Abstract
Recombinant adeno-associated viral (rAAV) vectors have gained attention for human gene therapy because of their high safety and clinical efficacy profile. For factor VIII gene delivery, splitting the coding region between two AAV vectors remains a viable strategy to avoid the packaging capacity limitation (∼5.0 kb). However, it is time-consuming and labor-intensive to produce two rAAV vectors in separate batches. Here we demonstrated successful production of dual rAAV vectors for hemophilia A gene therapy in a single preparation. When the AAV vector plasmids carrying the human factor VIII heavy chain (hHC) and the light chain (hLC) expression cassettes were cotransfected into 293 cells along with the AAV rep&cap and mini-adenovirus helper plasmids, both rAAV-hHC and rAAV-hLC were produced at the desired ratio and in high titer. Interestingly, the rAAV-hHC vectors always yielded higher titers than rAAV-hLC vectors as a result of more efficient replication of rAAV-hHC genomes. The resulting vectors were effective in transducing the tissue culture cells in vitro. When these vectors were administered to hemophilia A mice, factor VIII was detected in the mouse plasma by both the activated partial thromboplastin time assay and enzyme-linked immunosorbent assay. The functional activity as well as the antigen levels of secreted factor VIII were similar to those of vectors produced by the traditional method. The dual-vector production method has been successfully extended to both AAV2 and AAV8 serotypes. In conclusion, cotransfection of vector plasmids presents an efficient method for producing dual or multiple AAV vectors at significantly reduced cost and labor.
Introduction
Recombinant adeno-associated viral (rAAV) vectors have shown great promise for gene therapy because of their safety and clinical efficacy as demonstrated in a number of phase I/II clinical trials (Muramatsu et al., 2010; Nathwani et al., 2011; Bowles et al., 2012; Ferreira et al., 2013). Recently, the first gene therapy product, Glybera, utilizing rAAV1 as a vector, was approved by the European Commission for treatment of a rare disease, lipoprotein lipase deficiency (Miller, 2012; Ferreira et al., 2013; Gaudet et al., 2013). However, the primary limitation of the rAAV vector is that it only permits the packaging of ∼5.0 kb of exogenous DNA because of the small physical size (∼20 nm) of the virion (Dong et al., 2010; Lai et al., 2010; Wu et al., 2010). This limitation poses significant challenge for delivering large genes necessary for gene therapy of cystic fibrosis (Ostedgaard et al., 2005; Yan et al., 2013), Duchenne muscular dystrophy (Wang et al., 2000; Goncalves et al., 2005; Koo et al., 2013), hemophilia A (Gnatenko et al., 1999; McIntosh et al., 2013; Siner et al., 2013), and other genetic diseases with corresponding genes over this packaging limit.
The primary method to bypass the packaging limitation is to reduce the expression cassettes. Specifically for the 7.0 kb cDNA of the human coagulation factor VIII (hFVIII) gene, the nonessential B domain can be removed to get the size closer to the rAAV packaging capacity (Gnatenko et al., 1999; Lu et al., 2008). Delivery of the B-domain-deleted FVIII (hBDD-FVIII) using rAAV vectors (hBDD-FVIII) has successfully been demonstrated in mouse, canine, and nonhuman primate models (Sarkar et al., 2003, 2004; Ishiwata et al., 2006; Jiang et al., 2006; Scallan et al., 2006; Lu et al., 2008; Sabatino et al., 2011; McIntosh et al., 2013; Siner et al., 2013). However, the size of hBDD-FVIII (∼4.3 kb) is still close to the AAV packaging capacity, leaving few choices for regulatory elements required for high levels of transgene expression, including promoters, enhancers, introns, and polyadenylation sequences.
An alternative method to address packaging limitation is to split the oversized transgenes into two or more segments (Burton et al., 1999; Yan et al., 2000, 2007; Duan et al., 2001; Chao et al., 2002; Lai et al., 2005, 2006; Ghosh et al., 2006, 2011). The dual-vector strategy has been widely explored to express large genes, including hFVIII, dysferlin, dystrophin, and CFTR, using various strategies, including trans-splicing, overlapping, and hybrid of both (Ghosh et al., 2008; Trapani et al., 2013). To produce trans-splicing rAAV vectors, the split segments contain splicing signals, splice acceptor, and splice donor, respectively (Yan et al., 2000). The rAAV genomes first undergo recombination after coinfection and then the engineered splicing signals get removed during maturation of mRNA to restore the correct junctions. In the overlapping vectors, the two split segments share a common sequence (Duan et al., 2001; Halbert et al., 2002; Ghosh et al., 2006). These segments undergo homologous recombination to recover the full-length gene after coinfection. The hybrid vectors take the advantages of both trans-splicing and overlapping strategies that may further increase the expression efficiency of large genes (Yan et al., 2007; Ghosh et al., 2008, 2011; Trapani et al., 2013). For those genes working as heterodimers, such as hFVIII, they can be split into two expression cassettes and packaged separately. The combination of dual rAAV vectors can correct the hemophilia A phenotype in animal experiments by our and other labs (Burton et al., 1999; Chao et al., 2002; Sarkar et al., 2003; Scallan et al., 2003; Chen et al., 2007, 2009; Sabatino et al., 2011). Recently, this split strategy was further developed to produce three vectors to deliver even larger therapeutic gene, a 11 kb dystrophin (Koo et al., 2013).
