. All authors vouch for the completeness and accuracy of the data and analyses and the fidelity of the study to the protocol (available at NEJM.org). All authors made the decision to submit the manuscript for publication. Abbott Laboratories provided partial financial support and supplied the testosterone gel at no charge but had no role in the study design, data analysis, data interpretation, or manuscript preparation. AstraZeneca provided Zoladex and Arimidex at no cost but had no role in the study design, data analysis, data interpretation, or manuscript preparation.
TESTOSTERONE AND ESTRADIOL MEASUREMENTS
The serum level of total testosterone was measured by means of a solid-phase chemiluminescent immunoassay with the use of an automated analyzer (ADVIA Centaur XP, Siemens). The assay sensitivity was 20 ng per deciliter. The total testosterone level was remeasured by means of liquid chromatography–tandem mass spectroscopy at all time points in serum samples obtained from five randomly selected men in each of the five dose groups in cohort 1.9 The correlation between the testosterone assays was 0.93, and the assays had very similar results (Tradioimmunoassay = 0.98Tmass spectroscopy + 21 ng per deciliter). The serum level of estradiol was measured with the use of liquid chromatography–tandem mass spectroscopy, with a threshold for detection of 1.25 pg per milliliter.9
OUTCOMES AND ASSESSMENTS
The primary outcome variables were changes in the DXA-based measures of body fat and lean mass. The percentage of body fat and the total-body lean mass were determined by means of DXA (QDR 4500A, Hologic).10 Subcutaneous- and intraabdominal-fat areas were determined by means of CT at the L4 vertebral level with a LightSpeed Pro 16 scanner (General Electric Medical Systems).11 Cross-sectional thigh-muscle area was determined at the midpoint of the femur.12 Lower-extremity strength was assessed as the maximum weight lifted for one repetition with the use of a leg press (Air 300, Keiser).13
Sexual function, physical function, vitality, and overall health status were assessed at each visit with the use of a self-administered questionnaire on health-related quality of life that was previously validated in patients with prostate cancer who were undergoing androgen-deprivation therapy.14 Sexual function was divided into domains of sexual desire and erectile function.
The primary analysis was a modified intention-to-treat analysis. Because we were assessing changes in outcome variables, participants who completed only the baseline visit could not be included in any longitudinal analyses. Because changes in body composition are unlikely to occur within the first several weeks of hormonal manipulation, the protocol required that participants complete the first three visits (through week 8) to avoid exposing participants to additional radiation from repeat body-composition scans when it appeared unlikely that the results would be informative. In addition, the protocol required that participants miss no more than 20% of their study medication doses to be included in the analyses. Participants who discontinued the study medication after week 8 but before week 16 were asked to undergo follow-up body-composition and strength assessments at their final visit.
STATISTICAL ANALYSIS
The study was designed to have 80% power at an alpha level of 0.025, with the use of a one-way analysis of variance to detect mean changes from baseline to 16 weeks of at least 0.3 times the common standard deviation for body-composition measures on DXA and subcutaneous-fat area and thigh-muscle area on CT. These calculations were based on a sample of 40 participants per dose group, with the assumption that 80% of participants would have data that could be assessed.
To determine the testosterone dose needed to maintain body composition, strength, and sexual function, we compared changes in each outcome among dose groups with the use of Duncan’s multiple-range test. To control further for type I error, we adopted a significance level of 0.025, using the Bonferroni method to adjust for the two primary outcomes. Our primary analysis focused on comparisons of the group receiving the 5-g dose with the other dose groups, because this dose produced testosterone levels that were similar to baseline levels.
To determine whether the changes in each outcome were related to testosterone, estradiol, or both, we used the following approaches. To assess testosterone-related effects, we compared changes in each outcome among all dose groups in cohort 2 with the use of Duncan’s multiple-range test. Because anastrozole suppresses estradiol production dramatically, differences between groups within cohort 2 should reflect the effect of testosterone on each outcome. To assess estradiol-related effects, we used general linear model–based tests with 1 degree of freedom in which the effects were cohort (cohort 1 or cohort 2), the group-specific testosterone dose, and the cohort–testosterone dose interaction. The interaction compares the slopes of regression of the testosterone dose with the outcome values in cohort 1 and cohort 2. The effects of estradiol on each outcome were also inferred with the use of independent t-tests to compare the mean change in each outcome for all groups that received testosterone (1.25 g, 2.5 g, 5 g, or aromatase inhibitor in cohort 2. Because testosterone doses were identical in the two cohorts and estradiol synthesis was selectively inhibited in cohort 2, differences in outcomes between cohorts with the use of this approach should also reflect the effects of estradiol.
All reported P values are two-sided. P values of less than 0.025 were considered to indicate statistical significance, with the exception that for interaction tests, P values of less than 0.05 were considered to indicate statistical significance. Unless otherwise noted, data are presented as means ± SD.
