Abstract
Significance: MicroRNAs (miRNAs) are deregulated and play a causal role in numerous cardiovascular diseases, including myocardial infarction, coronary artery disease, hypertension, heart failure, stroke, peripheral artery disease, kidney ischemia–reperfusion. Recent Advances: One crucial component of ischemic cardiovascular diseases is represented by hypoxia. Indeed, hypoxia is a powerful stimulus regulating the expression of a specific subset of miRNAs, named hypoxia-induced miRNAs (hypoxamiR). These miRNAs are fundamental regulators of the cell responses to decreased oxygen tension. Certain hypoxamiRs seem to have a particularly pervasive role, such as miR-210 that is virtually induced in all ischemic diseases tested so far. However, its specific function may change according to the physiopathological context. Critical Issues: The discovery of HypoxamiR dates back 6 years. Thus, despite a rapid growth in knowledge and attention, a deeper insight of the molecular mechanisms underpinning hypoxamiR regulation and function is needed. Future Directions: An extended understanding of the function of hypoxamiR in gene regulatory networks associated with cardiovascular diseases will allow the identification of novel molecular mechanisms of disease and indicate the development of innovative therapeutic approaches. Antioxid. Redox Signal. 21, 1202–1219.
Introduction
Ischemic cardiovascular diseases are a leading cause of morbidity and mortality in developed countries worldwide (86). The cardiovascular system provides all the tissues of the body with blood, which delivers oxygen and nutrients that are required for cell survival. Decrease or blockade of local blood perfusion results in ischemia, which causes the cellular oxygen tension to fall and the carbon dioxide tension to rise. Ischemia also deprives cells of both metabolic nutrients, such as glucose, and growth factors. Cells can survive in ischemic conditions for limited time periods; after a critical threshold, the combined effects of tissue hypoxia, acidosis, and starvation, activate cell death by apoptosis and/or necrosis. Numerous interconnected factors trigger the initiation of cell death during ischemia and histological specificities and physiopathologic conditions, determine the sensitivity to ischemia of each tissue. Lack of oxygen is a crucial determinant of cell damage, but several other interlinked events are also important, such as acidosis, a paradoxical increase of oxidative stress, disturbance of calcium homeostasis, decreased adenosine triphosphate (ATP) levels, and mitochondrial and DNA damage (108, 125). Clinically, the treatment for ischemia is aimed at reestablishing blood flow to the ischemic area. However, reperfusion can paradoxically increase oxidative stress and tissue damage; this event, named ischemia–reperfusion (I/R), further aggravates the medical condition (83).
Different cell types show broadly variable sensitivity to ischemia. However, for each cell type, the vulnerability to cell death induced by ischemia can be opposed by ischemic preconditioning, a phenomenon whereby a sublethal ischemic stress gives protection against a later, longer lasting ischemic injury. Preconditioning occurs in two phases: an early phase that rapidly evolves and fades away within few hours and a longer lasting late period, requiring transcriptional activation (84).
Among many determinants dictating cell response to hypoxia and ischemia, an ever increasing number of studies have concurred to highlight a fundamental role of microRNAs (miRNAs). miRNAs are small noncoding RNA sequences, 20–25 nucleotide-long, that regulate gene expression mainly via suppression of specific target mRNAs. For a summary of miRNA biogenesis, see the Editorial of this Forum; for extensive reviews, see Refs. (18, 72, 156).
miRNAs originate from longer precursor RNAs, called primary miRNAs, that are generally transcribed by RNA polymerase II (77). Like mRNAs, miRNA expression is regulated by transcription factors and many of them exhibit a tissue-specific distribution or can be induced upon specific stimuli such as hypoxia or starvation. The sequential action of DROSHA/DGCR8 and DICER ribonucleases III yields mature miRNA sequences, which are loaded on the effector complex called RNA-induced silencing complex (RISC). Within the RISC, the mature miRNA associates with target mRNAs and, in most circumstances, acts as a negative regulator of gene expression.
The biological role of miRNA is particularly pervasive, regulating virtually all aspects of cell biology, and heart and blood vessels are no exceptions. Indeed, several reviews extensively illustrate miRNA regulation of cardiogenesis, angiogenesis, endothelial and myocyte growth, contractility, and cardiac rhythm (6, 41, 46, 114, 128, 129). Not surprisingly, the expression of specific miRNAs is altered in many cardiovascular diseases and experiments of gain- and loss of function in mice have shown that miRNA deregulation is a condition necessary and sufficient for multiple diseases. A huge amount of data have been accumulating linking miRNAs, such as let-7 family, miR-1, miR-133a/b, miR-19a/b, miR-150, miR-195, miR-199, miR-221, miR-23a/b, miR-29a/b, miR-30 family, and miR-320, to ischemic disease clinical data [for recent comprehensive reviews, see Refs. (41, 46, 56, 64, 75, 81, 128)].
Another element of interest is constituted by indications that miRNAs may represent excellent prognostic/diagnostic biomarkers. Indeed, mounting evidence accumulating in the last few years shows that miRNAs are present not only in tissues, but also in various biological fluids including blood, urine, and saliva (36, 135). Remarkably, miRNAs circulate in the peripheral blood in a highly stable, cell-free form that is protected from endogenous RNase activity and can be detected in both plasma and serum.
As previously pointed out, hypoxia constitutes a crucial pathogenetic element of ischemic cardiovascular diseases. This review will be focused on the regulation and role of hypoxia-induced miRNAs (hypoxamiRs). In particular, we will describe those miRNAs that not only are directly regulated by hypoxia, but also regulate cell responses to decreased oxygen tension. We will first review miRNAs involved in more general events such as hypoxia-regulated transcription, miRNA biogenesis, and angiogenesis. Next, we will describe hypoxamiR implication in some of the most common ischemic cardiovascular diseases. HypoxamiR deregulation, functions, and transcriptional regulation are summarized in Table 1.
Table 1.
Deregulated Hypoxamirs and Physiopathological Role
| miR | Biological effect/disease | Modulation | Tissue/cell | EFF | SMs | References |
|---|---|---|---|---|---|---|
| Let-7 | Angiogenesis | Up | EC | HIF1A | (27) | |
| miR-1 | Hypoxia | Up | CM | p38 MAPK | Tanshinone IIA | (160) |
| miR-1 | IPC (Mm) | Up | CM | nd | (120) | |
| miR-103 | Angiogenesis | Up | EC | HIF1A | (27) | |
| miR-107 | Angiogenesis | Up | EC | HIF1A | (27) | |
| miR-107 | Angiog/prolif | Up | CoCs | p53 | 102 | |
| miR-127 | Adhesion | Up | RC | HIF1A | (2) | |
| miR-132 | Angiogenesis | Up | EP | nd | (67) | |
| miR-185 | Angiogenesis | Up | EC | nd | (58) | |
| miR-199a | MI/CI (Mm) | Down | CM/brain | nd | 3-NPA | (117, 149) |
| miR-200b | Angiogenesis | Down | EC | nd | H2O2, BCNU | (22, 91) |
| miR-200c | Apopt/senescence | Down | EC/hUCB-MSCs | nd | 5-AzaC | (91, 130) |
| miR-21 | IP (Mm) | Up | CM | nd | (29) | |
| miR-21 | WH | Up | Skin | nd | (90, 104) | |
| miR-21 | Inflammation | Up | MØ | nd | (89) | |
| miR-21 | PH | Up | Lung | AKT2 | (103) | |
| miR-21 | PH | Down | Lung | TGF-β | (19) | |
| miR-21 | HF (Mm) | Down | Heart | AKT | (122) | |
| miR-21 | MI (Mm) | Down | CM | AKT | (122) | |
| miR-210 | MI | Up | Heart | nd | (12) | |
| miR-210 | MI (Mm) | Up | Heart | nd | (59) | |
| miR-210 | HF | Up | Heart | nd | (52) | |
| miR-210 | THR | Up | mSC | nd | (70) | |
| miR-210 | Hypoxia | Up | CM | AKT | (99) | |
| miR-210 | CI (Mm) | Up | Brain | nd | (88, 110) | |
| miR-210 | Stroke | Down | Blood | nd | (159) | |
| miR-210 | AO | Up | Blood | nd | (78) | |
| miR-210 | Kidney injury | Down | Blood | nd | (85) | |
| miR-210 | I/R (Mm) | Up | Kidney | nd | (80) | |
| miR-210 | IWH (Mm) | Up | Skin | HIF1A | (9) | |
| miR-210 | Angiogenesis | Up | EC | nd | WSS25 | (40, 80, 87, 147) |
| miR-210 | SC survival (Mm)/apoptosis/preclampsia | Up | PmSC/CNE/Trophoblast cells | HIF1A | DFOM | (57, 69, 98) |
| miR-210 | Proliferation | Up | Lung cancer | HIF1A | EGCG | (141) |
| miR-24 | Angiogenesis | Up | EC | nd | (45) | |
| miR-322 | MI (Mm) | Up | Heart | HIF1A | (48) | |
| miR-34a | Chronic hypoxia | Down | RC/EPC CAD | HIF1A | Atorvastatin | (38, 131) |
| miR-34a | MI (Mm) | Up | BM cells | nd | (148) | |
| miR-378 | MI (Mm) | Down | Heart | nd | (39) | |
| miR-378 | Apoptosis | Down | CM | nd | (39) | |
| miR-424 | Angiogenesis | Up | EC | SPI1/PU.1 | (48) | |
| miR-484 | MI (Mm) | Down | CM | FOXO3A | (142) |
3-NPA, nitropropionic acid; 5-AzaC, 5-azacytidine; AO, atherosclerosis obliterans; BCNU, 1,3-bis(2 chloroethyl)-1-nitrosourea; BM, bone marrow; CI, cerebral ischemia; CM, cardiomyocytes; CNE, nasopharyngeal carcinoma cells; CoCs, colon cancer cells; DFOM, deferoxamine mesylate; EC, endothelial cells; EGCG, epigallocatechin-3-gallate; EFF, effector (transcription factor/signaling pathway); EP, endothelial pericytes; EPC CAD, endothelial precursor cells from CAD patients; HF, heart failure; FOXO3A, forkhead box O3; hUCB-MSCs, human umbilical cord blood-derived multipotent stem cells; hypoxamiR, hypoxia-induced miRNA; IP, ischemic preconditioning; I/R, ischemia–reperfusion; IWH, ischemic wound healing; MI, myocardial infarction; Mm, mus musculus; MØ, macrophages; PH, pulmonary hypertension; PmSC, preconditioned mesenchymal stem cells; RC, renal cells; SC, stem cells; SMs, small molecules; THR, transplanted heart recovery; WH, wound healing.
Hypoxia-Inducible Factor 1: An miRNA-Mediated Modulator
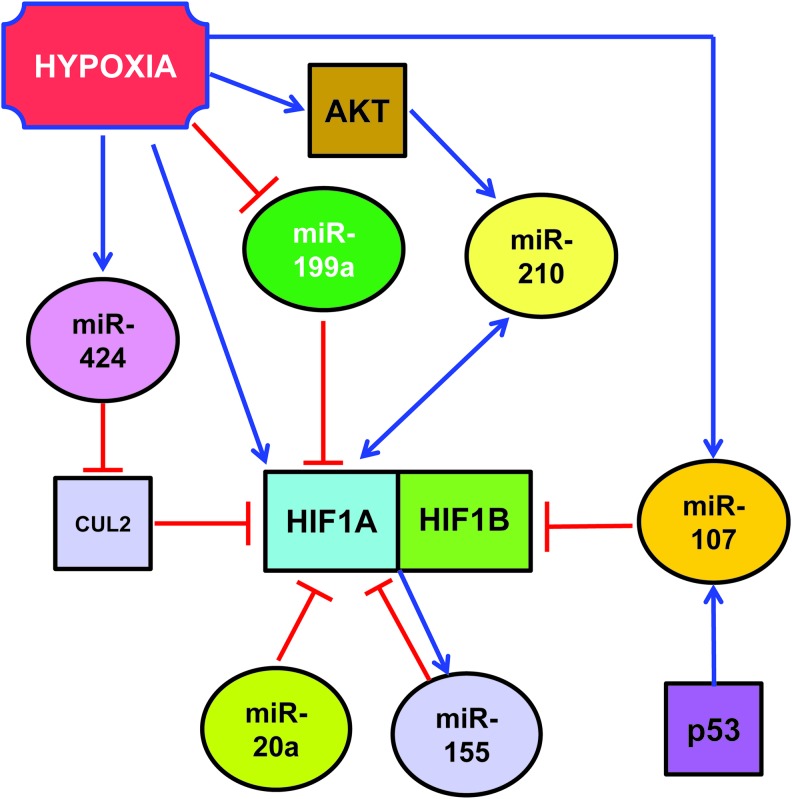
Hypoxia-inducible factor 1 alpha (HIF1A) is the first identified member of a family of oxygen-sensitive transcription factors that allows aerobic organisms and tissues to adapt to hypoxia (94, 124). This is mostly accomplished through the transcriptional activation of more than 200 genes, constituting a significant portion of the hypoxia-induced transcription. Moreover, stimuli other than hypoxia, such as nitric oxide and reactive oxygen species, can also activate HIF1A. Being a master transcription factor of the hypoxic response, HIF1A is implicated in virtually all ischemic diseases (101, 108).