Although the dual-vector strategy expands the utility of rAAV in the field of gene therapy, production of two independent rAAV vectors is a time- and labor-consuming task. The problem would only get worse if the size of genes dictates use of more than two rAAV vectors. In an attempt to solve this problem, Ma and colleagues (2010) reported a quadruple-plasmid transfection scheme that combined production of a conventional single-stranded rAAV2 vector with a self-complementary AAV2-protein phosphatase 5 (scAAV2-PP5) vector. The latter was used to improve the transduction of yet another single-stranded rAAV2 (Ma et al., 2010). In this study, we demonstrated simultaneous production of two rAAV vectors coding for fragments of an oversized hFVIII gene. These mixed vectors could express the functional hFVIII protein at a similar level as those vectors produced separately, both in vitro and in vivo. This novel approach could be applicable to package other large genes into rAAV vectors and dramatically reduce the labor and production costs.
Materials and Methods
Plasmids and vectors
The plasmids pAAV-hHC (10.5 kb, vector size 3.7 kb) and pAAV-hLC (10.6 kb, vector size 3.6 kb) containing hHC and hLC have been described in our previous studies (Chen et al., 2007, 2009). The expression of hHC and hLC was directed by either a human β-actin promoter with a CMV enhancer (CB) or a liver-specific promoter hAAT combined with an ApoE enhancer. rAAV vectors were generated by a triple-plasmid cotransfection method as described previously (Dong et al., 2010, 2013). To make rAAV-hHC and rAAV-hLC vectors separately, pAAV-Rep&Cap (8.0 kb), pAd helper (15 kb), and pAAV-hHC or pAAV-hLC plasmids were cotransfected into HEK293 cells cultured at a ratio of 1:1:1. About 4.5 μg DNA in total was used in each well of a 6-well plate and 0.45 mg DNA for each roller bottle. To make the rAAV-hHC&hLC mixture vector, different ratios of pAAV-hHC and pAAV-hLC plasmids were cotransfected with pAAV-Rep&Cap and pAd helper. The total amount of pAAV-hHC+pAAV-hLC is equal to those of pAAV-Rep&Cap and pAd helper. The transfected cells and medium were harvested 72 hr posttransfection. All rAAV vectors were purified by two rounds of cesium chloride gradient ultracentrifuge. The collected rAAV vectors were exchanged extensively against PBS buffer with 5% D-sorbitol. Vectors were characterized by silver staining, quantitative real-time PCR (qPCR), and Southern blot. The final vectors were stored at −80°C before infection or administration.
Silver staining
Purified virus (1–2×1010 particles) was mixed with protein loading dye and heated at 95°C for 10 min. The denatured samples were then separated on a 10% sodium dodecyl sulfate–polyacrylamide electrophoresis (SDS-PAGE) gel. After electrophoresis, the gel was stained by Pierce Silver Stain Kit (Thermo Scientific) according to the manufacturer's instructions.
Western blot
Total protein fraction was extracted with a lysis buffer consisting of 50 mM Tris at pH 8.0, 150 mM NaCl, 1% Triton X-100, 10 mM DTT, and 1× protein inhibitor (Roche). Cell lysates (50 μg protein) were resolved on 10% SDS-PAGE gel and transferred to a nitrocellulose membrane (Bio-Rad). After blocking the membrane with 5% nonfat dry milk in TBST buffer containing 25 mM Tris•HCl at pH 8.0, 150 mM NaCl, and 0.1% Tween 20, the membrane was incubated with the primary antibody, anti-AAV capsid (B1; American Research Products), or anti-AAV Rep (303.9; American Research Products), at a dilution of 1:500 at 4°C overnight. The membrane was washed and incubated with a horseradish peroxidase–conjugated sheep anti-mouse IgG antibody (Sigma) at a dilution of 1:2000. The membrane was developed using an enhanced chemiluminescent substrate (Amersham-Pharmacia Biotech).
Southern blot
The rAAV vectors were treated with DNaseI (NEB) at 37°C for 30 min, and the reactions were stopped by adding 10 mM EDTA plus heating at 75°C for 10 min. The mixture was then treated with Proteinase K (Denville Scientific) at 55°C for 2 hr and inactivated by heating the samples to 95°C for 20 min. The viral genome was purified using PCR purification Kit. Before loading, the purified DNA was heated at 95°C and snap-cooled in ice immediately. After electrophoresis in a 0.8% agarose gel, Southern blot was performed using either a hHC or hLC probe. A 1229 bp DNA fragment digested with AccI and KpnI from pAAV-hHC was used for hHC probe. A 733 bp DNA fragment digested with AleI and ApaLI fragment from pAAV-hLC was used for hLC probe. The SmaI-digested fragments from pAAV-hHC and pAAV-hLC were used as the viral genome standards for hHC and hLC, respectively. To examine the replication forms of the rAAV genome, HEK293 cells were transfected in a 6-well plate and harvested at 48 hr after transfection. Low-molecular-weight Hirt DNA was extracted and used for DpnI digestion overnight. The same amount of DNA was resolved in a 0.8% agarose gel. Replication-form viral genome was examined by hHC and hLC probes as described above.
Quantitative real-time PCR
About 10 μl of the vector was treated with DNaseI and proteinase K as described above. The mixture was diluted at a ratio of 1:500 or 1/5000. All qPCRs were carried out in an Eppendorf Mastercycler ep realplex machine using the Fast SYBR Green Master Mix and realplex software. In a 20 μl reaction system, the final concentrations of reagents were 0.4 μM of each primer, 5 mM MgCl2, 5 μl template, and 1× Master Mix. The PCR protocol was as follows: 1 cycle of 20 sec at 95°C followed by 40 cycles of 3 sec at 95°C and 30 sec at 60°C. A melting curve analysis was added after PCR. The following primers were used: hHC-forward: 5′-CTGAAATGGATGTGGTCAGG-3′; hHC-reverse: 5′-AGTCCCAGTCCTCCTCTTCA-3′; hLC-forward: 5′-CCAGATGGAAGATCCCACTT-3′; hLC-reverse: 5′-GCTGAGCAGATACCATCGAA-3′. The number of copies of hHC and hLC in each sample was determined by a standard curve made by serial dilutions of a plasmid carrying hBDD-FVIII.