RESULTS
BASELINE CHARACTERISTICS AND STUDY COMPLETION
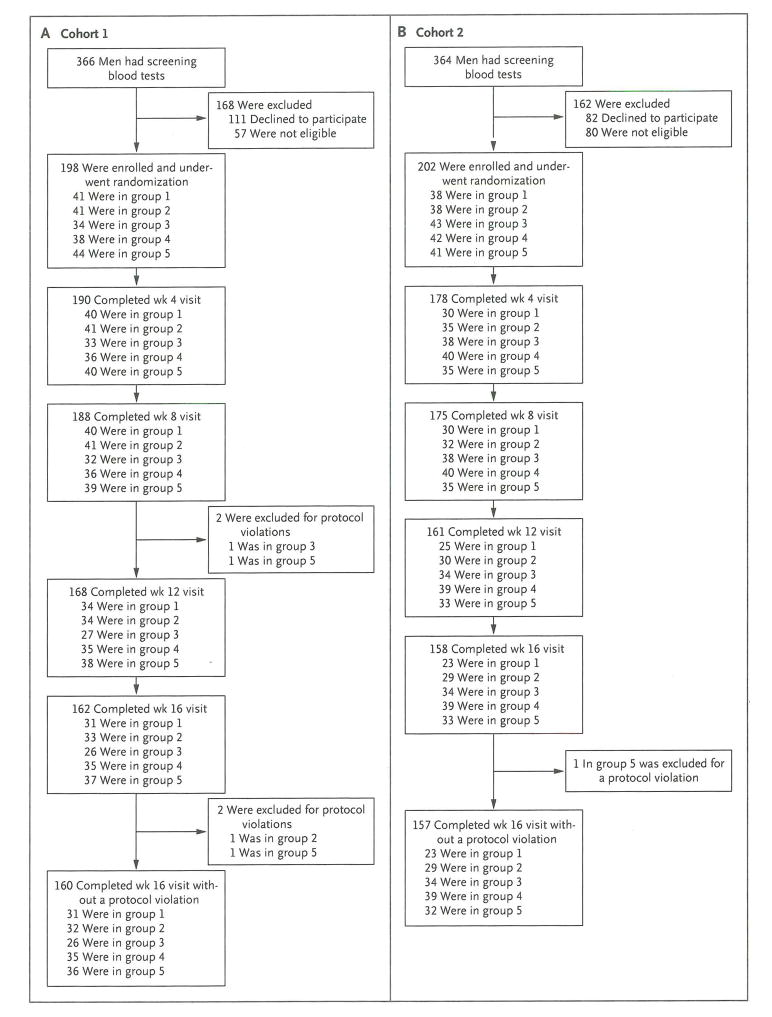
We enrolled 198 men in cohort 1 (Fig. 1A) and 202 men in cohort 2 (Fig. 1B). There were no significant differences in baseline testosterone levels among dose groups or between cohorts (Table 1). Ten men in cohort 1 and 27 in cohort 2 discontinued the study before week 8 and were included in analyses of baseline data only. We conducted a modified intention-to-treat analysis of the remaining data. In cohort 1, a total of 24 men discontinued participation between weeks 8 and 16, of whom 16 underwent follow-up body-composition and strength testing. Four additional participants were later excluded for protocol violations. In cohort 2, a total of 17 men discontinued participation between weeks 8 and 16, of whom 13 underwent follow-up body-composition and strength testing. One participant in cohort 2 was excluded for a protocol violation. Paired DXA, CT, or strength tests could not be completed in an additional 3, 5, and 10 men, respectively, in cohort 1 and in 1, 1, and 12 men, respectively, in cohort 2. Thus, the respective numbers of participants included in the analyses of sexual desire, body composition as measured by DXA, body composition as measured by CT, and strength were 184, 173, 171, and 166 for cohort 1 and 174, 169, 169, and 158 for cohort 2.
Figure 1. Recruitment of Participants and Study Completion.
Participants were recruited by sending letters to men in the local area who were identified with the use of commercially available mailing lists or by advertising in newspapers or on the Internet. A computerized program was used to randomly assign participants in permuted blocks. The block sizes were also randomly determined. Participants in cohort 1 (Panel A) were assigned to receive goserelin acetate plus placebo (group 1), 1.25 g of testosterone (group 2), 2.5 g of testosterone (group 3), 5 g of testosterone (group 4), or 10 g of testosterone (group 5) daily for 16 weeks. Participants in cohort 2 (Panel B) received the same study medications plus anastrozole at a dose of 1 mg per day. Participants who discontinued participation at week 8 or 12 were permitted to undergo repeat body-composition and strength testing that was planned for week 16. In cohort 1, eight men in group 1, five men in group 2, two men in group 3, and one man in group 4 underwent repeat body-composition and strength testing at week 8 or 12. In cohort 2, five men in group 1, two men in group 2, four men in group 3, one man in group 4, and one man in group 5 underwent repeat body-composition and strength testing at week 8 or 12.
Table 1.