Under normal oxygen tension, the hydroxylation of HIF1 at two specific proline residues, by the prolyl-4-hydroxylase domain-containing enzymes (PHD), targets HIF1A for polyubiquitination and proteosomal degradation by the von Hippel–Lindau tumor suppressor protein (62, 95). Under hypoxic conditions, PHDs are inhibited, saving HIF1A from degradation and allowing it to accumulate and translocate to the nucleus. Once in the nucleus, HIF1A dimerizes with HIF1 beta (HIF1B) and binds to specific hypoxia-responsive element sequences of target gene promoters. The transcriptional activity of the HIF1 heterodimer is also regulated by an asparaginyl hydroxylase, named factor inhibiting HIF1 (FIH-1) (92).
While the regulation of protein stability by the ubiquitin-proteasome pathway plays a fundamental role, other mechanisms of HIF1A regulation are present as well. Indeed, HIF1A mRNA levels dramatically increase in response to hypoxia or ischemia in heart, skeletal muscle, kidney, brain, and lung (7, 11, 76, 144). Albeit this may be primarily due to increased mRNA transcription, decreased mRNA degradation may play a role as well. In this respect, miRNAs seem to play an important role, by binding to the 3′-untranslated region (UTR) of HIF1A mRNAs and either blocking its translation or inducing its degradation (Fig. 1). For instance, miR-199a is downregulated in cardiac myocytes upon hypoxia and this reduction is required for the rapid upregulation of its target, HIF1A (117). The override of miR-199a decrease during hypoxia inhibits HIF1A expression and HIF1A-mediated stabilization of p53. As a consequence, apoptosis is attenuated as well. On the other hand, the blockade of miR-199a during normoxia results in the upregulation of HIF1A, both directly and indirectly. Indeed, miR-199a also targets sirtuin-1 that, in turn, negatively regulates PHD2, required for the de-stabilization of HIF1A. The relevance of this molecular circuitry is highlighted by the fact that, miR-199a inhibition reproduces hypoxia preconditioning in vitro (117).
FIG. 1.
MiRNAs regulating HIF1A. miRNA, microRNA. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Other miRNAs also target HIF1A transcript: Taguchi et al. found that HIF1A is a target of miR-17-92 cluster and in particular of its miR-20a component (Fig. 1) (132). In support of these findings, Kang et al. showed that a pyrrolopyrazine metabolite of the antiangiogenic drug Oltipraz inhibits HIF1A via miR-199a and miR-20a induction (66). However, both Taguchi et al. and Kang et al. used cancer cells as experimental systems, and the relevance of their findings in the cardiovascular system remains to be investigated.
HIF1A can be also targeted by miRNAs indirectly, that is, modulating fundamental regulators of its activity. Indeed, argonaute-1 (AGO1) and the miRNA machinery are necessary for Drosophila adaptation to hypoxia (35).
One way for miRNAs to regulate HIF1A is by targeting its ubiquitination machinery. Ghosh et al. found that miR-424 was induced in human endothelial cells exposed to hypoxia (Fig. 1) (48). miR-424, in turn, targets cullin 2, a scaffolding protein critical to the assembly of the ubiquitin ligase system, thereby stabilizing HIF1A. Hypoxia-induced miR-424 is not HIF1A dependent, but it relies on SPI1/PU.1 that, in turn, is increased in hypoxic endothelium by RUNX-1 and C/EBPα. The relevance of this pathway in post-ischemic vascular remodeling and angiogenesis is demonstrated by the fact that miR-424 promotes angiogenesis in vitro in mice. Moreover, mu-miR-322, the mouse homolog of human miR-424, is significantly upregulated along with HIF1A in experimental models of ischemia.
Another modifier of HIF1 is miR-31, which targets the 3′-UTR of FIH, an inhibitor of HIF under normoxic conditions. Thus, by targeting an HIF-inhibitor, miR-31 stimulates HIF function (81).
Other HIF1A-binding proteins can be miRNA target as well. For instance, p53 regulates hypoxic signaling in human colon cancer through the transcriptional induction of miR-107 that, in turn, suppresses the expression of HIF1B (Fig. 1) (151). Indeed, inhibition and overexpression of endogenous miR-107 enhances and inhibits, respectively, HIF1B expression and hypoxic signaling. Further, overexpression of miR-107 in tumor cells suppresses tumor angiogenesis, tumor growth, and tumor vascular endothelial growth factor (VEGF) expression in mice. Again, the relevance of these molecular circuitries in the cardiovascular system has not been demonstrated yet.
HIF1A can induce the expression of several miRNAs that, in turn, can regulate HIF1A with a feedback loop, either positive or negative (Fig. 2). Among HIF1A-induced miRNAs, miR-210 deserves a particular attention, since miR-210 induction is a virtually constant feature of the hypoxic response in both normal and transformed cells (17, 21, 40, 44, 61, 73, 109, 138). Indeed, HIF1A binds at specific sites of miR-210 promoter, activating its expression. Specifically, Kulshreshtha et al. (73) demonstrated in cancer cell lines, using a combination of promoter reporter and chromatin immunoprecipitation assays, the role of HIF1A in the upregulation of miR-210 by hypoxia. More recently, a specific functional HRE site was identified in hypoxic cancer cells (61) and in differentiating myoblasts (30). miR-210 expression is mainly stimulated by HIF1A, but an HIF1A-independent, AKT-dependent pathway has been described as well (99).
FIG. 2.
Positive and negative feedback loops regulating HIF1A. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
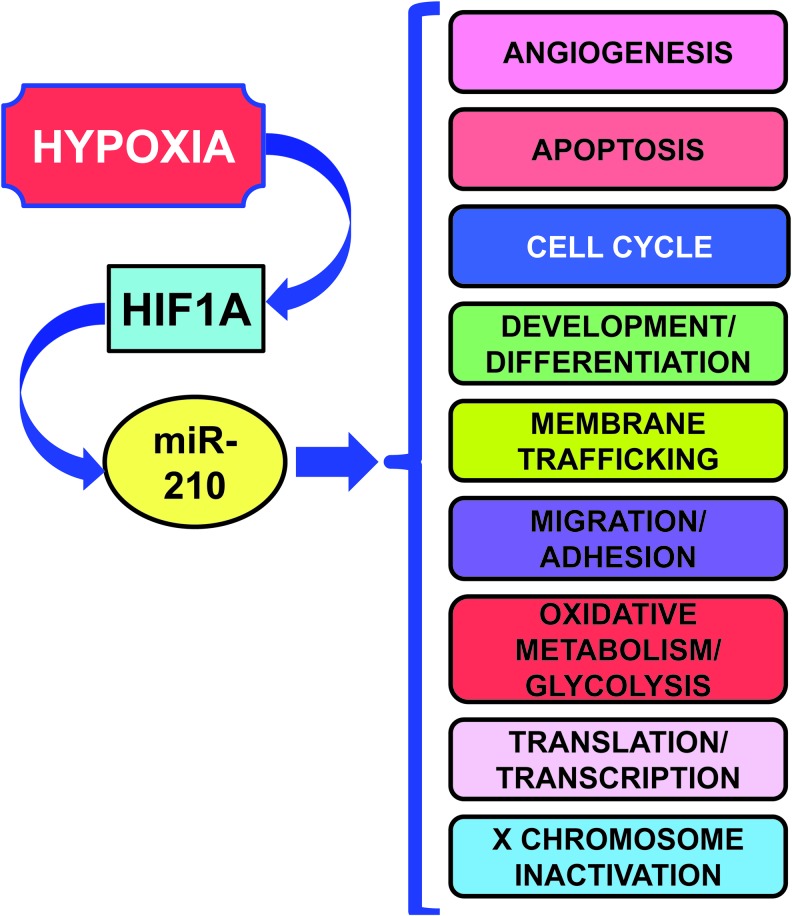
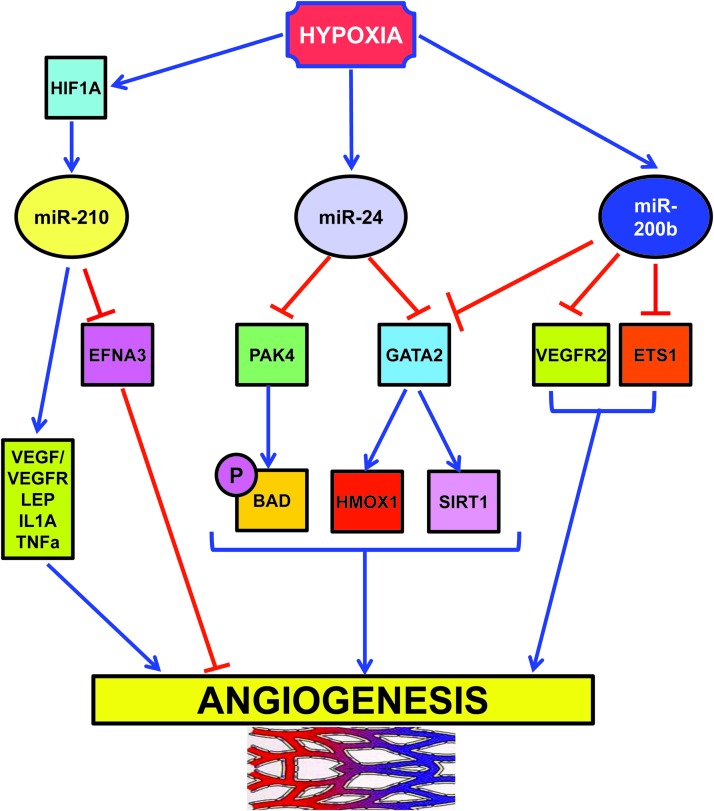
According to its consistent induction in different hypoxic situations, miR-210 regulates several key aspects of cardiovascular disease, summarized in Figure 3 and Table 2 (17, 40, 44, 61, 109, 161).
FIG. 3.
Gene ontology of miR-210 targets. miR-210 targets involved in each function are illustrated in Table 2. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Table 2.
miR-210 Experimental Validated Targets and Relevant Biological Functions
| Biological functions | Target | References |
|---|---|---|
| Angiogenesis | DDAH1/EGR3/ELK3/HYPB/HOXA3/EFNA3 | (28, 40, 42, 49) |
| Apoptosis | AcvR1b/AIFM3/APC/CASP8AP2/DAPK/MNT/NIPBL/RAD52/SHIP-1/UBQLN1/ROD1/BNIP3 | (33, 42, 43, 59, 69, 79, 97, 139, 154, 161) |
| Cell cycle | CDK10/CLASP2/CTGF/SEH1L/SERTAD2/E2F3/FGFRL1/BUB1B/CDC25B/CCNF/FAM83D/PLK1 | (9, 42, 57, 59, 61, 136) |
| Development/differentiation | BDNF/FGFRL1/HECTD1/HOXA1/HOXA3/HOXA9/KIAA1161/MDGA1/MIB1NP1/NPTX1/EFNA3/CTG/FSHH | (40, 42, 49, 61, 110, 136, 162) |
| Membrane trafficking | ABCB9/TNPO1 | (42) |
| Migration/adhesion | APC/EFNA3/DDAH1/GIT2/HOXA9/KIAA1161/NCAM1/PTPN1/PTPN2/TCF7L2/VMP1/CLASP2/P4HB | (26, 42, 54, 61, 71, 113, 157) |
| Oxidative/metabolism/glycolysis | AIFM3/ATP11C/COX10/FECH/GPD1L/ISCU/MID1IP1/NDUFA4/P4HB/SDHD/FECH/ | (8, 21, 28, 42, 44, 49, 109, 112, 154) |
| Translation/transcription | CBX1/CPEB2/CHD9/E2F3/ELK3/HOXA9/KCMF1/TCF7L2/UBQLN1/ZNF462 | (26, 42, 49, 61, 113) |
| X chromosome inactivation | SMCHD1/XIST | (42) |
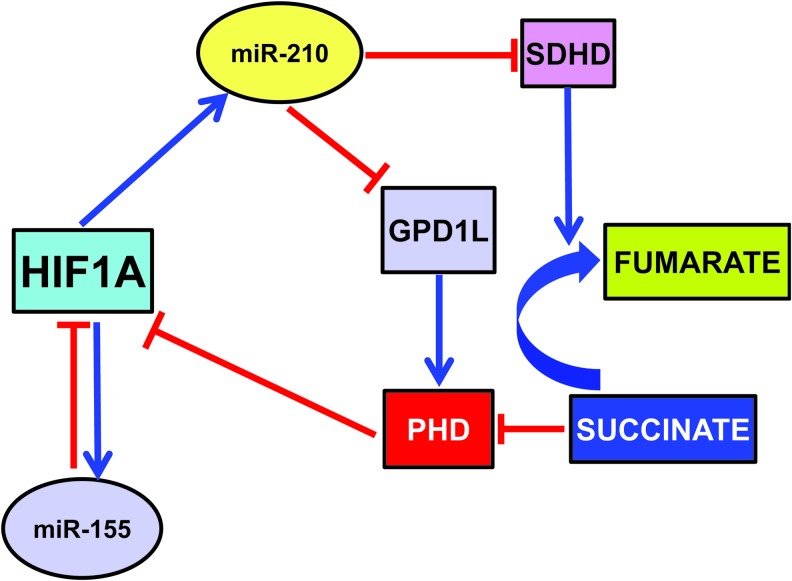
There is evidence of two mechanisms through which miR-210 triggers a positive feedback loop where HIF1A drives miR-210 expression, which further induces HIF1A protein stability. The first mechanism is via one of its direct targets, the enzyme glycerol-3-phosphate dehydrogenase 1-like (GPD1L) (42). Indeed, GPD1L increases proline hydroxylation of HIF1A, decreasing its stability. Thus, in normoxia, when HIF1A levels are low, small amounts of miR-210 result in high GPD1L protein levels and high activity of the PHDs, contributing to keep HIF1A levels at bay. When oxygen tension is lowered, decreased GPD1L protein potentiates the inhibition of PHD activity, leading to increased HIF1A stability (68).