rAAV vector transduction in vitro
HEK293 cells were purchased from the American Type Culture Collection. GM16095 cells were purchased from Coriell Institute for Medical Research. Cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) with 10% fetal bovine serum (FBS; HyClone), penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37°C in a moisturized environment supplied with 5% CO2. All vectors were generated in six-well plates. Quadruple-plasmid and triple-plasmid transfections were carried out using Polyjet following the protocol. After transfection overnight, the culture media were changed and the cells were continuously grown in the growth media. rAAV vectors were harvested at 72 hr posttransfection and freeze–thawed for three rounds. About 1×1010 vg vectors were used to infect 16,095 cells either with rAAV2-hHC&hLC or with rAAV2-hHC+rAAV2-hLC for 4 hr. The cells were then maintained in F10 media (Mediatech, Inc.) with 2% inactivated FBS for 68 hr before the cells and medium were collected. The secreted FVIII proteins were analyzed by the activated partial thromboplastin time (aPTT) assay and enzyme-linked immunosorbent assay (ELISA).
Animal procedures
Exon 16 FVIII knockout HA mice were obtained from Dr. Haig Kazazian (University of Pennsylvania, Philadelphia, PA). All mice were housed in a specific pathogen-free environment with a normal diet. All surgical procedures involving mice were in accordance with institutional guidelines under approved protocols at the Temple University. For tail vein injection, vectors were diluted with saline and the total volume was 200 μl. The mice were put above a heat lamp to increase blood flow in tail veins. Mouse plasma samples after vector administration were harvested by retro-orbital bleeding at regular intervals as described. For coagulation analysis, blood samples were collected using sodium citrate as an anticoagulant at a final concentration of 0.38% (w/v). The blood samples were then centrifuged at 4°C for 10 min at 10,000 rpm. The plasma samples were then collected and stored in −80°C before FVIII assays.
Quantitative analysis of FVIII antigen and activity
Biologically active FVIII in plasma was measured using the aPTT assay as previously described (Chen et al., 2007). ReFacto (Wyeth) was used as the standard. hHC and hLC antigen were determined using chain-specific ELISAs following typical sandwich ELISA protocol. All antibodies for ELISA were purchased from Green Mountain Antibodies. For measuring factor VIII heavy chain, a biotin-conjugated GMA-8015 was used as a detection antibody. For measuring the light chain, a biotin-conjugated GMA-8018 was used as a detection antibody. The final antibody used was horseradish peroxidase avidin D (Vector Labs). All antibodies were used at a concentration of 2 μg/ml. After the final wash, the antigen was detected using MTB substrate (Roche) and the absorbance was read at 450 nm.
Statistical analyses
Two-tailed Student's t-tests and one-way ANOVA with Bonferroni multiple comparison posttest were performed. The differences were considered significant when p was<0.05. The analysis was performed using SPSS 11.0.
Results
Dual-vector plasmid transfection method can produce fixed ratio of rAAV-hHC and rAAV-hLC vectors in one preparation
We recently reported that coadministration of rAAV vectors carrying enhanced hHC and hLC led to more biologically active hFVIII in vivo (Chen et al., 2007, 2009). These two vectors were generated separately using the standard triple-plasmid transfection protocol (Fig. 1A), a time-consuming and labor-intensive procedure requiring two independent vector preparations. To explore a possibility of generating the two vectors in one preparation, we transfected HEK293 cells with two vector plasmids (pAAV-hHC and pAAV-hLC) along with the two helper plasmids (pAd-Helper and pAAV2-Rep&Cap) at a ratio of 1:1 (Fig. 1B). As expected, both heavy chain and light chain vectors were generated in this small-scale setting. Both rAAV2-hHC and rAAV2-hLC vectors could be detected by quantitative PCR using the primers targeting hHC and hLC, respectively (Table 1).
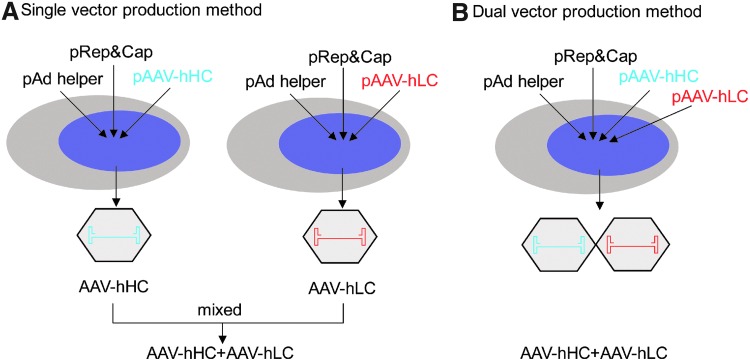
FIG. 1.
Schematic illustration of the production strategies for dual rAAV vectors. (A) Single-vector plasmid transfection method. Two independent triple-plasmid transfection production procedures are required. (B) Dual-vector plasmid cotransfection production method. The two vector plasmids get cotransfected into the host production cells at the same time. hHC, human factor VIII heavy chain; hLC, human factor VIII light chain; rAAV, recombinant adeno-associated viral. Color images available online at www.liebertpub.com/hgtb
Table 1.