Baseline Characteristics of the Study Participants.*
| Characteristic | Testosterone Dose | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 g/day | 1.25 g/day | 2.5 g/day | 5 g/day | 10 g/day | ||||||
| Cohort 1 (N = 41) | Cohort 2 (N = 38) | Cohort 1 (N = 41) | Cohort 2 (N = 38) | Cohort 1 (N = 34) | Cohort 2 (N = 43) | Cohort 1 (N = 38) | Cohort 2 (N = 42) | Cohort 2 (N = 44) | Cohort 2 (N = 41) | |
| Age (yr) | 32±9 | 34±7 | 34±7 | 33±7 | 32±8 | 33±7 | 34±8 | 33±6 | 33±8 | 34±6 |
| Height (cm) | 179±6† | 175±6 | 177±6 | 177±6 | 176±6 | 177±8 | 173±7‡ | 176±7 | 177±8 | 177±6 |
| Weight (kg) | 84±14 | 84±15 | 84±14 | 87±17 | 78±15 | 83±14 | 78±14‡ | 87±15 | 85±18 | 83±12 |
| Body-mass index§ | 26±4 | 27±5 | 27±4 | 28±5 | 25±4 | 26±4 | 26±4 | 28±5 | 27±5 | 26±4 |
| Testosterone (ng/dl) | 510±160 | 511±181 | 506±154 | 548±189 | 574±125 | 512±159 | 506±138 | 514±176 | 529±140 | 517±151 |
| Estradiol (pg/ml) | 27±8‡ | 32±10 | 27±8‡ | 32±10 | 32±10 | 30±13 | 27±8 | 30±10 | 29±9 | 27±9 |
| Percentage of body fat | 22.3±6.3 | 22.9±6.3 | 23.3±6.5 | 20.8±6.7 | 19.9±6.0 | 20.4±6.9 | 22.4±6.2 | 22.9±5.8 | 22.5±6.4 | 20.1±5.1 |
| Total-body lean mass (g) | 59,098±7073 | 56,896±8551 | 59,074±7834 | 59,263±8291 | 55,435±7130 | 57,418±7448 | 55,662±7523 | 59,103±9025 | 58,412±10,406 | 58,042±7658 |
| Subcutaneous-fat area (mm2) | 22,425±11,807 | 23,507±12,758 | 23,178±11,176 | 23,021±15,351 | 18,262±10,823 | 18,724±10,427 | 21,050±9868 | 25,339 ±11875 | 21,772±12,814 | 19,563±8740 |
| Intraabdominal-fat area (mm2)¶ | 10,987±6905 | 9504±4988 | 10,251±6916 | 8551±5431 | 7521±5153 | 8153±5352 | 10,023±6619 | 9996±6154 | 9582±5406 | 7964±5219 |
| Thigh-muscle area (mm2) | 17,082±2772 | 16,769±2668 | 16,810±2899 | 17,964±2926 | 16,478±2722 | 17,265±2711 | 16,579±2427 | 17,558±2654 | 16,905±3395 | 17,226±2361 |
| Leg press (lb) | 613±152 | 645±171 | 592±127† | 701±195 | 612±167 | 627±155 | 582±122† | 641±137 | 633±173 | 662±170 |
Plus–minus values are means ± SD. There were no significant differences between cohort 1 and cohort 2 for groups assigned to the same testosterone dose unless otherwise indicated. To convert the values for testosterone to nanomoles per liter, multiply by 0.03467. To convert the values for estradiol to picomoles per liter, multiply by 3.671. To convert the values for leg press to kilograms, multiply by 0.45.
P<0.01 with the use of a nonpaired t-test for the comparison with cohort 2.
P<0.05 with the use of a nonpaired t-test for the comparison with cohort 2.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
P<0.05 with the use of one-way analysis of variance for comparisons across dose groups in cohort 1.
HORMONE LEVELS
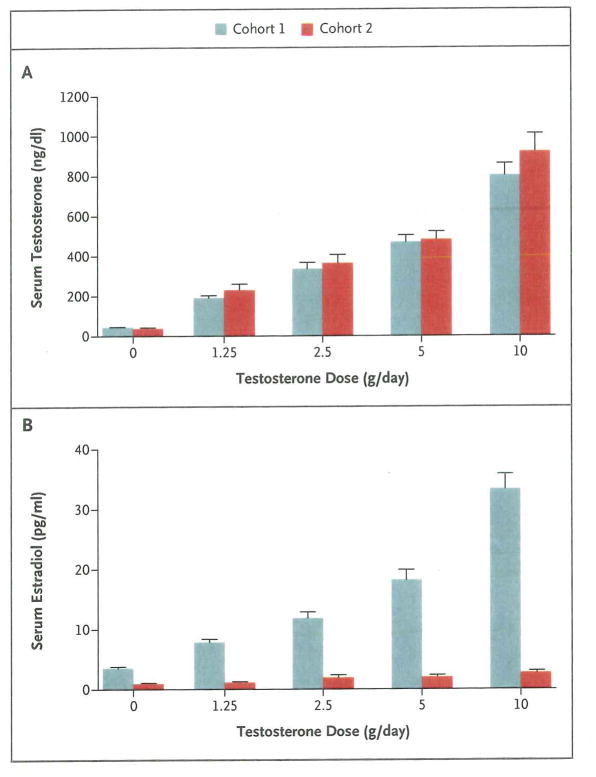
In men receiving goserelin acetate and 0 g (placebo), 1.25 g, 2.5 g, 5 g, or 10 g of testosterone gel daily (cohort 1), the mean testosterone levels were 44+13 ng per deciliter, 191+78 ng per deciliter, 337+173 ng per deciliter, 470+201 ng per deciliter, and 805+355 ng per deciliter, respectively (Fig. 2A). The corresponding mean estradiol levels were 3.6+1.4 pg per milliliter, 7.9+2.9 pg per milliliter, 11.9+5.7 pg per milliliter, 18.2+10.2 pg per milliliter, and 33.3+15.3 pg per milliliter (Fig. 2B). In men who also received anastrozole (cohort 2), the corresponding mean testosterone levels were 41+13 ng per deciliter, 231+171 ng per deciliter, 367+248 ng per deciliter, 485+240 ng per deciliter, and 924+521 ng per deciliter (Fig. 2A), and the corresponding mean estradiol levels were 1.0+0.4 pg per milliliter, 1.2+0.4 pg per milliliter, 2.0+2.3 pg per milliliter, 2.1+1.9 pg per milliliter, and 2.8+1.8 pg per milliliter (Fig. 2B).