Another mechanism is mediated by miR-210 modulation of oxidative phosphorylation (Cottrill et al., this issue). In particular, Puisségur et al. showed that the D subunit of the succinate dehydrogenase complex (SDHD) is a bona fide miR-210 target (109). Albeit more work is necessary, the current model proposes that miR-210-dependent demise in SDHD results in increased succinate accumulation, which is a by-product and natural inhibitor of PHD activity (123). However, miR-210-HIF1A positive feedback may not be present in all tissues and/or physiopathological conditions. Indeed, we found that miR-210 blockade did not affect HIF1A-pathway activation in a mouse model of acute hindlimb ischemia (158).
While the mechanism underpinning HIF activation is well understood, little is known about its resolution in cells exposed to prolonged hypoxia. Given their inhibitory nature, miRNAs are likely candidates for this role. miR-155 is an hypoxamiR directly targeting HIF1A transcript in gut epithelial cells (Fig. 2) (14). Overexpression of miR-155 decreases the HIF1A protein and transcriptional activity in hypoxia, and the blockade of endogenous miR-155 prevents the resolution of HIF1A stabilization and activity. Thus, miR-155 induction contributes to a negative feedback loop for the resolution of HIF1A activity in cells exposed to prolonged hypoxia, leading to the oscillatory behavior of HIF1A-dependent transcription.
Hypoxia Regulates the miRNA Biosynthesis Machinery
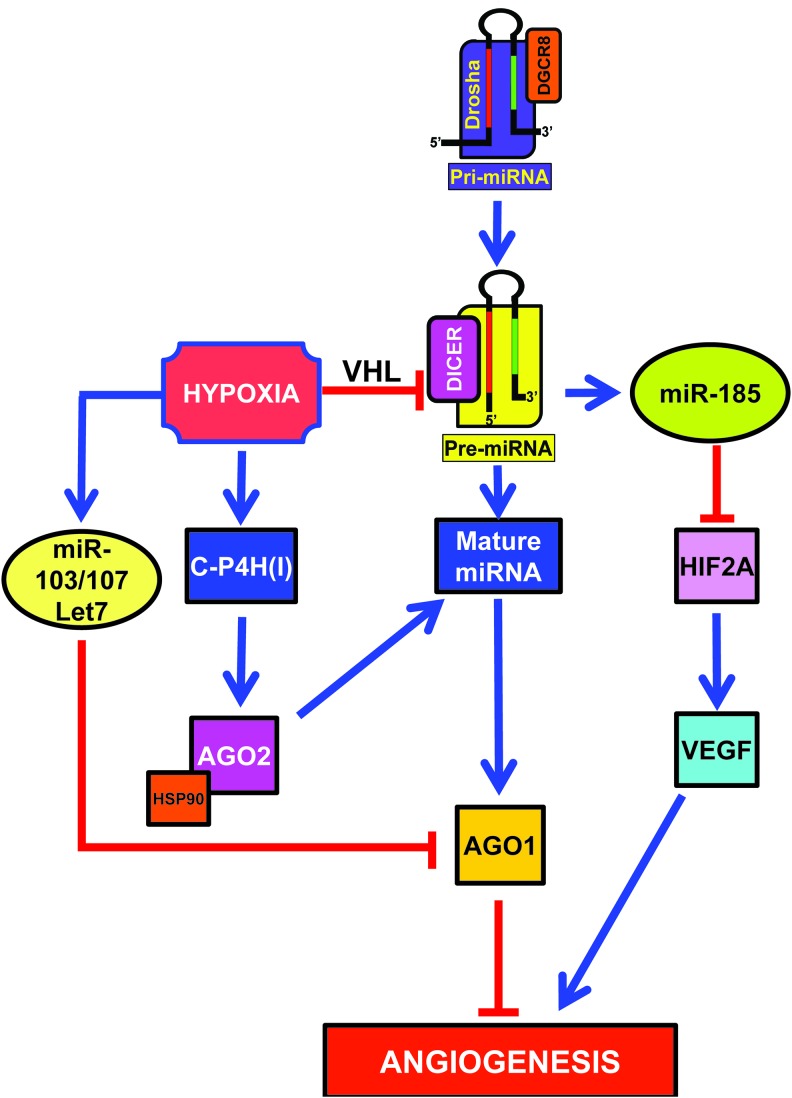
Hypoxia can modulate the miRNA biosynthesis machinery affecting miRNA levels and function, thereby eliciting broad effects (Fig. 4).
FIG. 4.
Hypoxia-induced miRNAs (hypoxamiRs) modulating miRNA biogenesis. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Data obtained in vitro and in vivo show that hypoxia impairs DICER expression and activity, resulting in global consequences on miRNA biogenesis (58). While most miRNAs remain unaffected, possibly due to DICER-independent maturation (31), miR-185 is decreased. This miRNA targets HIF2A, the main HIF species in endothelial cells. As a consequence, upon hypoxia HIF2A is de-repressed, increasing the expression of HIF-induced genes. Therefore, functional deficiency of DICER in hypoxia is relevant to both HIF and hypoxia-responsive/HIF target genes, especially in the vascular endothelium.
Hypoxia also induces type I collagen prolyl-4-hydroxylase [C-P4H(I)] (Fig. 4), which leads to prolyl-hydroxylation and accumulation of argonaute-2 (AGO2), a critical component of the RISC (111, 145). Hydroxylation of AGO2 is required for the association of AGO2 with heat shock protein 90, which, in turn, is necessary for the loading of miRNAs into the RISC, and translocation to stress granules. Moreover, hydroxylation of AGO2 increases miRNA levels and enhances the endonuclease activity of AGO2.
However, hypoxia regulation seems complex with both positive and negative elements. By deep sequencing of small RNA libraries derived from primary endothelial cells subjected to normoxia or hypoxia, Chen et al. found that Let-7, miR-103, and miR-107 are highly induced (Fig. 4) (27). Quite surprisingly, however, miR-210 is not reported as significantly induced as previously reported by many authors (17, 21, 40, 44, 61, 73, 109, 138). Let-7, miR-103, and miR-107 miRNAs are induced by HIF1A and target the RISC component AGO1. Targeting of AGO1 results in the translational de-repression of VEGF mRNA and stimulation of angiogenesis. Indeed, data from tumor xenografts and human cancer specimens indicate that AGO1-mediated increase of VEGF may be associated with tumor angiogenesis and poor prognosis. As described in the previous section, Yamakuchi et al. reported that miR-107 also targets HIF1B (151) and HIF1B targeting by miR-107 limits endothelial progenitor cell differentiation (96). However, this does not seem to be the case in mature endothelial cells or the inhibitory effect on HIF1B is overcome by the pro-angiogenic effect of AGO1 inhibition. Another element of apparent contradiction is that AGO1 silencing impairs Drosophila adaptation to hypoxia (35). However, differences in the experimental system and in the degree of AGO1 suppression may account for these apparently conflicting results.
HypoxamiR and Angiogenesis
Angiogenesis is a physiological process involving the growth of new blood vessels from pre-existing vessels. The regulation of angiogenesis by hypoxia is an important component of homeostatic mechanisms that link vascular oxygen supply to metabolic demand.
Several studies using quantitative polymerase chain reaction (21, 40) or RNA-sequencing (138) identified miR-210 as the prominent hypoxamiR in endothelial cells (Fig. 5). miR-210 overexpression in normoxic endothelial cells stimulates the formation of capillary-like structures, and VEGF-driven cell migration, whereas miR-210 blockade inhibits these events (40, 80, 87, 147). In keeping with these findings, anti-angiogenic effect of WSS25 sulfated polysaccharide is, at least in part, mediated by miR-210 down-modulation (147). Further, directed endothelial differentiation of embryonic stem cells is associated with miR-210 increased expression (65).
FIG. 5.
HypoxamiRs regulating angiogenesis. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
One critical direct target of miR-210 pro-angiogenic function is ephrin-A3 (EFNA3). Fasanaro et al. showed that the override of EFNA3 down-modulation either by miR-210 expression or by hypoxia prevents both tubulogenesis and chemotaxis stimulation (40). However, miR-210/EFNA3 relationship seems to be rather complex: hypoxia stimulates EFNA3 mRNA expression in endothelial cells, while miR-210 decreases EFNA3 protein levels (40). In this respect, suppression by miR-210 may represent a feedback loop to keep EFNA3 levels under control. In keeping with this model, Pulkkinen et al. found increased EFNA3 protein levels in post-ischemic mouse hippocampus (110). A possible differential expression in neuronal and endothelial compartments was not investigated.
It is also likely that miR-210 may act through other effectors as well, both direct and indirect. Accordingly, increased angiogenic factor signaling has been reported in miR-210-expressing cells (59, 80).
More in vivo evidence is needed to assess miR-210 pro-angiogenic role. Nevertheless, it is worth noting that tissue perfusion and capillary density in mouse ischemic hindlimb are significantly improved by the injection of umbilical cord blood CD34+ cells expressing miR-210 (4).
Another hypoxia-induced miRNA is miR-24 (73), which acts as a negative regulator of angiogenesis (Fig. 5) (45). Fiedler et al. found that miR-24 expression induces endothelial cell apoptosis, decreases endothelial capillary-like network formation on Matrigel, and inhibits cell sprouting from endothelial spheroids (45). These effects are mediated through direct targeting of the endothelium-enriched transcription factor GATA-binding protein 2 (GATA2) and the p21-activated kinase PAK4. These targets, in turn, impinge on BCL2-associated agonist of cell death and sirtuin-1 signaling cascades. The relevance of miR-24 modulation of angiogenesis is demonstrated by the impairment of zebrafish embryos angiogenesis upon overexpression of miR-24 or silencing of its targets. Moreover, the blockade of endothelial miR-24 limits mouse myocardial infarct size by preventing endothelial apoptosis and enhancing vascularity. These beneficial events, in turn, lead to preserved cardiac function and increased survival.
Hypoxia also negatively regulates miR-200b, a miRNA extensively studied in molecular oncology for its ability to regulate epithelial to mesenchymal transition (13). Overexpression of miR-200b in human microvascular endothelial cells inhibits and miR-200b blockade enhances angiogenesis in vitro, as evidenced by tube formation on Matrigel and cell migration (Fig. 5) (22). These events are mediated by miR-200b targeting of v-ets erythroblastosis virus E26 oncogene homolog 1 (ETS1) a crucial angiogenesis-related transcription factor: knocking down endogenous ETS1 is sufficient to inhibit angiogenesis, whereas overexpression of ETS1 rescues miR-200b-dependent impairment in angiogenic response and suppression of ETS1-associated gene expression. Interestingly, Magenta et al. found that miR-200c overexpression induces endothelial cell growth arrest, apoptosis, and senescence, indicating that the whole miR-200-family has an anti-angiogenic activity (91).
HypoxamiR and Heart Diseases
Myocardial infarction and hypoxamiR
Myocardial infarction (MI) is the irreversible damage of myocardial tissue caused by sustained hypoxia and ischemia. In most cases, MI occurs when a coronary artery becomes occluded upon the rupture of an atherosclerotic plaque, thereby leading to coronary thrombosis. When a vessel becomes completely occluded, the heart area perfused by that vessel will become ischemic and hypoxic causing myocardial cell death. Indeed, myocardial oxygenation measurement in infarcted rats by paramagnetic resonance oxymetry indicates that pO2 in the infarction site decreases from ∼20 to ∼3.0 mmHg (20). The damaged tissue is initially composed of a necrotic core surrounded by a border zone that, over time, can either recover to normal function or become irreversibly damaged (86).