Quantitative Real-Time PCR Analysis of hHC and hLC in the Vectors Produced by Single-Vector Plasmid Transfection Production Method (hHC and hLC) and Dual-Vector Plasmid Transfection Method (hHC&hLC)
| Vector types | Names | hHC (vg/cell) | hLC (vg/cell) | Total (vg/cell) | hHC&hLC ratio |
|---|---|---|---|---|---|
| AAV2 | hHC&hLC (1:1) | 0.43±0.12E+5 | 0.26±0.16E+5 | 0.69±0.14E+5 | 1.64±0.16 |
| hHC&hLC (4:1) | 0.58±0.09E+5 | 0.09±0.04E+5 | 0.67±0.08E+5 | 6.44+0.12 | |
| hHC | 0.69±0.16E+5 | — | 0.69±0.16E+5 | — | |
| hLC | —a | 0.53±0.05E+5 | 0.53±0.05E+5 | — | |
| AAV8 | hHC&hLC (1:1) | 0.94±0.32E+5 | 0.50±0.12E+5 | 1.44±0.22E+5 | 1.88±0.20 |
| hHC&hLC (4:1) | 1.59±0.19E+5 | 0.19±0.04E+5 | 1.78±0.12E+5 | 8.37+0.38 | |
| hHC | 1.72±0.56E+5 | — | 1.72±0.56E+5 | — | |
| hLC | — | 1.19±0.36E+5 | 1.19±0.36E+5 | — |
AAV, adeno-associated viral; hHC, human factor VIII heavy chain; hLC, human factor VIII light chain; qPCR, quantitative real-time PCR.
For the AAV2 vectors, the vectors released into the culture medium were used for the qPCR measurements. For the AAV8 vectors, purified vectors from large-scale production were used.
Very low titer or no reaction.
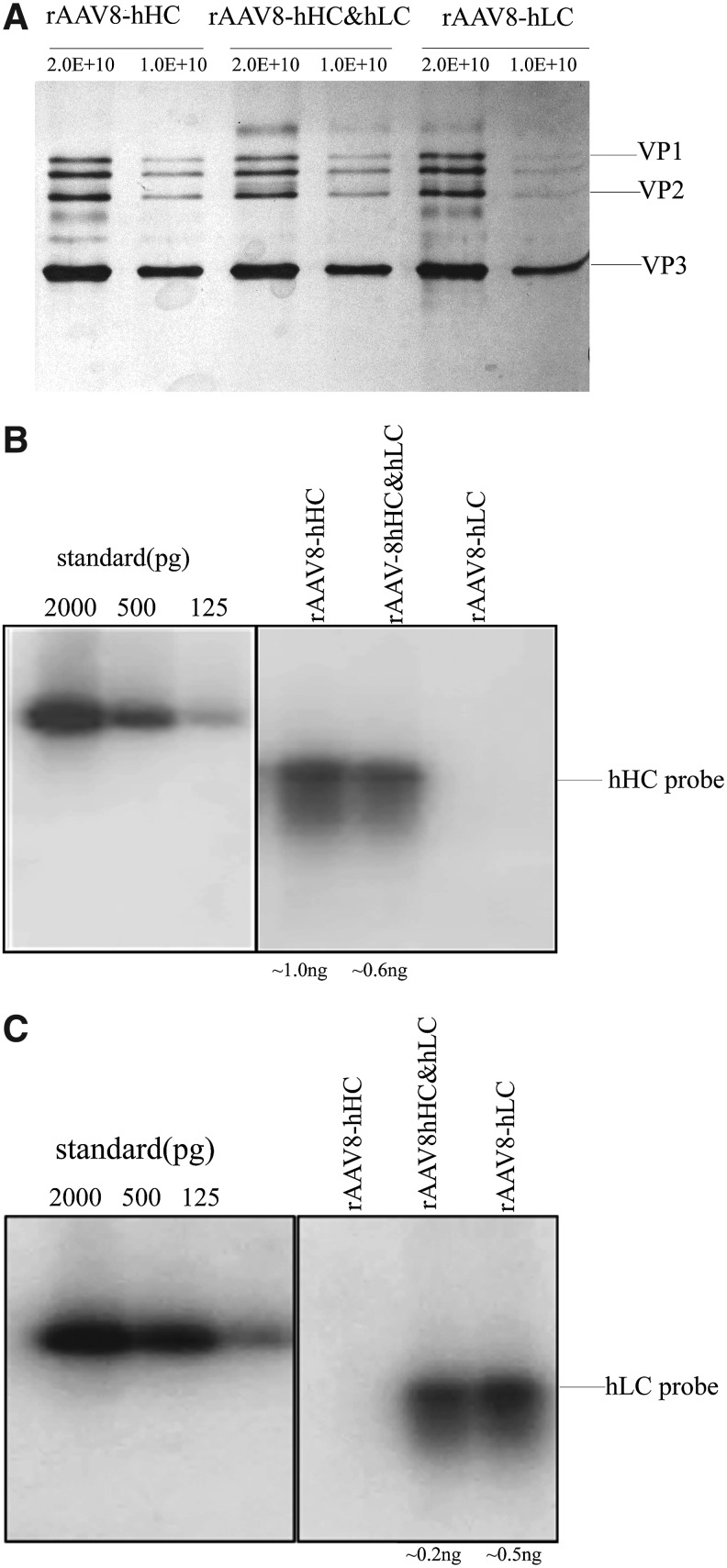
To further confirm the feasibility of producing rAAV-hHC and rAAV-hLC vectors in one preparation, we scaled it up by using 10 roller bottles. In addition, we switched to serotype AAV8 by replacing pAAV2-Rep&Cap with pAAV8-Rep&Cap. As expected, we successfully purified rAAV8-hHC and rAAV8-hLC vectors from the production system following the standard two rounds of CsCl gradient ultracentrifugation. Viral capsids revealed by silver staining showed that there was no alteration in the composition between the single-vector plasmid transfection method and the dual-vector plasmid transfection method (Fig. 2A). The viral particle yields were also similar. Southern blot analysis further confirmed that the vectors produced by the dual-vector plasmid transfection method contained both rAAV8-hHC and rAAV8-hLC DNA (Fig. 2B and C). Vector genome titer assayed by qPCR analysis confirmed the viral particle titers. These results indicated that dual-vector cotransfection method could produce rAAV-hHC and tAAV-hLC at a desired ratio.
