Abstract
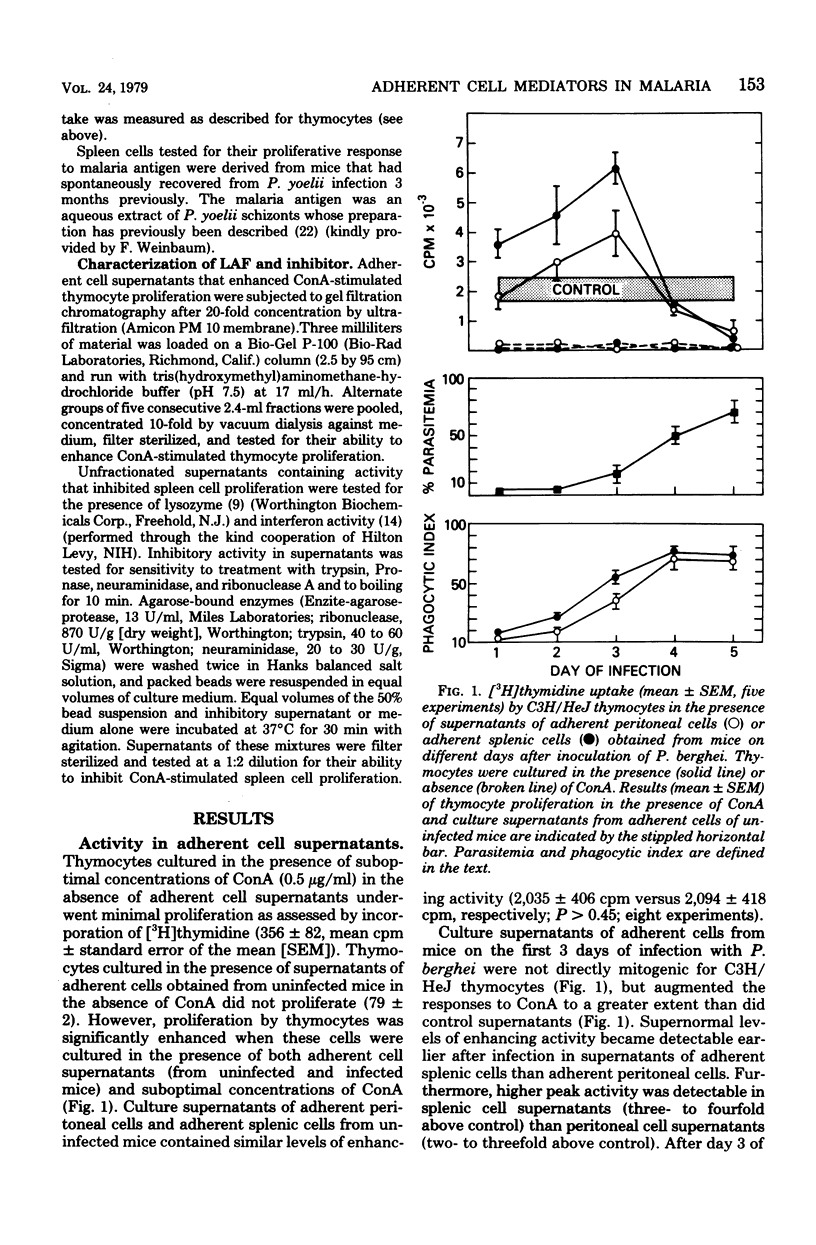
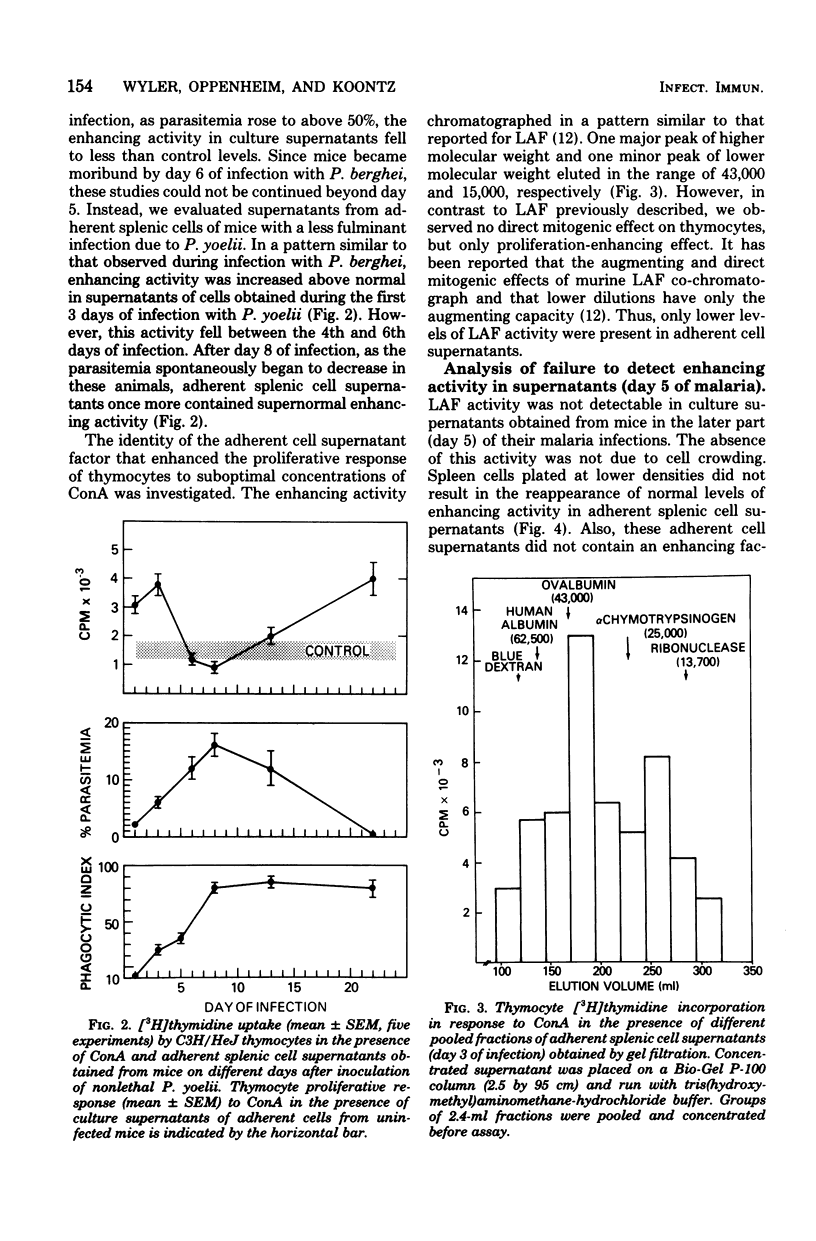
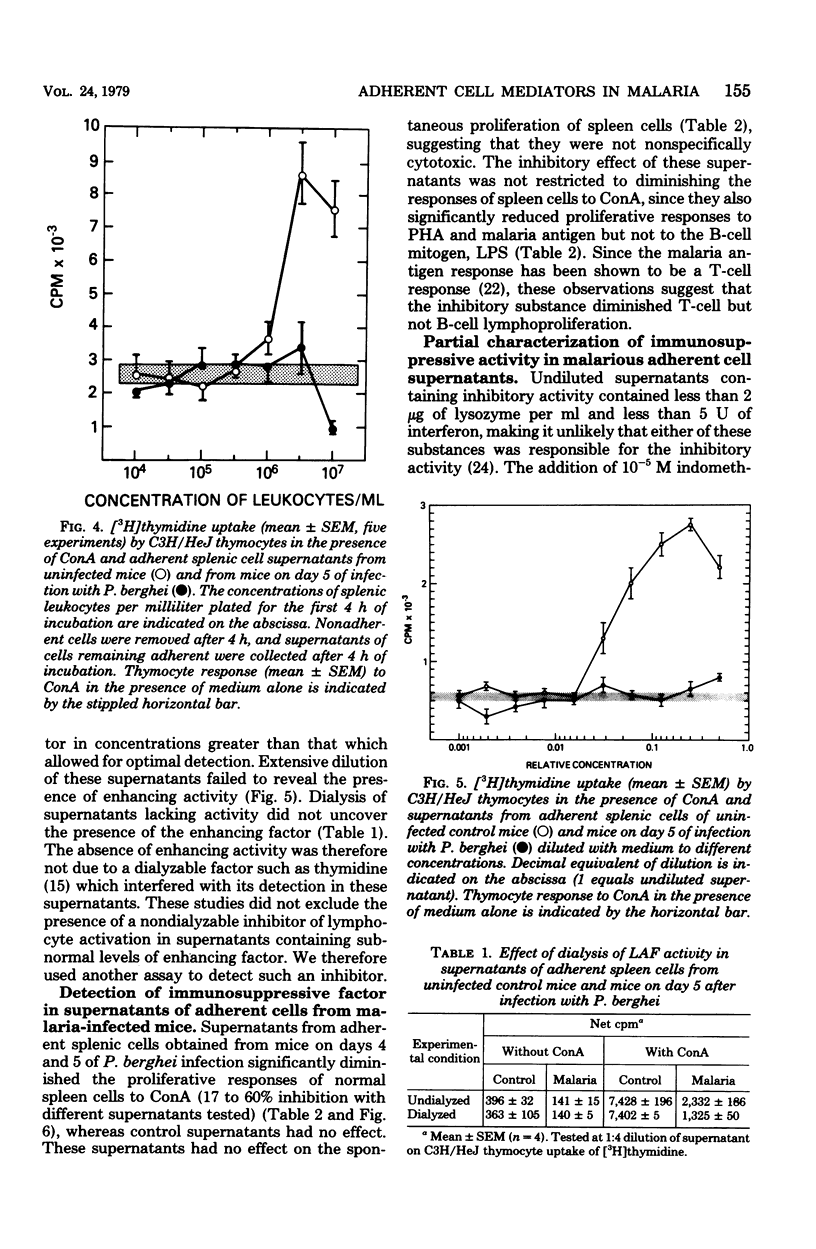
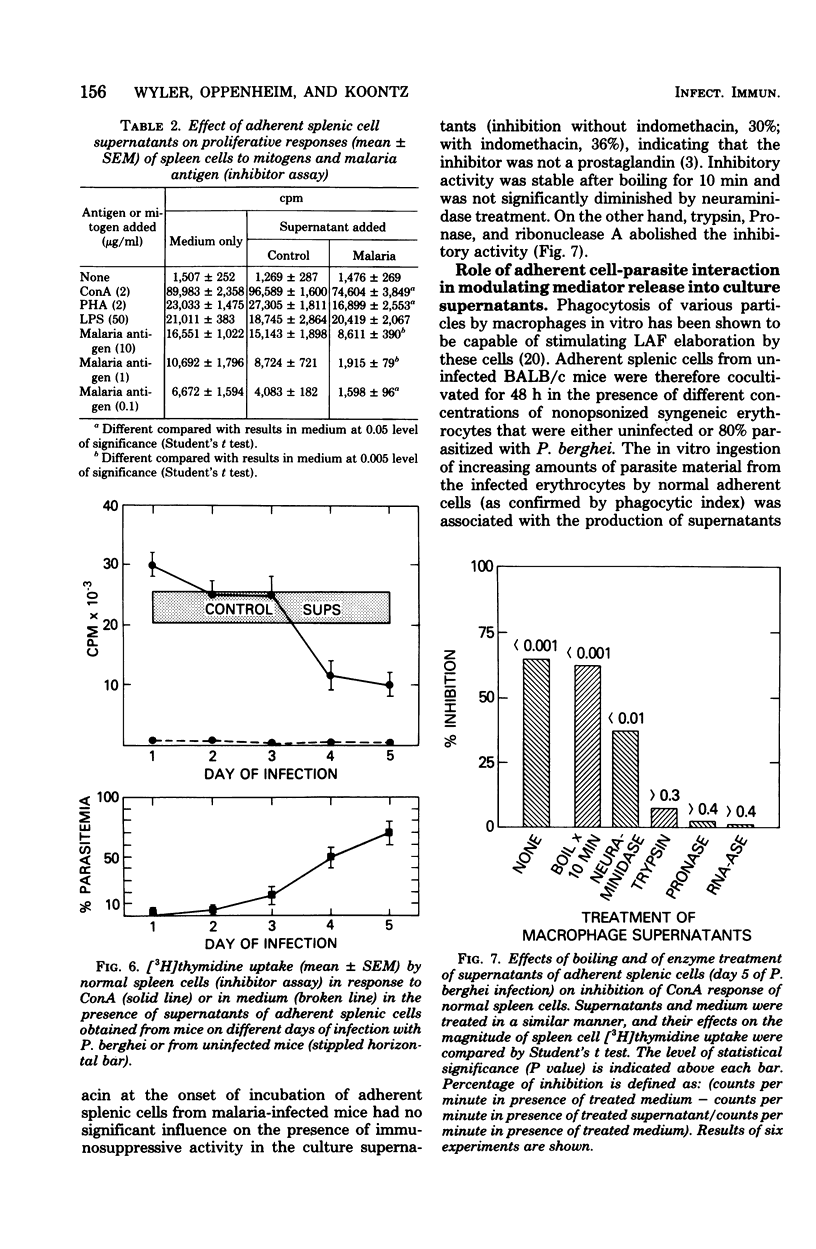
Malaria results in two seemingly paradoxical perturbations of the immune response: polyclonal B-cell activation and immunosuppression. To determine what immunoregulatory role mediators secreted by adherent cells might play in these alterations, we cultured adherent cells from uninfected mice and from mice at different times during infection with Plasmodium berghei or P. yoelii. Culture supernatants obtained from these cells were tested for their ability to enhance the in vitro proliferative responses of thymocytes to suboptimal concentrations of concanavalin A or to inhibit the mitogen-stimulated proliferation of normal spleen cells. Supernatants obtained from adherent cells of mice early in infection (days 1 to 3) contained significantly elevated levels of enhancing activity which on Bio-Gel P-100 chromatography resembled lymphocyte-activating factor. Later in infection (days 4 and 5), these supernatants contained inhibitory activity. Normal adherent cells, when cocultivated in vitro with parasitized erythrocytes, ingested parasite debris and were stimulated to produce the enhancing factor. At high parasite/adherent-cell ratios, cells elaborated an inhibitory factor. These findings suggest that during malaria, adherent cells are converted from a nonspecific helper role to a nonspecific