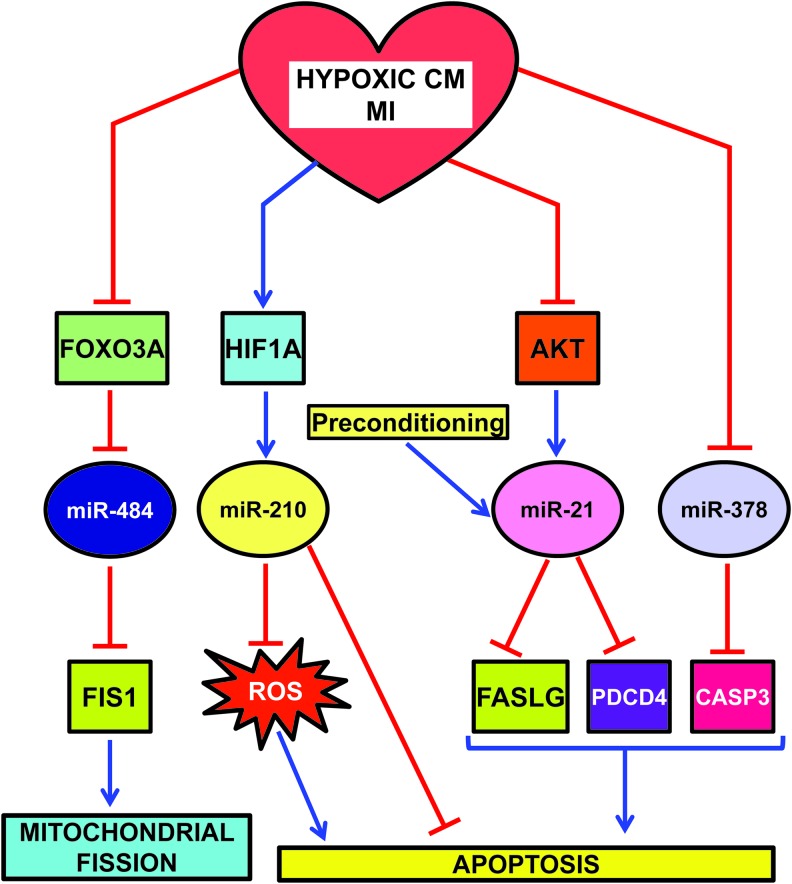
Given its prominent role as master hypoxamiR, miR-210 is one of the better characterized hypoxamiRs in MI (Fig. 6). In cardiomyocytes exposed to hypoxia in vitro, miR-210 is robustly upregulated through both HIF-dependent and -independent pathways (59, 99). miR-210 expression upregulates several angiogenic factors, inhibits caspase activity, and prevents cell apoptosis. Further, downregulation of miR-210 increases reactive oxygen species levels after hypoxia-reoxygenation. These functions are attributable to miR-210 ability to modulate mitochondrial metabolism and reactive oxygen species generation by the electron transport chain (Fig. 3) (Cottrill et al., this issue) and by the repression of pro-apoptotic genes, such as PTPN1. In keeping with in vitro data, miR-210 is also induced following MI in humans (12). To test the relevance of miR-210 induction, Hu et al. injected a minicircle vector carrying miRNA-210 precursor in the border zone of a mouse model of MI (59). The protective role of miR-210 is highlighted both by echocardiography showing a significant improvement of left ventricular fractional shortening and by histological analysis, displaying decreased cellular apoptosis and increased neovascularization.
FIG. 6.
HypoxamiRs regulating apoptosis and mitochondrial fission in hypoxic CM and/or MI. CM, cardiomyocytes. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
miR-210 involvement does seem to be limited to acute MI. Indeed, miR-210 is also induced in left ventricle biopsies of diabetic patients affected by post-ischemic heart failure (52).
miR-210 is not the only hypoxamiR modulating mitochondrial function and morphology (109). Indeed, mitochondria constantly undergo fusion and fission, two necessary processes for the maintenance of organelle fidelity. Wang et al. found that miR-484 can target mitochondrial fission protein FIS1, and inhibit FIS1-mediated fission and apoptosis in cardiomyocytes (Fig. 6) (142). During anoxia, miR-484 is downregulated and, as a consequence, FIS1 is de-repressed. Interestingly, miR-484 expression is regulated by forkhead box O3 (FOXO3A) transcription factor. Indeed, FOXO3A transgenic or knock-out mice exhibit, respectively, a high or low level of miR-484 and a reduced or enhanced mitochondrial fission, apoptosis, and MI damage.
Another miRNA playing an important role in MI is miR-21. miR-21 is positively regulated via an AKT-dependent pathway, which is down-modulated by sustained hypoxia (Fig. 6) (122). Accordingly, hypoxia-induced downregulation of miR-21 and upregulation of its target Fas ligand (FASLG) were reversed by activated AKT. More interestingly, the anti-apoptotic function of AKT, at least in part, requires miR-21, which is sufficient for inhibition of caspase-8 activity and mitochondrial damage. In keeping with these findings, overexpression of miR-21 in a transgenic mouse heart results in suppression of ischemia-induced upregulation of FASLG expression, a smaller infarct size, and less severe heart failure. Another target that may contribute to miR-21 anti-apoptotic role is programmed cell death 4 (PDCD4) (29).
In keeping with these data, Cheng et al. also found that miR-21 is also induced in heart after ischemic preconditioning (29). Accordingly, cardiac protection afforded by preconditioning against I/R injury is inhibited by knockdown of cardiac miR-21.
An additional miRNA down-modulated by both MI and hypoxia is miR-378 (Fig. 6) (39). In H9c2 cardiomyocyte cell line, miR-378 expression enhances cell viability, reduces lactate dehydrogenase release, and inhibits both apoptosis and necrosis. Conversely, miR-378 blockade aggravates hypoxia-induced apoptosis and cell injury. One interesting direct target is caspase-3, a key apoptosis executioner. However, more experiments are needed to ascertain the functional relevance of caspase-3 inhibition by miR-378.
Stem cells for MI therapy and hypoxamiR
miRNA induction by hypoxia also have repercussions in regenerative medicine for ischemic cardiac diseases. Preclinical studies in experimental animal models and some pioneering clinical trials have produced encouraging results that allow to envision angiogenic and myogenic repair of the ischemia-damaged heart by stem cell therapy (5). Despite these encouraging results, numerous problems still need to be solved. One of the most outstanding is massive death of donor cells in the ischemic myocardium after engraftment, since it greatly lowers the efficacy of the procedure.
Kim et al. reported that miR-210 is induced by ischemic preconditioning in bone marrow-derived mesenchymal stem cells. miR-210, in turn, improves their survival under subsequent longer exposure to anoxia and following engraftment in the infarcted myocardium (69). Accordingly, high miR-210 levels in mesenchymal stem cells, either by preconditioning or by miR-210 overexpression, accelerate the functional recovery of the ischemic heart after transplantation (69). Increased survival negatively correlates with the levels of caspase-8-associated protein-2 (CASP8AP2) (Fig. 3). Interestingly, miR-210/HIF1A positive feedback loop (Fig. 2) may also play an important role in increasing cell survival (25). Moreover, in vitro co-culture experiments show miR-210 transfer from mesenchymal stem cells to cardiomyocytes, indicating that mesenchymal cells may also act as a miRNA delivery system (70).
Another promising cell type is bone marrow mononuclear cells. Xu et al. found that bone marrow cells isolated from MI patients display increased miR-210 and miR-34a levels (148). The latter appears as particularly important for this cell type: inhibition of miR-34 significantly decreases hydrogen peroxide-induced cell death in vitro, whereas its overexpression reduces the bone marrow cell survival. Significantly, ex vivo blockade of miR-34a also increases the therapeutic benefit of transplanted bone marrow cells in mice after acute MI.
It is known that hypoxia maintains cultured skeletal myoblasts in an undifferentiated state by inhibiting myogenic differentiation by Notch pathway activation (55, 93). In this respect, hypoxia, downregulates miR-1 and increases the levels of its target gene Pax7, therefore inhibiting differentiation and promoting myoblasts satellite cell self-renewal (82). The negative regulation of miR-1 is mediated by the Notch signaling pathway, in particular, by the hypoxia-induced activation of Hes1 and Hey.
Interestingly, Hes1 and miR-1 seem to regulate one another. Indeed, in normoxic condition, miR-1 overexpression promotes the differentiation of mesenchymal stem cells toward the cardiac lineage by direct targeting of Hes1 (60).
Finally, pioneering studies identified a paracrine mechanism involving the secretion of miR-132 and inhibition of its target genes. Katare et al. assessed the regenerative potential of saphenous vein-derived pericyte progenitor cells (67). Transplantation of pericytes into the peri-infarct zone of mouse hearts attenuates left ventricular dilatation and improves ejection fraction. These beneficial effects are, at least in part, due to hypoxia-induced miR-132. Indeed, in vitro studies show that miR-132 is actively secreted by pericytes; moreover, pericyte conditioned medium stimulates endothelial cell tubulogenesis and reduces myofibroblast differentiation, through inhibition of methyl-CpG-binding protein 2 (MECP2) and RAS p21 protein activator (GTPase-activating protein) 1 (RASA1), which are both validated miR-132 targets. Further, miR-132 inhibition decreases pericyte ability to improve contractility, reparative angiogenesis, and interstitial fibrosis following MI.
HypoxamiR and Stroke
Stroke is a multifactorial disease with two major causal events: obstruction of a blood vessel in the brain (cerebral ischemia) or disruption of a cerebral vessel (hemorrhagic stroke). In cerebral ischemia, the most characterized hypoxamiR is, again, miR-210.
miR-210 is upregulated in adult rat ischemic brain cortexes, along with NOTCH1 signaling molecules that are also increased (88). This may be relevant for the compensatory angiogenesis occurring after cerebral ischemia that increases the blood flow to the injured area and limits extension of the ischemic penumbra. Indeed, NOTCH signaling regulates the vascularization process that occurs in ischemic tissues. In keeping with the in vivo findings, cultured hypoxic endothelial cells also display increased miR-210 and NOTCH1-pathway components. These events are tightly linked, since miR-210 overexpression causes the upregulation of NOTCH1 signaling molecules and induces endothelial cells to migrate and form capillary-like structures on Matrigel.
miR-210 is also a potential stroke marker. Zeng et al. measured miR-210 levels in blood samples from stroke patients harvested at different times (159). Compared with healthy controls, blood miR-210 was significantly decreased in stroke patients, with a minimum at 7 and 14 days after stroke onset. This is quite surprising since miR-210 is induced both by hypoxia and ischemia in multiple cell types and tissues, including the ischemic brain (88). However, in keeping with the anti-apoptotic function of miR-210, decreased miR-210 levels may relate to the severity of damage. Indeed, miR-210 level is significantly higher in stroke patients with good outcome than in patients with poor outcome (159).
HypoxamiR Peripheral Artery Disease and Wound Healing
Peripheral artery disease commonly involves the arteries supplying the leg and is mostly caused by atherosclerosis (105). Restriction of blood flow due to arterial stenosis or occlusion often leads to muscle pain on walking (intermittent claudication). Any further reduction in blood flow causes ischemic pain at rest, which affects the foot. Ulceration and gangrene may then supervene and can result in loss of the limb, if not treated. Although many patients with claudication remain stable, some progress to critical limb ischemia and need revascularization. Unfortunately, those who benefit from successful revascularization suffer from high rate of recurrent symptoms or revision surgery and many still require progressive amputations.
As for almost all ischemic diseases, miR-210 role has been investigated in peripheral artery disease and ischemic wounds. Indeed, we found that miR-210 is induced in a mouse model of hindlimb ischemia (158). Accordingly, Li et al. propose miR-210 as serum biomarker for atherosclerosis obliterans (78).
In a mouse model of ischemic wound healing, in vivo imaging shows significant HIF1A stabilization in ischemic wounds (9) (Fig. 7). HIF1A induces miR-210 expression that, in turn, silences its target E2F transcription factor 3 (E2F3), which is markedly downregulated in the wound-edge tissue of ischemic wounds. E2F3 transcription factor has a well established pro-proliferative effect. Indeed, knock down of E2F3 limits keratinocyte (HaCaTs) proliferation; accordingly, miR-210 overexpression and inhibition experiments demonstrate a key role of miR-210 in limiting keratinocyte proliferation. Interestingly, miR-210 does not seem to have the same role in all cell types. Indeed, miR-210 overexpression has no significant effect on endothelial cell proliferation (40).
FIG. 7.
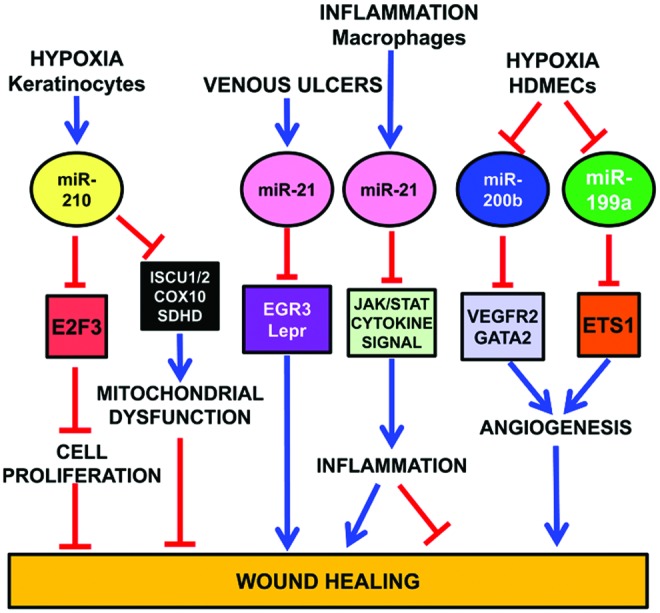
HypoxamiRs regulating wound healing. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
As previously discussed, miR-210 targets ISCU1/2 (21, 28), COX10 (28), and SDHD (109), slowing down mitochondrial respiration under a severe hypoxic insult. On the one hand, this response allows affected cells to survive longer. On the other hand, miR-210 also impairs functions necessary for active tissue regrowth that is required for wound healing (126) (Fig. 7).
Similar to miR-210, miR-21 also is a negative player in cutaneous wound healing (126). Indeed, miR-21 is upregulated in human venous ulcers and overexpression of miR-21 inhibits epithelialization and granulation tissue formation in a rat wound model. Interestingly, miR-21 directly targets early growth response factor 3 and the leptin receptor, which may mediate miR-21 effects, at least in part (104).
Further, miR-21 is TGF-β-inducible, contributing to its growth arrest properties in keratinocytes (143). However, conflicting reports have been published as well (155).