FIG. 2.
Comparison of vectors produced by single-vector plasmid transfection (rAAV8-hHC and rAAV8-hLC) and dual-vector plasmid transfection production method. (A) Silver staining of the purified vectors. About 1×1010 of purified viral particles were denatured, separated, and stained on 10% SDS-PAGE gel. (B and C) Southern blot analysis of DNA genome of AAV vector-probed hHC-specific (B) and hLC-specific (C) probes. DNA extracted from 1.0×109 purified viral particles was loaded to each lane. The SmaI fragments of hHC and hLC plasmids were used as the corresponding controls for viral genomes. The vector genome bands were scanned and quantified, and the amount in nanograms was indicated below the gels. SDS-PAGE, sodium dodecyl sulfate–polyacrylamide electrophoresis.
Effect of input vector plasmid ratio on the production of hHC and hLC vector
The disparity of hHC and hLC secretion can lead to a chain imbalance issue, thus decreasing the efficacy of rAAV-FVIII vector delivery and resulting in the majority of expressed transgene products being nonfunctional. We previously showed that the chain imbalance issue could be resolved by adjusting the ratio of hHC and hLC (Chen et al., 2007, 2009). The desired ratio of hHC and hLC vectors in vivo was found to be 4:1. Thus, we attempted to study the factors that affect the ratio of two vectors. As shown in Table 1, when pAAV-hHC and pAAV-hLC plasmids were cotransfected at 1:1 ratio, the ratio of rAAV8-hHC to rAAV8-hLC vector was 1.88. Repeated measurement ruled out the possibility of system error since the plasmid mixture of pAAV-hHC/pAAV-hLC(1:1) accurately came up with a ratio 1 in the same assay (data not shown).
To confirm that the input vector plasmid ratio may be different from the vector ratio, we performed another batch of vector production with pAAV-hHC and pAAV-hLC transfected at a 4:1 ratio. Interestingly, the vector ratio of AAV8-hHC to AAV8-hLC vectors became 7.37 (Table 1). Therefore, this system showed that the heavy chain vector can always be packaged approximately twofold more efficiently than the light chain vector. Knowing this ratio, the adjustment of the ratio of the input vector plasmids can result in the desired ratio of heavy chain and light chain vector.
Efficient hHC vector DNA replication led to the higher yield of hHC vector
To investigate the reasons behind the less efficient packaging of the hLC vector, we first attempted to determine whether it was because of differences in Cap and Rep expression. The Western blot analysis of the three Cap proteins did not show obvious difference between the dual- or single-vector plasmid transfection approaches (Fig. 3A). Likewise, Rep protein expression did not show significant difference either (Fig. 3B). These results suggested that the packaging difference between hHC and hLC was not because of the Cap and Rep expressions.
FIG. 3.
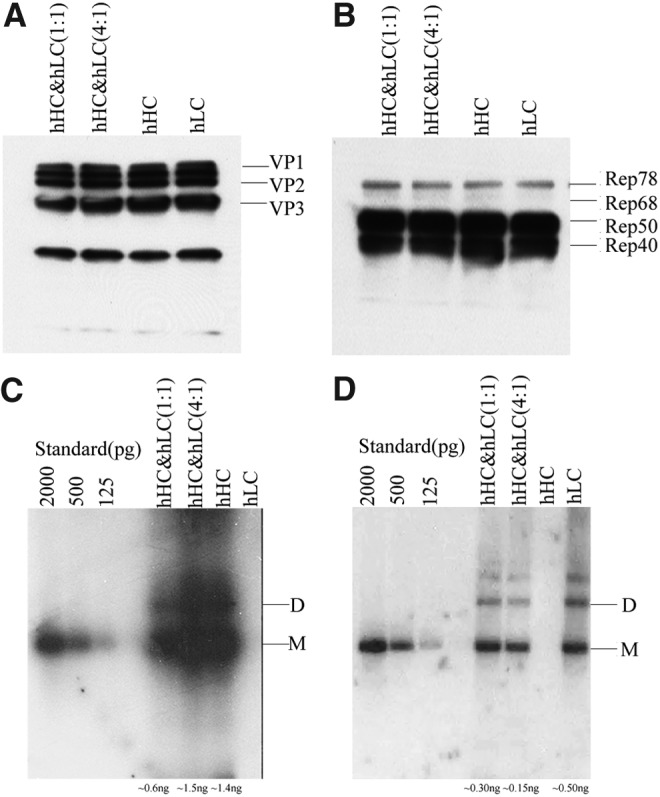
Profiles of expression of Cap and Rep and replication of hHC and hLC plasmids. HEK293 cells were transfected with a single-vector plasmid or dual-vector plasmid. For Western blot, 50 μg of protein samples was denatured and loaded in a 10% SDS-PAGE gel. Anti-AAV capsid (A) or anti-AAV Rep (B) was used to reveal the rep and cap expression profile. The membrane was incubated with a horseradish peroxidase (HRP)-conjugated sheep anti-mouse IgG antibody and developed with the substrate. For southern blot (C and D), 15 ng of Hirt DNAs was digested with DpnI overnight. After electrophoresis in a 0.8% agarose gel, Southern blot was performed using either a 32P-labeled hHC-specific probe (C) or hLC-specific probe (D). The SmaI fragments from pAAV-hHC and pAAV-hLC were used as the molecular weight controls of AAV genomes. “D” indicates dimer and “M” stands for monomer. The dimer band was quantified and the amount in nanograms was indicated below the gels.