suppressor role. This modulation in function may be due to the direct interaction between adherent cells and parasitized erythrocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose C. T. Regulation of the secondary antibody response in vitro. II. Chemical properties of an antibody inhibitory material (AIM) produced in antigen-stimulated rabbit lymph node organ culture. J Exp Med. 1973 Jun 1;137(6):1369–1392. doi: 10.1084/jem.137.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker L. R. Experimental malaria: effects upon the immune response to different antigens. J Infect Dis. 1971 Jan;123(1):99–101. doi: 10.1093/infdis/123.1.99. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Bankhurst A. D., Peake G. T., Saiki J. H., Williams R. C., Jr Prostaglandin-producing suppressor cells in Hodgkin's disease. N Engl J Med. 1977 Nov 3;297(18):963–968. doi: 10.1056/NEJM197711032971802. [DOI] [PubMed] [Google Scholar]
- Greenwood B. M. Possible role of a B-cell mitogen in hypergammaglobulinaemia in malaria and trypanosomiasis. Lancet. 1974 Mar 16;1(7855):435–436. doi: 10.1016/s0140-6736(74)92386-1. [DOI] [PubMed] [Google Scholar]
- Houck J. C., Kanagalingam K., Hunt C., Attallah A., Chung A. Lymphocyte and fibroblast chalones: some chemical properties. Science. 1977 May 20;196(4292):896–897. doi: 10.1126/science.140460. [DOI] [PubMed] [Google Scholar]