Figure 2. Mean Serum Testosterone and Estradiol Levels from Weeks 4 to 16, According to Testosterone Dose and Cohort.
I bars indicate standard errors.
EFFECTS OF TESTOSTERONE WITHOUT AROMATASE INHIBITION ON BODY COMPOSITION
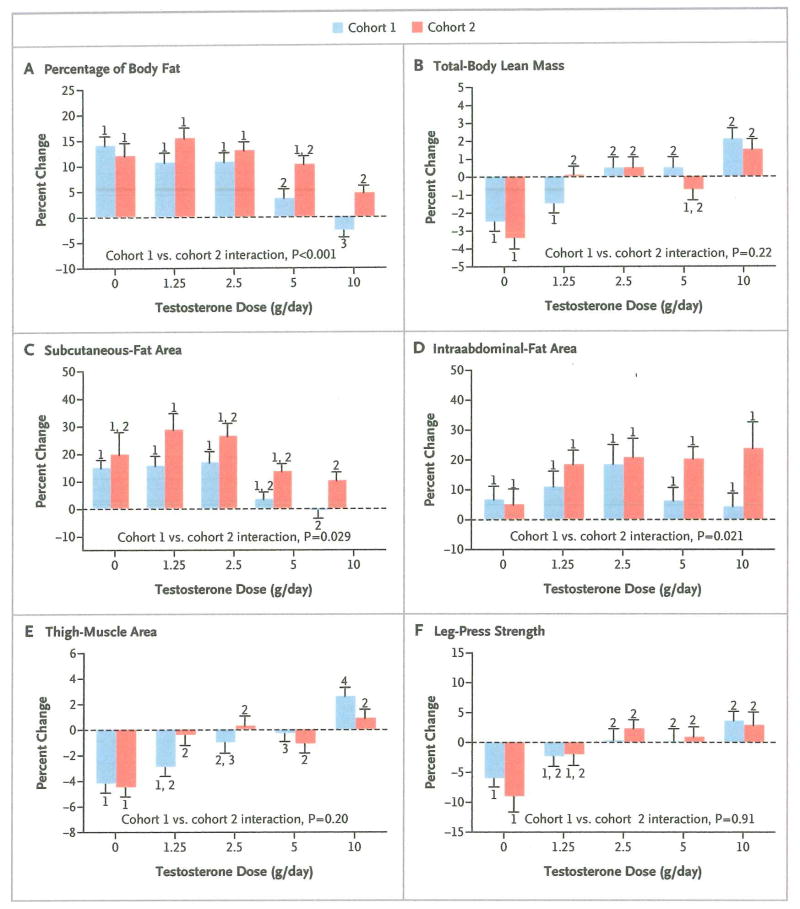
In cohort 1, the percentage of body fat increased significantly in men who received 0 g, 1.25 g, or 2.5 g of testosterone daily, as compared with men who received 5 g daily, and it decreased significantly in men who received 10 g of testosterone daily, as compared with each of the other groups (Fig. 3A). Lean mass decreased significantly in men who received placebo or 1.25 g of testosterone daily, as compared with men who received 2.5 g, 5 g, or 10 g of testosterone daily (Fig. 3B). Subcutaneous-fat area increased by a factor of 2 to 3 in men receiving 0 g, 1.25 g, or 2.5 g of testosterone daily, as compared with men receiving 5 g or 10 g daily, though only the comparisons with the 10-g dose group were significant (Fig 3C). Intraabdominal-fat area did not change significantly in any group (Fig. 3D). Thigh-muscle area decreased significantly in men who received placebo or 1.25 g of testosterone daily, as compared with men who received 5 g of testosterone daily, and it increased significantly in men who received 10 g of testosterone daily, as compared with all the other groups (Fig. 3E). Leg-press strength decreased significantly in men who received placebo, as compared with men receiving 2.5 g, 5 g, or 10 g of testosterone daily (Fig. 3F).
Figure 3. Mean Percent Change from Baseline in Percentage of Body Fat, Lean Body Mass, Subcutaneous- and Intra-abdominal-Fat Area, Thigh-Muscle Area, and Leg-Press Strength, According to Testosterone Dose and Cohort.