We then analyzed whether the vector plasmid replication played a role for causing the packaging difference. Indeed, Southern blot analysis of Hirt DNA extracted from production 293 cells revealed that the hLC vector DNA replicated less efficiently than the hHC vector DNA (Fig. 3C and D). Their replication disparities likely caused the difference in the final vector yield.
The hFVIII protein was expressed in vitro at a similar level by using rAAV2-hHC&hLC vectors and rAAV2-hHC+rAAV2-hLC
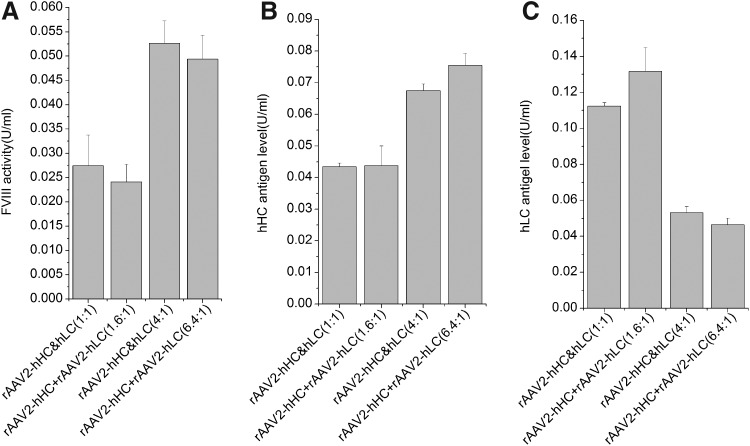
We then tested whether the vectors produced by the dual-vector method (the “rAAV2-hHC&hLC” mixture under CB promoter) could drive expression of biologically active FVIII. As a control, rAAV2-hHC and rAAV2-hLC made separately (the “rAAV2-hHC+rAAV2-hLC” mixture) were combined before transduction at the same ratio (based on qPCR measurements) as the rAAV2-hHC&hLC mixture (Table 1). About 1×1010 vg vectors of either mixture were used to infect GM16095 cells. As shown in Fig. 4A, cells infected with either rAAV2-hHC&hLC or rAAV2-hHC+rAAV2-hLC produced the protein of similar biological activities. Figure 4B and C shows that the antigen levels of hHC and hLC were also similar. This indicates that the coproduction method could generate the two vectors that yield biologically active protein at a similar level as the vectors produced separately.
FIG. 4.
Expression of hFVIII proteins in vitro. GM16095 cells were transduced with AAV2-hHC and AAV2-hLC that were generated by single-vector plasmid transfection or AAV2-hHC&hLC dual-vector plasmid transfection system. The hFVIII expression levels were detected at 72 hr posttransduction by aPTT (A), hHC-specific (B), and hLC-specific (C) ELISAs. A recombinant B-domain-deleted FVIII, ReFacto, was used as the standard. The ratio in hHC&hLC indicated the plasmids ratio of hHC and hLC during the transfection process. The ratios in hHC+hLC groups were adjusted according the qPCR results of AAV2-hHC&hLC vectors. rAAV2-hHC&hLC represents the dual-vector plasmid mode, and rAAV2-hHC+rAAV2-hLC the single-plasmid. aPTT, activated partial thromboplastin time; ELISA, enzyme-linked immunosorbent assay; qPCR, quantitative real-time PCR.
Vectors produced by the dual-vector plasmid transfection are equally potent as those from the single-vector plasmid transfection method
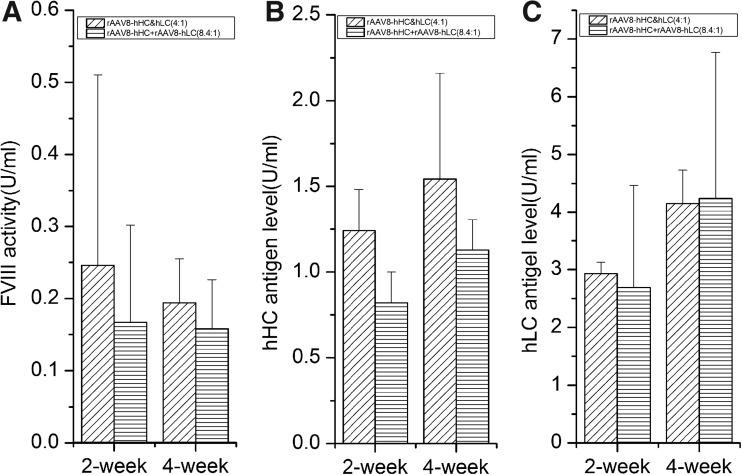
To demonstrate the performance of vectors produced by the dual-vector plasmid transfection method, we injected them into HA mice. In this study, both hHC and hLC were driven by a liver-specific hAAT promoter combined with an ApoE enhancer. One group of mice received the rAAV8-hHC&hLC vectors combined at 4:1 ratio of pAAV-hHC and pAAV-hLC, and the total dose was 5×1011 vg/mouse. Another group of mice were injected with rAAV8-hHC and rAAV8-hLC that were produced separately. In the second group, the ratio of two vectors was adjusted before injection to the same ratio as in rAAV8-hHC&hLC. Again, the two groups of mice produced similar levels of hFVIII, as measured by aPTT and ELISA (Fig. 5). These data showed that the dual-vector production method could generate the mixture of vectors without affecting their function in vivo.