Fibrosis is another important process that is induced by miR-21 (118). Indeed, miR-21 overexpression in wound healing in vitro assays increases ability of fibroblasts to migrate toward the wound area, whereas knocking down of miR-21 has the opposite effect (90). Concordant evidences come from different systems: in a mouse model of heart I/R, miR-21 regulates matrix metalloprotease (MMP-2) expression in cardiac fibroblasts of the infarct zone via a phosphatase and tensin homologue pathway (118). Moreover, miR-21 contributes to myocardial disease by stimulating MAP kinase signaling in fibroblasts of failing hearts (134).
Finally, miR-21 also regulates the inflammatory process (119). Indeed, miR-21 is induced by proinflammatory stimuli such as IL-6, TNF-α, and TLRs (89, 127). Moreover, miR-21 targets several genes involved in JAK/STAT and cytokine pathways (47, 126), and JAK-STAT signaling is directly implicated in cytokine-mediated inflammation. Thus, miR-21 may have an anti-inflammatory effect (16, 133) (Fig. 7).
Also miR-200 family plays an important role in wound healing. In human dermal microvascular endothelial cells (HDMECs), downregulation of miR-200b, which targets GATA2 and VEGFR2 expression, stimulates cutaneous wound angiogenesis (24) (Fig. 7). Accordingly, diabetic wounds exhibit high levels of miR-200b and complementarily low GATA2 and VEGFR2 expression. The impairment of miR-200b downregulation in the early phase of wound healing is mediated by elevated TNF-α. These data are consistent with the finding that diabetic wounds are refractory to resolution of inflammation and display a prolonged abundance of proinflammatory mediators.
Another hypoxamiR involved in wound healing is miR-199a (Fig. 7). Inhibition of endogenous miR-199a in HDMECs supports angiogenesis, while overexpression of miR-199a blocks the angiogenic response in Matrigel cultures (23). miR-199a targets Ets-1 in HMECs. Accordingly, MMP-1, one of the Ets-1 downstream mediators enabling endothelial migration (37), is negatively regulated by miR-199a. These data together indicate that the downregulation of miR-199a is involved in the induction of wound angiogenesis through derepressing the Ets-1-MMP-1 pathway.
HypoxamiR and Kidney I/R
I/R is one of the main causes of acute tubular necrosis, underpinning most of the cases of acute renal failure. Upon ischemia, apical actin cytoskeleton of tubule cell is rapidly reorganized and adhesion molecules change their localization. These events lead to impairment of cell-to-matrix and cell-to-cell adhesion structures and cell detachment, causing severe kidney dysfunction (10).
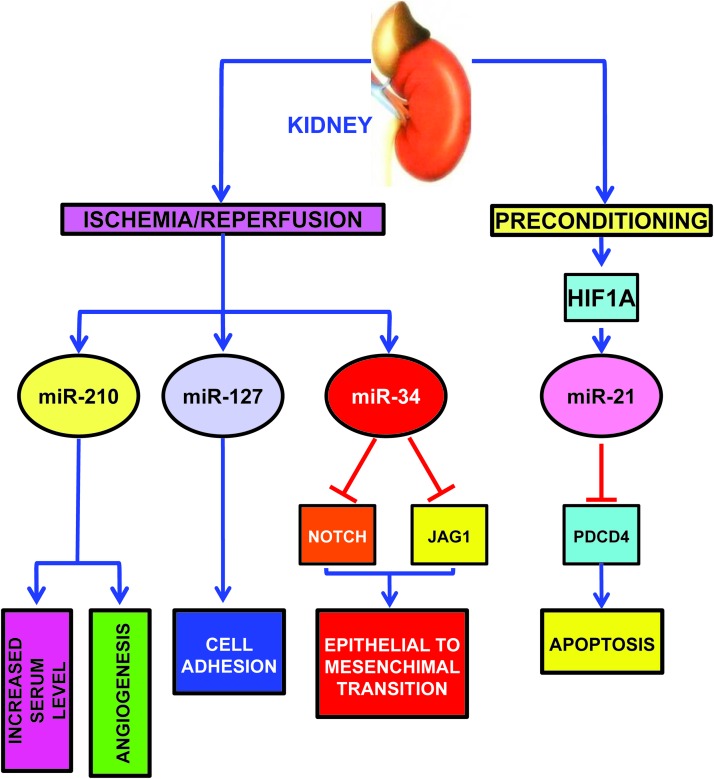
As expected, miR-210 is induced in a mouse model of kidney I/R, correlating with increased angiogenesis in the ischemic region (Fig. 8) (80). Interestingly, pathological changes on a cellular level are also detectable in more accessible specimens such as plasma: in patients with non post-renal acute kidney injury, circulating miR-210 is upregulated and predicts survival (85).
FIG. 8.
HypoxamiRs modulated in kidney I/R and preconditioning. I/R, ischemia–reperfusion. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Other hypoxamiRs seem to play a role in kidney ischemia as well. miR-127 is induced in proximal tubule cells cultured in conditions mimicking I/R with an HIF1A-dependent mechanism (Fig. 8) (2).
miR-127 is involved in cell–matrix and cell–cell adhesion maintenance, since its over-expression contributes to maintain the integrity of focal adhesion complexes and tight junctions. miR-127 targets kinesin family member 3B, which regulates cell trafficking; in keeping with this finding, miR-127 inhibition promotes dextran-FITC uptake.
Hypoxia-induced renal tubular cell epithelial-mesenchymal transition is another important pathogenetic mechanism, leading to renal fibrosis. In this respect, Du et al. found that hypoxia decreases miR-34a expression (Fig. 8) (38). miR-34a, in turn, promotes epithelial-mesenchymal transition of renal tubular epithelial cells by directly targeting NOTCH1 and JAG1, and subsequently, NOTCH downstream signaling.
Finally, in keeping with its function in ischemic heart preconditioning, miR-21 also plays a protective role in kidney delayed preconditioning (Fig. 8) (150). Indeed, ischemic preconditioning induces miR-21 with an HIF1A dependent mechanism and its blockade significantly exacerbates subsequent ischemia-reperfusion injury and tubular cell apoptosis in the mouse kidney. Accordingly, inhibition of miR-21 also results in the upregulation of its pro-apoptotic target PDCD4. Significantly, in the absence of ischemic preconditioning, blockade of miR-21 alone does not affect ischemia-reperfusion injury in the mouse kidney.
HypoxamiR and Pulmonary Hypertension
Pulmonary hypertension (PH) is a complex vascular disease with diverse etiologies, including hypoxia (115). Regardless the cause, PH induces a pathological dysregulation in the pulmonary vasculature and a maladaptive increase in pulmonary arterial pressure. PH involves numerous intersecting signaling pathways leading to deregulated vascular tone, cellular proliferation, and thrombosis.
Parikh et al. used a systems biology approach to integrate network analysis of genome-wide mRNA expression data, algorithmic prediction of miRNA targets and established concepts of biological pathways involved in PH, along with in vitro and in vivo experimentation (103). They identified miR-21 as the most compelling of a select group of miRNA predicted to control the expression of a convergent set of pathways in PH. Indeed, miR-21 is upregulated by both hypoxia and bone morphogenetic protein receptor 2 (BMPR2) signaling, two established PH triggers. In turn, miR-21 targets a fundamental vascular effector, Ras homolog family member B (RHOB), and induces molecular changes in pulmonary endothelial cells triggering vasodilation and decreased angiogenesis. Further, miR-21 is upregulated in the lung PH patients and in rodent models of PH. Significantly, in one of these models, the genetic absence of miR-21 causes accelerated disease progression.
However, it is worth noting that miR-21 expression pattern and functions are not entirely consistent throughout the literature. For instance, Caruso et al. report the downregulation, rather than upregulation, of miR-21 in both human and rodent PH lungs (19). Moreover, the administration of miR-21 complementary oligonucleotides in vivo, decreases pulmonary vascular remodeling in hypoxic rodents (153), in contradiction with the data obtained using genetically deleted miR-21 mice (103). Further, some studies have indicated that miR-21 can drive a pro-proliferative state in hypoxic human pulmonary artery smooth muscle cells in culture (121).
Clinical differences, and in particular the BMPR2 genotype, and technological differences, may explain these results, at least in part. However, albeit the reasons for the observed discrepancies are not fully clarified, it is evident that miR-21 carries a central function in controlling the progression of PH.
Finally, it is not surprising that miR-210 is induced in the lung of mice exposed to chronic hypoxia (51) and in human pulmonary artery smooth muscle and endothelial cells exposed to low oxygen tension in vitro (21, 51).
Potential Clinical Applications
Therapeutic perspectives
Understanding the pathogenetic meaning of hypoxamiR alterations represents a new frontier for both molecular biologists and clinicians. An expanded understanding of hypoxamiR function in gene regulatory networks associated with cardiovascular diseases will allow the identification of novel molecular mechanisms of disease and the development of innovative therapeutic strategies. This appears as particularly important considering that miRNAs are “druggable” targets. A phase IIa study has been recently published describing the use of Miravirsen, a chemically modified complementary locked nucleic acid (LNA) that binds to miR-122, disabling the binding and growth of the Hepatitis C virus (63). Interestingly, the drug was very well tolerated, suggesting that this may be a feature of this new class of LNA drugs. While much more work is needed, several promising preclinical studies indicate that this new therapeutic modality may be a viable approach also for cardiovascular diseases (114, 137).
In addition to miRNA inhibitors, there is also the possibility to mimic or re-express miRNAs by using synthetic RNA duplexes designed to mimic the endogenous functions of the miRNA of interest, with modifications for stability and cellular uptake. However, this approach has revealed to be technically challenging and is still in the preclinical stage. Moreover, even more so than for miRNA inhibitors, precursor delivery to the appropriate cell type or tissue is an important aspect of effective miRNA mimicry, to prevent unwanted side effects.
Another way to increase the level of a miRNA is by the use of viral vectors, such as of adeno-associated viruses (AAVs). These vectors display not only tissue specific expression, if opportune promoters are used, but also tropism toward different organs, according to AAV serotype (106). However, all the caveats and limitations that apply to the use of viral vectors for gene therapy should be kept in mind (53).
It is also possible to modulate miRNAs using small molecules (Table 1). One example is represented by Atorvastatin, a statin that inhibits endothelial senescence and enhances SIRT1 in HUVECs stimulated by oxidative stress (102). SIRT1 is a modulator of vascular endothelial cell homoeostasis and is a direct target of miR-34a (107). Interestingly, it has been shown that miR-34a negatively regulates SIRT1 expression in endothelial progenitor cells from coronary artery disease patients; atorvastatin can inhibit miR-34a and prevent SIRT1 decrease, providing an additional mechanism to the beneficial effects of atorvastatin on endothelial function in coronary artery disease (131).
miR-200 family is also modulated by small molecules. Indeed, oxidative stress-inducing drug 1,3-bis(2 chloroethyl)-1-nitrosourea (BCNU) upregulates miR-200c, and to a lower extent, miR-200a and b. These miRNAs, in turn, induce endothelial cell senescence and apoptosis (91). Accordingly, 5-azacytidine (5-AzaC) induces miR-200c and cellular senescence of human umbilical cord blood-derived multipotent stem cells (130).
Moreover, the administration of nitropropionic acid (3-NPA), an irreversible inhibitor of succinate dehydrogenase used to induce ischemic tolerance, down-modulates miR-199a in rat brains (149).
Finally, HIF is a promising target for a variety of diseases and an ever increasing number of small molecule agonists and inhibitors of HIF has been developed, some of which are progressing through preclinical and early clinical development (146). While no specific studies in this sense exist, it is reasonable to anticipate that pharmacological activation or inhibition of HIF will also lead to the up- or down-modulation of HIF-dependent hypoxamiR, respectively.
Diagnostic/prognostic perspectives
A new frontier has been opened by the recent discovery that miRNAs are present in the bloodstream and in other bodily fluids, in a remarkably stable form (36, 135). Because of their stability and their tissue- and disease-specific expression and the possibility to measure them with high specificity and sensitivity, miRNAs are emerging diagnostic biomarkers. Indeed, several miRNAs, including some hypoxamiR, display the potential to act as biomarkers for a wide range of cardiovascular diseases, including MI, coronary artery disease, hypertension, heart failure, viral myocarditis, and type-2 diabetes mellitus. For instance, several studies indicated an increase of circulating miR-1, miR-133a, miR-133b, and miR-499-5p following acute MI in both humans and mice (1, 3, 34, 50, 74, 140). Moreover, it has also been reported that circulating miR-208 is enhanced in some, but not all patients with acute MI (1, 32, 34, 100, 140).
Concluding Remarks
In 2007, the pioneering work of Mircea Ivan et al. identified the first group of hypoxia-induced miRNAs (73). Thereafter, evidence rapidly accumulated implicating hypoxamiR in many cardiovascular diseases.
Certain hypoxamiR seem to have a particularly pervasive role, such as miR-210. Indeed, miR-210 is modulated in virtually all ischemic diseases tested so far. We described studies implicating miR-210 in MI, stroke, peripheral ischemia, kidney I/R, PH, and wound healing. Moreover, miR-210 is also induced in the tissue around the necrotic area in human osteonecrosis (152) and in human atherosclerotic plaques (116), a finding implicating miR-210 in all thromboembolic diseases. Intriguingly behavioral and nutritional habits may also contribute to the modulation of miR-210. The HUNT-Study identified increased miR-210 levels in the serum of healthy subjects with low aerobic fitness, measured as maximal oxygen uptake, a good indicator of cardiovascular health (15). Moreover, in vitro experiments show that the green tea catechin epigallocatechin gallate can induce miR-210 with a HIF1A-dependent mechanism (141), suggesting that nutritional habits may influence miR-210 levels. However, several other hypoxamiR have been shown to be modulated in cardiovascular diseases, representing both adaptive and maladaptive mechanisms.