T bars indicate standard errors. Within each cohort, bars with the same number indicate no significant difference between dose groups. For example, the change in the percentage of body fat (Panel A) did not differ significantly among the groups that received 0 g, 1.25 g, or 2.5 g of testosterone daily in cohort 1 (all labeled “1”). The change in each of those three groups differed significantly from the change in the group that received 5 g per day (labeled “2”) and the change in the group that received 10 g per day (labeled “3”), and the change also differed significantly between these latter two groups. P values are for the cohort–testosterone dose interaction terms in analyses of variance comparing changes in each outcome measure between cohorts 1 and 2.
EFFECTS OF TESTOSTERONE WITH AROMATASE INHIBITION ON BODY COMPOSITION
In cohort 2, the percentage of body fat increased in all groups when the aromatization of testosterone to estradiol was inhibited. The magnitudes of these increases were similar with doses of 0 g, 1.25 g, 2.5 g, and 5 g of testosterone daily, a finding that suggests a predominantly estrogenic effect (Fig. 3A). Total-body lean mass decreased significantly in men who received placebo, as compared with those who received 1.25 g, 2.5 g, or 10 g of testosterone daily, a finding that implies an independent effect of testosterone (Fig. 3B). Subcutaneous-fat area increased in all groups in cohort 2, though only the comparison of changes between the 1.25-g and 10-g dose groups was significant (Fig. 3C). The increases in intraabdominal-fat area did not differ significantly among the dose groups (Fig. 3D). Thigh-muscle area decreased significantly in men who received placebo, as compared with men who received any of the four testosterone doses (Fig. 3E). As in cohort 1, leg-press strength declined significantly in men who received placebo, as compared with men who received the three highest testosterone doses (Fig. 3F).
EFFECTS OF TESTOSTERONE WITH AND WITHOUT AROMATASE INHIBITION ON SEXUAL FUNCTION
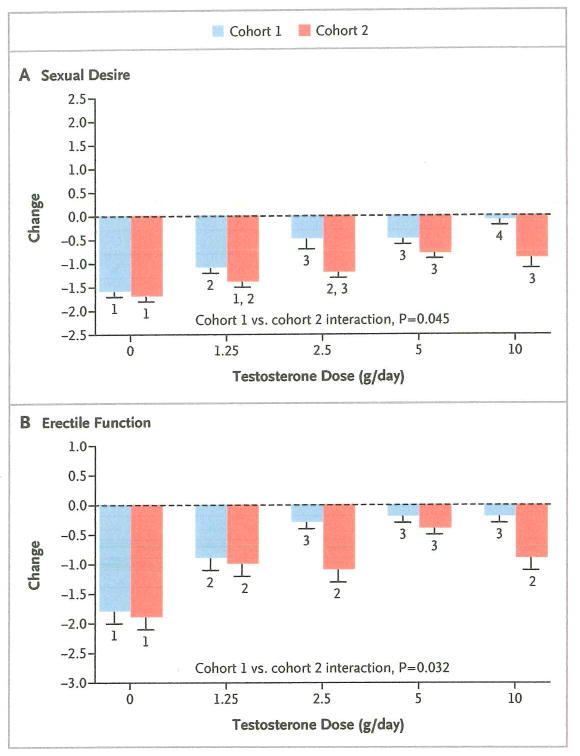
In cohort 1, sexual desire decreased progressively with declining testosterone doses, from 10 g to 0 g of testosterone daily, and all dose groups differed significantly from one another except for the 2.5-g and 5-g dose groups (Fig. 4A). Erectile function worsened significantly in men who received placebo, as compared with men who received testosterone, and declined more in men who received 1.25 g of testosterone daily than in men in the three highest dose groups (Fig. 4B).
Figure 4. Mean Absolute Change from Baseline in Sexual Desire and Erectile Function, According to Testosterone Dose and Cohort.
Sexual desire (Panel A) was assessed at each visit by asking participants to rate their sex drive as compared with their sex drive before the study began (−2 indicates much less, −1 somewhat less, 0 the same, 1 somewhat more, and 2 much more). Erectile function (Panel B) was evaluated by asking each man to consider the prior month and rate the degree to which each of the following three statements most closely applied to himself: “I had difficulty becoming sexually aroused,” “I had difficulty getting or maintaining an erection,” and “I had difficulty reaching orgasm,” with 1 indicating not at all, 2 a little, 3 some, 4 quite a bit, and 5 a great deal. For each man, the mean value at the final visit was then subtracted from the mean value at the baseline visit. T bars indicate standard errors. Within each cohort, bars with the same number indicate no significant difference between dose groups. P values are for the cohort–testosterone dose interaction terms in analyses of variance comparing changes in each outcome measure between cohorts 1 and 2.
In cohort 2, sexual desire declined significantly in men who received placebo, as compared with men in the three highest dose groups, and declined more in men who received 1.25 g of testosterone daily than in men in the two highest dose groups (Fig. 4A). Erectile function decreased more in men who received placebo than in men who received testosterone (Fig. 4B). Results for other self-reported measures are available in the Supplementary Appendix.