- KUVIN S. F., TOBIE J. E., EVANS C. B., COATNEY G. R., CONTACOS P. G. Fluorescent antibody studies on the course of antibody production and serum gamma globulin levels in normal volunteers infected with human and simian malaria. Am J Trop Med Hyg. 1962 Jul;11:429–436. doi: 10.4269/ajtmh.1962.11.429. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Chused T. M., Herberman R. B., Holden H. T., Lavrin D. H. Evidence of suppressor cell activity in spleens of mice bearing primary tumors induced by Moloney sarcoma virus. J Exp Med. 1974 Jun 1;139(6):1473–1487. doi: 10.1084/jem.139.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman W. J., Farrar J. J., Oppenheim J. J., Fuller-Bonar J., Dougherty S. Association of a low molecular weight helper factor(s) with thymocyte proliferative activity. J Immunol. 1977 Jul;119(1):55–60. [PubMed] [Google Scholar]
- LITWACK G. Photometric determination of lysozyme activity. Proc Soc Exp Biol Med. 1955 Jul;89(3):401–403. doi: 10.3181/00379727-89-21824. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S., Oppenheim J. J. Bidirectional amplification of macrophage-lymphocyte interactions: enhanced lymphocyte activation factor production by activated adherent mouse peritoneal cells. J Immunol. 1977 Jan;118(1):77–82. [PubMed] [Google Scholar]
- Namba Y., Jegasothy B. V., Waksman B. H. Regulatory substances produced by lymphocytes. V. Production of inhibitor of DNA synthesis (IDS) by proliferating T lymphocytes. J Immunol. 1977 Apr;118(4):1379–1384. [PubMed] [Google Scholar]