FIG. 5.
Expression of hFVIII proteins in vivo. HA mice in Balb/C background were injected with a total amount of 5×1011 vg/mouse of AAV8-hHC&hLC or AAV8-hHC+AAV8-hLC (n=4 for each group). The ratio of AAV8-hHC and AAV8-hLC was adjusted to 8.4:1 according the qPCR results of AAV8-hHC&hLC vectors. The hFVIII expression levels at 2 and 4 weeks after injection were detected by aPTT (A) and hHC-specific (B), and hLC-specific (C) ELISA. A recombinant B-domain-deleted FVIII, ReFacto, was used as the standard.
Discussion
Splitting genes into two or more vectors represents a sound strategy to deal with AAV packaging limitation. This strategy expands the application scope of the rAAV vector. Taking hFVIII as an example, the gene can be split into a functional heterodimer of hHC and hLC. The combination of two rAAV vectors thus represents a promising strategy for treating hemophilia A. However, as mentioned above, this strategy also increases the time and labor costs. In this study, we demonstrated that two vectors can be produced in the same time by using the dual-vector plasmid transfection method. The resulting vectors showed similar performance in vitro and in vivo (Figs. 4 and 5). Thus, this strategy would significantly improve vector productions for the split-gene strategy.
Since hHC gets secreted at a much lower rate than hLC, it is important to control the ratio of the two vectors to achieve a balanced expression of both chains. We found that the desired ratio of two rAAV vectors could be achieved by adjusting the ratio of the transgene plasmids. Interestingly, our results showed that the final vector ratio could also be different from the input vector plasmid ratio. Nevertheless, the hHC and hLC genome ratios in the final products were very consistent (Table 1). Our Southern blot analysis confirmed that replication difference between the transfected vector plasmid was the primary cause for the vector ratio deviating from the input vector plasmid ratio. Although the input plasmid ratio will not predict the final vector ratio, the fixed ratio of the output vector arising from their replication properties could represent a useful innate property of the dual system that could potentially be utilized to control or fine-tune the ratio of the final vector products. On the other hand, this property also shows an inconvenience in that it would require certain amount of effort beforehand to figure out the proper ratio of the two cis-plasmid DNA for transfection in order to get the desired ratio of the two vectors in the final product, and such ratio is unlikely to be applied to other vectors for other applications because of sequence differences. Nevertheless, for applications that require large amount of vectors such as for large animal studies, this method could still be cost-effective.
In summary, we showed a simplified method to generate two rAAV vectors for the dual-chain strategy for Hemophilia A, which is also potentially applicable to any other large gene. Moreover, this method can be further expanded to generate multiple AAV vectors in a single incubation. One potential envisioned use of this approach could lie in the induced pluripotent stem cell induction field where all necessary vectors get produced simultaneously by transfecting all vector plasmids at once into the production host cell lines. In addition to the reduction in time and labor costs discussed here, the system also represents a significant simplification for clinical production and release testing of such vectors, since the need to characterize two separate vectors for a single drug product would be eliminated.
Acknowledgments
This work was supported by the National Institutes of Health (NIH; R01HL114152 and R01HL084381 to W.X.) and NIH under Ruth L. Kirschstein National Research Service Award T32-HL-007777 from the National Heart Lung and Blood Institute (to A.R.M.).
Author Disclosure Statement
No competing financial interests exist.
References
- Bowles D.E., McPhee S.W., Li C., et al. (2012). Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol. Ther. 20, 443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton M., Nakai H., Colosi P., et al. (1999). Coexpression of factor VIII heavy and light chain adeno-associated viral vectors produces biologically active protein. Proc. Natl. Acad. Sci. USA 96, 12725–12730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao H., Sun L., Bruce A., et al. (2002). Expression of human factor VIII by splicing between dimerized AAV vectors. Mol. Ther. 5, 716–722 [DOI] [PubMed] [Google Scholar]
- Chen L., Zhu F., Li J., et al. (2007). The enhancing effects of the light chain on heavy chain secretion in split delivery of factor VIII gene. Mol. Ther. 15, 1856–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Lu H., Wang J., et al. (2009). Enhanced factor VIII heavy chain for gene therapy of hemophilia A. Mol. Ther. 17, 417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B., Nakai H., and Xiao W. (2010). Characterization of genome integrity for oversized recombinant AAV vector. Mol. Ther. 18, 87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B., Moore A.R., Dai J., et al. (2013). A concept of eliminating nonhomologous recombination for scalable and safe AAV vector generation for human gene therapy. Nucleic Acids Res. 41, 6609–6617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D., Yue Y., and Engelhardt J.F. (2001). Expanding AAV packaging capacity with trans-splicing or overlapping vectors: a quantitative comparison. Mol. Ther. 4, 383–391 [DOI] [PubMed] [Google Scholar]
- Ferreira V., Twisk J., Kwikkers K.L., et al. (2013). Immune responses to intramuscular administration of alipogene tiparvovec (AAV1-LPLS447X) in a phase II clinical trial of Lipoprotein Lipase deficiency (LPLD) gene therapy. Hum. Gene Ther. 25, 180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet D., Methot J., Dery S., et al. (2013). Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 20, 361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Yue Y., and Duan D. (2006). Viral serotype and the transgene sequence influence overlapping adeno-associated viral (AAV) vector-mediated gene transfer in skeletal muscle. J. Gene Med. 8, 298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Yue Y., Lai Y., and Duan D. (2008). A hybrid vector system expands adeno-associated viral vector packaging capacity in a transgene-independent manner. Mol. Ther. 16, 124–130 [DOI] [PubMed] [Google Scholar]