COMPARISONS OF CHANGES IN BODY COMPOSITION AND SEXUAL FUNCTION WITH AND WITHOUT AROMATASE BLOCKADE
The cohort–testosterone dose interaction was significant for the percentage of body fat (P=0.001), intraabdominal-fat area (P = 0.021), subcutaneous-fat area (P = 0.029), sexual desire (P = 0.045), and erectile function (P = 0.032); these findings indicate that estradiol exerted an independent effect on these variables (Fig. 3 and 4). In the groups that received testosterone, inhibition of estrogen synthesis (cohort 2), as compared with intact estrogen synthesis (cohort 1), was associated with significant increases in the percentage of body fat (P<0.001), subcutaneous-fat area (P<0.001), and intraabdominal-fat area (P = 0.002) and with significant decreases in sexual desire (P<0.001) and erectile function (P = 0.022); these findings provide additional evidence of an independent effect of estradiol on these measures. The cohort–testosterone dose interaction was not significant for total-body lean mass (P = 0.22), thigh-muscle area (P = 0.20), or leg-press strength (P = 0.91); among the men who received testosterone, there were no significant differences between cohorts in changes from baseline for total-body lean mass (P = 0.52), thigh-muscle area (P = 0.19), or leg-press strength (P = 0.90).
DISCUSSION
Although the sensitivity of various androgen target tissues is known to vary,15 the diagnosis of androgen deficiency is typically based on a single laboratory criterion: a testosterone level at least 2 SD below the mean value in normal young men. In this study, we found that the dose of testosterone required to prevent adverse changes in a variety of measures varies considerably. When aromatization was intact, fat accumulation began with mild gonadal steroid deficiency (a testosterone level of approximately 300 to 350 ng per deciliter), whereas lean mass, thigh-muscle area, and muscle strength were preserved until gonadal steroid deficiency was more marked (a testosterone level ≤200 ng per deciliter). Sexual desire and erectile function, the two major domains of sexual function, showed distinct patterns of change as serum testosterone levels were reduced. The variation in tissue sensitivity to androgens could be due to polymorphisms affecting polyglutamine repeat length in the androgen-receptor gene, tissue-specific differences in androgen-receptor expression or local hormone metabolism, or, as shown in the present study, variation in the roles of androgens and estrogens in the regulation of target-tissue responses.
Observational studies have shown that lean mass and strength are reduced and fat mass is increased in men with low testosterone levels.5,10,11 Men with hypogonadism report less sexual activity, fewer sexual thoughts, and fewer spontaneous erections than men with normal testosterone levels. Moreover, testosterone replacement increases lean mass, decreases fat mass, and can improve sexual function in men with hypogonadism.11,16–22 These observations have led to the widespread belief that undesirable changes in body composition and sexual dysfunction in men with hypogonadism are due to androgen deficiency. However, because estradiol is a metabolite of testosterone, it is difficult to distinguish the effects of androgens from those of estrogens in observational studies, or even in randomized, controlled trials if aromatizable androgens are used without the administration of an aromatase inhibitor.
By administering a variety of testosterone doses with and without concomitant aromatase inhibition, we found that changes in lean mass, thigh-muscle area, and leg-press strength were attributable to changes in testosterone levels, whereas changes in fat measures were primarily related to changes in estradiol levels. Both androgens and estrogens contributed to the maintenance of normal libido and erectile function. Although these results may be surprising, they are consistent with studies showing that body fat is increased in humans and male mice with null mutations of the aromatase gene or the estrogen-receptor α gene and that sexual function is markedly impaired in mice and humans with these genetic defects.23–26
Our observations may have important clinical implications. First, they provide a physiological basis for interpreting testosterone levels in young and middle-aged men and identifying the adverse consequences that are most likely to occur at various gonadal steroid levels. Second, because increases in visceral fat reduce insulin sensitivity and are associated with diabetes and the metabolic syndrome,27 the marked increase in intraabdominal fat with aromatase inhibition could portend an increase in cardiovascular disease with long-term estrogen deficiency. Finally, because lean mass, thigh-muscle area, and erectile function were reduced at a testosterone dose (1.25 g per day) that elicited a mean serum level of approximately 200 ng per deciliter, testosterone supplementation seems justified in men with testosterone levels in this range. However, some men have alterations in these measures at lower or higher testosterone levels, and other consequences of hypogonadism, such as increases in body fat and loss of sexual desire, routinely develop at higher mean testosterone levels. Thus, each person’s specific clinical scenario should be considered when interpreting the clinical significance of the circulating testosterone level.
These findings may also have implications for older men. Serum testosterone levels decline modestly as men age, such that 20% of men older than 60 years of age and 50% of men older than 80 years of age have testosterone levels at least 2 SD below the mean level in young men.2,28 Aging in men is also accompanied by declines in bone mineral density,29 lean mass,30,31 muscle strength,32,33 energy, and sexual function34 and by increases in fat mass31,35 — features that collectively are reminiscent of organic hypogonadism in young men.33 Decreases in muscle mass and strength are strong predictors of falls, fractures, and loss of the ability to live independently.36 Thus, if young and old men have similar responses to a decline in testosterone levels, as they do to an increase in testosterone levels,37,38 these findings suggest that some of the changes observed in aging men may be related to age-associated changes in gonadal steroids and may be preventable with appropriate replacement. A direct determination of the relationships between gonadal steroid levels and clinical measures in elderly men is needed to confirm this hypothesis.