- Oie H. K., Buckler C. E., Uhlendorf C. P., Hill D. A., Baron S. Improved assays for a variety of interferons. 1. Proc Soc Exp Biol Med. 1972 Sep;140(4):1178–1181. doi: 10.3181/00379727-140-36636. [DOI] [PubMed] [Google Scholar]
- Opitz H. G., Niethammer D., Jackson R. C., Lemke H., Huget R., Flad H. D. Biochemical characterization of a factor released by macrophages. Cell Immunol. 1975 Jul;18(1):70–75. doi: 10.1016/0008-8749(75)90037-4. [DOI] [PubMed] [Google Scholar]
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. 3. Suppression of plaque-forming cell responses in vitro by supernatant fluids from concanavalin A-activated spleen cell cultures. J Immunol. 1974 Apr;112(4):1360–1368. [PubMed] [Google Scholar]
- Rosenberg Y. J. Autoimmune and polyclonal B cell responses during murine malaria. Nature. 1978 Jul 13;274(5667):170–172. doi: 10.1038/274170a0. [DOI] [PubMed] [Google Scholar]
- Shaper A. G., Kaplan M. H., Mody N. J., McIntyre P. A. Malarial antibodies and autoantibodies to heart and other tissues in the immigrant and indigenous peoples of Uganda. Lancet. 1968 Jun 22;1(7556):1342–1346. doi: 10.1016/s0140-6736(68)92037-0. [DOI] [PubMed] [Google Scholar]
- Stobo J. D. Immunosuppression in man: suppression by macrophages can be mediated by interactions with regulatory T cells. J Immunol. 1977 Sep;119(3):918–924. [PubMed] [Google Scholar]
- Warren H. S., Weidanz W. P. Malarial immunodepression in vitro: adherent spleen cells are functionally defective as accessory cells in the response to horse erythrocytes. Eur J Immunol. 1976 Nov;6(11):816–819. doi: 10.1002/eji.1830061112. [DOI] [PubMed] [Google Scholar]