- Ghosh A., Yue Y., and Duan D. (2011). Efficient transgene reconstitution with hybrid dual AAV vectors carrying the minimized bridging sequences. Hum. Gene Ther. 22, 77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnatenko D.V., Saenko E.L., Jesty J., et al. (1999). Human factor VIII can be packaged and functionally expressed in an adeno-associated virus background: applicability to haemophilia A gene therapy. Br. J. Haematol. 104, 27–36 [DOI] [PubMed] [Google Scholar]
- Goncalves M.A., Van Nierop G.P., Tijssen M.R., et al. (2005). High-capacity hybrid viral vector with site-specific integration ability. J. Virol. 79, 3146–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert C.L., Allen J.M., and Miller A.D. (2002). Efficient mouse airway transduction following recombination between AAV vectors carrying parts of a larger gene. Nat. Biotechnol. 20, 697–701 [DOI] [PubMed] [Google Scholar]
- Ishiwata A., Mimuro J., Kashiwakura Y., et al. (2006). Phenotype correction of hemophilia A mice with adeno-associated virus vectors carrying the B domain-deleted canine factor VIII gene. Thromb. Res. 118, 627–635 [DOI] [PubMed] [Google Scholar]
- Jiang H., Pierce G.F., Ozelo M.C., et al. (2006). Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol. Ther. 14, 452–455 [DOI] [PubMed] [Google Scholar]
- Koo T., Popplewell L.J., Athanasopoulos T., and Dickson G. (2014). Triple trans-splicing AAV vectors capable of transferring the coding sequence for full-length dystrophin protein into dystrophic mice. Hum. Gene Ther. 25, 98–108 [DOI] [PubMed] [Google Scholar]
- Lai Y., Yue Y., Liu M., et al. (2005). Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors. Nat. Biotechnol. 23, 1435–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y., Yue Y., Liu M., and Duan D. (2006). Synthetic intron improves transduction efficiency of trans-splicing adeno-associated viral vectors. Hum. Gene Ther. 17, 1036–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y., Yue Y., and Duan D. (2010). Evidence for the failure of adeno-associated virus serotype 5 to package a viral genome>or=8.2 kb. Mol. Ther. 18, 75–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Chen L., Wang J., et al. (2008). Complete correction of hemophilia A with adeno-associated viral vectors containing a full-size expression cassette. Hum. Gene Ther. 19, 648–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Yu C., Zhang W., and Hu J. (2010). A simple template-dependent ligase ribozyme as the RNA replicase emerging first in the RNA world. Astrobiology 10, 437–447 [DOI] [PubMed] [Google Scholar]
- McIntosh J., Lenting P.J., Rosales C., et al. (2013). Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood 121, 3335–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N. (2012). Glybera and the future of gene therapy in the European Union. Nat. Rev. Drug Discov. 11, 419. [DOI] [PubMed] [Google Scholar]
- Muramatsu S., Fujimoto K., Kato S., et al. (2010). A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson's disease. Mol. Ther. 18, 1731–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani A.C., Tuddenham E.G., Rangarajan S., et al. (2011). Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 365, 2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostedgaard L.S., Rokhlina T., Karp P.H., et al. (2005). A shortened adeno-associated virus expression cassette for CFTR gene transfer to cystic fibrosis airway epithelia. Proc. Natl. Acad. Sci. USA 102, 2952–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino D.E., Lange A.M., Altynova E.S., et al. (2011). Efficacy and safety of long-term prophylaxis in severe hemophilia A dogs following liver gene therapy using AAV vectors. Mol. Ther. 19, 442–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar R., Xiao W., and Kazazian H.H., Jr (2003). A single adeno-associated virus (AAV)-murine factor VIII vector partially corrects the hemophilia A phenotype. J. Thromb. Haemost. 1, 220–226 [DOI] [PubMed] [Google Scholar]
- Sarkar R., Tetreault R., Gao G., et al. (2004). Total correction of hemophilia A mice with canine FVIII using an AAV 8 serotype. Blood 103, 1253–1260 [DOI] [PubMed] [Google Scholar]
- Scallan C.D., Liu T., Parker A.E., et al. (2003). Phenotypic correction of a mouse model of hemophilia A using AAV2 vectors encoding the heavy and light chains of FVIII. Blood 102, 3919–3926 [DOI] [PubMed] [Google Scholar]
- Scallan C.D., Jiang H., Liu T., et al. (2006). Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood 107, 1810–1817 [DOI] [PubMed] [Google Scholar]
- Siner J.I., Iacobelli N.P., Sabatino D.E., et al. (2013). Minimal modification in the factor VIII B-domain sequence ameliorates the murine hemophilia A phenotype. Blood 121, 4396–4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani I., Colella P., Sommella A., et al. (2014). Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol. Med. 6, 194–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Li J., and Xiao X. (2000). Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc. Natl. Acad. Sci. USA 97, 13714–13719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Yang H., and Colosi P. (2010). Effect of genome size on AAV vector packaging. Mol. Ther. 18, 80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Zhang Y., Duan D., and Engelhardt J.F. (2000). Trans-splicing vectors expand the utility of adeno-associated virus for gene therapy. Proc. Natl. Acad. Sci. USA 97, 6716–6721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Lei-Butters D.C., Zhang Y., et al. (2007). Hybrid adeno-associated virus bearing nonhomologous inverted terminal repeats enhances dual-vector reconstruction of minigenes in vivo. Hum. Gene Ther. 18, 81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Keiser N.W., Song Y., et al. (2013). A novel chimeric adenoassociated virus 2/human bocavirus 1 parvovirus vector efficiently transduces human airway epithelia. Mol. Ther. 21, 2181–2194 [DOI] [PMC free article] [PubMed] [Google Scholar]