Our finding that estrogens have a fundamental role in the regulation of body fat and sexual function, coupled with evidence from prior studies of the crucial role of estrogen in bone metabolism,6–8 indicates that estrogen deficiency is largely responsible for some of the key consequences of male hypogonadism and suggests that measuring estradiol might be helpful in assessing the risk of sexual dysfunction, bone loss, or fat accumulation in men with hypogonadism. For example, in men with serum testosterone levels of 200 to 400 ng per deciliter, sexual-desire scores decreased by 13% if estradiol levels were 10 pg per milliliter or more and by 31% if estradiol levels were below 10 pg per milliliter. Our findings also suggest that treatment with aromatizable androgens would be preferable to treatment with nonaromatizable androgens in most men with hypogonadism.
Our study has limitations. First, to avoid clinically significant changes in healthy men, such as bone loss, the study was limited to 16 weeks.39,40 Because changes in body composition may progress over time, greater changes might have been seen at higher testosterone and estradiol levels if gonadal steroids had been suppressed over a longer period. Second, although most circulating estradiol is derived from the aromatization of circulating testosterone, a small portion is directly secreted by the testes in normal men and may not be restored with exogenous testosterone administration.41 Third, changes induced by aromatase inhibition could primarily reflect the effects on local aromatase activity; therefore, circulating estradiol levels may not reflect estrogen effects reliably. Changes seen in our model of acute gonadal steroid deprivation may also differ from those seen when gonadal steroids decline gradually over a period of years. Finally, it seems likely that the relationship between declining gonadal steroid levels and the risk of adverse consequences is more accurately represented as a continuum than as a rigid threshold above which clinical measures are normal and below which adverse changes occur. However, clinicians ultimately must decide how to treat each patient on the basis of his individual data, of which the testosterone level is generally the principal component.
In summary, we conducted a dose-ranging study to determine the relative testosterone doses and associated serum levels at which body composition, strength, and sexual function initially decline. By examining these relationships with and without suppression of estrogen synthesis, we found that lean mass, muscle size, and strength are regulated by androgens; fat accumulation is primarily a consequence of estrogen deficiency; and sexual function is regulated by both androgens and estrogens. Delineation of the degrees of hypogonadism at which undesirable consequences develop and of the relative roles of androgens and estrogens in each outcome should facilitate the development of more rational approaches to the diagnosis and treatment of hypogonadism in men.
Supplementary Material
Acknowledgments
Funded by the National Institutes of Health and others; ClinicalTrials.gov number, NCT00114114.
Supported by grants from the National Institutes of Health (R01 AG030545, K24 DK02759, and RR-1066) and an investigator-initiated grant from Abbott Laboratories.
We thank Mr. Alex Linker, Ms. Christine Kim, Dr. Jonathan Youngner, Mr. Christopher Hahn, Mr. Andrew Servais, Mr. Nicholas Perros, and Mr. Matthew Webb for their dedicated administration of the study protocol and assistance with data management; the staff of the Mallinckrodt General Clinical Research Center for their care of the study participants; the staff of the Massachusetts General Hospital Bone Density Center for performing the measurements of bone density and body composition; Drs. Robert M. Neer and Henry M. Kronenberg for their scientific guidance; and Mrs. Deborah Fitzgerald for her administrative support.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Liverman CT, Blazer DG. Testosterone and aging: clinical research directions. Washington, DC: National Academy of Sciences; 2004. [PubMed] [Google Scholar]
- 2.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 3.Longcope C, Kato T, Horton R. Conversion of blood androgens to estrogens in normal adult men and women. J Clin Invest. 1969;48:2191–201. doi: 10.1172/JCI106185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khosla S, Melton LJ, III, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83:2266–74. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- 5.van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85:3276–82. doi: 10.1210/jcem.85.9.6825. [DOI] [PubMed] [Google Scholar]
- 6.Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106:1553–60. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khosla S, Melton LJ, III, Riggs BL. Estrogens and bone health in men. Calcif Tissue Int. 2001;69:189–92. doi: 10.1007/s00223-001-1044-8. [DOI] [PubMed] [Google Scholar]
- 8.Leder BZ, LeBlanc KM, Schoenfeld DA, Eastell R, Finkelstein JS. Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab. 2003;88:204–10. doi: 10.1210/jc.2002-021036. [DOI] [PubMed] [Google Scholar]
- 9.Khosla S, Amin S, Singh RJ, Atkinson EJ, Melton LJ, III, Riggs BL. Comparison of sex steroid measurements in men by immunoassay versus mass spectroscopy and relationships with cortical and trabecular volumetric bone mineral density. Osteoporos Int. 2008;19:1465–71. doi: 10.1007/s00198-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 11.Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81:4358–65. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- 12.Grinspoon S, Corcoran C, Rosenthal D, et al. Quantitative assessment of cross-sectional muscle area, functional status, and muscle strength in men with the acquired immunodeficiency syndrome wasting syndrome. J Clin Endocrinol Metab. 1999;84:201–6. doi: 10.1210/jcem.84.1.5375. [DOI] [PubMed] [Google Scholar]