- Weinbaum F. I., Evans C. B., Tigelaar R. E. An in vitro assay for T cell immunity to malaria in mice. J Immunol. 1976 May;116(5):1280–1283. [PubMed] [Google Scholar]
- Weinbaum F. I., Weintraub J., Nkrumah F. K., Evans C. B., Tigelaar R. E., Rosenberg Y. J. Immunity to Plasmodium berghei yoelii in mice. II. Specific and nonspecific cellular and humoral responses during the course of infection. J Immunol. 1978 Aug;121(2):629–636. [PubMed] [Google Scholar]
- Weinstein Y., Brodeur B. R., Melmon K. L., Merigan T. C. Interferon inhibition of lymphocyte mitogenesis. Immunology. 1977 Sep;33(3):313–319. [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Remington J. S. Studies on the regulation of lymphocyte reactivity by normal and activated macrophages. Cell Immunol. 1977 Apr;30(1):108–121. doi: 10.1016/0008-8749(77)90052-1. [DOI] [PubMed] [Google Scholar]
- Wyler D. J., Gallin J. I. Spleen-derived mononuclear cell chemotactic factor in malaria infections: a possible mechanism for splenic macrophage accumulation. J Immunol. 1977 Feb;118(2):478–484. [PubMed] [Google Scholar]
- Wyler D. J., Herrod H. G., Weinbaum F. I. Response of sensitized and unsensitized human lymphocyte subpopulations to Plasmodium falciparum antigens. Infect Immun. 1979 Apr;24(1):106–110. doi: 10.1128/iai.24.1.106-110.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