In conclusion, the described studies have convincingly demonstrated the robust and complex nature of hypoxamiR in the control of the hypoxic and ischemic responses. Further investigation of their regulation, their targets, and their physiological and/or pathogenic effects in cardiovascular diseases are anticipated in the coming years.
Abbreviations Used
- 3-NPA
nitropropionic acid
- 5-AzaC
5-azacytidine
- AAVs
adeno-associated viruses
- AGO1
argonaute-1
- AGO2
argonaute-2
- AKT
v-akt murine thymoma viral oncogene homolog 1
- AO
atherosclerosis obliterans
- ATP
adenosine triphosphate
- BCNU
1,3-bis(2 chloroethyl)-1-nitrosourea
- BM
bone marrow
- BMPR2
bone morphogenetic protein receptor 2
- CASP8AP2
caspase-8-associated protein-2
- C/EBPα
CCAAT/enhancer binding protein (C/EBP), alpha
- CI
cerebral ischemia
- CM
cardiomyocytes
- CNE
nasopharyngeal carcinoma cells
- CoCs
colon cancer cells
- C-P4H(I)
type I collagen prolyl-4-hydroxylase
- DFOM
deferoxamine mesylate
- DGCR8
DiGeorge syndrome critical region gene 8
- E2F3
E2F transcription factor 3
- EC
endothelial cells
- EFF
effector
- EFNA3
ephrin-A3
- EGCG
epigallocatechin-3-gallate
- EP
endothelial pericytes
- EPC CAD
endothelial precursor cells from CAD patients
- ETS1
v-ets erythroblastosis virus E26 oncogene homolog 1
- FASLG
Fas ligand (TNF superfamily)
- FIH-1
factor inhibiting HIF1
- FIS1
fission 1 (mitochondrial outer membrane) homolog
- FOXO3A
forkhead box O3
- GATA2
GATA-binding protein 2
- GPD1L
glycerol-3-phosphate dehydrogenase 1-like
- HDMECs
human dermal microvascular endothelial cells
- HF
heart failure
- HIF1A/HIF1B
hypoxia-inducible factor 1 alpha/hypoxia-inducible factor 1 beta
- hUCB-MSCs
human umbilical cord blood-derived multipotent stem cells
- hypoxamiR
hypoxia-induced miRNAs
- IP
ischemic preconditioning
- I/R
ischemia–reperfusion
- IWH
ischemic wound healing
- JAG1
JAGged 1
- LNA
locked nucleic acid
- MECP2
methyl-CpG-binding protein 2 (Rett syndrome)
- MI
myocardial infarction
- miRNA
microRNA
- Mm
mus musculus
- MMP
matrix metalloprotease
- MØ
macrophages
- PAK4
p21 protein (Cdc42/Rac)-activated kinase 4
- PDCD4
programmed cell death 4 (neoplastic transformation inhibitor)
- PH
pulmonary hypertension
- PHD
prolyl-4-hydroxylase domain-containing enzymes
- PmSC
preconditioned mesenchymal stem cells
- PTPN1/2
protein tyrosine phosphatase, nonreceptor type 1/protein tyrosine phosphatase, nonreceptor type 2
- PU.1/SPI1
spleen focus forming virus proviral integration oncogene
- RASA1
RAS p21 protein activator (GTPase-activating protein) 1
- RC
renal cells
- RHOB
Ras homolog family member B
- RISC
RNA-induced silencing complex
- RUNX-1
Runt-related transcription factor 1
- SC
stem cells
- SDHD
succinate dehydrogenase complex, subunit D
- SMs
small molecules
- THR
transplanted heart recovery
- UTR
untranslated region
- VEGF
vascular endothelial growth factor
- WH
wound healing
Acknowledgments
F.M. and S.G. are supported by Ministero della Salute and Associazione Italiana per la Ricerca sul Cancro (Grant AIRC IG-11436). Dr. Biagina Maimone, IRCCS Policlinico San Donato, Milan, is acknowledged for her critical reading of the article.
References
- 1.Adachi T, Nakanishi M, Otsuka Y, Nishimura K, Hirokawa G, Goto Y, Nonogi H, and Iwai N. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clin Chem 56: 1183–1185, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Aguado-Fraile E, Ramos E, Saenz-Morales D, Conde E, Blanco-Sanchez I, Stamatakis K, del Peso L, Cuppen E, Brune B, and Bermejo ML. miR-127 protects proximal tubule cells against ischemia/reperfusion: identification of kinesin family member 3B as miR-127 target. PLoS One 7: e44305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ai J, Zhang R, Li Y, Pu J, Lu Y, Jiao J, Li K, Yu B, Li Z, Wang R, Wang L, Li Q, Wang N, Shan H, Li Z, and Yang B. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem Biophys Res Commun 391: 73–77, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Alaiti MA, Ishikawa M, Masuda H, Simon DI, Jain MK, Asahara T, and Costa MA. Up-regulation of miR-210 by vascular endothelial growth factor in ex vivo expanded CD34+ cells enhances cell-mediated angiogenesis. J Cell Mol Med 16: 2413–2421, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anversa P, Kajstura J, Rota M, and Leri A. Regenerating new heart with stem cells. J Clin Invest 123: 62–70, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Bauersachs J. and Thum T. Biogenesis and regulation of cardiovascular microRNAs. Circ Res 109: 334–347, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Bergeron M, Yu AY, Solway KE, Semenza GL, and Sharp FR. Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur J Neurosci 11: 4159–4170, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Bindra RS, Gibson SL, Meng A, Westermark U, Jasin M, Pierce AJ, Bristow RG, Classon MK, and Glazer PM. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res 65: 11597–11604, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Biswas S, Roy S, Banerjee J, Hussain SR, Khanna S, Meenakshisundaram G, Kuppusamy P, Friedman A, and Sen CK. Hypoxia inducible microRNA 210 attenuates keratinocyte proliferation and impairs closure in a murine model of ischemic wounds. Proc Natl Acad Sci USA 107: 6976–6981, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonventre JV. Pathophysiology of AKI: injury and normal and abnormal repair. Contrib Nephrol 165: 9–17, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Bosch-Marce M, Okuyama H, Wesley JB, Sarkar K, Kimura H, Liu YV, Zhang H, Strazza M, Rey S, Savino L, Zhou YF, McDonald KR, Na Y, Vandiver S, Rabi A, Shaked Y, Kerbel R, Lavallee T, and Semenza GL. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res 101: 1310–1318, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Bostjancic E, Zidar N, and Glavac D. MicroRNA microarray expression profiling in human myocardial infarction. Dis Markers 27: 255–268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brabletz S. and Brabletz T. The ZEB/miR-200 feedback loop—a motor of cellular plasticity in development and cancer? EMBO Rep 11: 670–677, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruning U, Cerone L, Neufeld Z, Fitzpatrick SF, Cheong A, Scholz CC, Simpson DA, Leonard MO, Tambuwala MM, Cummins EP, and Taylor CT. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Mol Cell Biol 31: 4087–4096, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bye A, Rosjo H, Aspenes ST, Condorelli G, Omland T, and Wisloff U. Circulating microRNAs and aerobic fitness—the HUNT-Study. PLoS One 8: e57496, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell IL. Cytokine-mediated inflammation, tumorigenesis, and disease-associated JAK/STAT/SOCS signaling circuits in the CNS. Brain Res 48: 166–177, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, and Ragoussis J. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res 14: 1340–1348, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Carthew RW. and Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell 136: 642–655, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, Greig JA, Robertson KE, Masson R, Denby L, Dempsie Y, Long L, Morrell NW, and Baker AH. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol 30: 716–723, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Chacko SM, Khan M, Kuppusamy ML, Pandian RP, Varadharaj S, Selvendiran K, Bratasz A, Rivera BK, and Kuppusamy P. Myocardial oxygenation and functional recovery in infarct rat hearts transplanted with mesenchymal stem cells. Am J Physiol 296: H1263–H1273, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, and Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab 10: 273–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan YC, Khanna S, Roy S, and Sen CK. miR-200b targets Ets-1 and is down-regulated by hypoxia to induce angiogenic response of endothelial cells. J Biol Chem 286: 2047–2056, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan YC, Roy S, Huang Y, Khanna S, and Sen CK. The microRNA miR-199a-5p down-regulation switches on wound angiogenesis by derepressing the v-ets erythroblastosis virus E26 oncogene homolog 1-matrix metalloproteinase-1 pathway. J Biol Chem 287: 41032–41043, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan YC, Roy S, Khanna S, and Sen CK. Downregulation of endothelial microRNA-200b supports cutaneous wound angiogenesis by desilencing GATA binding protein 2 and vascular endothelial growth factor receptor 2. Arterioscler Thromb Vasc Biol 32: 1372–1382, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang W, Lee CY, Park JH, Park MS, Maeng LS, Yoon CS, Lee MY, Hwang KC, and Chung YA. Survival of hypoxic human mesenchymal stem cells is enhanced by a positive feedback loop involving miR-210 and hypoxia-inducible factor 1. J Vet Sci 14: 69–76, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen WY, Liu WJ, Zhao YP, Zhou L, Zhang TP, Chen G, and Shu H. Induction, modulation and potential targets of miR-210 in pancreatic cancer cells. Hepatobiliary Pancreat Dis Int 11: 319–324, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Lai TC, Jan YH, Lin FM, Wang WC, Xiao H, Wang YT, Sun W, Cui X, Li YS, Fang T, Zhao H, Padmanabhan C, Sun R, Wang DL, Jin H, Chau GY, Huang HD, Hsiao M, and Shyy JY. Hypoxia-responsive miRNAs target argonaute 1 to promote angiogenesis. J Clin Invest 123: 1057–1067, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, Li Y, Zhang H, Huang P, and Luthra R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene 29: 4362–4368, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Cheng Y, Zhu P, Yang J, Liu X, Dong S, Wang X, Chun B, Zhuang J, and Zhang C. Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc Res 87: 431–439, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cicchillitti L, Di Stefano V, Isaia E, Crimaldi L, Fasanaro P, Ambrosino V, Antonini A, Capogrossi MC, Gaetano C, Piaggio G, and Martelli F. Hypoxia-inducible factor 1-alpha induces miR-210 in normoxic differentiating myoblasts. J Biol Chem 287: 44761–44771, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cifuentes D, Xue H, Taylor DW, Patnode H, Mishima Y, Cheloufi S, Ma E, Mane S, Hannon GJ, Lawson ND, Wolfe SA, and Giraldez AJ. A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science 328: 1694–1698, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corsten MF, Dennert R, Jochems S, Kuznetsova T, Devaux Y, Hofstra L, Wagner DR, Staessen JA, Heymans S, and Schroen B. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circulation 3: 499–506, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Crosby ME, Kulshreshtha R, Ivan M, and Glazer PM. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res 69: 1221–1229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Alessandra Y, Devanna P, Limana F, Straino S, Di Carlo A, Brambilla PG, Rubino M, Carena MC, Spazzafumo L, De Simone M, Micheli B, Biglioli P, Achilli F, Martelli F, Maggiolini S, Marenzi G, Pompilio G, and Capogrossi MC. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J 31: 2765–2773, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dekanty A, Romero NM, Bertolin AP, Thomas MG, Leishman CC, Perez-Perri JI, Boccaccio GL, and Wappner P. Drosophila genome-wide RNAi screen identifies multiple regulators of HIF-dependent transcription in hypoxia. PLoS Genet 6: e1000994, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Stefano V, Zaccagnini G, Capogrossi MC, and Martelli F. microRNAs as peripheral blood biomarkers of cardiovascular disease. Vasc Pharmacol 55: 111–118, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Dittmer J. The biology of the Ets1 proto-oncogene. Mol Cancer 2: 29, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du R, Sun W, Xia L, Zhao A, Yu Y, Zhao L, Wang H, Huang C, and Sun S. Hypoxia-induced down-regulation of microRNA-34a promotes EMT by targeting the Notch signaling pathway in tubular epithelial cells. PLoS One 7: e30771, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang J, Song XW, Tian J, Chen HY, Li DF, Wang JF, Ren AJ, Yuan WJ, and Lin L. Overexpression of microRNA-378 attenuates ischemia-induced apoptosis by inhibiting caspase-3 expression in cardiac myocytes. Apoptosis 17: 410–423, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, and Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem 283: 15878–15883, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fasanaro P, Greco S, Ivan M, Capogrossi MC, and Martelli F. microRNA: emerging therapeutic targets in acute ischemic diseases. Pharmacol Ther 125: 92–104, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Fasanaro P, Greco S, Lorenzi M, Pescatori M, Brioschi M, Kulshreshtha R, Banfi C, Stubbs A, Calin GA, Ivan M, Capogrossi MC, and Martelli F. An integrated approach for experimental target identification of hypoxia-induced miR-210. J Biol Chem 284: 35134–35143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fasanaro P, Romani S, Voellenkle C, Maimone B, Capogrossi MC, and Martelli F. ROD1 is a seedless target gene of hypoxia-induced miR-210. PLoS One 7: e44651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Favaro E, Ramachandran A, McCormick R, Gee H, Blancher C, Crosby M, Devlin C, Blick C, Buffa F, Li JL, Vojnovic B, Pires das Neves R, Glazer P, Iborra F, Ivan M, Ragoussis J, and Harris AL. MicroRNA-210 regulates mitochondrial free radical response to hypoxia and krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU. PLoS One 5: e10345, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiedler J, Jazbutyte V, Kirchmaier BC, Gupta SK, Lorenzen J, Hartmann D, Galuppo P, Kneitz S, Pena JT, Sohn-Lee C, Loyer X, Soutschek J, Brand T, Tuschl T, Heineke J, Martin U, Schulte-Merker S, Ertl G, Engelhardt S, Bauersachs J, and Thum T. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation 124: 720–730, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Fiedler J. and Thum T. MicroRNAs in myocardial infarction. Arterioscler Thromb Vasc Biol 33: 201–205, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Gao W, Xu J, Liu L, Shen H, Zeng H, and Shu Y. A systematic-analysis of predicted miR-21 targets identifies a signature for lung cancer. Biomed Pharmacother 66: 21–28, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Ghosh G, Subramanian IV, Adhikari N, Zhang X, Joshi HP, Basi D, Chandrashekhar YS, Hall JL, Roy S, Zeng Y, and Ramakrishnan S. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-alpha isoforms and promotes angiogenesis. J Clin Invest 120: 4141–4154, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giannakakis A, Sandaltzopoulos R, Greshock J, Liang S, Huang J, Hasegawa K, Li C, O'Brien-Jenkins A, Katsaros D, Weber BL, Simon C, Coukos G, and Zhang L. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer Biol Ther 7: 255–264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gidlof O, Andersson P, van der Pals J, Gotberg M, and Erlinge D. Cardiospecific microRNA plasma levels correlate with troponin and cardiac function in patients with ST elevation myocardial infarction, are selectively dependent on renal elimination, and can be detected in urine samples. Cardiology 118: 217–226, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Gou D, Ramchandran R, Peng X, Yao L, Kang K, Sarkar J, Wang Z, Zhou G, and Raj JU. miR-210 has an antiapoptotic effect in pulmonary artery smooth muscle cells during hypoxia. Am J Physiol Lung Cell Mol Physiol 303: L682–L691, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greco S, Fasanaro P, Castelvecchio S, D'Alessandra Y, Arcelli D, Di Donato M, Malavazos A, Capogrossi MC, Menicanti L, and Martelli F. MicroRNA dysregulation in diabetic ischemic heart failure patients. Diabetes 61: 1633–1641, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grieger JC. and Samulski RJ. Adeno-associated virus vectorology, manufacturing, and clinical applications. Methods Enzymol 507: 229–254, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Grosso S, Doyen J, Parks SK, Bertero T, Paye A, Cardinaud B, Gounon P, Lacas-Gervais S, Noel A, Pouyssegur J, Barbry P, Mazure NM, and Mari B. MiR-210 promotes a hypoxic phenotype and increases radioresistance in human lung cancer cell lines. Cell Death Dis 4: e544, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, and Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell 9: 617–628, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Hata A. Functions of microRNAs in cardiovascular biology and disease. Annu Rev Physiol 75: 69–93, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He J, Wu J, Xu N, Xie W, Li M, Li J, Jiang Y, Yang BB, and Zhang Y. MiR-210 disturbs mitotic progression through regulating a group of mitosis-related genes. Nucleic Acids Res 41: 498–508, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ho JJ, Metcalf JL, Yan MS, Turgeon PJ, Wang JJ, Chalsev M, Petruzziello-Pellegrini TN, Tsui AK, He JZ, Dhamko H, Man HS, Robb GB, Teh BT, Ohh M, and Marsden PA. Functional importance of Dicer protein in the adaptive cellular response to hypoxia. J Biol Chem 287: 29003–29020, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu S, Huang M, Li Z, Jia F, Ghosh Z, Lijkwan MA, Fasanaro P, Sun N, Wang X, Martelli F, Robbins RC, and Wu JC. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation 122: S124–S131, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang F, Tang L, Fang ZF, Hu XQ, Pan JY, and Zhou SH. miR-1-mediated induction of cardiogenesis in mesenchymal stem cells via downregulation of Hes-1. Biomed Res Int 2013: 216286, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, Story M, Le QT, and Giaccia AJ. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell 35: 856–867, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, and Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, and Hodges MR. Treatment of HCV infection by targeting microRNA. N Engl J Med 368: 1685–1694, 2013 [DOI] [PubMed] [Google Scholar]
- 64.Jiang X, Tsitsiou E, Herrick SE, and Lindsay MA. MicroRNAs and the regulation of fibrosis. FEBS J 277: 2015–2021, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kane NM, Meloni M, Spencer HL, Craig MA, Strehl R, Milligan G, Houslay MD, Mountford JC, Emanueli C, and Baker AH. Derivation of endothelial cells from human embryonic stem cells by directed differentiation: analysis of microRNA and angiogenesis in vitro and in vivo. Arterioscler ThrombVasc Biol 30: 1389–1397, 2013 [DOI] [PubMed] [Google Scholar]
- 66.Kang SG, Lee WH, Lee YH, Lee YS, and Kim SG. Hypoxia-inducible factor-1alpha inhibition by a pyrrolopyrazine metabolite of oltipraz as a consequence of microRNAs 199a-5p and 20a induction. Carcinogenesis 33: 661–669, 2012 [DOI] [PubMed] [Google Scholar]
- 67.Katare R, Riu F, Mitchell K, Gubernator M, Campagnolo P, Cui Y, Fortunato O, Avolio E, Cesselli D, Beltrami AP, Angelini G, Emanueli C, and Madeddu P. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res 109: 894–906, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kelly TJ, Souza AL, Clish CB, and Puigserver P. A hypoxia-induced positive feedback loop promotes hypoxia-inducible factor 1alpha stability through miR-210 suppression of glycerol-3-phosphate dehydrogenase 1-like. Mol Cell Biol 31: 2696–2706, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim HW, Haider HK, Jiang S, and Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem 284: 33161–33168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim HW, Jiang S, Ashraf M, and Haider KH. Stem cell-based delivery of Hypoxamir-210 to the infarcted heart: implications on stem cell survival and preservation of infarcted heart function. J Mol Med (Berl) 90: 997–1010, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim JH, Park SG, Song SY, Kim JK, and Sung JH. Reactive oxygen species-responsive miR-210 regulates proliferation and migration of adipose-derived stem cells via PTPN2. Cell Death Dis 4: e588, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim VN, Han J, and Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10: 126–139, 2009 [DOI] [PubMed] [Google Scholar]
- 73.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, and Ivan M. A microRNA signature of hypoxia. Mol Cell Biol 27: 1859–1867, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, Watanabe S, Baba O, Kojima Y, Shizuta S, Imai M, Tamura T, Kita T, and Kimura T. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circulation 4: 446–454, 2011 [DOI] [PubMed] [Google Scholar]
- 75.Latronico MV. and Condorelli G. microRNAs in hypertrophy and heart failure. Exp Biol Med (Maywood) 236: 125–131, 2011 [DOI] [PubMed] [Google Scholar]
- 76.Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, and Thistlethwaite PA. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med 342: 626–633, 2000 [DOI] [PubMed] [Google Scholar]
- 77.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, and Kim VN. MicroRNA genes are transcribed by RNA polymerase II. Embo J 23: 4051–4060, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li T, Cao H, Zhuang J, Wan J, Guan M, Yu B, Li X, and Zhang W. Identification of miR-130a, miR-27b and miR-210 as serum biomarkers for atherosclerosis obliterans. Clin Chim Acta 412: 66–70, 2011 [DOI] [PubMed] [Google Scholar]
- 79.Liang WC, Wang Y, Wan DC, Yeung VS, and Waye MM. Characterization of miR-210 in 3T3-L1 adipogenesis. J Cell Biochem 114: 2699–2707, 2013 [DOI] [PubMed] [Google Scholar]
- 80.Liu F, Lou YL, Wu J, Ruan QF, Xie A, Guo F, Cui SP, Deng ZF, and Wang Y. Upregulation of microRNA-210 regulates renal angiogenesis mediated by activation of VEGF signaling pathway under ischemia/perfusion injury in vivo and in vitro. Kidney Blood Press Res 35: 182–191, 2012 [DOI] [PubMed] [Google Scholar]
- 81.Liu N. and Olson EN. MicroRNA regulatory networks in cardiovascular development. Dev Cell 18: 510–525, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu W, Wen Y, Bi P, Lai X, Liu XS, Liu X, and Kuang S. Hypoxia promotes satellite cell self-renewal and enhances the efficiency of myoblast transplantation. Development 139: 2857–2865, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Logue SE, Gustafsson AB, Samali A, and Gottlieb RA. Ischemia/reperfusion injury at the intersection with cell death. J Mol Cell Cardiol 38: 21–33, 2005 [DOI] [PubMed] [Google Scholar]
- 84.Loor G. and Schumacker PT. Role of hypoxia-inducible factor in cell survival during myocardial ischemia-reperfusion. Cell Death Differ 15: 686–690, 2008 [DOI] [PubMed] [Google Scholar]
- 85.Lorenzen JM, Kielstein JT, Hafer C, Gupta SK, Kumpers P, Faulhaber-Walter R, Haller H, Fliser D, and Thum T. Circulating miR-210 predicts survival in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol 6: 1540–1546, 2011 [DOI] [PubMed] [Google Scholar]
- 86.Loscalzo J. Harrison's Cardiovascular Medicine. New York: McGraw-Hill Medical, 2010 [Google Scholar]
- 87.Lou Y, Gao F, Xie A, Guo F, Deng Z, and Wang Y. [MicroRNA-210 modified human umbilical vein endothelial cells induce capillary formation]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 26: 587–591, 2012 [PubMed] [Google Scholar]
- 88.Lou YL, Guo F, Liu F, Gao FL, Zhang PQ, Niu X, Guo SC, Yin JH, Wang Y, and Deng ZF. miR-210 activates notch signaling pathway in angiogenesis induced by cerebral ischemia. Mol Cell Biochem 370: 45–51, 2012 [DOI] [PubMed] [Google Scholar]
- 89.Lu TX, Munitz A, and Rothenberg ME. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J Immunol 182: 4994–5002, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Madhyastha R, Madhyastha H, Nakajima Y, Omura S, and Maruyama M. MicroRNA signature in diabetic wound healing: promotive role of miR-21 in fibroblast migration. Int Wound J 9: 355–361, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Magenta A, Cencioni C, Fasanaro P, Zaccagnini G, Greco S, Sarra-Ferraris G, Antonini A, Martelli F, and Capogrossi MC. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ 18: 1628–1639, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mahon PC, Hirota K, and Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev 15: 2675–2686, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Majmundar AJ, Skuli N, Mesquita RC, Kim MN, Yodh AG, Nguyen-McCarty M, and Simon MC. O(2) regulates skeletal muscle progenitor differentiation through phosphatidylinositol 3-kinase/AKT signaling. Mol Cell Biol 32: 36–49, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Majmundar AJ, Wong WJ, and Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 40: 294–309, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, and Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399: 271–275, 1999 [DOI] [PubMed] [Google Scholar]
- 96.Meng S, Cao J, Wang L, Zhou Q, Li Y, Shen C, Zhang X, and Wang C. MicroRNA 107 partly inhibits endothelial progenitor cells differentiation via HIF-1beta. PLoS One 7: e40323, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mizuno Y, Tokuzawa Y, Ninomiya Y, Yagi K, Yatsuka-Kanesaki Y, Suda T, Fukuda T, Katagiri T, Kondoh Y, Amemiya T, Tashiro H, and Okazaki Y. miR-210 promotes osteoblastic differentiation through inhibition of AcvR1b. FEBS Lett 583: 2263–2268, 2009 [DOI] [PubMed] [Google Scholar]
- 98.Muralimanoharan S, Maloyan A, Mele J, Guo C, Myatt LG, and Myatt L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta 33: 816–823, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mutharasan RK, Nagpal V, Ichikawa Y, and Ardehali H. microRNA-210 is upregulated in hypoxic cardiomyocytes through Akt- and p53-dependent pathways and exerts cytoprotective effects. Am J Physiol 301: H1519–H1530, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Olivieri F, Antonicelli R, Lorenzi M, D'Alessandra Y, Lazzarini R, Santini G, Spazzafumo L, Lisa R, La Sala L, Galeazzi R, Recchioni R, Testa R, Pompilio G, Capogrossi MC, and Procopio AD. Diagnostic potential of circulating miR-499-5p in elderly patients with acute non ST-elevation myocardial infarction. Int J Cardiol 167: 531–536, 2013 [DOI] [PubMed] [Google Scholar]
- 101.Ong SG. and Hausenloy DJ. Hypoxia-inducible factor as a therapeutic target for cardioprotection. Pharmacol Ther 136: 69–81, 2012 [DOI] [PubMed] [Google Scholar]