- 13.Stone MH, O’Bryant H, Garhammer J. A hypothetical model for strength training. J Sports Med Phys Fitness. 1981;21:342–51. [PubMed] [Google Scholar]
- 14.Cleary PD, Morrissey G, Oster G. Health-related quality of life in patients with advanced prostate cancer: a multinational perspective. Qual Life Res. 1995;4:207–20. doi: 10.1007/BF02260860. [DOI] [PubMed] [Google Scholar]
- 15.Simanainen U, Brogley M, Gao YR, et al. Length of the human androgen receptor glutamine tract determines androgen sensitivity in vivo. Mol Cell Endocrinol. 2011;342:81–6. doi: 10.1016/j.mce.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson JM, Camargo CA, Smith ER. Effects of androgen on sexual behavior in hypogonadal men. J Clin Endocrinol Metab. 1979;48:955–8. doi: 10.1210/jcem-48-6-955. [DOI] [PubMed] [Google Scholar]
- 17.Kwan M, Greenleaf WJ, Mann J, Crapo L, Davidson JM. The nature of androgen action on male sexuality: a combined laboratory-self-report study on hypogonadal men. J Clin Endocrinol Metab. 1983;57:557–62. doi: 10.1210/jcem-57-3-557. [DOI] [PubMed] [Google Scholar]
- 18.Page ST, Amory JK, Bowman FD, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502–10. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- 19.Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–53. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- 20.Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, Bachand R. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88:2673–81. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Swerdloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2839–53. doi: 10.1210/jcem.85.8.6747. [DOI] [PubMed] [Google Scholar]
- 22.Woodhouse LJ, Gupta N, Bhasin M, et al. Dose-dependent effects of testosterone on regional adipose tissue distribution in healthy young men. J Clin Endocrinol Metab. 2004;89:718–26. doi: 10.1210/jc.2003-031492. [DOI] [PubMed] [Google Scholar]
- 23.Callewaert F, Venken K, Ophoff J, et al. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-α. FASEB J. 2009;23:232–40. doi: 10.1096/fj.08-113456. [DOI] [PubMed] [Google Scholar]
- 24.Carani C, Granata AR, Rochira V, et al. Sex steroids and sexual desire in a man with a novel mutation of aromatase gene and hypogonadism. Psychoneuroendocrinology. 2005;30:413–7. doi: 10.1016/j.psyneuen.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Jones ME, Thorburn AW, Britt KL, et al. Aromatase-deficient (ArKO) mice accumulate excess adipose tissue. J Steroid Biochem Mol Biol. 2001;79:3–9. doi: 10.1016/s0960-0760(01)00136-4. [DOI] [PubMed] [Google Scholar]
- 26.Maffei L, Rochira V, Zirilli L, et al. A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clin Endocrinol (Oxf) 2007;67:218–24. doi: 10.1111/j.1365-2265.2007.02864.x. [DOI] [PubMed] [Google Scholar]
- 27.Raji A, Seely EW, Arky RA, Simonson DC. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab. 2001;86:5366–71. doi: 10.1210/jcem.86.11.7992. [DOI] [PubMed] [Google Scholar]
- 28.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2002;87:589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 29.Cawthon PM, Ewing SK, McCulloch CE, et al. Loss of hip BMD in older men: the Osteoporotic Fractures in Men (MrOS) study. J Bone Miner Res. 2009;24:1728–35. doi: 10.1359/JBMR.090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhasin S, Buckwalter JG. Testosterone supplementation in older men: a rational idea whose time has not yet come. J Androl. 2001;22:718–31. [PubMed] [Google Scholar]
- 31.Guo SS, Zeller C, Chumlea WC, Siervogel RM. Aging, body composition, and lifestyle: the Fels Longitudinal Study. Am J Clin Nutr. 1999;70:405–11. doi: 10.1093/ajcn/70.3.405. [DOI] [PubMed] [Google Scholar]
- 32.Lindle RS, Metter EJ, Lynch NA, et al. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol. 1997;83:1581–7. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci. 2002;57:M76–M99. doi: 10.1093/gerona/57.2.m76. [DOI] [PubMed] [Google Scholar]
- 34.Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts Male Aging Study. J Urol. 2000;163:460–3. [PubMed] [Google Scholar]
- 35.Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009;8:339–48. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Vermeulen A. Androgen replacement therapy in the aging male — a critical evaluation. J Clin Endocrinol Metab. 2001;86:2380–90. doi: 10.1210/jcem.86.6.7630. [DOI] [PubMed] [Google Scholar]
- 37.Bhasin S, Woodhouse L, Casaburi R, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–E1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 38.Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–88. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- 39.Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–55. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 40.Stoch SA, Parker RA, Chen L, et al. Bone loss in men with prostate cancer treated with gonadotropin-releasing hormone agonists. J Clin Endocrinol Metab. 2001;86:2787–91. doi: 10.1210/jcem.86.6.7558. [DOI] [PubMed] [Google Scholar]
- 41.MacDonald PC, Madden JD, Brenner PF, Wilson JD, Siiteri PK. Origin of estrogen in normal men and in women with testicular feminization. J Clin Endocrinol Metab. 1979;49:905–16. doi: 10.1210/jcem-49-6-905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.