- 102.Ota H, Eto M, Kano MR, Kahyo T, Setou M, Ogawa S, Iijima K, Akishita M, and Ouchi Y. Induction of endothelial nitric oxide synthase, SIRT1, and catalase by statins inhibits endothelial senescence through the Akt pathway. Arterioscler Thromb Vasc Biol 30: 2205–2211, 2010 [DOI] [PubMed] [Google Scholar]
- 103.Parikh VN, Jin RC, Rabello S, Gulbahce N, White K, Hale A, Cottrill KA, Shaik RS, Waxman AB, Zhang YY, Maron BA, Hartner JC, Fujiwara Y, Orkin SH, Haley KJ, Barabasi AL, Loscalzo J, and Chan SY. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation 125: 1520–1532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pastar I, Khan AA, Stojadinovic O, Lebrun EA, Medina MC, Brem H, Kirsner RS, Jimenez JJ, Leslie C, and Tomic-Canic M. Induction of specific microRNAs inhibits cutaneous wound healing. J Biol Chem 287: 29324–29335, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peach G, Griffin M, Jones KG, Thompson MM, and Hinchliffe RJ. Diagnosis and management of peripheral arterial disease. BMJ 345: e5208, 2012 [DOI] [PubMed] [Google Scholar]
- 106.Poller W, Hajjar R, Schultheiss HP, and Fechner H. Cardiac-targeted delivery of regulatory RNA molecules and genes for the treatment of heart failure. Cardiovasc Res 86: 353–364, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Potente M. and Dimmeler S. Emerging roles of SIRT1 in vascular endothelial homeostasis. Cell Cycle 7: 2117–2122, 2008 [DOI] [PubMed] [Google Scholar]
- 108.Prabhakar NR. and Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev 92: 967–1003, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Puissegur MP, Mazure NM, Bertero T, Pradelli L, Grosso S, Robbe-Sermesant K, Maurin T, Lebrigand K, Cardinaud B, Hofman V, Fourre S, Magnone V, Ricci JE, Pouyssegur J, Gounon P, Hofman P, Barbry P, and Mari B. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ 18: 465–478, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pulkkinen K, Malm T, Turunen M, Koistinaho J, and Yla-Herttuala S. Hypoxia induces microRNA miR-210 in vitro and in vivo ephrin-A3 and neuronal pentraxin 1 are potentially regulated by miR-210. FEBS Lett 582: 2397–2401, 2008 [DOI] [PubMed] [Google Scholar]
- 111.Qi HH, Ongusaha PP, Myllyharju J, Cheng D, Pakkanen O, Shi Y, Lee SW, Peng J, and Shi Y. Prolyl 4-hydroxylation regulates Argonaute 2 stability. Nature 455: 421–424, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qiao A, Khechaduri A, Kannan Mutharasan R, Wu R, Nagpal V, and Ardehali H. MicroRNA-210 decreases heme levels by targeting ferrochelatase in cardiomyocytes. J Am Heart Assoc 2: e000121, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qin L, Chen Y, Niu Y, Chen W, Wang Q, Xiao S, Li A, Xie Y, Li J, Zhao X, He Z, and Mo D. A deep investigation into the adipogenesis mechanism: profile of microRNAs regulating adipogenesis by modulating the canonical Wnt/beta-catenin signaling pathway. BMC Genomics 11: 320, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Quiat D. and Olson EN. MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment. J Clin Invest 123: 11–18, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 122: 4306–4313, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Raitoharju E, Lyytikainen LP, Levula M, Oksala N, Mennander A, Tarkka M, Klopp N, Illig T, Kahonen M, Karhunen PJ, Laaksonen R, and Lehtimaki T. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis 219: 211–217, 2011 [DOI] [PubMed] [Google Scholar]
- 117.Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, Vatner DE, Vatner SF, and Abdellatif M. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res 104: 879–886, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ, and Sen CK. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res 82: 21–29, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roy S. and Sen CK. miRNA in wound inflammation and angiogenesis. Microcirculation 19: 224–232, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salloum FN, Yin C, and Kukreja RC. Role of microRNAs in cardiac preconditioning. J Cardiovasc Pharmacol 56: 581–588, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sarkar J, Gou D, Turaka P, Viktorova E, Ramchandran R, and Raj JU. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am J Physiol Lung Cell Mol Physiol 299: L861–L871, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sayed D, He M, Hong C, Gao S, Rane S, Yang Z, and Abdellatif M. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J Biol Chem 285: 20281–20290, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, and Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 7: 77–85, 2005 [DOI] [PubMed] [Google Scholar]
- 124.Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med 2: 336–361, 2010 [DOI] [PubMed] [Google Scholar]
- 125.Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med 365: 537–547, 2011 [DOI] [PubMed] [Google Scholar]
- 126.Sen CK. and Roy S. OxymiRs in cutaneous development, wound repair and regeneration. Semin Cell Dev Biol 23: 971–980, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sheedy FJ. and O'Neill LA. Adding fuel to fire: microRNAs as a new class of mediators of inflammation. Ann Rheum Dis 67(Suppl 3): iii50–iii55, 2008 [DOI] [PubMed] [Google Scholar]
- 128.Small EM, Frost RJ, and Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation 121: 1022–1032, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Small EM. and Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature 469: 336–342, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.So AY, Jung JW, Lee S, Kim HS, and Kang KS. DNA methyltransferase controls stem cell aging by regulating BMI1 and EZH2 through microRNAs. PLoS One 6: e19503, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tabuchi T, Satoh M, Itoh T, and Nakamura M. MicroRNA-34a regulates the longevity-associated protein SIRT1 in coronary artery disease: effect of statins on SIRT1 and microRNA-34a expression. Clin Sci (Lond) 123: 161–171, 2012 [DOI] [PubMed] [Google Scholar]
- 132.Taguchi A, Yanagisawa K, Tanaka M, Cao K, Matsuyama Y, Goto H, and Takahashi T. Identification of hypoxia-inducible factor-1 alpha as a novel target for miR-17–92 microRNA cluster. Cancer Res 68: 5540–5545, 2008 [DOI] [PubMed] [Google Scholar]
- 133.Terrell AM, Crisostomo PR, Wairiuko GM, Wang M, Morrell ED, and Meldrum DR. Jak/STAT/SOCS signaling circuits and associated cytokine-mediated inflammation and hypertrophy in the heart. Shock 26: 226–234, 2006 [DOI] [PubMed] [Google Scholar]
- 134.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, and Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456: 980–984, 2008 [DOI] [PubMed] [Google Scholar]
- 135.Tijsen AJ, Pinto YM, and Creemers EE. Circulating microRNAs as diagnostic biomarkers for cardiovascular diseases. Am J Physiol 303: H1085–H1095, 2012 [DOI] [PubMed] [Google Scholar]
- 136.Tsuchiya S, Fujiwara T, Sato F, Shimada Y, Tanaka E, Sakai Y, Shimizu K, and Tsujimoto G. MicroRNA-210 regulates cancer cell proliferation through targeting fibroblast growth factor receptor-like 1 (FGFRL1). J Biol Chem 286: 420–428, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Rooij E. and Olson EN. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov 11: 860–872, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Voellenkle C, Rooij J, Guffanti A, Brini E, Fasanaro P, Isaia E, Croft L, David M, Capogrossi MC, Moles A, Felsani A, and Martelli F. Deep-sequencing of endothelial cells exposed to hypoxia reveals the complexity of known and novel microRNAs. RNA 18: 472–484, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang F, Xiong L, Huang X, Zhao T, Wu LY, Liu ZH, Ding X, Liu S, Wu Y, Zhao Y, Wu K, Zhu LL, and Fan M. miR-210 suppresses BNIP3 to protect against the apoptosis of neural progenitor cells. Stem Cell Res 11: 657–667, 2013 [DOI] [PubMed] [Google Scholar]
- 140.Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, Qin YW, and Jing Q. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J 31: 659–666, 2010 [DOI] [PubMed] [Google Scholar]
- 141.Wang H, Bian S, and Yang CS. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1alpha. Carcinogenesis 32: 1881–1889, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang K, Long B, Jiao JQ, Wang JX, Liu JP, Li Q, and Li PF. miR-484 regulates mitochondrial network through targeting Fis1. Nat Commun 3: 781, 2012 [DOI] [PubMed] [Google Scholar]
- 143.Wang T, Zhang L, Shi C, Sun H, Wang J, Li R, Zou Z, Ran X, and Su Y. TGF-beta-induced miR-21 negatively regulates the antiproliferative activity but has no effect on EMT of TGF-beta in HaCaT cells. Int J Biochem Cell Biol 44: 366–376, 2012 [DOI] [PubMed] [Google Scholar]
- 144.Wiener CM, Booth G, and Semenza GL. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun 225: 485–488, 1996 [DOI] [PubMed] [Google Scholar]
- 145.Wu C, So J, Davis-Dusenbery BN, Qi HH, Bloch DB, Shi Y, Lagna G, and Hata A. Hypoxia potentiates microRNA-mediated gene silencing through posttranslational modification of Argonaute2. Mol Cell Biol 31: 4760–4774, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xia Y, Choi HK, and Lee K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur J Med Chem 49: 24–40, 2012 [DOI] [PubMed] [Google Scholar]
- 147.Xiao F, Qiu H, Zhou L, Shen X, Yang L, and Ding K. WSS25 inhibits Dicer, downregulating microRNA-210, which targets Ephrin-A3, to suppress human microvascular endothelial cell (HMEC-1) tube formation. Glycobiology 23: 524–535, 2013 [DOI] [PubMed] [Google Scholar]
- 148.Xu Q, Seeger FH, Castillo J, Iekushi K, Boon RA, Farcas R, Manavski Y, Li YG, Assmus B, Zeiher AM, and Dimmeler S. Micro-RNA-34a contributes to the impaired function of bone marrow-derived mononuclear cells from patients with cardiovascular disease. J Am Coll Cardiol 59: 2107–2117, 2012 [DOI] [PubMed] [Google Scholar]
- 149.Xu WH, Yao XY, Yu HJ, Huang JW, and Cui LY. Downregulation of miR-199a may play a role in 3-nitropropionic acid induced ischemic tolerance in rat brain. Brain Res 1429: 116–123, 2012 [DOI] [PubMed] [Google Scholar]
- 150.Xu X, Kriegel AJ, Liu Y, Usa K, Mladinov D, Liu H, Fang Y, Ding X, and Liang M. Delayed ischemic preconditioning contributes to renal protection by upregulation of miR-21. Kidney Int 82: 1167–1175, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yamakuchi M, Lotterman CD, Bao C, Hruban RH, Karim B, Mendell JT, Huso D, and Lowenstein CJ. P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc Natl Acad Sci USA 107: 6334–6339, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yamasaki K, Nakasa T, Miyaki S, Yamasaki T, Yasunaga Y, and Ochi M. Angiogenic microRNA-210 is present in cells surrounding osteonecrosis. J Orthop Res 30: 1263–1270, 2012 [DOI] [PubMed] [Google Scholar]
- 153.Yang S, Banerjee S, Freitas A, Cui H, Xie N, Abraham E, and Liu G. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 302: L521–L529, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang W, Sun T, Cao J, Liu F, Tian Y, and Zhu W. Downregulation of miR-210 expression inhibits proliferation, induces apoptosis and enhances radiosensitivity in hypoxic human hepatoma cells in vitro. Exp Cell Res 318: 944–954, 2012 [DOI] [PubMed] [Google Scholar]
- 155.Yang X, Wang J, Guo SL, Fan KJ, Li J, Wang YL, Teng Y, and Yang X. miR-21 promotes keratinocyte migration and re-epithelialization during wound healing. Int J Biol Sci 7: 685–690, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yeo JH. and Chong MM. Many routes to a micro RNA. IUBMB Life 63: 972–978, 2011 [DOI] [PubMed] [Google Scholar]
- 157.Ying Q, Liang L, Guo W, Zha R, Tian Q, Huang S, Yao J, Ding J, Bao M, Ge C, Yao M, Li J, and He X. Hypoxia-inducible microRNA-210 augments the metastatic potential of tumor cells by targeting vacuole membrane protein 1 in hepatocellular carcinoma. Hepatology 54: 2064–2075, 2011 [DOI] [PubMed] [Google Scholar]
- 158.Zaccagnini G, Maimone B, Di Stefano V, Fasanaro P, Greco S, Perfetti A, Capogrossi MC, Gaetano C, and Martelli F. Hypoxia-induced miR-210 modulates tissue response to acute peripheral ischemia. Antioxid Redox Signal 21: 1177–1188, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zeng L, Liu J, Wang Y, Wang L, Weng S, Tang Y, Zheng C, Cheng Q, Chen S, and Yang GY. MicroRNA-210 as a novel blood biomarker in acute cerebral ischemia. Front Biosci (Elite Ed) 3: 1265–1272, 2011 [DOI] [PubMed] [Google Scholar]
- 160.Zhang Y, Zhang L, Chu W, Wang B, Zhang J, Zhao M, Li X, Li B, Lu Y, Yang B, and Shan H. Tanshinone IIA inhibits miR-1 expression through p38 MAPK signal pathway in post-infarction rat cardiomyocytes. Cell Physiol Biochem 26: 991–998, 2010 [DOI] [PubMed] [Google Scholar]
- 161.Zhang Z, Sun H, Dai H, Walsh RM, Imakura M, Schelter J, Burchard J, Dai X, Chang AN, Diaz RL, Marszalek JR, Bartz SR, Carleton M, Cleary MA, Linsley PS, and Grandori C. MicroRNA miR-210 modulates cellular response to hypoxia through the MYC antagonist MNT. Cell Cycle 8: 2756–2768, 2009 [DOI] [PubMed] [Google Scholar]
- 162.Zheng G, Tao Y, Yu W, and Schwartz RJ. SRF dependent Mir-210 silencing of the sonic hedgehog signaling pathway during cardiopoesis. Stem Cells 2013. DOI: 10.1002/stem.1464 [DOI] [PubMed] [Google Scholar]